Abstract
Introduction: To explore the effect of cigarette smoking on the risk for developing diabetic macular edema (DME) among patients with diabetes. Methods: This retrospective exactly-matched cohort study used claims data for patients from all 50 states in the United States from 2010 through 2020. Patients with an initial diagnosis of diabetes were stratified into 3 cohorts as follows: active smokers, never smokers, and former smokers. After exact matching based on demographics and comorbidities, Kaplan-Meier survival functions for the 3 cohorts were compared using pairwise log-rank tests. Results: After matching, there were 42 298 patients in each cohort. Over 6 years of follow-up, the cumulative risk for DME was significantly higher among never smokers (1.18%) than among active smokers (0.88%) and former smokers (0.90%) (both P < .001). Conclusions: Among patients with diabetes, smoking may decrease the risk for developing DME. Although the harms of smoking far outweigh any potential protective benefits, further investigation into the mechanisms behind these findings has potential to uncover new therapeutic targets.
Keywords: diabetes, diabetic retinopathy, diabetic macular edema, tobacco, smoking
Introduction
Nearly 30 million individuals in the United States have diabetes mellitus (DM), and it has been estimated that up to 33% of the US population will have diabetes by 2050.1,2 Approximately one third of patients with diabetes will develop diabetes-related eye damage, which is the leading cause of blindness in working-age adults globally.3–5 The most common mechanism of vision loss in patients with diabetes is macular edema (ME).5–7
Several researchers have studied risk factors for developing diabetic ME (DME).8–12 There is consensus across these studies that hypertension, chronic kidney disease, a longer duration of diabetes, insulin use, and higher hemoglobin A1c (HbA1c) are all risk factors for developing DME.
Studies have shown that smokers are 30% to 40% more likely than nonsmokers to develop type 2 DM.13,14 Furthermore, smoking is known to hasten the onset of other health complications linked to diabetes, including heart disease, chronic lower respiratory disease, and cerebrovascular disease.13,15 Several mechanisms contribute to the harmful effects of smoking. Tobacco smoke is comprised of more than 4000 different compounds, which are humidified in the respiratory tract before condensing in the lungs, leaving an estimated 50% to 95% deposited in the bronchi, bronchioles, and alveoli. 16 This leads to an increase in endogenous free radicals and oxidative stress, increased inflammation, vasomotor dysfunction, and smooth muscle proliferation. However, whether smoking is associated with the development of DME has not been well elucidated. Many of the first studies of this topic found no association between smoking and DME,8–10 while 2 more recent studies suggest a possible decreased risk for DME among smokers.11,12
The primary objective of this study was to leverage an extensive administrative claims database with patient records from all 50 states in the US to investigate the relationship between tobacco smoking and the development of DME. A secondary objective was to use the same database to validate previous findings in the literature that show insulin use, hypertension, and chronic kidney disease to be risk factors for the development of DME.
Methods
Data Source
This retrospective exactly-matched cohort study used the MARINER database (PearlDiver Inc), an all-payer claims database that includes claims from all 50 states in the US. 17 The database contains longitudinal analytic files containing all inpatient, outpatient, drug, and laboratory claims for more than 53 million patients from 2010 through 2020. The database was queried using Current Procedural Terminology (CPT), International Classification of Diseases (ICD)-9, and ICD-10 procedure codes. DME was defined as (1) having an ICD code for DME or (2) having ICD codes for retinal edema or cystoid macular degeneration in conjunction with ICD codes for diabetes. The use of ICD codes to identify DME in this manner was validated by Bearelly et al 18 with a specificity of 96% and a sensitivity of 88%, and this method has been used in several other publications.19–21 ICD and CPT codes were also used to define comorbidities, cohorts, and outcomes; these codes are delineated in Supplemental Table 1.
