Abstract
Objective
Metabolic syndrome is a serious and costly health condition that is increasingly prevalent in the United States. Current treatment standards, which include lifestyle modification and medication, do not consistently yield sustainable improvements. High rates of co-occurrence with psychiatric disorders suggest that understanding psychological factors associated with metabolic syndrome may be important for enhancing interventions. The current study examines the relations between the psychological construct of “dysregulation” and metabolic risk in children, adolescents, and adults.
Method
Participants were 95 family triads comprising 158 youth aged 7 to 17 years and 127 biological parents. Dysregulation was measured using a bifactor model comprising symptoms from the Anxious/Depressed, Attention Problems, and Aggressive Behavior subscales of the Child Behavior Checklist and Adult Self Report for children and adults, respectively. Metabolic risk was measured using confirmatory factor analysis, which included waist circumference, mean arterial pressure, insulin resistance, and triglyceride-to-HDL ratio.
Results
Higher levels of dysregulation were associated with increased metabolic risk in adults. In children, this association was moderated by age, such that dysregulation and metabolic risk were positively associated only for older youth.
Conclusion
The findings of this study suggest that the association between dysregulation and metabolic risk may become stronger with age and development. This highlights that early detection and intervention of dysregulation may help prevent metabolic comorbidities later in life.
Key words: metabolic, dysregulation, mood disorders, insulin resistance, child behavior
Plain language summary
Psychiatric disorders frequently co-occur with metabolic syndrome. Understanding psychological factors associated with metabolic syndrome may help enhance interventions for both conditions. This study examined the relation between the psychological construct of “dysregulation” and metabolic risk in 95 families. Results showed that higher levels of dysregulation were associated with increased metabolic risk in both adults and their children. Dysregulation was positively associated with metabolic risk but only for older youth. Early identification and intervention of dysregulation may help prevent metabolic comorbidities later in life.
Metabolic syndrome is a cluster of co-occurring conditions, including central obesity, hyperlipidemia, hypertension, and insulin resistance, that significantly increase the risk of coronary heart disease, cardiovascular disease, and type 2 diabetes.1 The prevalence of metabolic syndrome in the United States remains extremely high among adults over 60 years of age and is increasing in younger adults, with almost 1 in 5 adults between the ages of 20 to 39 years meeting the criteria.2 The high prevalence and morbidity of metabolic syndrome contributes significantly to health care use and expenditures.3,4 In adults, research suggests that beyond adverse cardiovascular effects and chronic health problems, the components of metabolic syndrome are also correlated with psychiatric disorders. In particular, metabolic syndrome has been linked with anxiety, depression, and attention-deficit/hyperactivity disorder (ADHD),5,6 which are all disorders that most often present in childhood and adolescence.7 However, little is known about mechanisms connecting psychiatric and metabolic conditions, particularly in youth. Identification and examination of these mechanisms across the lifespan has the potential to improve early detection and intervention and to enhance management of both metabolic syndrome and psychiatric disorders.
Previous literature has suggested that self-regulation may be a key psychological process that contributes to or exacerbates metabolic disorders. Self-regulation is defined as the ability to control emotional responses within the self and is influenced by genetic,8 environmental,9 and physiological10 factors. Poor self-regulation or “dysregulation” impairs emotional, cognitive, and behavioral responses. We and others have measured dysregulation using measures that tap into these 3 domains. It is possible that dysregulation may manifest in behaviors that increase metabolic risk, such as binge eating. Within the literature, emotion dysregulation in children and adolescents moderated the association between youth loss-of-control eating and adiposity.11 The role of self-regulation in metabolic disorders has also been supported by epigenetic research, which suggests that negative environmental factors, such as poverty and chronic adversity, may impair self-regulatory abilities via post-genomic modifications, potentially leading to emotional–behavioral disturbances and metabolic problems.12,13 Finally, some research has suggested that adults with better emotion regulation have higher subjective experience of health and better physiological regulation systems, leading to a reduced risk for metabolic syndrome.14
Despite the behavioral and biological relations between self-regulation and metabolic syndrome, the current gold standard for treating metabolic syndrome does not include a psychological component but, rather, emphasizes early diagnosis, prevention through lifestyle changes including nutrition, increasing physical activity, smoking cessation, and, often, aggressive multifactorial medical management or metabolic and bariatric surgery.15, 16, 17 However, these intervention strategies in adults have demonstrated limited long-term efficacy, with weight re-gain occurring in over half of all individuals who undergo lifestyle changes only, medication only, or a combination of the standard treatments.18 Separate from the recommended early diagnosis and prevention, metabolic and bariatric surgeries have been shown to demonstrate long-term efficacy; however, there could be limited availability and accessibility to patients, especially youth.
Developmentally, these processes have not been explored among children and adolescents, leaving a gap in understanding the relationship between metabolic syndrome and dysregulation across the lifespan. Further identification of the mechanisms that connect psychological and metabolic symptoms is critical to facilitating the development of novel methods of treatment, identification, and prevention and to reducing the health burden associated with these highly comorbid conditions. To further refine our understanding of self-regulation as a factor related to metabolic syndrome and to address the lack of research pertaining to youth, this paper examines the associations with dysregulation across the lifespan. Namely, we aim to study the relationship between dysregulation and metabolic risk, and whether metabolic risk increases with age. We hypothesized that dysregulation would be associated with increased metabolic health risks in both children and adults.
