Abstract
Background
The Woven EndoBridge (WEB) is a treatment modality available for the treatment of intracranial aneurysms, specifically beneficial in wide-necked bifurcation aneurysms. Conventional sizing methods rely on the manipulation of aneurysm width and height measurements. This results in frequent need for re-sizing after initial WEB insertion attempts. Previous studies have suggested that volume-based sizing may decrease this rate.
Methods
We conducted a multicenter retrospective cohort study in three complex vascular centers in the United States from 1 January 2020 to 30 June 2023. All patients who underwent attempted aneurysmal WEB embolization were included. Using three-dimensional angiogram reconstructions, we measured the aneurysm volume. We calculated the WEB volume and measured the WEB-aneurysm volume (WAVe) ratio. The primary outcome was whether a WEB required re-sizing.
Results
A total of 133 cases were identified, 114 correctly sized and 19 incorrectly sized. Twelve patients (9.0%) required additional stent placement during WEB insertion. One patient (0.8%) had WEB abandonment. There were no differences in demographic or baseline characteristics between the size/re-sizing cohorts aside from aneurysm location (“other” and basilar locations increased the rate of re-sizing). The median WAVe ratio in our appropriately sized cohort was 0.997 (interquartile range (IQR) 0.826, 1.30) versus 1.14 in our re-sizing cohort (IQR 0.734, 1.51; p = 0.728). Using logistic regression, we identified a WAVe ratio ranging from 0.76 to 1.24 yielding > 80% probability of a successful sizing with 95% confidence.
Conclusions
Incorporating volume-based measurements in aneurysm embolization with WEBs may improve rates of re-sizing but has an unclear effect on aneurysm occlusion. A WAVe ratio of 0.76–1.24 provides the greatest probability of appropriate initial WEB sizing.
Keywords: Intracranial aneurysm, embolization, Woven EndoBridge (WEB), WEB-aneurysm volume (WAVe) ratio, angiography
Introduction
The Woven EndoBridge (WEB; Microvention/Terumo, Aliso Viejo, California, USA) is one of several treatment modalities available for the treatment of intracranial aneurysms. It has specific utility in the treatment of wide-necked bifurcation aneurysms (WNBAs). 1 It is an intra-saccular device that reconstructions the parent artery-aneurysm neck interface and disrupts flow within the aneurysm to initiate thrombosis and act as a conductive surface for endothelization at the artery-aneurysm interface.2–4
The ability of the WEB device to properly treat aneurysms is dependent on several factors, most importantly adequate sizing and placement. If the device is too large, the WEB device will protrude through the neck and cause parent vessel thromboses with risk for or even complete prolapse. If undersized, endoleak and aneurysm persistence is a risk.2,3,5,6 This is in addition to the cost incurred to the patient or healthcare system should a device have to be replaced or an aneurysm re-treated. 7 Traditional sizing recommendations are to select the width first by adding 1–2 mm to the width of the aneurysm (without including lobules in spaces the device will not occupy) and then to select the device height by subtracting from the aneurysm height the amount added to the width. This recommendation is to mitigate the risk of device prolapse, intussusception, or irregular shaping due to height expansion during lateral wall apposition; this is termed the “+1/–1 rule.”2,6,7
Previous papers suggest a 20%–30% rate for WEB re-sizing.7–9 If improved, this could lower procedural time, lower complication risk, increase occlusion rates, and reduce costs. Two groups previously worked to develop sizing thresholds based on three-dimensional (3D) volume sizing as opposed to the standard “+1/–1 rule.” Tanabe et al. that WEB volume/aneurysm volume should be between 0.90 and 1.16 to secure > 80% probability of successful sizing at 95% confidence. Ansari et al. found an ideal ratio of 0.6–0.8 through a slightly different methodology. Namely, Ansari et al. 8 used automated volumetric segmentation on 3-D DSA volumes versus manual measurements on 3D angiographic images (Tanabe et al.). We principally sought to validate volume-based sizing techniques on a multi-institutional cohort. We secondarily aimed to find other factors that might be related to successful initial WEB sizing as well as evaluate the impact of WEB sizing on aneurysm occlusion in follow-up.
Methods
Study design and population
After institutional review board approval, we conducted a multicenter retrospective cohort study in three complex vascular centers in the United States from 1 January 2020 to 30 June 2023. We included all consecutive patients who underwent attempted WEB placement for embolization of intracranial aneurysm. This was defined by the opening of a WEB device at any point during the procedure, whether or not it was implanted. The decision to attempt treatment with WEB was based on institutional and proceduralist preference; on-label usage of the WEB was not a requisite. We included both WEB single-layer (SL) and SL sphere (SLS) devices.
