Abstract
Background
Malaria continues to be among the leading causes of mortality in Africa including Uganda, with the emergence of parasite resistance to the first-line therapeutics (Artemisinin- based Combination Therapy). To find new therapeutics, this study has reported an in vivo antimalarial efficacy of combinations of Artemisia annua (Aa), Vernonia amygdalina (Va), and Microglossa pyrifolia (Mp) in mice model using factorial design.
Methods
The Aa and Va were extracted by hot infusion, and Mp by cold maceration using distilled water. The dry extracts were screened for different phytochemicals, and later subjected to in vivo antimalarial activity using Peter’s 4-day suppressive test. The 23 factorial design used Aa, Va, and Mp aqueous extracts as independent variables at two levels (-1 and 1), and the percentage chemo suppression and survival time as response variables. The data was analyzed using Design Expert 13 and GraphPad Prism employing ANOVA linear regression modelling and t-test respectively.
Results
All the extracts had alkaloids, phenols, saponins, terpenoids, cardiac glycosides, tannins, steroids, and carbohydrates. The various combinations showed chemo suppression from 41.5 to 91.0% and survival time of 19 to 23 days. The first three combinations having lower levels of Aa (200 mg/kg) exhibited higher chemo suppression (> 90%) compared to Artemisinin-Lumefantrine positive control at 4 mg/kg with 87.5%. Lower levels of Aa in the combinations contributed to high chemo suppression while higher levels of Va prolonged survival times. Interactions between Aa and Mp showed higher chemo suppression, and that between Aa and Va increased survival time. An optimized prediction of 94.4% chemo suppression was made by the ANOVA model at lower levels of Aa and Va, and a higher level of Mp, which is similar to an experimental run which gave a response of 90. 6%.
Conclusion
An optimum combination of the three plants as a natural herbal antimalarial therapy was obtained using factorial design, and it offers an alternative to first line Artemisinin based Combination Therapy (ACTs) as parasite resistance looms. This combination could be further developed into a standard phytopharmaceutical and subjected to Randomized Controlled Trials (RCTs).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-024-04691-z.
Keywords: Artemisia annua, Vernonia amygdalina, Microglossa pyrifolia, Antimalarial, Herbal, Factorial Design
Introduction
Malaria is an infectious disease caused in humans by a number of plasmodium parasites of various species with Plasmodium falciparum being the most predominant in Sub-Saharan Africa. Despite decades of global efforts articulated in Global Technical Strategy (GTS) to end this endemic by 2030, the disease continues to claim lives most especially in developing countries in Sub-Saharan Africa. According to the current reports of WHO [1], 3.2 billion people close to half of the world’s population are at risk, 247 million cases were registered in 2021 with 619,000 mortality. Sub-Saharan Africa accounted for 95% of these cases and 96% of the death tolls. Uganda is among the African countries with high malaria burden ranking 3rd globally with 12 million annual morbidity [2]. The infection accounts for 32.1% of all Outpatient Department (OPD) attendances in the country with the prevalence of the disease increasing by 5% from 13.6 million cases in 2020/2021 to 14.9 million cases in 2021/2022 [3, 4]. The north and north eastern regions of Uganda are the highest malaria endemic areas due to stagnant water and cessation of indoor residual spraying (IRS) notable in Kole District [5].
The increase in malaria incidence has been exacerbated by climatic changes as demonstrated by a powerful cyclone in Mozambique and as simulated using artificial neural networks (ANNs) [6]. The projected increase in malaria infection may be worsened by the emerging resistance to the first -line drugs for malaria treatment as reported in Uganda and neighboring Rwanda [7–9]. This is likely to hamper realization of the Sustainable Development Goal (SDG) goal of completely eradicating malaria by 2023 if no robust and clinically effective alternative interventions are developed. Several interventions including artemisinin-based therapies and seasonal malaria chemoprevention (SMC) approaches using sulfadoxine– pyrimethamine (SP) and amodiaquine (AQ) have also shown appreciable success [10]. The approval of the RTS, S/ AS01 malaria vaccine for malaria prevention by WHO is one of the latest interventions but its efficacy stands at 30% in protecting people from severe malaria and reduces hospitalization rates due to malaria by only 21% [11]. The effectiveness of the vaccine also wanes off after 6 months which necessitates continuous dosing, thus making it expensive for most developing African countries.
Based on the low effectiveness of the new vaccine and the increasing resistance to ACTs in Africa, the fight to end malaria epidemic by 2030 is far-fetched. For example, a recent Ugandan study reported partial resistance of over 20% in 11 of 16 districts surveyed [12]. The different resistance markers showed varying patterns across the country—the PfK13 469Y and 675 V mutations reached a prevalence of 54% in Northern Uganda, both 561H and 441L mutations had 23% prevalence in southwestern and western regions respectively by 2022. A previous study in northern Uganda had also profiled 19.8% resistance to artesunate injections due to A675V or C469Y allele in the kelch13 gene [13]. Compared to the study by Conrad and co-workers [12], it is evident that the magnitude of parasite resistance to ACTs is increasing. Similar cases of resistance to ACTs have also been reported in Eritrea [14], Niger [15], Pakistan [16], and other parts of Africa.
Therefore, the available interventions are not enough to end the malaria endemic by 2030 as projected based on the above analysis. Thus, there is the need for more robust and practically implementable innovations. The need for alternative effective antimalarial therapeutics in Africa in the near future as evidences of ACT resistance increase is a discussion shaping the efforts in new antimalarial drug development. Herbal medicines with a long history of use with no known parasite resistance seem to be the future for new antimalarials.
In an effort to prioritize medicinal plants for antimalarial product development, we previously reviewed efficacy and safety of Ugandan antimalarial plants using RITAM (The Research Initiative on Traditional and Antimalarial Methods) score [17]. Among the forty one (41) plants with data on antimalarial activity, Artemisia annua (Aa), and Vernonia amygdalina (Va) were the most extensively studied up to the level of clinical trial. The aqueous leaf extract of Aa has a potent antiplasmodial activity with IC50 of 0.88–1.11 µg/ml [18], and a sesquiterpeine lactone artemisinin is known as the main antimalarial compound in the plant. Other active compounds such as arteannuic acid, scopoletin, arteannuin B, and flavonoids present in the plant have also been reported to work synergistically with artemisinin in enhancing its antimalarial effect [19]. Clinical studies also revealed 70 – 77% efficacy of Aa infusion in curing malaria [20], but the effectiveness was affected by parasite recrudescence after 7 days. Vernonia amygdalina also has promising in vitro antiplasmodial activity with IC50 of 11.2–13.6 µg/ml [21], and an in vivo chemo suppression of 75.15% in mice [22]. Over 67% adequate clinical response with marked symptomatic improvement was also reported in a randomized clinical trials on the plant for malaria treatment [23, 24]. Unfortunately, the clinical outcomes of the plant was also affected by parasite recrudescence just like the Artemisia annua trials. In an attempt to enhance the antimalarial activity of the Va, its combination with Aa showed 100% clearance of the parasite in mice by the fifth day, but the animals had shorter survival times of 10 days [25]. Therefore there was still a need for another plant to be included in the combination to eliminate the impeding challenge of parasite recrudescence or relapse. Microglossa pyrifolia leaf aqueous extract, with an IC50 of 0.05 µg/ml [26] and a potential to penetrate liver cells [27, 28] was identified as a potential candidate to ameliorate this unsatisfactory antimalarial efficacy posed by recrudescence or relapse if carefully added to a combination of Aa and Va.