Method
Participants, Procedures, and Measures
Participants
Children were recruited from the Pediatric Psychiatry Clinic at the University of Vermont Medical Center. As the larger study aimed to examine genetic relatedness among family members, children were accompanied by 2 biological family members (eg 1 parent and a sibling or 2 parents). Participants were 95 family triads comprising 158 youth aged 7 to 17 years (mean age =11.43 years; SD = 2.82 years; 64% boys) and 127 biological parents (mean age = 43.46 years, SD = 6.45 years; 69% mothers). Age, sex, race, ethnicity, and socioeconomic status were collected through self-report. Socioeconomic status was coded in accordance with the Hollingshead scale.19 Exclusion criteria included child IQ lower than 70, severe uncorrected visual impairment, and parent or child incarceration. No participant was excluded for metabolic or cardiovascular reasons. To construct a sample with heterogeneous psychiatric symptoms, families were recruited through both clinician referrals at an outpatient child and adolescent psychiatry clinic as well as through advertisements in the community.
Procedures
All study procedures were approved by the University of Vermont Institutional Review Board. Prior to participation, families were provided with a detailed verbal explanation of the study and completed written consent and assent documentation. Study participation consisted of 3 visits to a university-based laboratory, during which children and parents met separately with trained research assistants to complete interviews, online questionnaires, and computerized assessments.
In addition, during 1 appointment, scheduled in the morning, participants completed an overnight (at least 8 hours) fasting blood draw performed by a trained phlebotomist, and morphometric and blood pressure measurements were also obtained. Families were compensated $20 per hour for their time.
Child Behavior Checklist/ 6-18
The Child Behavior Checklist/ 6-18 (CBCL)20 consists of 113 self-report items for caregivers assessing their children’s emotional, behavioral, and social problems during the past 6 months. Caregivers rated each problem on a 3-point scale (0 = never true or not at all true, 1 = somewhat or sometimes true, 2 = very true or often true). The CBCL assesses 8 factor-analytically derived syndrome scales that are shown to be consistent across age, informant, and culture, with Cronbach alphas ranging from 0.79 to 0.97 and test–retest reliabilities ranging from 0.74 to 0.95.20 The CBCL yields a Dysregulation Profile (DP), a widely used and reliable measure of general self-regulatory issues.21, 22, 23, 24 This profile is formed from items from the Anxious/Depressed (AD), Attention Problems (AT), and Aggressive Behavior (AG) syndrome scales.25
Diagnostic Interviews
Psychiatric diagnoses were obtained using the Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime version (KSADS-PL)26 for children and the Composite International Diagnostic Interview (CIDI)27 for adults.
Adult Self-Report
Analogous to the CBCL, the Adult Self-Report (ASR)28 is a self-report measure for adults to rate the extent to which they have experienced 126 psychological problems during the past 6 months on a 3-point scale (0 = not true, 1= somewhat or sometimes true, 2 = very true or often true). Items are factor-analytically reduced to form 8 empirically-derived syndrome scales. These scales are normed by age, sex/gender, and culture. The psychometrics for these scales have shown good test–retest reliability ranging from 0.87 to 0.91, and Cronbach alphas ranging from 0.83 to 0.88.28
Metabolic Measures
Using the National Cholesterol Education Program (NCEP) guidelines,29 morphometric measurements of weight (kg), height (cm), diastolic and systolic blood pressure, resting heart rate, and waist circumference (cm; measured halfway between the iliac crest and the lowest rib) were collected. Fasting blood samples were analyzed for plasma glucose (mg/dL), triglycerides (mg/dL), high-density lipoprotein (mg/dL), low-density lipoprotein (mg/dL), and insulin (μU/mL) concentrations.
From these measures, we computed metrics of insulin resistance (homeostasis model assessment–estimated insulin resistance [HOMA-IR]), mean arterial pressure, and triglyceride-to-HDL ratio, consistent with the approach used by Pladevall et al. (2006).30
Medication Status
Medication data that corresponded to the study timeframe were extracted from the Electronic Health Record by the University of Vermont Health Network Data Management Office and transmitted to the study team. Medications were classified by medical doctors into the following groups: metabolic medications, stimulants, antidepressants, antipsychotics, anxiolytics, antiepileptics and mood stabilizers, non-stimulants prescribed for ADHD, other psychotropics, and other medications (not metabolic or psychiatric) described in sample frequencies in Table S1, available online.