Demographic variables and aneurysm measurement
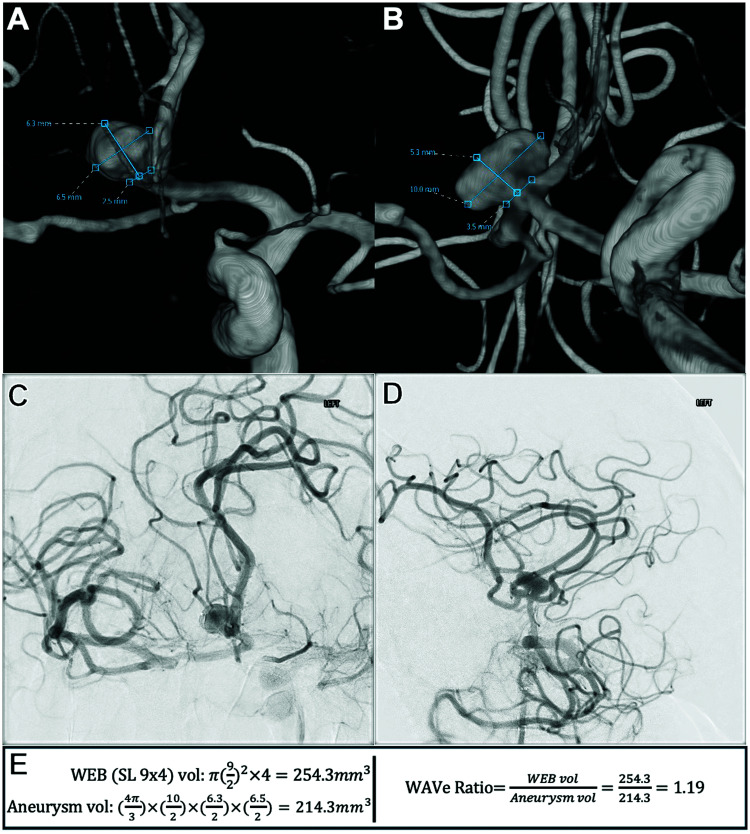
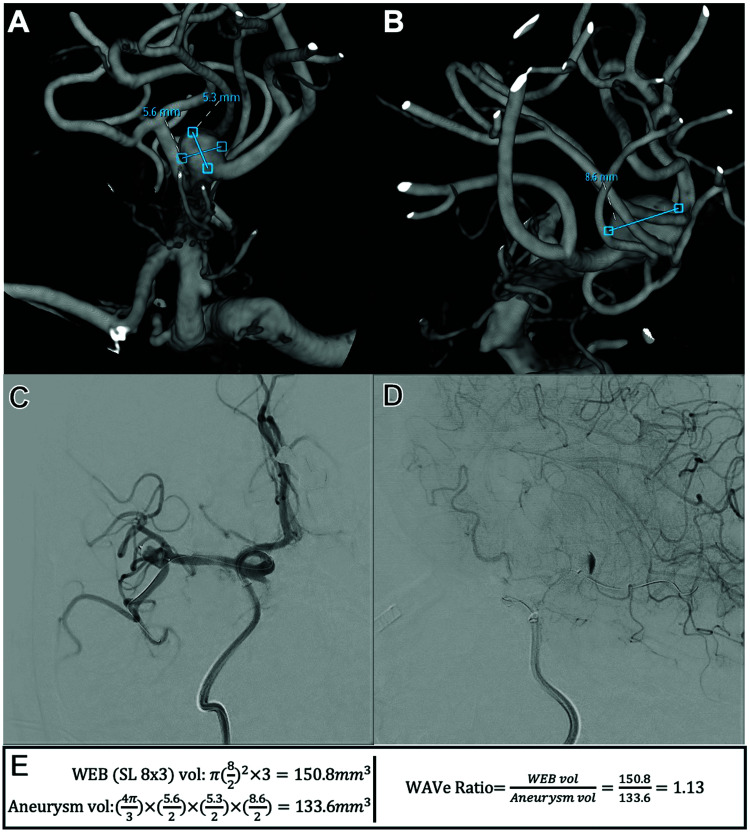
We collected demographics such as age, sex, and aneurysm rupture status from the electronic health record. Aneurysm morphometric details were collected from commercially available 3D reconstruction software at each institution. Aneurysm maximum diameter, width, depth, and height were measured manually (examples can be seen in Figures 1 and 2). This was measured either during treatment or if not present from the treatment records, they were re-measured by investigators not involved in the treatment procedure. Aneurysm volume was then calculated using the formula for an ellipsoid (Figures 1 and 2). Similarly, aneurysm neck diameter was measured in two orthogonal dimensions (Figures 1 and 2). These were also then averaged to calculate the average neck diameter.
Figure 1.
Sample patient demonstrating measurements, calculations, and formulae used: (A) three-dimensional reconstructed image from internal carotid artery angiographic injection. The view is close to an anteroposterior projection. Unobstructed view of anterior communicating artery aneurysm being embolized, measuring its dimensions. (B) Three-dimensional reconstructed image from internal carotid artery angiographic injection. The view is close to a lateral projection and is orthogonal to panel A. Unobstructed view of anterior communicating artery aneurysm being embolized, measuring its dimensions. (C) Working anteroposterior projection from internal carotid artery injection after WEB SL insertion demonstrating its adequate position within the aneurysmal sac without prolapse. (D) Working lateral projection from internal carotid artery injection after WEB SL insertion demonstrating its adequate position within the aneurysmal sac without prolapse. (E) Formulae using the measurements in this case to demonstrate WEB SL cylindric volume calculation, aneurysm ellipsoid volume calculation, and WAVe ratio calculation.