Participants
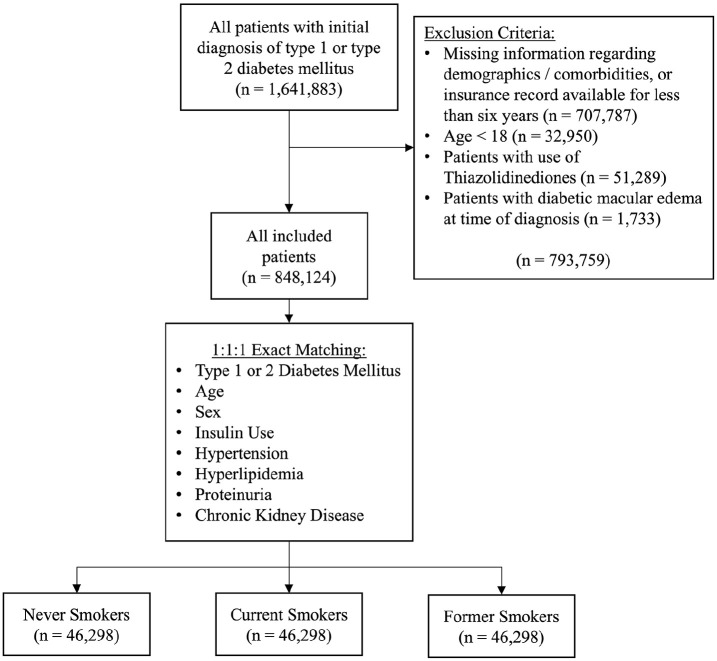
For inclusion in this study, patients (1) received their initial diagnosis of DM (type 1 or type 2) within the study period (2010–2020) and (2) had at least 6 years of continuous enrollment in the database after the initial diagnosis. Exclusion criteria were age less than 18 years, missing information regarding demographics or comorbidities of interest, the use of thiazolidinediones at any time during the study period, and the presence of DME at the time of initial diagnosis of diabetes (Figure 1). Comorbidities of interest included hypertension, hyperlipidemia, proteinuria, chronic kidney disease, and insulin use. Thiazolidinediones are insulin-sensitizing medications that are known to cause fluid retention and increase the risk for DME. 22
Figure 1.
Patient selection diagram.
Statistical Analysis
To assess the relationship between smoking status and the risk for developing DME, patients were first categorized into 3 cohorts as follows: current smokers, never smokers, and former smokers. Descriptive characteristics between cohorts were compared using χ2 tests. The 3 cohorts were exactly matched on a 1:1:1 basis according to age group, sex, and comorbidities of interest (ie, hypertension, hyperlipidemia, proteinuria, chronic kidney disease, and insulin use). Patients were followed for 6 years after the date of their initial diagnosis of diabetes. Kaplan-Meier survival functions were plotted, and pairwise log-rank tests were used to compare the 3 cohorts.
To determine the minimum sample size, a power analysis for log-rank tests was conducted according to the methodology outlined by Yung and Liu 23 and using a relative hazard of 0.7 and an annual incidence of 0.2%. The results indicated a minimum sample size of 11 452 in each cohort. Bonferroni correction was applied to the pairwise log-rank tests to set the significance level at α < .0167.
Finally, to validate previous findings that insulin use, hypertension, proteinuria, and chronic kidney disease are associated with DME and to validate the results of the primary analysis internally, a Cox proportional hazards model was applied to the unmatched data. All comorbidities and smoking status were used as covariates for this model.
All statistical analyses were completed using R software (R Project for Statistical Computing). The significance level was set at α < .05 unless otherwise specified. 24
Results
After the inclusion and exclusion criteria were applied, 848 124 patients remained in the unmatched population. Patients ranged from in age from 18 to 79 years, with nearly half (49.1%) falling between 60 years and 74 years. Among the study population, 16.1% were current smokers (Supplemental Table 2).
After exact matching, there were 46 298 patients in each of the 3 cohorts, for a total of 138 894 patients. The matched study population was predominantly male (54.1%) and 60 to 74 years old (62.7%). The most prevalent comorbidities were hypertension and hyperlipidemia (94.4% and 91.4%, respectively). There was no difference in age, sex, or any measured comorbidity between the 3 cohorts (Table 1). Over 6 years of follow-up, 1.0% of the matched population received a diagnosis of DME. Figure 2 shows the Kaplan-Meier survival curves for each cohort. The cumulative risk for DME in 6 years of follow-up among never smokers (1.18%) was significantly higher than the cumulative risk for current smokers (0.88%) and for former smokers (0.90%) (both P < .001). The cumulative risk was not significantly different between current smokers and never smokers (P = .84).