Analytic Plan
The main statistical analyses were conducted using MPlus Version 8.1. Unless otherwise noted, age, sex, and socioeconomic status were included as covariates, and familial clusters were embedded within models to account for non-independence of sibling pairs. Statistical significance was defined as alpha values of less than 0.05, and the following criteria were used to evaluate the fit of measurement and structural models: root mean square error of approximation (RMSEA; ≤0.08 = acceptable, ≤0.05 = good), comparative fit index (CFI; ≥0.90 = acceptable, ≥0.95 = good), Tucker–Lewis index (TLI; ≥0.90 = acceptable, ≥0.95 = good), and standardized root mean square residual (SRMR; ≤0.08 = good). χ2 Statistics were also examined; however, because of bias toward statistical significance in large samples and complex models, this was considered to be a less reliable index of model fit.
Bifactor Model of Dysregulation
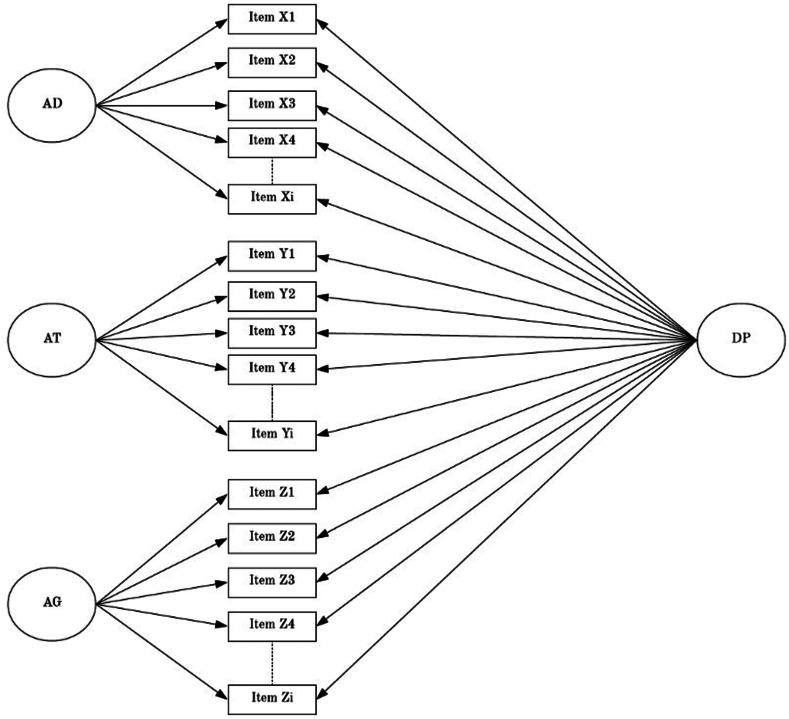
A bifactor model23 was used to measure dysregulation separately in children and adults. Bifactor models are an extension of traditional confirmatory factor analysis (CFA) techniques and are valuable for representing constructs comprising multiple distinct, yet related, facets (eg, psychopathology, intelligence). Bifactor models specify both a general factor that accounts for covariance among items, as well as specific factors that account for unique variance over and above the general factor.31 As illustrated in Figure 1, the bifactor model of dysregulation consisted of a general dysregulation factor (DP), which accounts for significant covariance among problem items from the AD, AT, and AG syndrome scales of the CBCL. In addition, the model specifies 3 scale-specific factors that account for unique variance in the AD, AT, and AG domains, over and above the general DP factor. All factors were set orthogonal to each other and CBCL items were dichotomized, such that 0 = not present and 1= present.
Figure 1.
Bifactor Model of Dysregulation Adapted from Deutz et al.23
CFA of Metabolic Risk
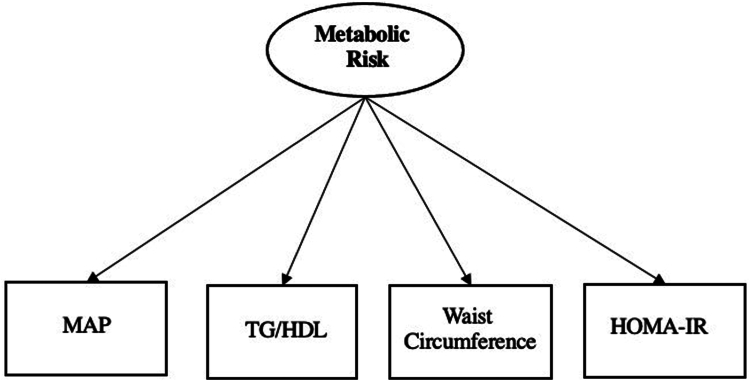
A CFA model was used to create a continuous latent factor of metabolic risk separately for in children and adults using HOMA-IR, mean arterial pressure, triglycerides-to-HDL ratio, and waist circumference as indicators in each model, as shown in Figure 2.
Figure 2.
Latent Factor Model of Metabolic Risk
Structural Models
Separate structural equation models were then tested for children and adults, with DP severity as the independent variable and metabolic risk as the dependent variable. Finally, within the child sample, an interaction effect between DP and age on metabolic risk was also examined.