WEB SL: Woven EndoBridge single-layer; WAVe: Woven EndoBridge-aneurysm volume.
Figure 2.
Second sample patient demonstrating measurements, calculations, and formulae used: (A) three-dimensional reconstructed image from internal carotid artery angiographic injection. Unobstructed view of middle cerebral artery bifurcation aneurysm being embolized, measuring its dimensions. (B) Three-dimensional reconstructed image from internal carotid artery angiographic injection. Orthogonal to view in panel A. Unobstructed view of middle cerebral artery bifurcation aneurysm being embolized, measuring its dimensions. (C) Working anteroposterior projection from internal carotid artery injection after WEB SL insertion demonstrating its adequate position within the aneurysmal sac without prolapse into either M2 segment. The view is with significant submental rotation with some right anterior oblique via the AP plane. (D) Working lateral projection from internal carotid artery injection after WEB SL insertion demonstrating contrast stasis in the dome. (E) Formulae using the measurements in this case to demonstrate WEB SL cylindric volume calculation, aneurysm ellipsoid volume calculation, and WAVe ratio calculation.
WEB SL: Woven EndoBridge single-layer; WAVe: Woven EndoBridge-aneurysm volume.
WEB placement and volume ratio calculation
All WEBs were deployed using the corresponding VIA catheters. On the first attempt of aneurysm embolization, WEB size was selected based on the traditional sizing recommendations (the “+1/–1” rule): width was selected by adding 1–2 mm to the width of the aneurysm; device height was selected by subtracting the same amount added to the width from the height of aneurysm. On the second attempt, devices were selected by the proceduralist based on angiographic information from the first.2,6,7 Of note, pre-procedure and post-procedure antiplatelet regimens were dictated by proceduralist preference.
WEB devices were collected from electronic health records. Using manufacturer information, the volume for each WEB device was calculated by investigators not involved in the treatment procedure. For WEB SL devices, cylindric volumes were ascertained using the listed device specifications (Figure 1). For SLS, spheric volumes were similarly calculated. The WEB aneurysm-volume “WAVe” ratio was then calculated, by investigators not involved in the treatment procedure, by dividing the WEB volume by the calculated aneurysm volume as previously specified in hopes of finding an “ideal” (iWAVe) ratio (Figures 1 and 2). 7
Outcomes measured
The primary outcome of the measure was whether a WEB required re-sizing. This was defined as the opening of a second WEB device that was then placed in lieu of (or in addition to) the initial WEB attempted. We also created a composite outcome comprised of either WEB re-sizing or adjunctive stent placement. These were each performed under the judgment of the physician performing the procedure. This additional outcome was chosen as in our cohort we viewed stenting as a tool that was used when WEBs were not adequately sized (composite WEB sizing failure). Lastly, aneurysm occlusion using a digital subtraction angiogram at the most recent angiographic follow-up was assessed. The ordinal Bicêtre Occlusion Scale Score (BOSS) was retrospectively applied. 10 Given the variability of previous definitions for aneurysm occlusion we defined adequate occlusion as BOSS 0, 0′, and 1 or as BOSS 0–2 in separate analyses.7,11,12 Aneurysmal occlusion was graded at the latest angiogram available up to one-year post-procedure.
Statistical analysis
Demographic and baseline variables for the correctly sized and incorrectly sized groups were summarized as median (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. Differences between groups for demographic/baseline variables were assessed using a Mann-Whitney U-test for continuous variables and a chi-square test for categorical variables. For the primary analysis of assessing the relationship between correct WEB sizing and iWAVe ratio, logistic regression with restricted cubic spline was performed. The model with the most appropriate number of knots was decided by using the Akaike information criterion (AIC). A similar procedure was carried out for our secondary outcome, composite WEB sizing failure.
Results
Demographics
A total of 133 cases were included, 114 correctly sized and 19 incorrectly sized. Twelve patients (9.0%) required additional stent placement at the time of WEB insertion. In the original cohort comparison, stenting was not used as an outcome to suggest incorrect WEB sizing. One patient (0.8%) had complete WEB abandonment and was embolized with coils instead. There were no differences in demographic or baseline characteristics between the two groups aside from aneurysm location (Table 1). Specifically, “other” and basilar apex locations increased the likelihood of incorrect WEB sizing (p = 0.009). Other locations included the posterior communicating artery (N = 3), the posterior inferior cerebellar artery (N = 3), the pericallosal artery (N = 2), the vertebral artery (N = 2), and the ophthalmic artery (N = 1).
Table 1.