Table 1.
Demographics and Comorbidities of the Matched Population.
| Parameter | Number (%) | P Value | ||
|---|---|---|---|---|
| Never Smokers (n = 46 298) |
Current Smokers (n = 46 298) |
Former Smokers (n = 46 298) |
||
| Age range (y) | >.999 | |||
| 15–19 | 21 (0.05) | 21 (0.05) | 21 (0.05) | |
| 20–24 | 121 (0.26) | 121 (0.26) | 121 (0.26) | |
| 25–29 | 284 (0.61) | 284 (0.61) | 284 (0.61) | |
| 30–34 | 554 (1.2) | 554 (1.2) | 554 (1.2) | |
| 35–39 | 812 (1.8) | 812 (1.8) | 812 (1.8) | |
| 40–44 | 1425 (3.1) | 1425 (3.1) | 1425 (3.1) | |
| 45–49 | 2387 (5.2) | 2387 (5.2) | 2387 (5.2) | |
| 50–54 | 4235 (9.2) | 4235 (9.2) | 4235 (9.2) | |
| 55–59 | 6029 (13.0) | 6029 (13.0) | 6029 (13.0) | |
| 60–64 | 7732 (16.7) | 7732 (16.7) | 7732 (16.7) | |
| 65–69 | 9677 (20.9) | 9677 (20.9) | 9677 (20.9) | |
| 70–74 | 11 634 (25.1) | 11 634 (25.1) | 11 634 (25.1) | |
| 75–79 | 1387 (3.0) | 1387 (3.0) | 1387 (3.0) | |
| Sex | >.999 | |||
| Female | 21 253 (45.9) | 21 253 (45.9) | 21 253 (45.9) | |
| Male | 25 045 (54.1) | 25 045 (54.1) | 25 045 (54.1) | |
| Comorbidities | ||||
| Diabetes, type 1 | 167 (0.36) | 167 (0.36) | 167 (0.36) | >.999 |
| Diabetes, type 2 | 46 135 (99.6) | 46 135 (99.6) | 46 135 (99.6) | >.999 |
| Insulin | 10 754 (23.2) | 10 754 (23.2) | 10 754 (23.2) | >.999 |
| Hypertension | 43 711 (94.4) | 43 711 (94.4) | 43 711 (94.4) | >.999 |
| Hyperlipidemia | 42 320 (91.4) | 42 320 (91.4) | 42 320 (91.4) | >.999 |
| Proteinuria | 8322 (18.0) | 8322 (18.0) | 8322 (18.0) | >.999 |
| Chronic kidney disease | 15 819 (34.2) | 15 819 (34.2) | 15 819 (34.2) | >.999 |
Figure 2.
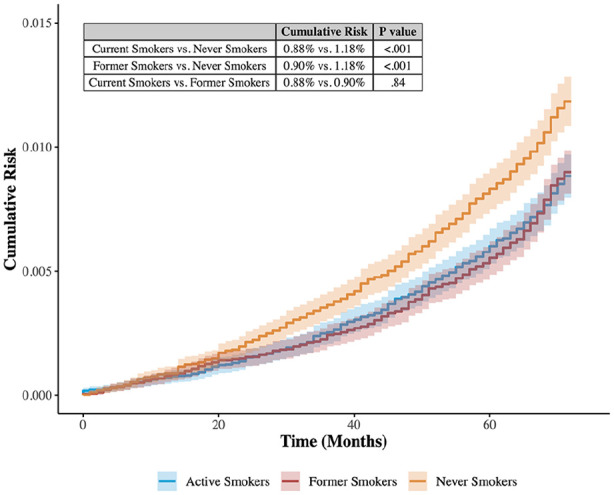
Smoking and the development of diabetic macular edema: Kaplan-Meier estimates.