Results
As shown in Table 1, 93.7% of families identified as White, which is consistent with the racial distribution of the study catchment area, and most families were of middle-class socioeconomic status (mean = 64.80, SD = 22.38) as assessed by the Hollingshead scale. In addition, 36.7% of children and 21.3% of adults met current diagnostic criteria for a psychiatric illness based on DSM criteria. Descriptive statistics for indicators of metabolic syndrome are presented in Table 2. Mean values for both children and adults fell within the normal range for all indicators.
Table 1.
Demographic Characteristics of Parents and Children
| Parents (n = 127) |
Children (n = 158) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Male | 39 | 30.7 | 101 | 63.9 |
| Female | 88 | 69.3 | 57 | 36.1 |
| Race | ||||
| American Indian or Alaska Native | 3 | 2.4 | 7 | 4.4 |
| Asian or Asian American | 2 | 1.6 | 5 | 3.2 |
| Black or African American | 1 | 0.8 | 2 | 1.3 |
| White | 119 | 93.7 | 144 | 91.1 |
| Hawaiian or Pacific Islander | 0 | 0 | 0 | 0 |
| Other or not reported | 2 | 1.6 | 0 | 0 |
| Ethnicity | ||||
| Hispanic or Latinx | 5 | 3.9 | 11 | 6.9 |
| Not Hispanic or Latinx | 122 | 96.1 | 147 | 93.0 |
| Household SES | ||||
| 0-9 (lowest) | 4 | 3.1 | 8 | 5.1 |
| 10-19 | 1 | 0.8 | 2 | 1.3 |
| 20-29 | 4 | 3.1 | 7 | 4.4 |
| 30-39 | 3 | 2.4 | 3 | 1.9 |
| 40-49 | 9 | 7.1 | 12 | 7.6 |
| 50-59 | 13 | 10.2 | 11 | 7.0 |
| 60-69 | 20 | 15.7 | 22 | 13.9 |
| 70-79 | 23 | 18.1 | 29 | 18.4 |
| 80-89 | 24 | 18.9 | 33 | 20.9 |
| 90-100 (highest) | 24 | 18.9 | 30 | 19.0 |
| Current Psychiatric Diagnosisa | ||||
| SMDb | -- | -- | 20 | 12.6 |
| MDD | 6 | 4.7 | 9 | 5.6 |
| Dysthymia | 3 | 2.4 | 4 | 2.5 |
| Bipolar disorder | 0 | 0 | 0 | 0 |
| Separation anxiety | -- | -- | 7 | 4.4 |
| Social phobia | -- | -- | 14 | 8.9 |
| GAD | 3 | 2.4 | 27 | 17.7 |
| OCD | 9 | 7.1 | 5 | 3.2 |
| PTSD | 8 | 6.3 | 4 | 2.5 |
| ADHD | 2 | 1.6 | 45 | 28.4 |
| Conduct | 0 | 0 | 0 | 0 |
| ODD | 0 | 0 | 29 | 18.3 |
| Substance abuse | 1 | 0.1 | 0 | 0 |
| Any diagnosis | 24 | 21.3 | 58 | 36.7 |
Note: ADHD = attention-deficit/hyperactivity disorder; GAD = generalized anxiety disorder; MDD = major depressive episode; OCD = obsessive compulsive disorder; ODD = oppositional defiant disorder; PTSD = post-traumatic stress disorder; SES = socioeconomic status; SMD = severe mood dysregulation.
Diagnoses were determined using the Kiddie Schedule for Affective Disorders and Schizophrenia- Present Lifetime Version (K-SADS-PL)26 administered by graduate- or doctoral-level clinicians trained to reliability.
SMD is a research precursor to the current DSM-5 diagnosis of disruptive mood dysregulation disorder.
Table 2.
Descriptive Statistics for Metabolic Syndrome Criteria
| Children |
Adults |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Waist circumference | 69.99 | 14.24 | 92.00 | 20.07 |
| Triglycerides | 82.88 | 51.63 | 94.06 | 24.63 |
| HDL | 54.03 | 14.41 | 57.49 | 17.13 |
| Glucose | 87.87 | 15.62 | 109.64 | 34.31 |
| Systolic BP | 111.33 | 11.35 | 118.96 | 12.29 |
| Diastolic BP | 64.04 | 6.56 | 73.21 | 8.87 |
Note: BP = blood pressure; HDL = high-density lipoprotein.
Measurement Models
Bifactor Model of Dysregulation
The bifactor model of dysregulation showed excellent fit to the data in both children (χ2 = 922.205, df= 738, p < .0001; RMSEA=0.035 [90% CI = 0.27-0.042]; CFI = 0.978; TLI =0.976) and adults (χ2 = 1085.111, df = 943, p < .0001; RMSEA = 0.037 [90% CI = 0.25-0.047]; CFI = 0.954; TLI = 0.949). As detailed in Table S2, available online, all problem items loaded significantly onto the general DP factor in both samples, whereas scale-specific loadings were significant, albeit generally of smaller magnitude, for 31 of 41 problem items in children and 27 of 47 problem items for adults. In the adult sample, 2 items (no. 37 fights, no. 57 attacks people) were removed from the AG subscale because of having having zero variance.