Demographic characteristics as observed between the correctly and incorrectly sized cohorts.
| Variable | Correctly sized (n = 114) | Incorrectly sized (n = 19) | p-value |
|---|---|---|---|
| Age (years, IQR) | 64.00 (56.75, 70.25) | 63.00 (49.00, 76.00) | 0.941 |
| Male | 34 (29.8%) | 5 (26.3%) | 0.756 |
| Aneurysm location | – | – | 0.009* |
| ACOM | 42 (36.8%) | 3 (15.8%) | – |
| Basilar apex | 19 (16.7%) | 7 (36.8%) | – |
| Distal ICA | 8 (7.0%) | 2 (10.5%) | – |
| MCA | 35 (30.7%) | 2 (10.5%) | – |
| Other | 10 (8.8%) | 5 (26.3%) | – |
| Ruptured | 19 (16.7%) | 4 (21.1%) | 0.640 |
| Aspirin use | 108 (94.7%) | 17 (89.5%) | 0.372 |
| Dual antiplatelet therapy | 70 (61.4%) | 11 (57.9%) | 0.772 |
IQR: interquartile range; ACOM: anterior communicating artery; ICA: internal carotid artery; MCA: middle cerebral artery.
* Significance at a pre-determined level, 0.05.
Aneurysm and WEB dimensional analysis
Many aneurysm size variables measured in our cohort were associated with an increased risk of re-sizing (Table 2). Namely, the larger the aneurysm, the larger the neck, and the larger the WEB device, the greater the risk of needing to re-size (Table 2). The median WAVe ratio in our appropriately sized cohort was 0.997 (IQR 0.826, 1.30) versus our re-sizing cohort which was 1.14 (IQR 0.734, 1.51; p = 0.728; Table 2). Our final WAVe ratio for completed procedures was 0.985 (IQR 0.802, 1.30). When a WEB was re-sized, the WAVe ratio was changed by a median of 0.314 (IQR 0.218, 0.470). Sixteen of the patients requiring re-sizing (84.2%) received a smaller WEB device than initially attempted versus only three patients (15.8%) who required a larger device. Notably, when using the thresholds set by Tanabe et al. or Ansari et al. for iWAVe (0.90–1.16 and 0.6–0.8, respectively) we did not find a statistical agreement that it improved the rate of re-sizing (p = 0.433, p = 0.548, respectively; Table 2).
Table 2.
Radiographic characteristics of measured aneurysms, WEB used, WEB-aneurysm volume ratios, and aneurysm occlusion at follow-up.
| Variable | Correctly sized (n = 114) | Incorrectly sized (n = 19) | p-value |
|---|---|---|---|
| Max aneurysm diameter (mm, IQR) | 7.10 (5.50, 8.80) | 7.80 (6.30, 8.90) | 0.010* |
| Aneurysm height (mm, IQR) | 6.40 (5.20, 8.50) | 7.00 (5.40, 8.70) | 0.082 |
| Aneurysm width (mm, IQR) | 5.70 (4.60, 7.30) | 6.30 (4.50, 7.80) | 0.026* |
| Average neck diameter (mm, IQR) | 3.70 (3.10, 4.35) | 3.55 (3.25, 4.35) | 0.036* |
| Average aneurysm volume (mm3, IQR) | 117.0 (73.9, 204) | 197 (96.7, 312) | 0.007* |
| WEB volume (mm3, IQR) | 115 (58.9, 251) | 201 (113, 382) | 0.001* |
| WAVe ratio (IQR) | 0.997 (0.826, 1.30) | 1.14 (0.734, 1.51) | 0.728 |
| WAVe ratio 0.90–1.16 | 30 (26.3%) | 4 (21.1%) | 0.433 |
| WAVe ratio 0.60–0.80 | 15 (13.2%) | 2 (10.5%) | 0.548 |
| Final WAVe ratio (IQR) | 0.997 (0.826, 1.30) | 0.926 (0.604, 1.30) | 0.218 |
| Final BOSS grade | – | – | 0.309 |
| 0 | 45 (53.6%) | 7 (70.0%) | – |
| 0′ | 8 (9.5%) | 0 (0.0%) | – |
| 1 | 19 (22.6%) | 3 (30.0%) | – |
| 2 | 8 (9.5%) | 0 (0.0%) | – |
| 3 | 3 (3.6%) | 0 (0.0%) | – |
| 1 + 3 | 1 (1.2%) | 0 (0.0%) | – |
| Angiography follow-up (months, IQR) | 7.52 (4.4, 10.9) | 6.51 (2.8, 9.3) | 0.282 |
IQR: interquartile range, WEB: Woven EndoBridge, WAVe: WEB-aneurysm volume, BOSS: Bicêtre Occlusion Scale Score.
* Significance at a pre-determined level, 0.05.