For robust evaluation of combined efficacy of these plants against malaria parasite, multivariate experimental design approach was deemed more appropriate compared to univariate methods that have dominated most pharmacological efficacy studies over the years. Factorial design is one of Design of Experiment (DoE) techniques with the capacity to measure and understand relationship between cause and outcomes (independent and dependent factors). To understand the possible interaction among the three plants in suppressing malaria parasite proliferation and also determining the optimum antimalarial combination, 23 factorial design was considered the most appropriate. This current work therefore reports the in vivo antimalarial effect of various combinations of the three plant extracts on suppression of malaria parasite in a mice model, and their impact on survival time of infected animals using factorial design.
Materials and methods
Collection and processing of plant material
The fresh leaves of the plants Artemisia annua L, Microglossa pyrifolia (Lam.) Kuntze, and Vernonia amygdalina Del. (Asteraceae) were collected within Uganda from Fort portal—0.6668◦ N, 30.2854◦ E; Bushenyi—0.524◦ N, 30.2173◦ E; and Mbarara—0.6152◦ S, 30.6522◦ E respectively. The plant materials were authenticated at the National Herbarium of Makerere University, Department of Botany. Accession numbers 51145, 51,146, and 51,147 were issued for M. pyrifolia (Mp), V. amygdalina (Va), and A. annua (Aa) respectively. The leaves were sorted, cleaned, shade-dried for two weeks, and then milled to a coarse powder and stored in amber bottles till extraction. The leaf powders of the different plants were extracted as follows: Aa and Va were infused in hot water as described previously [13, 14], while the Mp was cold macerated in distilled water according to a previous work [26]. The mixture were filtered using a muslin cloth and thereafter using Whatman No. 1 filter papers. The filtrates were concentrated in vacuo using a rotary evaporator (IKA, Germany) at 50 °C and finally freeze-dried (FD-1CL, USA). The percentage yield of the extracts obtained was calculated as:
| 1 |
Phytochemical screening
This was conducted to detect the presence of alkaloids, flavonoids, phenols, saponins, terpenoids, cardiac glycosides, tannins, steroids, carbohydrates and anthraquinones for all the extracts (5% in distilled water) according to the following procedures previously described [29, 30]:
Alkaloids (Dragendorff’s Test): The extract (2 mL) was mixed in equal volumes with 1% HCl in a test tube and heated gently followed by filtering. To the filtrate, 3 drops of dragendorff’s solution was added by the side of the test tube. A persistence of yellow colour was indicative of a positive test.
Flavonoids: To the extract (1 mL) in a test tube, a dilute ammonia (5 mL) was added followed by concentrated sulphuric acid (2 mL), and the mixture heated for 2 min. Appearance of a yellow showed presence of flavonoids.
Phenols: To the extract (1 mL) in a test tube, 2 drops of 2% w/v FeCl3 were added. A black coloration was indicative of the presence of phenols.
Saponins (Frothing Test): Distilled water (5 mL) was added to the extract (1 mL) in a test tube and the solution shaken vigorously to observe for stable persistent froth within 15 min.
Terpenoids (Salkowski Test): Ethanol (2 mL, 99%) was added to the extract (1 mL). Acetic anhydride (2 mL) was also added, followed by conc. H2SO4. A change in colour from pink to violet shows the presence of terpenoids.
Cardiac glycosides: The extract (5 mL) was treated with 2 mL of glacial acetic acid and one drop of ferric chloride solution. Then, 1 mL conc. H2SO4 was added. An appearance of a brown ring at the interface due to formation of a de-oxysugar is characteristic of cardenolides.
Tannins: To the extract (2 mL), 3 drops of 1% ferric chloride solution were added and occurrence of a blue-black, green, or blue-green precipitate was indicative of the presence of tannins.
Steroids: To the extract (2 mL), an equal amount of chloroform was added followed by conc. sulphuric acid and the mixture was shaken. An appearance of a red chloroform layer and a greenish-yellow fluorescence confirms presence of steroids.
Carbohydrates: To the extract, 1 mL of Molisch’s reagent was added, then along the walls of the test tube conc. H2SO4 was carefully added. Formation of a brown ring at the junction of two liquids indicates presence of carbohydrates.
Anthraquinones: To the extract (1 mL), dilute HCl (5 mL) was added and boiled in a water bath for 10 min and filtered. Then the filtrate was extracted with carbon tetrachloride and an equal amount of ammonia. After shaking, the reaction mixture was observed for the formation of pink– red colour in the ammonia layer.
Experimental animals
Sixty (60) swiss albino mice of both sexes in equal numbers (weighing 18–20 g), were obtained from the Animal Research Facility of Mbarara University of Science and Technology, Mbarara. The animals were housed under a 12 h light/dark cycle with access to water ad libitum and were fed with standard pellets. They were acclimatized for two weeks prior to commencement of the experiment. All the animals were cared for as per National Institute for Health (NIH) guidelines for care and use of laboratory animals in teaching and research [31].
Preparation of malarial parasite inoculum
The chloroquine – sensitive Plasmodium berghei, ANKA strain of malaria parasite was obtained from BEI Resources (USA). The vial containing 0.5 mL of the parasite in dried ice was activated by thawing frozen cryovial in a water bath at 35 °C for 2 min. The outside surface of the vial was wiped with 70% ethanol before opening for injection intraperitoneally into the mouse for continuous passage. The growth of parasites in mice was monitored by tail vein blood sampling and Giemsa-stained thin blood smear microscopy daily, starting from day 3 post-inoculation. The parasites were maintained in continuous blood passage in mice. A standard inoculum of 1 × 107 parasitized erythrocytes was prepared by diluting the blood harvested from a donor mouse (> 30% parasitaemia) with normal saline for the subsequent chemo suppressive test.
Factorial design for combinations of the three (3) plants
A 23 full factorial design was used where Aa, Va, and Mp were three independent variables with experimental levels coded as -1 and 1 for low and high values respectively. The dose levels selected for each plant in the combinations were based on the previous efficacy and safety studies for the plants as reviewed earlier [17]. Design Expert® software version 13 was used for generating the various combinations or runs as shown in Table 1. The percentage chemo suppression and survival time were the key response or dependent variables.
Table 1.
Experimental design for optimization of antimalarial efficacy
| Run order | Aa (mg/kg) | Mp (mg/kg) | Va (mg/kg) |
|---|---|---|---|
| 1 | 1.00 | 1.00 | -1.00 |
| 2 | -1.00 | 1.00 | 1.00 |
| 3 | 1.00 | 1.00 | 1.00 |
| 4 | 1.00 | -1.00 | 1.00 |
| 5 | 1.00 | -1.00 | -1.00 |
| 6 | -1.00 | -1.00 | 1.00 |
| 7 | -1.00 | -1.00 | -1.00 |
| 8 | -1.00 | 1.00 | -1.00 |
Key: Aa = Artemisia annua, Mp = Microglossa pyrifolia, Va = Vernonia amygdalina, -1.00 = code referring to lower independent level (representing 200 mg/kg for Aa and Va, and 50 mg/kg for Mp), + 1.00 = code referring to higher level of the independent variable (representing 400 mg/kg for Aa and Va, and 100 mg/kg for Mp)
Peter’s four (4) day chemo suppressive test
The antimalarial activity of the different combinations of the plants was conducted using the four-day suppressive mice model as described previously [22]. Swiss albino mice were inoculated with 1 × 107 parasite (2 h prior) before randomizing them into ten (10) groups consisting of six(6) animals (3 females and 3 males) per group for test samples (Table 1) – Groups 1–8, positive control (4 mg/kg Artemisinin-Lumefantrine) – Group 9, and negative control (distilled water) – Group 10. Test samples including negative and positive controls were administered orally once daily in the morning for 4 days. The parasitaemia levels were assessed by examining and counting the parasitized and total red blood cells in 10% Giemsa-stained (Giemsa in Phosphate buffer) blood smears on day 5. The tail vein was used to collect a drop of blood used for the preparation of a film on the microscopic slides. The RBCs were fixed using methanol, and then stained for 10 min before gentle washing with distilled water and air drying at room temperature. Finally, the slides were examined under a light microscope (CX21FS1, Tokyo Japan) at an oil immersion objective (× 100 magnification power) by recording the number of parasitized red blood cells out of every 500 RBCs counted in at least six (6) random fields per slide. Percentage parasitaemia and average percentage chemo suppression of parasitaemia was calculated using the following equations [32].