CFA of Metabolic Risk
The 1-factor model of metabolic risk showed good fit in both the child and adult samples. All variables were log or square root transformed to conform to a normal distribution. Goodness-of-fit statistics and standardized factor loadings are presented in Tables S3 and S4, respectively, available online.
Structural Models
Because of problems with model convergence, factor scores from the latent metabolic risk factor were extracted and used as the dependent variable structural models. In children, only the general DP (β = 0.11, p = .09) factor was marginally positively associated with metabolic risk in children. Being older (β = 0.53, p < .001 and of lower socioeconomic status (β = −0.26, p < .002) were also significant predictors of higher metabolic risk. In adults, the general DP factor was the only component of the bifactor model that significantly predicted metabolic risk (β = 0.19, p = .008). Being older (β = 0.19, p = .05), male (β = −0.37, p < .001), and of lower socioeconomic status (β = −0.26, p = .001) were also significant predictors of higher metabolic risk.
Interaction With Child Age
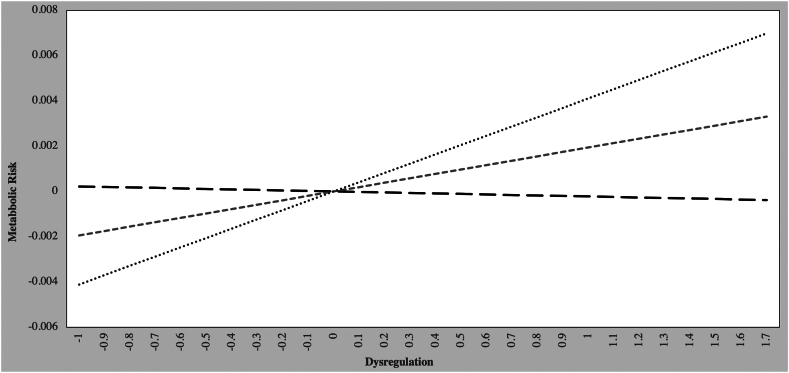
Finally, possible moderating effects of child age on the relations between DP severity and metabolic risk were tested. Analyzing the data using age as a continuous variable showed that child age moderated the interaction between DP and metabolic risk. This was followed by simple slope analyses in which we created 3 age groups (older, average, and younger) based on being greater or less than 1 SD above or below the mean (11.42 years). The older group comprised individuals older than 14.25 years; the younger group comprised those younger than 8.61 years. Results showed that child age significantly moderated the relations between DP and metabolic risk (b = 0.195, p = .005). Follow-up simple slope analyses were calculated for different values of age32 (Figure 3). (As seen in Figure 3, there was a significant, positive association between dysregulation and metabolic risk for both older children (b = 0.004, p = .005) and children of average age (b = 0.002, p = .037). Dysregulation and metabolic risk were not significantly related for younger children.
Figure 3.
Interaction Effect of Age and DP Severity on Metabolic Risk Among Children
Effects of Medication Status
In the adult sample, we regressed each class of medications on all latent factors (DP, AP, AB, and AD). Our results indicated that antidepressants were significantly and positively associated with the DP factor (b = 0.35, SE = 0.096, p < 0.001), whereas anxiolytics were significantly and negatively associated with the AD factor (b = −0.55, SE = 0.20, p = .005). When antidepressant medications were added to our regression model as a covariate, the effect of antidepressants on metabolic risk remained significant (b = 0.22, SE = 0.10, p = .04), in addition to sex (b = −0.43, SE = 0.08, p < .001), whereas the effect of DP on metabolic risk was rendered nonsignificant (b = 0.09, SE = 0.10, p = .39).
In the child sample, when we regressed each class of medications on metabolic risk, no class of medication was significantly associated with metabolic risk. When each class of medications was regressed on all latent factors, our results indicated that antidepressants (b = 0.025, SE = 0.08, p = .001), stimulants (b = 0.26, SE = 0.07, p < .001), and psychotropics (b = 0.29, SE = 0.08, p < .001) were positively and significantly associated with the DP factor. After adding antidepressants, psychotropics, and stimulants as covariates to our initial regression model, the effect of DP on metabolic risk remained nonsignificant (b = 0.14, SE = 0.09, p = .10).
Discussion
We have characterized broad dysregulation as a latent factor that encompasses dysregulated affect, cognition, and behavior. To measure dysregulation, we used items of the CBCL-DP in a bifactor model that allowed us to derive a specific dysregulation factor. Previous research has shown that dysregulation places youth and adults at risk for poor outcomes including risk for depression, suicidality, and substance use.24,33,34 For the first time, the findings presented here extend this risk to metabolic symptoms. In these analyses, there was a significant association between dysregulation and metabolic risk in adults. The same metabolic risk was associated with dysregulation in youth, but only when moderated by age, such that older youth with higher dysregulation also showed higher metabolic risk.