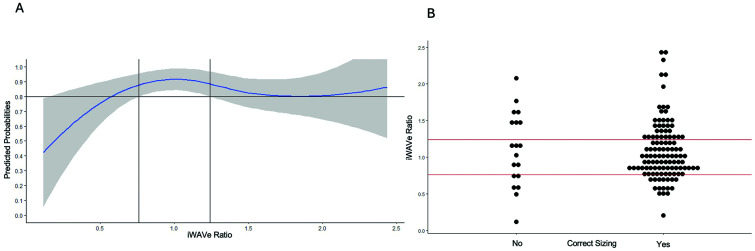
We then sought to define an iWAVE ratio based on our cohort. Based on the AIC value, a model with four knots was selected. This included two boundary knots (0.1179050 and 2.4367816) and two internal knots (0.8579926, 1.2111468). Using predicted probabilities calculated from this model, a WAVe ratio ranging from 0.76 to 1.24 gives at least an 80% probability of successful sizing based on the lower limit of the 95% confidence interval (CI; (Figure 3). This model also suggested that changes in smaller WAVe ratios will have a larger impact on successful sizing than changes in higher WAVe ratios. This means that each unit increase in the WAVe ratio increases the probability of successful sizing up to the first internal knot (0.858). This is shown in the model summary with the first segment of the spline model being significantly associated with outcome (Figure 4B).
Figure 3.
Re-sizing data outcomes: (A) logistic regression demonstrating the relationship of the WAVe ratio and predicted probability of correct initial sizing. The shaded area represents the 95% confidence interval. The model and curve were created using splinic knots (0.8579926, 1.2111468). Using predicted probabilities calculated from this model, a WAVe ratio ranging from 0.76 to 1.24 gives at least an 80% probability of successful sizing based on the lower limit of the 95% confidence interval. (B) Dot plot representing the distribution of correct and incorrect initial WEB sizing in our patients on the first attempt. The lines across the dot plot depict the same splinic knots.
WAVe: Woven EndoBridge-aneurysm volume; WEB: Woven EndoBridge.
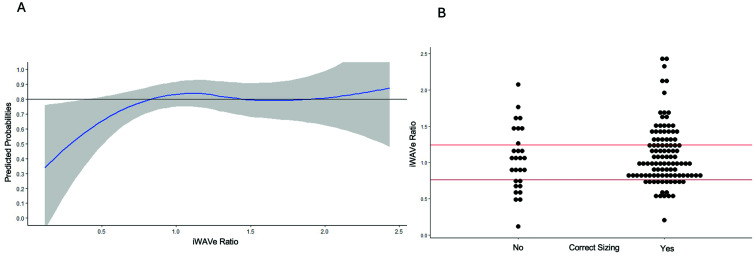
Figure 4.
Composite re-sizing data outcomes (re-sizing required or concomitant stent placed): (A) logistic regression demonstrating the relationship of the WAVe ratio and predicted probability of correct initial sizing. The shaded area represents the 95% confidence interval. The model and curve were created using the same methodology as the first regression model however using predicted probabilities calculated from this model, no WAVe ratio yielded at least an 80% probability of successful sizing due to the lower limit of the 95% confidence interval. (B) Dot plot representing the distribution of correct and incorrect initial WEB sizing in our patients on the first attempt. The lines across the dot plot depict the same splinic knots as Figure 2 for comparison due to the inability to find appropriate ranges in this outcome.
WAVe: Woven EndoBridge-aneurysm volume; WEB: Woven EndoBridge.
We undertook the same analysis for our composite outcome (re-sizing or stent placement required). Due to the 12 cases where concomitant stents were placed, the cohort sizes in this analysis were 105 patients who had correct sizing and 28 who did not (three patients with stent placement had already required re-sizing prior to stent placement and as such were initially in the incorrectly sized cohort). For this composite outcome, the median WAVe in correctly sized was 1.00 (IQR 0.830, 1.31), and in incorrectly sized was 1.03 (0.701, 1.39) yielding p = 0.566. For this composite outcome, there was no WAVe ratio which would yield > 80% probability of successful sizing (Figure 4).
Aneurysm occlusion
Lastly, we assessed aneurysm occlusion. This was determined at the patient's most recent angiogram up to one year. When adequate occlusion was defined as BOSS 0, 0′, or 1, we obtained an 87.2% occlusion rate (Table 2). When defined as BOSS 0, 0′, 1, or 2, we obtained a rate of 95.7% (Table 2). The median time of angiography follow-up was 7.4 months. Of those with a BOSS higher than 2, the median WAVe was 0.962 (IQR 0.834, 1.22). We did not find that dual antiplatelet therapy was associated with a shift in BOSS scores (p = 0.204) nor a change in the rate of BOSS 0–1 (p = 0.924) or BOSS 0–2 (p = 0.373). We also found no difference in aneurysm occlusion rates based on differences between final WEB width and average aneurysm neck diameter (BOSS 0–1 median 2.9 mm (IQR 2.2, 3.74 mm); BOSS ≥ 2 median 2.95 mm (IQR 1.50, 5.03 mm).