In the Cox proportional hazards model applied to the unmatched population, hypertension (hazard ratio [HR], 2.14; 95% CI, 1.34-3.44), proteinuria (HR, 2.08; 95% CI, 1.84-2.34), chronic kidney disease (HR, 1.81; 95% CI, 1.59-2.04), and insulin use (HR, 3.49; 95% CI, 3.12-3.91) were significantly associated with an increased risk for developing DME. On the other hand, current smoking status (HR, 0.74; 95% CI, 0.65-0.85) and former smoking status (HR, 0.76; 95% CI, 0.66-0.86) were significantly associated with a decreased risk for developing DME (Supplemental Table 3).
Conclusions
Using a large all-payer claims database with longitudinal analytic files from 2010 through 2020, we retrospectively identified 848 124 patients with an initial diagnosis of diabetes and 6 years of available follow-up data. Of these patients, 89.2% had comorbid hypertension. The prevalence of hypertension among adults with type 2 diabetes has been estimated at 73.6% according to National Health and Nutrition Examination Survey (NHANES) data from the US Centers for Disease Control and Prevention (CDC). 25 The relatively higher proportion of patients with hypertension in our study population may be attributable to their older age, with more than 20% being between the ages of 70 years and 74 years. In our unmatched population, 16.1% were identified as current smokers, which corresponds well with CDC estimates that 15.1% of adults were current smokers in 2015, the midpoint of our study period. 26 After 1:1:1 exact matching, this survival analysis found that never smokers were more likely than current smokers and former smokers to be diagnosed with DME over 6 years. This finding adds to mounting evidence that smoking may have a protective effect against the development of DME.
The literature before 2010 was ambiguous on the relationship, or lack thereof, between smoking and DME. Among the first studies to investigate the relationship between smoking and DME was the Wisconsin Epidemiologic Study of Diabetic Retinopathy. This 1984 cross-sectional review of 2990 patients found no association between smoking and DME. 9 In 2009, Klein et al 8 followed up with 955 patients from their original cohort with type I diabetes. They found that smoking was positively correlated with DME in univariable analysis but not in a multivariable analysis. Kramer et al 27 reported similar results in 2008. In 2007, Romero et al 10 reported no significant relationship between smoking and DME among 112 patients with Type 1 diabetes.
Since 2010, 2 large-scale studies reported on the relationship between smoking and DME, and both found a negative relationship. In 2014, Varma et al 11 studied 1038 patients with type 1 or type 2 diabetes from the NHANES data who had complete retinal imaging. Using multivariable logistic regression, they found that current smokers were less likely to have DME (odds ratio [OR], 0.33; 95% CI, 0.15-0.74). In 2022, Lin et al. reported lower rates of DME among current smokers than among never smokers in a population of 1893 patients with type 2 diabetes, again using multivariable logistic regression (OR, 0.61; 95%. CI 0.40-0.94). 12 In the present study, the largest to date with 848 124 patients in the unmatched population, we report an HR of 0.74 for current smokers and 0.76 for former smokers. These results suggest that smoking may have a protective benefit against developing DME; however, the physiologic mechanism for such an effect remains unclear.
Although the pathophysiology of DME is complicated, its hallmark is alteration of the blood–retinal barrier. Upregulation of growth factors and cytokines alters the blood–retinal barrier through the breakdown of endothelial cell junctions, pericyte loss, and leukostasis. These growth factors and cytokines include vascular endothelial growth factor (VEGF), tumor necrosis factor, intercellular adhesion molecule, interleukins, and angiopoietins. 7 In fact, the mainstay of treatment for DME is anti-VEGF therapy, and many pipeline therapeutics for DME target these other factors.
There are several possible mechanisms by which smoking may decrease the risk for DME. For example, several studies suggest that smoking may inhibit the secretion of VEGF and subsequent endothelial cell migration.28–30 However, there is also some conflicting evidence that suggests nicotine increases secretion of VEGF. 31 Thus, evidence supporting the hypothesis that smoking may decrease the risk for DME by inhibiting VEGF is inconclusive. There are over 4000 active substances in cigarette smoke, any number of which may have pharmacologic effects that decrease the risk for DME.32,33 As such, further investigation has the potential to unveil novel therapeutic targets.