| 2 |
| 3 |
Mean survival time and experimental endpoint
The animals in all the in vivo experimental groups including the negative and positive controls were monitored post-inoculation of the parasite for mortality. The observation was conducted for 28 days which was set as the study endpoint. The number of days from the time of inoculation to death of each mouse was recorded and those that were alive after 28 days were assigned survival time of 29 days. On the 29th day, the animals were euthanized using a high dose of sodium pentobarbital (100 mg/kg) injected intraperitoneally. Death was confirmed by loss of breath and heart beat supplemented by percutaneous cardiac puncture after the animal becoming unconscious. Failure of needle attached to syringe to move after insertion into the heart indicated absence of cardiac muscle movement and death [33]. The carcasses of the animals were appropriately disposed by incineration.
Statistical analysis
The average percentage parasitaemia in each group were calculated using Microsoft excel version 2019. Design Expert® version 13 was used for statistical analysis of optimization data employing ANOVA/ linear regression modelling, Shapiro–Wilk test, Pareto chart, model graphs, and prediction of optimum factors and response. GraphPad Prism version 8.0.2. 263 was used for comparison of the means of test groups by one way ANOVA and Tukey’s multiple comparison t-test.
Results
Extraction and phytochemical screening
The percentage yields of aqueous extracts were 12.14, 18.5, and 15.5% for Mp, Va, and Aa respectively. According to the preliminary phytochemical screening, all the three plant extracts showed presence of alkaloids, flavonoids, terpenoids, phenols, tannins, steroids, and carbohydrates as shown in Table 2. Anthraquinones were present in Aa, but in traces in the Mp and Va extracts.
Table 2.
Phytochemical Screening of the plant extracts
| SN | Phytochemical | Aa | Mp | Va |
|---|---|---|---|---|
| 1 | Alkaloids | + | + | + |
| 2 | Flavonoids | + | + | + |
| 3 | Phenols | + | + | + |
| 4 | Saponins | + | + | + |
| 5 | Terpenoids | + | + | + |
| 6 | Cardiac glycosides | + | + | + |
| 7 | Tannins | + | + | + |
| 8 | Steroids | + | + | + |
| 9 | Carbohydrates | + | + | + |
| 10 | Anthraquinones | + | ± | ± |
+ = present,—= absent, ± = traces; Aa – Artemisia annua, Va – Vernonia amygdalina, and Mp – Microglossa pyrifolia
Antimalarial efficacy (chemo suppression and survival time)
The 23 factorial optimization antimalarial efficacy of the combined plant extracts was conducted using Aa, Mp, and Va as independent variables at two levels (-1 and 1) as shown in Table 3. The percentage chemo suppression and survival time from the day of inoculation to death were considered as response or dependent variables. The various runs gave varying responses ranging from 41.5 to 90.98% and 19 to 23 days for chemo suppression and survival time respectively (Table 3). The positive control group (Artemisinin-Lumefantrine) had the highest survival time which was only statistically significant compared to the negative control group (p ≤ 0.05) but similar to extract combination treatment groups. The first three combinations (1, 2, and 3) exhibited higher chemo suppression (> 90%) compared to the positive control though not statistically significant.