Discussion
In our multicenter analysis of 133 patients with WEB device placement for the treatment of WNBA, a ratio of WEB volume/aneurysm volume between 0.76 and 1.24 resulted in at least an 80% probability of successful sizing on initial device insertion attempts. Further, we found that this ratio had a greater impact on the success of device placement when the ratio was < 0.858, beyond this ratio, it appeared to have less impact on the probability of success. Increasing aneurysm size, increasing aneurysm neck diameter, and increasing WEB volumes increased the risk of incorrect WEB sizing. Lastly, no matter the need to re-size or the final WEB volume/aneurysm volume ratio, we obtained good rates of aneurysm occlusion at follow-up (87.2% and 95.4% depending on the definition used).
Our results stand in difference to two prior studies of note. First, Tanabe et al. 7 found an ideal device-to-aneurysm volume ratio to be between 0.90 and 1.16. Second, Ansari et al. 8 found an ideal ratio to be between 0.60 and 0.80. It's interesting to note how disparate these two studies’ results are. Our findings are more closely in line with Tanabe et al. however even still, we did not find any statistically significantly improved accuracy of sizing within their designated ideal threshold. Aside from the iWAVe ratio in our cohort being slightly between the two prior studies, we also had a wider interval identified than either study suggesting there is a greater variability in WEB volume/aneurysm volume ratios that can be tolerated for successful treatment. This suggests that volume-based sizing methods may only partly contribute to successful WEB sizing when used.
In the prior two studies, only Ansari et al.'s study had access to 3D automated segmentation software, which other studies have assessed to be a more accurate measure of aneurysm sizing.8,9,13 Unfortunately, this technology is not readily accessible at all centers and the difference in r values between 3D automated versus manual measurements is typically between 0.01 and 0.1 depending on the study, suggestive of questionable clinical relevance. We felt it was thus a reasonable comparison to use manual measurements on 3D angiographic runs. We believe our study also has additional relevance due to its multi-institutional nature (the previous studies are single-center) and large patient population (our study consists of 3–4× as many patients as previous studies).
For practical consideration, our results suggest that one should attempt to match the WEB device volume to the aneurysm volume. However, there are some limitations to this approach. Mainly, the WEB device only fills the main aneurysm compartment, the presence of daughter sacs or irregularities to the aneurysm shape will affect the need to perfectly match the WEB volume to the aneurysm volume. It is also possible that for a given aneurysm volume, there are multiple WEBs of different height/width configurations (for SL) with a matching volume. In this case height/width measurements are invaluable. It is also clear from our study that this becomes more challenging as we work to treat larger aneurysms. This is exemplified by our findings that the larger the aneurysm, the neck, or the WEB, the greater the risk for re-sizing. As the scale increases, device selection becomes harder due to the increased variety of options (as well as lessened maneuverability) though volume-based sizing may help with this. How to best reconcile the balance of sizing remains to be seen.
Based on the BOSS definition used, we achieved either an 87.2% or 95.7% occlusion rate at a median of 7.4 months via angiography. The occlusion rates were not affected by the need to re-size WEBs, the WAVe ratio, or dual antiplatelet therapy administration. It is worth noting that the principal outcome of our study (and the other two volume-measurement-based studies) is based on real-time practitioner assessment of adequate device placement, lateral wall compression, intussusception, and prolapse (and possibly other factors). No study so far has found these volume-based sizing methods to be associated with aneurysm occlusion in follow-up.7,8 While this may prompt questions about its clinical relevance, a volume-assisted approach does appear to lower rates of re-sizing compared to the previously reported 20%–30% which is at least important from a cost standpoint given the device at cost is between $15,000 and $20,000.7–9 These results are in contrast to other studies. One by Kewlani et al. 14 found that higher WAVe ratios increased the likelihood of aneurysm occlusion. Nawka et al. 11 found that increasing differences between WEB width and aneurysm diameter increased rates of early aneurysm occlusion, something we also did not find. Of note, Nawka et al.'s study assessed this at a six-month angiogram whereas our median angiogram post-procedure for determination of occlusion was at seven months. It is also important to note that there are other factors found to be important for aneurysm occlusion which may not be well factored into simply a volume-based sizing method such as neck wall apposition. 15 Irregularly-shaped aneurysms which may either be difficult to reliably measure or also may result in devices sitting askew, also appear to be risks for non-occlusion and may explain the lack of consistency of effect from volume-based measurements. 16 New automated technology may be helpful in picking appropriate WEB sizes and avoiding some of these pitfalls. 13
Limitations
Our study features several limitations. First, it is doubtful that the physical WEB size is the only factor involved with the need for WEB re-sizing. Given the flexibility of the WEB device, the angle with which it is inserted, or the forces applied during insertion (e.g. pull-out versus push-in) may have an impact on the WEB device's position or final shape. This may impact a proceduralist's view that a WEB should be re-sized. Second, the measurement of aneurysm volume to be treated can be challenging. Appropriately selecting where to measure the aneurysm dimensions and where a daughter sac or irregularity irrelevant to the WEB final location starts or ends can be difficult. Another challenge in volume measurement was we treated each aneurysm as a spheroid, which may have been an oversimplification to accurately measure volumes. These factors may have led to some inconsistencies in aneurysm volumes and thus WEB-aneurysm volume ratios. It is unclear if using automated software would assist with this aspect. Perhaps most importantly, the need to re-size was based on proceduralist discretion. While we performed a multicenter study to attempt to mitigate this bias, it likely still had an impact on our results. This is exemplified by our findings and the two prior studies’ findings that none of the studied factors altered occlusion rates.7,8 This raises some controversy as to the clinical relevance of aneurysm volume-based matching for WEB sizing. However, even if it does not impact aneurysmal occlusion, if this methodology is successful in reducing re-sizing needs, it will lower procedural times, complications for patients, radiation exposure, and costs (which can be sizeable).