Moreover, we demonstrated some specificity of the association between this general dysregulation vs its component parts. Because the dysregulation factor was derived as part of a bifactor model, we specifically examined the contributions of dysregulation as well as the CBCL subscales of which it is composed. Metabolic risk was not associated with attention problems, aggressive behavior, or anxiety/depression in isolation. This adds to the literature demonstrating that dysregulation is associated with physiological effects over and above its component parts. For example, it has been shown that although children with ADHD demonstrate electroencephalographic findings separable from those of controls, this association is seen only when they also have dysregulation.35 Our group has demonstrated that children with dysregulation show a lack of physiological adaptation to situational demands.36 Taken together, the associations among dysregulation and neurophysiology, behavior, and metabolic indicators suggests that this phenotype needs continued examination separate from its underlying components.
In a novel analytic approach, we examine the dysregulation profile for the first time in adults. We demonstrated that the dysregulation factor is associated with metabolic risk in both youth and adults, directly associated with age. The important question remains as to why this association is observed. It could be argued that having more dysregulated affect, behavior, and cognition could make it more difficult to maintain weight, glucose control, or other metabolic risk factors. For example, it is possible that those who make impulsive choices during development, such as impulsive decisions about food, exercise, or substance use, would have an increased later metabolic risk. We did have stop-signal reaction time task data available in this sample and examined whether the association between dysregulation and metabolic risk was mediated by impulsivity, but no significant mediation was found.36
The data presented here demonstrated that in younger children there was no association between metabolic risk and dysregulation, whereas in older children there was; the association would seem to support an additional developmental factor that might lead to metabolic risk. However, it is also possible that metabolic regulation and emotional/behavioral regulation are affected by a common process. For example, there could be common epigenetic changes associated with dysregulation and metabolic pathways such that changes in 1 system affect the other. It is also possible that medications being provided for the disorders associated with dysregulation could have affected the metabolic pathways, given that some antidepressants, antipsychotics, and mood stabilizers have been associated with weight gain and other metabolic effects.37 In addition, because this is a cross sectional analysis, we can also consider a mechanism in which metabolic risk drives further dysregulation via biological pathways, for example, inflammation,38 such that severe or prolonged metabolic symptoms could have a negative impact on emotional functioning. Further longitudinal assessments of these relationships are needed to clarify the directionality.
There are some limitations to the interpretation of these results. During the study, there was no attempt made to have participants withhold medication. However, it would seem less likely that the effect of medication on metabolic symptoms would be specific to the dysregulation factor and not to symptoms of aggressive behavior, anxiety/depression, or attention problems, which are presumably what are being targeted with medication. Finally, although this is a sizeable sample of youth and adults to measure metabolic symptoms and emotional behavior, there is the possibility that with larger samples, the scale-specific factors may have been significantly associated with metabolic risk. Examination of the beta weights (Table 3) suggest that this is unlikely, but possible. Examination of potential mediators and moderators of the association is warranted.
Table 3.
Standardized coefficients for effects of bifactor components and covariates on metabolic risk in children and adults
| Children |
Adults |
|||
|---|---|---|---|---|
| B | p | b | p | |
| AD | 0.11 | 0.18 | 0.11 | 0.34 |
| AT | −0.09 | 0.33 | 0.08 | 0.47 |
| AG | 0.15 | 0.12 | 0.14 | 0.11 |
| DP | 0.11 | 0.09 | 0.19∗∗ | 0.008 |
| Age | 0.53∗∗∗ | <0.001 | 0.19 | 0.05 |
| Sex | −0.05 | 0.42 | −0.37∗∗∗ | <0.001 |
| SES | −0.26∗∗ | 0.002 | −0.26∗∗ | 0.001 |
Note: AD = Anxious/Depressed; AG = Aggressive Behavior; AT = Attention Problems; DP = Dysregulation; SES = socioeconomic status.
∗∗p < .01, ∗∗∗cp < .001.
The post hoc medication analysis was included to test possible associations and effects of medications between psychiatric symptoms and the metabolic factor. There are some limitations to the interpretation of these results. Although these data were retrieved from the largest health network in the state, medication information was unavailable for patients who had not been seen in the health network (n = 23). In addition, the accuracy of medications prescribed by providers outside of the network (ie, private practice) is unclear. Finally, these data were retrospectively collected from 2014 to 2017, during a time that the University of Vermont Health Network was using a different electronic health record system.
Finally, this sample was 93.7% White in the parents group and 91.1% White in the children group, and was largely not Hispanic/Latinx. The youth were also more likely to be identified as male, whereas the adults were more likely to be identified as female. The household socioeconomic status of this group was also higher than in some other studies, placing them mostly in a middle-class range. These characteristics are a function of the population from which this sample was drawn (clinic and community sample in Vermont) and the type of protocol used, which required a significant, multi-day commitment on the part of the family. Replication within more diverse samples is critical to understand whether these findings can be generalized to other populations.
Recently, there has been a focus on early life constructs, such as adverse childhood experiences (ACEs), that can have later significant medical risks.39 These data provide another aspect to the development of metabolic problems. In this demonstration of potential metabolic risks associated with dysregulation, we offer the opportunity of early lifestyle intervention in youth that may help prevent later morbidity and mortality. Earlier identification and a focus on the treatment of dysregulation or a general psychologic treatment component has the potential to lower the risk of metabolic problems across the lifespan. Given the high health care costs in the management of diabetes, hypertension, and heart disease, along with the immeasurable human cost of these disorders, the discovery of alternative pathways of risk, and further investigation of how to intervene and prevent risk, seem necessary.