Table 3.
Experimental design for optimization of antimalarial efficacy
| Independent variables | Dependent variables | ||||
|---|---|---|---|---|---|
| Run order | Aa (mg/kg) | Mp (mg/kg) | Va (mg/kg) | % Chemo suppression | Survival Time (Days, Mean ± SD) |
| 1 | -1.00 | -1.00 | -1.00 | 91.0 | 23 ± 5 |
| 2 | -1.00 | 1.00 | 1.00 | 90.7 | 19 ± 3 |
| 3 | -1.00 | 1.00 | -1.00 | 90.6 | 23 ± 6 |
| 4 | -1.00 | -1.00 | 1.00 | 81.5 | 19 ± 6 |
| 5 | 1.00 | -1.00 | 1.00 | 71.3 | 20 ± 7 |
| 6 | 1.00 | 1.00 | -1.00 | 67.0 | 24 ± 6 |
| 7 | 1.00 | -1.00 | -1.00 | 58.0 | 22 ± 6 |
| 8 | 1.00 | 1.00 | 1.00 | 41.5 | 23 ± 3 |
| Positive Control (4 mg/kg Artemisinin-Lumefantrine) | 87.1 | 28 ± 2* | |||
| Negative Control (distilled water) | - | 14 ± 3 | |||
*Significant at p ≤ 0.05 (compared to negative control), Aa = Artemisia annua, Mp = Microglossa pyrifolia, Va = Vernonia amygdalina, -1.00 = code referring to lower independent level (representing 200 mg/kg for Aa and Va, and 50 mg/kg for Mp), + 1.00 = code referring to higher level of the independent variable (representing 400 mg/kg for Aa and Va, and 100 mg/kg for Mp)
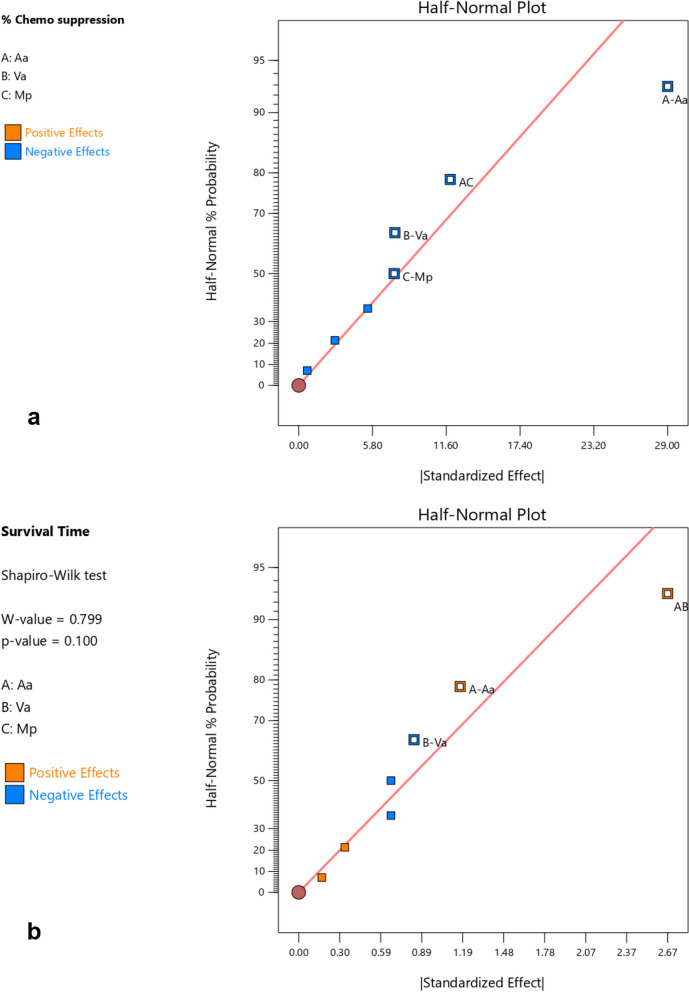
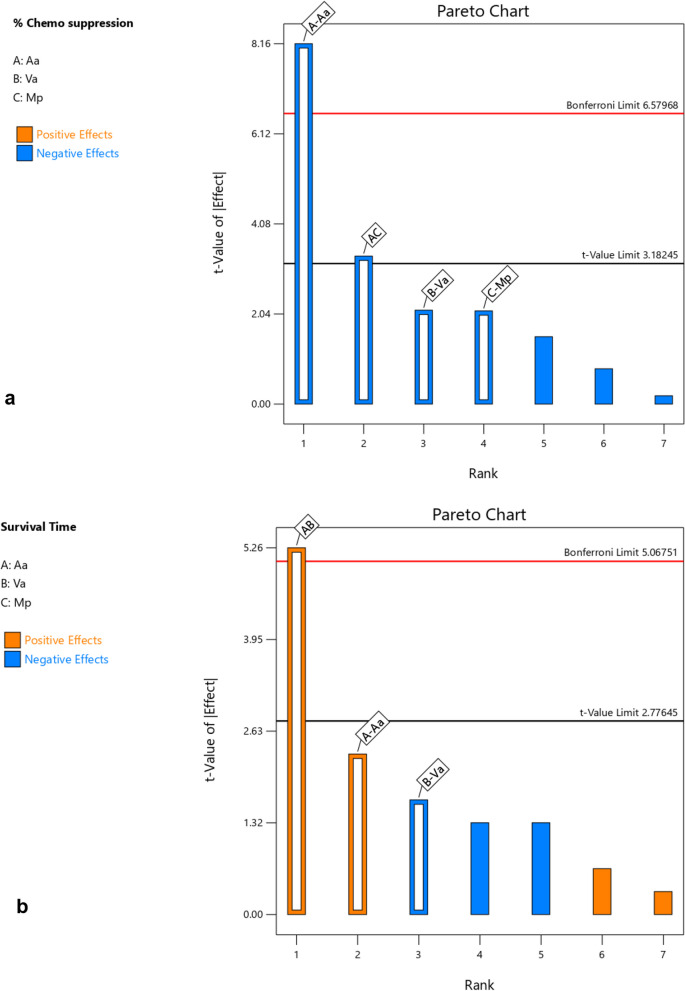
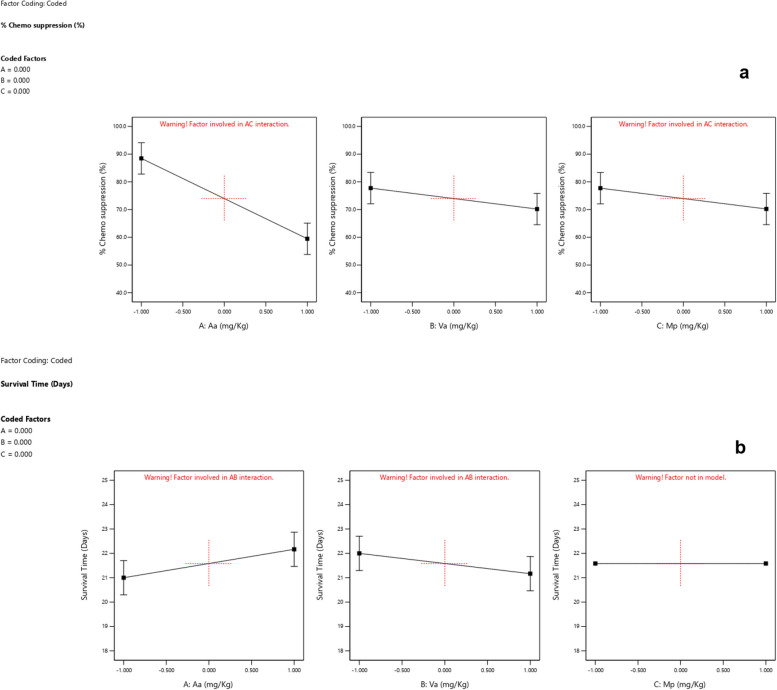
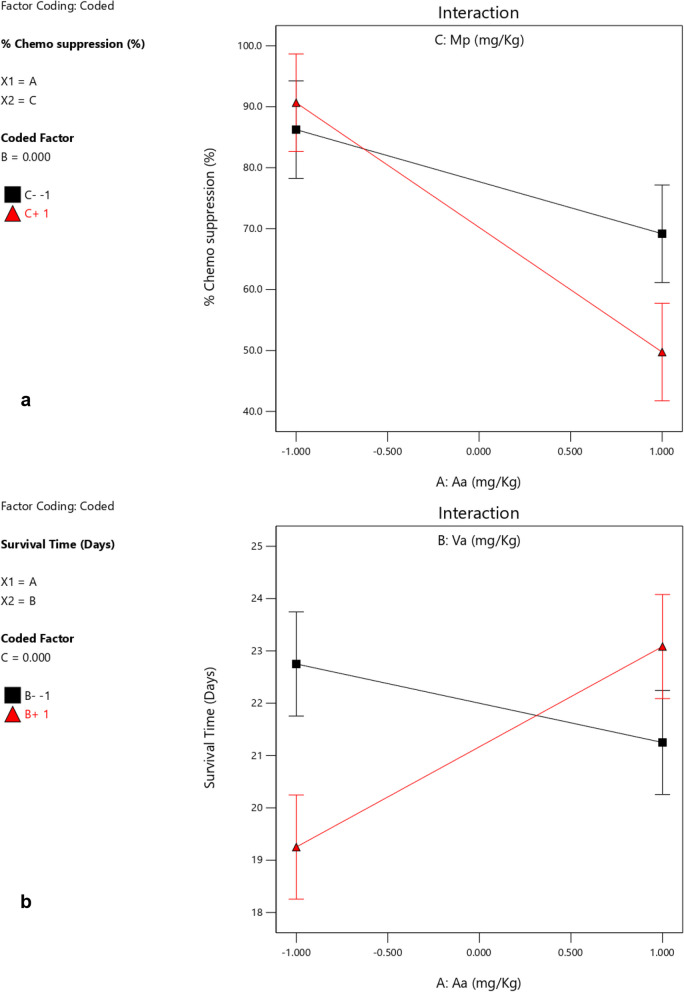
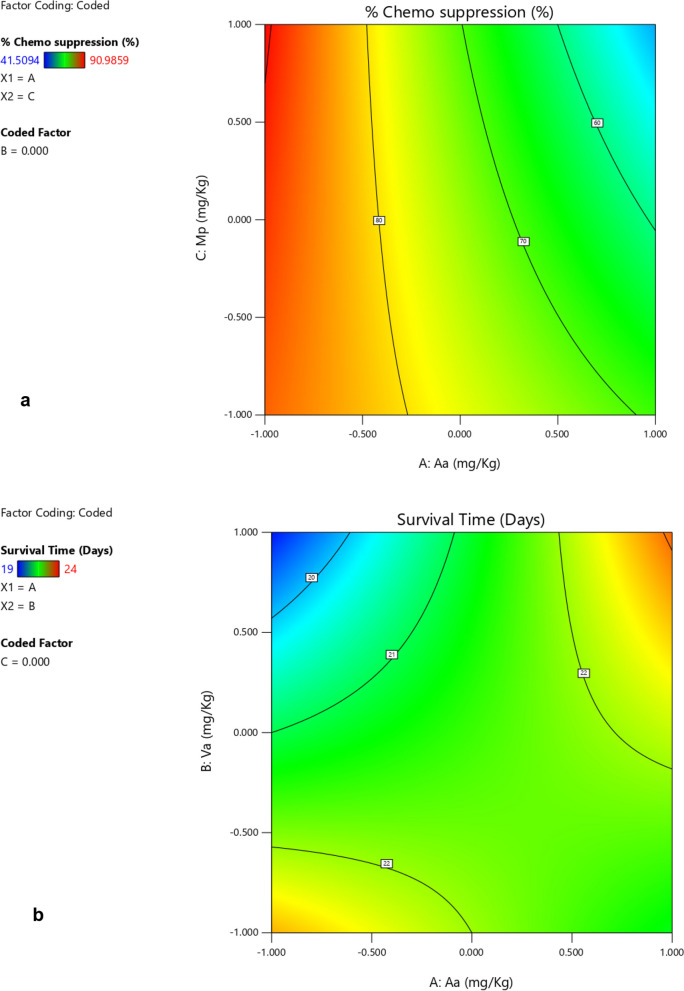
Based on the interactions of the different independent factors leading to the observed responses presented in Table 3 and Figs. 1, 2, 3 and 4 Aa exhibited the highest effect in increasing the chemo suppressive effects as shown in Figs. 1 and 3. This contribution of factor Aa was confirmed to be statistically significant by ANOVA test at P ≤ 0.05 (P = 0.0039) as indicated in Table 4. Interaction between Aa and Mp also resulted into a significant antimalarial effect (P = 0.0440). Interestingly, the colors which signify either negative or positive effects in both the half normal plot (Shapiro Wilk test) and Pareto chart (Fig. 2a) indicate that reducing the levels of the factors leads to higher effects. This is further illustrated by the interaction plots in Figs. 3 and 4. According to Fig. 3a which illustrates the influence of each independent factor on chemo suppression, there is a sharp increase in parasite suppression dependent on the decrease in the level of Aa. The same trend is also noted with Va and Mp but the increase in chemo suppression independently by these factors is not significant at P ≤ 0.05. The significant interaction between Aa and Mp in increasing chemo suppression illustrated in Fig. 4a shows that a higher level of chemo suppression is influenced by a low level of Aa and high level of Mp, though the difference in Mp level (-1 to + 1) is not significant as evidenced by the overlapping ends of the red and black colors of factor C (Mp).
Fig. 1.