Although these results suggest that smoking may have a protective effect with regard to the development of DME, the harms of smoking far outweigh any protective benefits it may confer. Smoking is a major cause of all 4 leading causes of death in the US, which include heart disease, cancer, chronic lower respiratory diseases, and cerebrovascular disease. An estimated 480 000 deaths every year are attributable to smoking. 15
Regarding other risk factors associated with DME, studies assessing these have found HbA1c, the duration of diabetes, insulin use, hypertension, proteinuria, and chronic kidney disease to be risk factors.8–12 Our Cox regression analysis results support previous studies, finding hypertension, proteinuria, chronic kidney disease, and insulin use to be risk factors for developing DME. In addition, Romero et al 10 found a relationship between DME and hyperlipidemia. However, the only previous study to investigate hyperlipidemia as a risk factor found no relationship. 12 We did not find any statistically significant association between hyperlipidemia and the development of DME in our large patient cohort (Supplemental Table 3).
Ours was a retrospective cohort study; thus, the possibility of an unmeasured confounding variable influencing the results cannot be excluded. For example, HbA1c is a well-known risk factor for developing DME. One study found that the odds of developing DME rose sharply for patients with HbA1c greater than 7.0%, 11 and another study found that HbA1c greater than 8.0% was associated with increased macular thickness. 34 Yet another study found significantly increased rates of DME among those with HbA1c greater than 7.0%. 12 If patients in the current smokers and former smokers cohorts in our study had significantly lower HbA1c than never smokers, this could account for the associations measured in our study. However, previous studies have shown that among patients with diabetes, smoking is associated with higher, not lower, HbA1c.35–38 Another possible confounding variable is body mass index (BMI). If smoking is associated with lower BMI and lower BMI is associated with a decreased risk for developing DME, this could also explain our results. 39 Notably, however, many confounders that were not explicitly measured in this analysis, including HbA1c, BMI, ethnicity, educational level, health insurance status, history of cardiovascular disease, and ethnicity, were measured in previous studies. These previous analyses produced similar results, finding lower rates of DME among smokers than among never smokers.11,12
In addition, although exact matching was used in this study to reduce the risk for confounders, it cannot be entirely ruled out that it could introduce unintended selection bias. For instance, there is a well-established association between smoking and hyperlipidemia.40,41 Matching the rates of hyperlipidemia among current smokers and never smokers may select a subset of never smokers with greater baseline susceptibility to hyperlipidemia. Similar selection bias could be introduced as a result of matching for any of the covariates, including hypertension, proteinuria, chronic kidney disease, and insulin use. Such selection bias could result in a nonrepresentative subset of never smokers being included in the primary analysis. Notably, in the supplemental analysis, a Cox proportional hazards model was applied to the entire unmatched population and was thus not subject to the same risk for selection bias. The results of the Cox model corroborated the findings of the primary analysis that current smoking status and former smoking status conferred a decreased risk for DME. Furthermore, exact matching was not used in either of the 2 most recent studies of this topic, both of which produced similar results.11,12 This consistency in results across studies using slightly different study populations, covariates, and statistical techniques strengthens the evidence of a true association between smoking and a decreased risk for DME.
Administrative claims data are primarily collected for billing purposes. Although studies have validated the use of ICD codes for identifying DME, smoking status, and many of the comorbidities measured in this study, these codes are not 100% accurate.18,42–45 As such, it is possible that incomplete coding, coding errors, or upcoding may have affected the results. Another limitation is the level of detail available in the database. Variables such as HbA1c and number of cigarettes smoked per day could not be measured and analyzed. Further, information regarding the patients’ ethnicity was not available for analysis in the database used in this study. Finally, the follow-up period of this study was limited to 6 years. Diabetic eye disease is a chronic condition that may take several years to develop, and a longer follow-up may provide more robust results.