Conclusion
Optimal WEB sizing is essential for successful aneurysm treatment, reduction of costs, and avoidance of complications. In our multi-institutional cohort, we found that a ratio of WEB volume to aneurysm volume of 0.76–1.24 provides the greatest probability of successful initial sizing when using a volume-matching-based model. We recommend further studies assessing the impact of volume-based sizing on successful and timely aneurysm occlusion perhaps compared to the traditional “+1/–1 rule.”
Consent to participate: A multicenter retrospective study that received institutional review board approval.
Consent for publication: Not applicable, no identifiable data included.
Data availability: Data available on request from the authors.
Competing interests: EP: None. JHW: None. AAB: None. GJR: None. SS: None. JVJ: None. TS: None. PY: None. JMD: Stock options: Synchron, Cerebrotech, QAS.AI; Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: Medtronic, Rapid Medical; Support for attending meetings and/or travel: Medtronic, Rapid Medical; Patents planned, issued or pending: QAS.AI; Participation on a Data Safety Monitoring Board or Advisory Board: NIH NIHDS Strokenet. AHS: Financial Interest/Investor/Stock Options/Ownership: Adona Medical, Bend IT Technologies, BlinkTBI, Borvo Medical, Cerebrotech Medical Systems, Code Zero Medical, Cognition Medical, Collavidence, CVAID, E8, Endostream Medical, Galaxy Therapeutics, Hyperion Surgical, Imperative Care, InspireMD, Instylla, Launch NY, Neurolutions, NeuroRadial Technologies (sold to Medtronic in 2021), Neurovascular Diagnostics, Peijia Medical, PerFlow Medical, Piraeus Medical, Q’Apel Medical, QAS.ai, Radical Catheter Technologies, Rebound Therapeutics Corp (purchased 2019 by Integra Lifesciences Corp), Rist Neurovascular (purchased 2020 by Medtronic), Sense Diagnostics, Serenity Medical, Silk Road Medical, Sim & Cure, Spinnaker Medical, StimMed, Synchron, Tulavi Therapeutics, Vastrax, Viseon, Whisper Medical, Willow Medtech, Consultant/Advisory Board: Amnis Therapeutics, Apellis Pharmaceuticals, Boston Scientific, Canon Medical Systems USA, Cardinal Health 200, Cerebrotech Medical Systems, Cerenovus, Cordis, Corindus, Endostream Medical, Hyperfine Operations, Imperative Care, InspireMD, Integra, IRRAS AB, Medtronic, MicroVention, Minnetronix Neuro, Peijia Medical, Penumbra, Piraeus Medical, Q’Apel Medical, Rapid Medical, Serenity Medical, Silk Road Medical, StimMed, Stryker Neurovascular, VasSol, Viz.ai; National PI/Steering Committees: Cerenovus EXCELLENT and ARISE II Trial; Medtronic SWIFT PRIME, VANTAGE, EMBOLISE and SWIFT DIRECT Trials; MicroVention FRED Trial & CONFIDENCE Study; MUSC POSITIVE Trial; Penumbra 3D Separator Trial, COMPASS Trial, INVEST Trial, MIVI Neuroscience EVAQ Trial; Rapid Medical SUCCESS Trial; InspireMD C-GUARDIANS IDE Pivotal Trial; Patent: Patent No. US 11,464,528 B2, Date: October 11, 2022, Clot Retrieval System for Removing Occlusive Clot from a Blood Vessel, Applicant and Assignee: Neuravi Limited (Galway), Role: Co-Inventor. EIL: Consulting fees: Clarion, GLG Consulting, Guidepoint Global, Imperative Care, Medtronic, StimMed, Misionix, Mosiac; Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: Medtronic, Penumbra, MicroVention, Integra; Patents planned, issued or pending: Ultrasonic Surgical Blade; Participation on a Data Safety Monitoring Board or Advisory Board: Stryker (AIS Clinical Advisory Board), NeXtGen Biologics, MEDX, Cognition Medical; Endostream Medical, IRRAS AB; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: CNS, ABNS, UBNS: Stock or stock options (shareholder or ownership interest): NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care, Rebound Therapeutics, StimMed, Three Rivers Medical; Other financial or non-financial interests: Haniva Medical Technology (Chief Medical Officer); Medtronic (National PI: Steering Committee for SWIFT Prime and SWIFT Direct trials; Site PI: STRATIS Study Sub 1), MicroVention (Site PI: CONFIDENCE Study). WRG: None. MM: Grants: NIH; Consultant: Balt, Cerenovus, Medtronic, Rapid Pulse; Stock options: Bendit Technologies, Borvo Medical, BrainQ, Endostream, Radical Catheter Technologies, Serenity Medical, Synchron, Sim&Cure, QAS.AI, Quantanosis.AI; Payment for expert testimony: Foley Mansfield, Huff Powell Bailey. KV: Consultant: Imperative Care, MicroVention.