Half normal plot a for percentage chemo suppression and b for survival time. The colors denote different effects i.e. low levels of Aa and Mp in a (blue) exhibit higher chemo suppression, while the orange colors as demonstrated by AB show that higher levels of Aa and Va led to longer survival times
Fig. 2.
Pareto chart for a chemo suppression and b survival time
Fig. 3.
Influence of independent factors on chemo suppression (a), and survival time (b)
Fig. 4.
Interaction between Aa and Mp on chemo suppression (a), and between Aa and Va on survival time (b)
Table 4.
ANOVA analysis for chemo suppression
| Source | Sum of Squares | df | Mean Squares | F-value | p-value | Status |
|---|---|---|---|---|---|---|
| Model | 18.33 | 3 | 6.11 | 11.89 | 0.0184 | significant |
| A-Aa | 2.72 | 1 | 2.72 | 5.30 | 0.0828 | |
| B-Va | 1.39 | 1 | 1.39 | 2.70 | 0.1755 | |
| AB | 14.22 | 1 | 14.22 | 27.68 | 0.0063 | |
| Residual | 2.06 | 4 | 0.5139 | |||
| Cor Total | 20.39 | 7 |
Regarding the survival time, the interaction between Aa and Va has shown the highest level of effect as shown in Fig. 1b and 2b. This interaction has also been confirmed to be significant by ANOVA test at P ≤ 0.05 (Table 5), but the effect is positive meaning the survival time increases as the dose levels increase. This interaction is clearly illustrated by Figs. 3b and 4b. The survival time increases with rise in the level of Aa while Va shows the opposite according to Fig. 4b. Additionally, at constant value (0.00) of factor Mp, a higher survival time is observed at lower values of both Aa and Va,
Table 5.
ANOVA analysis for survival time
| Source | Sum of Squares | df | Mean Squares | F-value | p-value | Status |
|---|---|---|---|---|---|---|
| Model | 2192.54 | 4 | 548.14 | 21.68 | 0.0150 | significant |
| A-Aa | 1682.11 | 1 | 1682.11 | 66.54 | 0.0039 | |
| B-Va | 114.15 | 1 | 114.15 | 4.52 | 0.1236 | |
| C-Mp | 112.47 | 1 | 112.47 | 4.45 | 0.1254 | |
| AC | 283.80 | 1 | 283.80 | 11.23 | 0.0440 | |
| Residual | 75.83 | 3 | 25.28 | |||
| Cor Total | 2268.38 | 7 |
The design space for chemo suppression established by the ANOVA model based on the factors with significant contributions to the response, shows that > 80% parasite suppression can be achieved at a constant Va (0.000) by the levels of—0.273 and – 1.000 of Aa, and -1.000 and 1.000 of Mp (Fig. 5a). However, the model predictions indicated two design spaces for a survival time ≥ 22 days at a constant value of Mp (0.000) as shown in red color (Fig. 5b). These include levels of Aa from – 0.65 to -1.000 and Va from -0.996 to – 1.000, and the second having Aa levels from 0.6878 to 1.000 and Va ranging from 0.323 to 1.000.
Fig. 5.
Design space a for chemo suppression based on Aa and Mp at constant Va, and b for survival time based Aa and Va at constant Mp
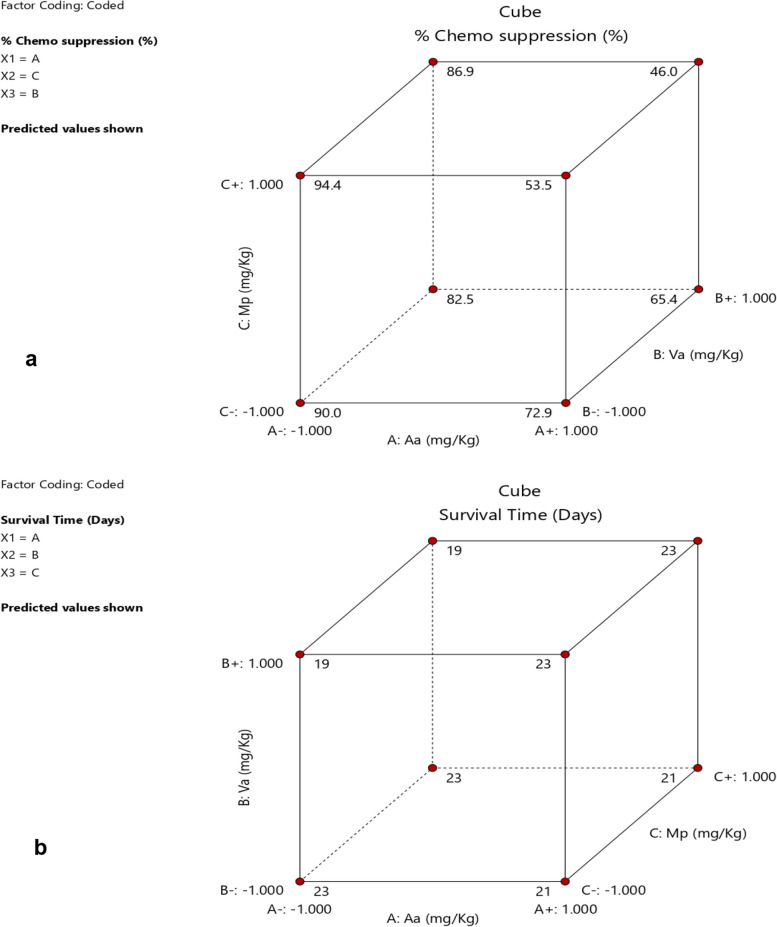
Additional predictions in Fig. 6a indicate that, 94.4% of chemo suppression can be achieved at lower levels of Aa and Va, and a higher level of Mp, which is similar to experimental run 4 that gave a response of 90. 6%. Similar predictions of survival time of 23 days are shown in cube 3-dimensional plot (Fig. 6b).