In conclusion, DME is a leading cause of vision loss among working-age adults globally. The present study provides evidence that among patients with diabetes, smoking may decrease the risk for developing DME. Although this result is counterintuitive, thousands of active chemicals in cigarette smoke may have pharmacologic effects that protect against the development of DME. Thus, further investigation into the physiologic mechanisms behind these findings has the potential to uncover novel therapeutic targets.
Supplemental Material
Supplemental material, sj-docx-1-vrd-10.1177_24741264241269479 for Association Between Tobacco Smoking and the Development of Diabetic Macular Edema by Kyle B. Thomson, Syed I. Khalid, Naryan Sabherwal and Michael J. Heiferman in Journal of VitreoRetinal Diseases
Footnotes
Disclaimers: The claims expressed in the submitted article are based on independent research and are not the official position of the affiliated institutions.
Ethical Approval: This study was approved by the University of Illinois at Chicago Institutional Review Board. All study protocols and procedures were conducted in accordance with the Declaration of Helsinki.
Statement of Informed Consent: The Institutional Review Board approved this study with a waiver of patient informed consent because the nature of this analysis posed minimal risk to participating individuals and the data were presented in aggregate to minimize any risk for loss of confidentiality of medical data.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kyle B. Thomson  https://orcid.org/0000-0002-0009-9851
https://orcid.org/0000-0002-0009-9851
Supplemental Material: Supplemental material is available online with this article.
References
- 1. Holekamp NM. Overview of diabetic macular edema. Suppl Featur Publ. 2016;22(10 Suppl):s284-s291. Accessed May 12, 2022. https://www.ajmc.com/view/overview-of-diabetic-macular-edema [PubMed] [Google Scholar]
- 2. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8(1):29. doi: 10.1186/1478-7954-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruta LM, Magliano DJ, Lemesurier R, Taylor HR, Zimmet PZ, Shaw JE. Prevalence of diabetic retinopathy in type 2 diabetes in developing and developed countries. Diabet Med J Br Diabet Assoc. 2013;30(4):387-398. doi: 10.1111/dme.12119 [DOI] [PubMed] [Google Scholar]
- 4. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35(3):556-564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CDC. Diabetes and Vision Loss. Centers for Disease Control and Prevention. 2021. Accessed May 19, 2022. https://www.cdc.gov/diabetes/managing/diabetes-vision-loss.html [Google Scholar]
- 6. Mitchell P, Annemans L, Gallagher M, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol. 2012;96(5):688-693. doi: 10.1136/bjophthalmol-2011-300726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122(7):1375-1394. doi: 10.1016/j.ophtha.2015.03.024 [DOI] [PubMed] [Google Scholar]
- 8. Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BEK. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXIII. The twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497-503. doi: 10.1016/j.ophtha.2008.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464–1474. doi: 10.1016/S0161-6420(84)34102-1 [DOI] [PubMed] [Google Scholar]
- 10. Romero P, Baget M, Mendez I, Fernández J, Salvat M, Martinez I. Diabetic macular edema and its relationship to renal microangiopathy: a sample of type I diabetes mellitus patients in a 15-year follow-up study. J Diabetes Complications. 2007;21(3):172-180. doi: 10.1016/j.jdiacomp.2006.07.008 [DOI] [PubMed] [Google Scholar]
- 11. Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334-1340. doi: 10.1001/jamaophthalmol.2014.2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin Z, Wang FH, Wen L, et al. Prevalence of and risk factors for diabetic macular edema in a northeastern Chinese population. Int J Ophthalmol. 2022;15(2):320-326. doi: 10.18240/ijo.2022.02.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CDC. Smoking and Diabetes. Centers for Disease Control and Prevention. 2022. Accessed October 4, 2023. https://www.cdc.gov/diabetes/library/features/smoking-and-diabetes.html [Google Scholar]