Footnotes
Drs. Ametti and Cheaito contributed equally to this work.
This article was reviewed under and accepted by Ad Hoc Editor Benjamin I. Goldstein, MD, PhD.
This research was supported in part by a COBRE grant from the National Institute of General Medical Sciences (NIGMS, grant P20GM103644).
The research was performed with permission from the University of Vermont Institutional Review Board.
This study was presented as a new research poster at the American Academy of Child and Adolescent Psychiatry 69th Annual Meeting; October 17-22, 2022; Toronto, Ontario, Canada.
Author Contributions
Conceptualization: Ades, Althoff
Data curation: Ametti, Althoff
Formal analysis: Ametti, Cheaito
Fundingacquisition: Althoff
Investigation: David
Methodology: Ametti, Cheaito, Althoff
Project administration: Ametti
Supervision: Althoff
Visualization: Frering, Ades
Writing – original draft: Ametti, Cheaito, Frering, Ades, Althoff
Writing – review and editing: Ametti, Cheaito, Frering, Ades, David, Althoff
Disclosure: Dr. Althoff has received grant or research support from NIMH, NIGMS, NIDA, and the Klingenstein Third Generation Foundation. He has served on the editorial board of Child Psychiatry and Human Development, as consulting editor of the Journal of Clinical Child and Adolescent Psychology, and is the Associate Editor of the Journal of the American Academy of Child and Adolescent Psychiatry for which he receives an honorarium. He has received honoraria from the Massachusetts General Hospital Psychiatry Academy and Frontline Medical Communications, Inc. for CME programs and publications. He is a partner of WISER Systems, LLC. Drs. Ametti, Ades, and David and Mss. Cheaito and Frering have reported no biomedical financial interests or potential conflicts of interest.
Supplemental Material
References
- 1.Ginsberg H.N., MacCallum P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2009;4(2):113–119. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirode G., Wong R.J. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020;323(24):2526–2528. doi: 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers J., Kokkinos P., Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients. 2019;11(7):1652. doi: 10.3390/nu11071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fappa E., Yannakoulia M., Pitsavos C., Skoumas I., Valourdou S., Stefanadis C. Lifestyle intervention in the management of metabolic syndrome: could we improve adherence issues? Nutrition. 2008;24(3):286–291. doi: 10.1016/j.nut.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Mansur R.B., Brietzke E., McIntyre R.S. Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neurosci Biobehav Rev. 2015;52:89–104. doi: 10.1016/j.neubiorev.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q., Hartman C.A., Haavik J., et al. Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: a population-based cross-sectional study. PLoS One. 2018;13(9) doi: 10.1371/journal.pone.0204516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merikangas K.R., He J-p, Burstein M., et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson Linnér R., Mallard T.T., Barr P.B., et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self-regulation and addiction. Nat Neurosci. 2021;24(10):1367–1376. doi: 10.1038/s41593-021-00908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao H., Liang Y., Zhou N. Early tobacco smoke exposure, preschool cool/hot inhibitory control, and young adolescents’ externalizing/internalizing problems. J Fam Psychol. 2021;35(3):311. doi: 10.1037/fam0000739. [DOI] [PubMed] [Google Scholar]
- 10.Ayer L., Greaves-Lord K., Althoff R.R., et al. Blunted HPA axis response to stress is related to a persistent dysregulation profile in youth. Biol Psychol. 2013;93(3):343–351. doi: 10.1016/j.biopsycho.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly N.R., Tanofsky-Kraff M., Vannucci A., et al. Emotion dysregulation and loss-of-control eating in children and adolescents. Health Psychol. 2016;35(10):1110. doi: 10.1037/hea0000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagot R.C., Meaney M.J. Epigenetics and the biological basis of gene × environment interactions. J Am Acad Child Adolesc Psychiatry. 2010;49(8):752–771. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnunen M.-L., Kokkonen M., Kaprio J., Pulkkinen L. The associations of emotion regulation and dysregulation with the metabolic syndrome factor. J Psychosom Res. 2005;58(6):513–521. doi: 10.1016/j.jpsychores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Sherling D.H., Perumareddi P., Hennekens C.H. Metabolic syndrome: clinical and policy implications of the new silent killer. J Cardiovasc Pharmacol Ther. 2017;22(4):365–367. doi: 10.1177/1074248416686187. [DOI] [PubMed] [Google Scholar]
- 16.Grundy S.M., Cleeman J.I., Daniels S.R., et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/circulationaha.105.169405. [DOI] [PubMed] [Google Scholar]