Fig. 6.
Cube showing predicted chemo suppression (a) and survival time (b) values based on their respective ANOVA models
Discussion
The study investigated the in vivo antimalarial efficacy and optimization of combinations of the best three selected antimalarial plants (Artemisia annua, Vernonia amygdalina, and Microglossa pyrifolia) in Uganda based on RITAM scores reviewed previously [17]. The aqueous extracts of the three plants were mixed at two dose levels using 23 factorial design approach in mice model using Plasmodium berghei ANKA strain. Three of the combinations exhibited a chemo suppression of over 90% higher than the standard drug (Artemisinin-Lumefantrine) but all the groups (including the positive control) in the various runs showed similar mice survival time at P ≤ 0.05. The ANOVA model developed using Design Expert® (version 13) indicated that Artemisia annua (Aa) was the main factor that contributed significantly to the parasite chemo suppression, in addition to Microglossa pyrifolia (Mp). Vernonia amygdalina (Va) contributed to increasing the survival times of the animals. The model further predicted 94.4% chemo suppression at lower levels of Aa and Va, and a higher level of Mp, which is similar to experimental run 4 which gave a response of 90. 6%. These findings offer hopes for a natural combination therapy a potential alternative to ACTs that are currently under treats of parasite resistance.
The percentage yield for Aa crude aqueous extract was lower than the 27% reported for a similar solvent obtained by a study in Saudi Arabia [34]. This difference could be attributed to variations in the methods of extraction (soxhlet Vs maceration) and geographical differences (Saudi Arabia Vs Uganda). Conversely, Va demonstrated a higher yield compared to 11.89% and 10.11% obtained in Nigerian studies [26, 27, 35, 36] that both used cold maceration method as opposed to our hot infusion. Omotola [37], reported even lower yields for the aqueous crude extract of Va (5.1%). However, another study in Ethiopia [38] reported similar yields of aqueous extract of Va leaf (14%) using maceration method. The yield of the extract from Mp was 12.1% which is close but lower than 13.4% percent obtained in Kenya [39]. In the fractionation of active components from non-polar to polar solvents for bioassay-guided extraction in Uganda [26], an aqueous yield of 3.8% was reported for Mp. This is expected since the Mp plant leaves are known to be rich in short chain fatty acids and sesquiterpene lactones which may have partitioned into the non-polar solvents, thus reducing the final concentration of total extract in water fraction [40]. In Kenya [41], a lower aqueous extract yield of 3.974% was obtained from the plant leaf powder without prior fractionation as undertaken by Adia and co-workers [26].
The phytochemical composition of a plant extract is very critical and responsible for its pharmacological or toxicological effects on the body. Therefore, the phytochemistry of the different extracts directly influences their therapeutic activities. The higher levels of terpenoids in both Va and Mp observed in this study are very useful for their antimalarial efficacy. Sesquiterpene lactones such as Vernodalol, Vernodalin, and Hydroxyvernolide have already been isolated and reported to be responsible for the antimalarial activity of V. amygdalina [42]. Similarly, terpenoids have also been reported to be the major phytochemical groups in Microglossa pyrifolia responsible for its antimalarial activity. Compounds such as E-phyto l, 1,3- hydroxyoctadeca-9Z, 11E, 15-trien-oic-acid and 6E-geranylgeraniol-19-oic-acid (all terpenoids in M. pyrifolia) have demonstrated good anti-plasmodial activities though not as potent as the crude extract [40]. Therefore, the presence and quantity of total terpenoids in these extracts could be used as a potential chemical marker in the standardization of herbal products based on V. amydalina and M. pyrifolia as active ingredient. The Artemisia annua extract is known to contain artemisinin (a sesquiterpene lactone) as the major active compound for antimalarial efficacy of the plant. The infusion of the plant also showed presence of the terpenoids, though a study [18] reported lower levels of artemisinin (0.18%) in the infusion but both crude aqueous extract and pure artemisinin demonstrated comparable in vitro antiplasmodial activities. It is therefore clear that the antimalarial activity of the Artemisia infusion does not solely depend on artemisinin (sesquiterpene lactones), but its combination with other phytochemicals such as phenolic compounds, flavonoids, and polysaccharides (all readily present in our extract) [43, 44]. Therefore, the various phytochemical compounds present in our extracts are responsible for the observed therapeutic effects presented.
The combinations containing lower levels of Artemisia annua and Vernonia amygdalina showed better parasite suppression (> 90%), and even though the higher level of Microglossa pyrifolia in the combinations showed higher activity, the difference in chemo suppression between -1 and 1 levels was not statistically significant. This is good, demonstrating the joint potency of the plant extracts in the suppression of malaria plasmodium parasite. The lower level of Aa showing higher chemo suppression in the combination implies that the extract is more effective at such doses and less effective at higher doses. Conversely, the higher survival time associated with higher level of Va could mean that it is less potent. Interactions among the extracts in the combination is the reason for these observed effects. Beneficial or desirable interactions are those that result into high chemo suppression or longer survival times (especially those that gave chemo suppression above 90%). In polyherbal practice, such interactions are often referred to as synergistic, additive or potentiative, but it was beyond the scope of our study to understand the exact type of interactions. To understand the pharmacological mechanisms underlying these observations or outcomes of the interactions among the plant extracts in the combination, more studies will be warranted. Previously, a combination of Aa with Va non polar extracts (petroleum ether) had demonstrated 100% parasite clearance by day five [45], but the survival time for the animals was short (10 days) compared to the artemisinin group (over a month). Our study unveiled that, the combination of the aqueous extracts of the two plants with Microglossa pyrifolia showed a higher parasite suppression than the standard drug (Arteminisin-Lumefantrine), and it also increased the survival time similar to the positive control. This is even safer and cheaper since petroleum ether and other non-polar solvents are expensive and associated with toxicity. Aqueous extracts are also commercially practical for large-scale production and closely replicate the traditional uses of these plants in Ugandan local communities for the management of malaria.
Antimalarial in vivo studies on the individual aqueous extracts of the three plants have never exhibited any chemo suppression as shown by the combinations. The highest efficacy ever reported for V. amygdalina was 75.15% with a survival period of 14 days at the dose of 400 mg/kg [22]. For Artemisia annua infusion, a parasite inhibition of close to 80% with 1000 mg/kg was reported but survival did not exceed 2 weeks [46]. No studies so far available for the in vivo antimalarial efficacy of Microglossa pyrifolia, creating the need to profile its activity in future studies at lower doses though it has been linked to liver toxicity.