- 14. Chang SA. Smoking and type 2 diabetes mellitus. Diabetes Metab J. 2012;36(6):399-403. doi: 10.4093/dmj.2012.36.6.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonnie RJ, Stratton K, Kwan LY, Products C; on the PHI of R the MA for PT, Practice B on PH and PH, Medicine I of. The Effects of Tobacco Use on Health. National Academies Press (US); 2015. Accessed April 9, 2023. https://www.ncbi.nlm.nih.gov/books/NBK310413/ [Google Scholar]
- 16. Adams TN, Morris J. Smoking. [Updated 2023 May 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Accessed October 4, 2023. https://www.ncbi.nlm.nih.gov/books/NBK537066/ [Google Scholar]
- 17. PearlDiver. 2021. http://www.pearldiverinc.com/researchinfo.html
- 18. Bearelly S, Mruthyunjaya P, Tzeng JP, et al. Identification of patients with diabetic macular edema from claims data: a validation study. Arch Ophthalmol. 2008;126(7):986-989. doi: 10.1001/archopht.126.7.986 [DOI] [PubMed] [Google Scholar]
- 19. Holekamp NM, Campbell J, Almony A, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83-91. doi: 10.1016/j.ajo.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 20. Lundeen EA, Andes LJ, Rein DB, et al. Trends in prevalence and treatment of diabetic macular edema and vision-threatening diabetic retinopathy among Medicare part B fee-for-service beneficiaries. JAMA Ophthalmol. 2022;140(4):345-353. doi: 10.1001/jamaophthalmol.2022.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. VanderBeek BL, Shah N, Parikh PC, Ma L. Trends in the care of diabetic macular edema: analysis of a national cohort. PLoS One. 2016;11(2):e0149450. doi: 10.1371/journal.pone.0149450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med. 2012;172(13):1005-1011. doi: 10.1001/archinternmed.2012.1938 [DOI] [PubMed] [Google Scholar]
- 23. Yung G, Liu Y. Sample size and power for the weighted log-rank test and Kaplan-Meier based tests with allowance for nonproportional hazards. Biometrics. 2020;76(3):939-950. doi: 10.1111/biom.13196 [DOI] [PubMed] [Google Scholar]
- 24. R Core Team. R: a language and environment for statistical computing. 2021. Accessed April 8, 2023. https://www.R-project.org/
- 25. Naha S, Gardner MJ, Khangura D, Kurukulasuriya LR, Sowers JR. Hypertension in diabetes. In: Feingold KR, Anawalt B, Blackman MR, et al. , eds. Endotext. MDText.com, Inc; 2000. Accessed April 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK279027/ [Google Scholar]
- 26. Jamal A. Current cigarette smoking among adults — United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205-1211. doi: 10.15585/mmwr.mm6544a2 [DOI] [PubMed] [Google Scholar]
- 27. Kramer CK, de Azevedo MJ, da Costa Rodrigues T, Canani LH, Esteves J. Smoking habit is associated with diabetic macular edema in type 1 diabetes mellitus patients. J Diabetes Complications. 2008;22(6):430. doi: 10.1016/j.jdiacomp.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 28. Michaud SÉ, Dussault S, Groleau J, Haddad P, Rivard A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: role of NO and reactive oxygen species. J Mol Cell Cardiol. 2006;41(2):275-284. doi: 10.1016/j.yjmcc.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 29. Thaikoottathil JV, Martin RJ, Zdunek J, Weinberger A, Rino JG, Chu HW. Cigarette smoke extract reduces VEGF in primary human airway epithelial cells. Eur Respir J. 2009;33(4):835-843. doi: 10.1183/09031936.00080708 [DOI] [PubMed] [Google Scholar]
- 30. Tuder RM, Wood K, Taraseviciene L, Flores SC, Voekel NF. Cigarette smoke extract decreases the expression of vascular endothelial growth factor by cultured cells and triggers apoptosis of pulmonary endothelial cells. Chest. 2000;117(5):241S-242S. doi: 10.1378/chest.117.5_suppl_1.241S [DOI] [PubMed] [Google Scholar]