- 17.Shuai X., Tao K., Mori M., Kanda T. Bariatric surgery for metabolic syndrome in obesity. Metab Syndr Relat Disorders. 2015;13(4):149–160. doi: 10.1089/met.2014.0115. [DOI] [PubMed] [Google Scholar]
- 18.Wadden T.A., Butryn M.L., Byrne K.J. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(S12):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 19.Hollingshead A.B. Yale University; 1975. Four Factor Index of Social Status. [Google Scholar]
- 20.Achenbach T.M., Dumenci L., Rescorla L.A. University of Vermont; 2001. Ratings of Relations Between DSM-IV Diagnostic Categories and Items of the CBCL/6-18, TRF, and YSR; pp. 1–9. [Google Scholar]
- 21.Aitken M., Battaglia M., Marino C., Mahendran N., Andrade B.F. Clinical utility of the CBCL Dysregulation Profile in children with disruptive behavior. J Affect Disord. 2019;253:87–95. doi: 10.1016/j.jad.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Althoff R.R., Verhulst F.C., Rettew D.C., Hudziak J.J., van der Ende J. Adult outcomes of childhood dysregulation: a 14-year follow-up study. J Am Acad Child Adolesc Psychiatry. 2010;49(11):1105–1116. doi: 10.1016/j.jaac.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deutz M.H., Geeraerts S.B., van Baar A.L., Deković M., Prinzie P. The Dysregulation Profile in middle childhood and adolescence across reporters: factor structure, measurement invariance, and links with self-harm and suicidal ideation. Eur Child Adolesc Psychiatry. 2016;25(4):431–442. doi: 10.1007/s00787-015-0745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtmann M., Buchmann A.F., Esser G., Schmidt M.H., Banaschewski T., Laucht M. The Child Behavior Checklist—Dysregulation Profile predicts substance use, suicidality, and functional impairment: a longitudinal analysis. J Child Psychol Psychiatry. 2011;52(2):139–147. doi: 10.1111/j.1469-7610.2010.02309.x. [DOI] [PubMed] [Google Scholar]
- 25.Rescorla L.A., Blumenfeld M.C., Ivanova M.Y., Achenbach T.M., International ASEBA Consortium International comparisons of the dysregulation profile based on reports by parents, adolescents, and teachers. J Clin Child Adolesc Psychol. 2019;48(6):866–888. doi: 10.1080/15374416.2018.1469090. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman J., Birmaher B., Brent D., et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization . World Health Organization; 1994. Composite International Diagnostic Interview (CIDI) Researcher's Manual: Version 1.1. [Google Scholar]
- 28.Achenbach T.M., Dumenci L., Rescorla L. Research Center for Children, Youth and Families; 2003. Ratings of relations between DSM-IV diagnostic categories and items of the Adult Self-Report (ASR) and Adult Behavior Checklist (ABCL) [Google Scholar]
- 29.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Pladevall M., Singal B., Williams L.K., et al. A single factor underlies the metabolic syndrome: a confirmatory factor analysis. Diabetes Care. 2006;29(1):113–122. doi: 10.2337/dc06-0800. [DOI] [PubMed] [Google Scholar]
- 31.Chen F.F., Hayes A., Carver C.S., Laurenceau J.P., Zhang Z. Modeling general and specific variance in multifaceted constructs: a comparison of the bifactor model to other approaches. J Personal. 2012;80(1):219–251. doi: 10.1111/j.1467-6494.2011.00739.x. [DOI] [PubMed] [Google Scholar]
- 32.Aiken L.S., West S.G., Reno R.R. Sage; 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- 33.Aebi M., Winkler Metzke C., Steinhausen H.-C. Predictors and outcomes of self-reported dysregulation profiles in youth from age 11 to 21 years. Eur Child Adolesc Psychiatry. 2020;29(10):1349–1361. doi: 10.1007/s00787-019-01444-z. [DOI] [PubMed] [Google Scholar]
- 34.Deutz M.H., Geeraerts S.B., Belsky J., et al. General psychopathology and dysregulation profile in a longitudinal community sample: stability, antecedents and outcomes. Child Psychiatry Hum Dev. 2020;51(1):114–126. doi: 10.1007/s10578-019-00916-2. [DOI] [PubMed] [Google Scholar]
- 35.McGough J.J., McCracken J.T., Cho A.L., et al. A potential electroencephalography and cognitive biosignature for the Child Behavior Checklist—Dysregulation Profile. J Am Acad Child Adolesc Psychiatry. 2013;52(11):1173–1182. doi: 10.1016/j.jaac.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ametti M.R., Crehan E.T., O’Loughlin K., et al. Frustration, cognition, and psychophysiology in dysregulated children: a Research Domain Criteria approach. J Am Acad Child Adolesc Psychiatry. 2022;61(6):796–808. doi: 10.1016/j.jaac.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solmi M., Fornaro M., Ostinelli E.G., et al. Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry. 2020;19(2):214–232. doi: 10.1002/wps.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gianaros P.J., Marsland A.L., Kuan D.C.-H., et al. An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biol Psychiatry. 2014;75(9):738–745. doi: 10.1016/j.biopsych.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webster E.M. The impact of adverse childhood experiences on health and development in young children. Glob Pediatr Health. 2022;9 doi: 10.1177/2333794x221078708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.