The significant increase in survival time by the interaction between Aa and Va (P = 0.0063) is not in agreement with the work of Cissy [25] who reported 10 days survival time for combinations of Aa and Va petroleum ether extracts. This difference could be attributed to the difference in the extraction solvents (non-polar vs aqueous), and it also indicates that petroleum ether extract combinations of the plants exhibit fast chemo-suppression but short survival times compared to aqueous extracts. This is also similar to a study that reported faster reduction of parasitemia (93%) by combinations of Arteminisin with Arteanuin B, Scopoletin, and Arteanuinic acid, but also exhibited shorter survival time [19].
Therefore, the optimum combination of the aqueous extracts of the three plants have so far demonstrated better antimalarial activity and longer survival time compared to any other known study regarding these selected plants. The complexity of phytochemical compositions of herbal extracts has rendered them immune to parasite resistance compared to one-bullet synthetic drugs that have suffered from resistance over the years (ranging from quinine, chloroquine, amodioaquine, and now artemisinin-based therapies). Therefore, an optimum combination of the three plants as a natural herbal antimalarial therapy offers an alternative to first line Artemisinin based Combination Therapy (ACTs) as parasite resistance looms.
Conclusion
The factorial design approach is useful in optimization of various combinations of plant extracts to understand possible interactions and obtaining the best or most efficacious set of dose levels. Based on this, our study has demonstrated that, an optimum combination of aqueous extracts of Artemisia annua, Vernonia amygdalina, and Microglossa pyrifolia suppressed the malaria parasite and improved the survival time comparable to the standard Artemisinin-Lumefantrine at 4 mg/kg. This offers a potential natural polyherbal therapy as an alternative to ACTs (already showing signs of parasite resistance in some parts of sub-Saharan Africa) if further developed into a standard phytopharmaceutical and subjected to Randomized Controlled Trials (RCTs).
Supplementary Information
Acknowledgements
Our heartfelt gratitude goes to Pharm-Biotechnology and Traditional Medicine Center (PHARMBIOTRAC), Mbarara University of Science and Technology (MUST) for providing the funds without which this research work would not be possible. We are also grateful to the tireless efforts of our research assistants (Mr. Muganga Gershom and Ms. Madinah Nantege) who helped during the conduct of the study experiments.
Abbreviations
- Aa
Artemisia annua
- ACTs
Artemisinin based Combination Therapy
- ANNs
Artificial Neural Networks
- ANOVA
Analysis of Varriance
- AQ
Amodiquine
- DoE
Design of Experiments
- HMIS
Health Management Information System
- IRS
Indoor Residual Spraying
- Mp
Microglossa pyrifolia
- NIH
National Institute of Health
- RCTs
Randomized Clinical Trials
- RITAM
The Research Initiative on Traditional and Antimalarial Methods
- SMC
Seasonal Malaria Chemosuppression
- SP
Sulfadoxine-pyrimethamine
- Va
Vernonia amygdalina
- WHO
World Health Organization
Author’s contributions
AJR birthed the research idea, designed the experiment protocols, participated in conduct of the studies and analysis of research data, and drafted the manuscript. CA participated in conducting the in vivo antimalarial studies and reviewed the manuscript. JT and NCN participated in conceptualization, provided technical guidance and mentorship throughout the study period and reviewed the manuscript.
Funding
The research work was funded by Government of Uganda through PHARMBIOTRAC (Pharm-Biotechnology and Traditional Medicine Center) Africa Center of Excellence hosted by Mbarara University of Science and Technology.
Data availability
Data is provided within the manuscript and supplementary information files.
Declarations
Ethics approval and consent to participate
The research and animal experimental protocols used in this study were reviewed and approved at various levels at Mbarara University of Science and Technology (Uganda). The technical approvals were obtained at the department of pharmaceutical sciences and pharmacy, and Faculty Research Committee (FRC). The final research ethical approval/ permission for use of laboratory animals was obtained from Mbarara University of Science and Technology Research Ethical Committee (MUST-REC, MUST-2021–171). All the research assistants and technical staff who participated in conduct of the experiments received appropriate trainings in animal care and use, then on management of pain and distress in laboratory mice and rats. All the approved procedures in the protocol were performed and reported in accordance with Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines and regulations.
Consent for publication
The work does not contain any individual or personal data in form of images or videos that requires consent before publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World malaria report. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- 2.WHO, Guidelines for malaria. Geneva: World Health Organization; 2023. WHO/UCN/GMP/2023.01. https://www.who.int/teams/global-malaria-programme
- 3.Mwebembezi BE. Uganda’s Ntungamo District tackles through various interventions.UNICEF Uganda. https://www.unicef.org/uganda/stories/ugandas-ntungamo-district-tackles-through-various-interventions (2023). Accessed on September 21, 2023.
- 4.Epstein A, Namuganga JF, Nabende I, Kamya EV, Kamya MR, Dorsey G, et al. Mapping malaria incidence using routine health facility surveillance data in Uganda. BMJ Glob Heal. 2023;8: e011137. 10.1136/bmjgh-2022-011137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabatanzi M, Ntono V, Kamulegeya J, Kwesiga B, Bulage L, Lubwama B, et al. Malaria outbreak facilitated by increased mosquito breeding sites near houses and cessation of indoor residual spraying, Kole district, Uganda, January-June 2019. BMC Public Health. 2022;22:1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben F. How climate change is giving the world’s worst diseases a deadly boost. https://www.telegraph.co.uk/global-health/science-and-disease/climate-change-malaria-diseases-pandemic/ (2023). Accessed on September 21, 2023.
- 7.Uwimana A, Legrand E, Stokes BH, Ndikumana J-LM, Warsame M, Umulisa N, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med. 2020;26:1602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tumwebaze PK, Katairo T, Okitwi M, Byaruhanga O, Orena S, Asua V, et al. (2021) Drug susceptibility of Plasmodium falciparum in eastern Uganda: a longitudinal phenotypic and genotypic study. The Lancet Microbe. 2021;2:e441–9. 10.1016/s2666-5247(21)00085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward KE, Fidock DA, Bridgford JL. Plasmodium falciparum resistance to artemisinin-based combination therapies. Curr Opin Microbiol. 2022;69: 102193. 10.1016/j.mib.2022.102193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuwa A, Baker K, Bonnington C, Odongo M, Kyagulanyi T, Bwanika JB, et al. (2023) A non-randomized controlled trial to assess the protective effect of SMC in the context of high parasite resistance in Uganda. Malar J. 2023;22:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Full evidence report on the RTS,S/ AS01 Malaria Vaccine. 2021. https://terrance.who.int/mediacentre/data/sage/SAGE_eYB_Oct2021.