Ethical approval and informed consent: A multicenter retrospective study that received institutional review board approval.
Ethical consideration: Received institutional review board approval.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Elliot Pressman https://orcid.org/0000-0002-5160-802X
Ammad A Baig https://orcid.org/0000-0002-8241-6864
Teagen Smith https://orcid.org/0000-0002-5573-3178
Jason M Davies https://orcid.org/0000-0002-5225-3072
Adnan H Siddiqui https://orcid.org/0000-0002-9519-0059
Samantha Schimmel BA https://orcid.org/0000-0001-9860-8820
References
- 1.Muskens IS, Senders JT, Dasenbrock HH, et al. The Woven EndoBridge device for treatment of intracranial aneurysms: a systematic review. World Neurosurg 2017; 98: 809–817.e1. [DOI] [PubMed] [Google Scholar]
- 2.Goyal N, Hoit D, DiNitto J, et al. How to WEB: a practical review of methodology for the use of the Woven EndoBridge. J Neurointerv Surg 2020; 12: 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rooij SBT, van Rooij WJ, Peluso JP, et al. WEB treatment of ruptured intracranial aneurysms: a single-center cohort of 100 patients. AJNR Am J Neuroradiol 2017; 38: 2282–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierot L, Liebig T, Sychra V, et al. Intrasaccular flow-disruption treatment of intracranial aneurysms: preliminary results of a multicenter clinical study. AJNR Am J Neuroradiol 2012; 33: 1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubicz B, Mine B, Collignon L, et al. WEB device for endovascular treatment of wide-neck bifurcation aneurysms. AJNR Am J Neuroradiol 2013; 34: 1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierot L. Ten years of clinical evaluation of the Woven EndoBridge: a safe and effective treatment for wide-neck bifurcation aneurysms. Neurointervention 2021; 16: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanabe J, Nakahara I, Ishihara T, et al. Decision-making tree for optimal Woven EndoBridge device sizing with ideal Woven EndoBridge-aneurysm volume (iWAVe) ratio. J Clin Neurosci 2023; 114: 55–61. [DOI] [PubMed] [Google Scholar]
- 8.Ansari S, Zevallos CB, Farooqui M, et al. Optimal Woven EndoBridge (WEB) device size selection using automated volumetric software. Brain Sci 2021; 11: 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah KA, White TG, Teron I, et al. Volume-based sizing of the Woven EndoBridge (WEB) device: a preliminary assessment of a novel method for device size selection. Interv Neuroradiol 2021; 27: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caroff J, Mihalea C, Tuilier T, et al. Occlusion assessment of intracranial aneurysms treated with the WEB device. Neuroradiology 2016; 58: 887–891. [DOI] [PubMed] [Google Scholar]
- 11.Nawka MT, Fiehler J, Bester M, et al. Impact of Woven EndoBridge shape modification on aneurysm recanalization at short-term follow-up digital subtraction angiography. Neurosurgery 2022; 90: 597–604. [DOI] [PubMed] [Google Scholar]
- 12.Simgen A, Weyrich A, Dietrich P, et al. Treatment of wide-necked cerebral aneurysms using the WEB device including flow alteration assessment with color-coded imaging: a single center experience. World Neurosurg X 2023; 17: 100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cagnazzo F, Marnat G, Ferreira I, et al. Comparison of Woven EndoBridge device sizing with conventional measurements and virtual simulation using the Sim&Size software: a multicenter experience. J Neurointerv Surg 2021; 13: 924–929. [DOI] [PubMed] [Google Scholar]
- 14.Kewlani B, Ryan DJ, Henry J, et al. A single centre retrospective analysis of short- and medium-term outcomes using the Woven EndoBridge (WEB) device and identification of the device-to-aneurysm volume ratio as a potential predictor of aneurysm occlusion status. Interv Neuroradiol 2023; 29: 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortese J, Juhasz J, Rodriguez-Erazú F, et al. Neck apposition is a key factor for aneurysm occlusion after Woven EndoBridge device embolization. J Neurointerv Surg 2024: jnis-2024-022155. [DOI] [PubMed] [Google Scholar]
- 16.Cortese J, Caroff J, Chalumeau V, et al. Determinants of cerebral aneurysm occlusion after embolization with the WEB device: a single-institution series of 215 cases with angiographic follow-up. J Neurointerv Surg 2023; 15: 446–451. [DOI] [PubMed] [Google Scholar]