- 31. Maugeri G, D’Amico AG, Rasà DM, et al. Nicotine promotes blood retinal barrier damage in a model of human diabetic macular edema. Toxicol In Vitro. 2017;44:182-189. doi: 10.1016/j.tiv.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 32. Solberg Y, Rosner M, Belkin M. The association between cigarette smoking and ocular diseases. Surv Ophthalmol. 1998;42(6): 535-547. doi: 10.1016/S0039-6257(98)00002-2 [DOI] [PubMed] [Google Scholar]
- 33. Cai X, Chen Y, Yang W, Gao X, Han X, Ji L. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes: a meta-analysis. Endocrine. 2018;62(2):299-306. doi: 10.1007/s12020-018-1697-y [DOI] [PubMed] [Google Scholar]
- 34. Chou TH, Wu PC, Kuo JZC, Lai CH, Kuo CN. Relationship of diabetic macular oedema with glycosylated haemoglobin. Eye. 2009;23(6):1360-1363. doi: 10.1038/eye.2008.279 [DOI] [PubMed] [Google Scholar]
- 35. Nilsson P, Gudbjörnsdottir S, Eliasson B, Cederholm J. Smoking is associated with increased HbA1c values and microalbuminuria in patients with diabetes — data from the National Diabetes Register in Sweden. Diabetes Metab. 2004;30(3):261-268. doi: 10.1016/S1262-3636(07)70117-9 [DOI] [PubMed] [Google Scholar]
- 36. Sia HK, Kor CT, Tu ST, Liao PY, Wang JY. Association between smoking and glycemic control in men with newly diagnosed type 2 diabetes: a retrospective matched cohort study. Ann Med. 2022;54(1):1385-1394. doi: 10.1080/07853890.2022.2075559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vlassopoulos A, Lean ME, Combet E. Influence of smoking and diet on glycated haemoglobin and ‘pre-diabetes’ categorisation: a cross-sectional analysis. BMC Public Health. 2013;13:1013. doi: 10.1186/1471-2458-13-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alqudah S, Jarab AS, Alefishat EA, Mayyas F, Khdour M, Pinto S. Factors associated with poor hemoglobin A1c control in patients with type 2 diabetes. Curr Diabetes Rev. 2019;15(2):164-170. doi: 10.2174/1573399814666180510144858 [DOI] [PubMed] [Google Scholar]
- 39. Bush T, Lovejoy JC, Deprey M, Carpenter KM. The effect of tobacco cessation on weight gain, obesity and diabetes risk. Obes Silver Spring Md. 2016;24(9):1834-1841. doi: 10.1002/oby.21582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jeong W. Association between dual smoking and dyslipidemia in South Korean adults. PLoS One. 2022;17(7):e0270577. doi: 10.1371/journal.pone.0270577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aminullah, Shah J, Alsubaie ASR, et al. Association of cigarette smoking with hyperlipidemia in male individuals. Food Nutr Sci. 2021;12(10):937-949. doi: 10.4236/fns.2021.1210069 [DOI] [Google Scholar]
- 42. Chi GC, Li X, Tartof SY, Slezak JM, Koebnick C, Lawrence JM. Validity of ICD-10-CM codes for determination of diabetes type for persons with youth-onset type 1 and type 2 diabetes. BMJ Open Diabetes Res Care. 2019;7(1):e000547. doi: 10.1136/bmjdrc-2018-000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schroeder EB, Donahoo WT, Goodrich GK, Raebel MA. Validation of an algorithm for identifying type 1 diabetes in adults based on electronic health record data. Pharmacoepidemiol Drug Saf. 2018;27(10):1053-1059. doi: 10.1002/pds.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quan H, Li B, Duncan Saunders L, et al. Assessing Validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424-1441. doi: 10.1111/j.1475-6773.2007.00822.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wiley LK, Shah A, Xu H, Bush WS. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20(4):652-658. doi: 10.1136/amiajnl-2012-001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-vrd-10.1177_24741264241269479 for Association Between Tobacco Smoking and the Development of Diabetic Macular Edema by Kyle B. Thomson, Syed I. Khalid, Naryan Sabherwal and Michael J. Heiferman in Journal of VitreoRetinal Diseases