- 12.Conrad MD, Asua V, Garg S, Giesbrecht D, Niaré K, Smith S, et al. Evolution of partial resistance to artemisinins in malaria parasites in Uganda. N Engl J Med. 2023;389:722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana S-I, Yamauchi M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med. 2021;385:1163–71. [DOI] [PubMed] [Google Scholar]
- 14.Mihreteab S, Platon L, Berhane A, Stokes BH, Warsame M, Campagne P, et al. Increasing prevalence of artemisinin-resistant HRP2-negative malaria in eritrea. N Engl J Med. 2023;389:1191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arzika II, Lobo NF, Lamine MM, Tidjani IA, Sandrine H, Sarrasin-Hubert V, et al. Plasmodium falciparum kelch13 polymorphisms identified after treatment failure with artemisinin-based combination therapy in Niger. Malar J. 2023;22:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan AQ, Pernaute-Lau L, Khattak AA, Luijcx S, Aydin-Schmidt B, Hussain M, et al. Surveillance of genetic markers associated with Plasmodium falciparum resistance to artemisinin-based combination therapy in Pakistan, 2018–2019. Malar J. 2020;19:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angupale JR, Tusiimire J, Ngwuluka NC. A review of efficacy and safety of Ugandan anti-malarial plants with application of RITAM score. Malar J. 2023;22:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Donno A, Grassi T, Idolo A, Guido M, Papadia P, Caccioppola A, Villanova L, Merendino A, Bagordo F, Fanizzi FP. First-time comparison of the in vitro antimalarial activity of Artemisia annua herbal tea and artemisinin. Trans R Soc Trop Med Hyg. 2012;106:696–700. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Zhang C, Gong M, Wang M. Combination of artemisinin-based natural compounds from Artemisia annua L for the treatment of malaria: pharmacodynamic and pharmacokinetic studies. Phyther Res. 2018;32:1415–20. [DOI] [PubMed] [Google Scholar]
- 20.Mueller MS, Runyambo N, Wagner I, Borrmann S, Dietz K, Heide L. Randomized controlled trial of a traditional preparation of Artemisia annua L. (Annual Wormwood) in the treatment of malaria. Trans R Soc Trop Med Hyg. 2004;98:318–21. [DOI] [PubMed] [Google Scholar]
- 21.Sha’a KK, Oguche S, Watila IM, Ikpa TF. Antimalarial activity of the extracts of vernonia amygdalina commonly used in traditional medicine in Nigeria: an in vitro study. Curr Approaches Sci Technol Res. 2021;6:113–9. [Google Scholar]
- 22.Ajayi CO, Elujoba AA, Okella H, Oloro J, Raymond A, Weisheit A, Tolo CU, Ogwang PE. In vivo antimalarial activities of five Ugandan medicinal plants on Plasmodium berghei in mice. European J Med Plants. 2020;31:1–13. [Google Scholar]
- 23.Challand S, Willcox M. A clinical trial of the traditional medicine Vernonia amygdalina in the treatment of uncomplicated malaria. J Altern Complement Med. 2009;15:1231–7. [DOI] [PubMed] [Google Scholar]
- 24.Willcox ML. A clinical trial of “AM”, a Ugandan herbal remedy for malaria. J Public Health Med. 1999;21:318–24. [DOI] [PubMed] [Google Scholar]
- 25.Cissy N, Engeu O, Berna O, Norbert A, Esther M. Artemisia annua L.- Vernonia amygdalina Del: a potential herbal artemisinin combination treatment against malaria. Br J Pharm Res. 2016;14:1–7. [Google Scholar]
- 26.Adia MM, Emami SN, Byamukama R, Faye I, Borg-Karlson AK. Antiplasmodial activity and phytochemical analysis of extracts from selected Ugandan medicinal plants. J Ethnopharmacol. 2016;186:14–9. [DOI] [PubMed] [Google Scholar]
- 27.Muganga R, Angenot L, Tits M, Frédérich M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J Ethnopharmacol. 2010;128:52–7. [DOI] [PubMed] [Google Scholar]
- 28.de Magalhães PM, Figueira GM, de Souza JM, Ventura AMR, de Oliveira Ohnishi MD, da Silva DR, et al. A new version of a traditional tea under randomized, controlled clinical trial for the treatment of malaria. Adv Biosci Biotechnol. 2016;07:545–63. [Google Scholar]
- 29.El-Kamali HH, Elshikh AA. Preliminary Phytochemical Screening of 27 Plants Species Use in Ethnoveterinary in Khartoum State, Sudan. Adv Life Sci. 2015;5(2):48–52.
- 30.Guevara BQ. A Guidebook to Plant Screening: Phytochemical and Biological. 3rd ed. Manila: University of Santo Tomas Pub. House; 2005. [Google Scholar]
- 31.National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. American Physiological Society; 2011.
- 32.Alehegn AA, Yesuf JS, Birru EM. Antimalarial activity of crude extract and solvent fractions of the leaves of Bersama abyssinica Fresen (Melianthaceae) against Plasmodium berghei Infection in Swiss albino mice. Evidence-based Complement Altern Med. 2020;2020:9467359. 10.1155/2020/9467359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Association AVM (2020) American Veterinary Medical Association Guidelines For The Euthanasia of Animals: 2020 Edition.
- 34.Allemailem KS. Aqueous extract of artemisia annua shows in vitro antimicrobial activity and an in vivo chemopreventive effect in a small-cell lung cancer model. Plants. 2022;11:3341. 10.3390/plants11233341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makinde OO, Olabanji OB, Ibisanmi TA. Evaluation of the bioactive compounds of Vernonia amygdalina Delile extracts and their antibacterial potentials on water - related bacteria. Bull Natl Res Cent. 2021;45:191. 10.1186/s42269-021-00651-6. [Google Scholar]
- 36.Omede A, Suleiman MS, Atanu FA, Momoh S, Friday ET, Sheneni Vd, et al. Evaluation of antioxidant and cytotoxic properties of Vernonia amygdalina. Int J Cell Sci & Mol Biol. 2018;2018(4):4. [Google Scholar]
- 37.Abdulmalik O, Oladapo OO. Bolaji MO (2016) Effect of aqueous extract of Vernonia amygdalina on atherosclerosis in rabbits. ARYA atherosclerosis. 2016;12:35. [PMC free article] [PubMed] [Google Scholar]
- 38.Abay SM, Lucantoni L, Dahiya N, Dori G, Dembo EG, Esposito F, et al. Plasmodium transmission blocking activities of Vernonia amygdalina extracts and isolated compounds. Malar J. 2015;14:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odongo GA. Anti-Asthmatic and immunomodulatory properties of extracts of acacia xanthophloea, strychnos henningsii and microglossa pyrifolia in asthma-induced mice. Jomo Kenyatta University of Agriculture and Technology; 2015
- 40.Köhler I, Jenett-Siems K, Kraft C, Siems K, Abbiw D, Bienzle U, et al. Herbal remedies traditionally used against malaria in Ghana: Bioassay-guided fractionation of Microglossa pyrifolia (Asteraceae). Zeitschrift für naturforschung C. 2002;57:1022–7. [DOI] [PubMed] [Google Scholar]
- 41.Onyoyo SG. Antiplasmodial activities and in vivo safety of extracts and compounds of seven indeginous Kenyan medicinal plants used for treatment of malaria. Kenyatta University; 2020.
- 42.Zakaria Y, Azlan NZ, Fakhuruddin N, Hassan N. Phytochemicals and acute oral toxicity studies of the aqueous extract of Vernonia amygdalina from state of Malaysia. J Med Plants Stud. 2016;4:1–5. [Google Scholar]
- 43.Ogwang PE, Ogwal JO, Kasasa S, Ejobi F, Kabasa D, Obua C. Use of Artemisia annua L. Infusion for malaria prevention: mode of action and benefits in a Ugandan community. Br J Pharm Res. 2011;1:124–32. [Google Scholar]
- 44.Cai TY, Zhang YR, Ji JB, Xing J. Investigation of the component in Artemisia annua L. leading to enhanced antiplasmodial potency of artemisinin via regulation of its metabolism. J Ethnopharmacol. 2017;207:86–91. [DOI] [PubMed] [Google Scholar]
- 45.Weathers PJ, Towler M, Hassanali A, Lutgen P, Engeu PO. Dried-leaf Artemisia annua: a practical malaria therapeutic for developing countries? World J Pharmacol. 2014;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zime-diawara H, Ganfon H, Gbaguidi F, Yemoa A, Bero J, Jansen O, et al. The antimalarial action of aqueous and hydro alcoholic extracts of Artemisia annua L. cultivated in Benin : In vitro and in vivo studies. J Chem Pharma Res. 2015;7:817–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript and supplementary information files.