Abstract
Background
The objective of this research was to test whether efficient tinnitus suppression could be achieved by electrical stimulation of the single most basal electrode contact of a cochlear implant. This approach simulates the effects of electrical stimulation using a round-window electrode.
Methods
The study was performed in 10 adult cochlear implant patients showing complete or almost complete tinnitus suppression during electrical stimulation with their standard fitting-MAP. In all patients, tinnitus appeared again when the implant was switched off. Five Nucleus implant (1 CI532, 4 CI24RE CA) users and 5 Mi12xx series with FLEX28 electrodes with at least 6 months of CI experience were included. Two types of stimulation were presented at the most basal CI contact: a constant pulse train and a modulated pulse train. The variation in pulse rates was low rate (100-300 pps) and high (≥900 pps), and the current level ranged from the C-level to less than the T-level for both stimulation types. The effect of acute electrical stimulation at the most basal electrode contact was compared to the effect obtained with multichannel stimulation with the patient’s current fitting MAP. Electrical stimulation was paused between tests with different stimulation types until tinnitus returned to baseline intensity. Patients reported Visual Analog Scale (VAS) scores for tinnitus loudness and intrusiveness during normal CI use and for each single contact stimulation type.
Results
Eight participants perceived complete suppression with one or more stimulation patterns. In 2 patients, suppression was less efficient than full-band CI stimulation. Louder stimuli are generally perceived as annoying and less effective in reducing tinnitus. In FLEX28 patients, it was also possible to obtain full tinnitus suppression with current amplitudes under the thresholds for auditory perception (this was not tested in patients with the Nucleus device).
Conclusion
In 8 of the 10 included patients, we were able to obtain complete or almost complete tinnitus suppression with electrical stimulation at only 1 most basal electrode contact. Therefore, round-window stimulation with a single electrode may be a potential treatment for tinnitus in patients with significant residual hearing. The long-term effects of this therapy should be confirmed in future studies.
Keywords: Cochlear Implant, tinnitus, electrical stimulation
Introduction
Tinnitus, being a perception of sound appearing without the presence of an external source of sound, remain a clinical and scientific obstacle. The perceived sensations include hissing, sizzling, cicada-like sounds, and ringing1 and cause different degrees of annoyance in affected persons.
According to population surveys, tinnitus affects 10-25% of adults aged >18 years from different nations.2 The prevalence of severe tinnitus is 2.3% (95% CI, 1.7-3.1%).3 Numerous aspects of daily life can be affected by tinnitus. People who experience severe tinnitus report having trouble sleeping, paying attention, enjoying social interactions, and hearing conversational dialogues. Tinnitus has been linked in cross-sectional research to higher probabilities of anxiety disorder and depressive symptoms.4
However, the pathogenesis of tinnitus is not fully understood. There is evidence linking hearing loss and tinnitus in several different ways, and the vast majority of people with tinnitus have some degree of hearing loss.5 According to Roberts et al (2008), people with tinnitus have higher hearing thresholds than age-matched controls; however, those with normal audiograms may exhibit minimal cochlear deafferentation too.6 Additionally, it has been reported that the slopes of audiograms of people with both noise-induced hearing loss and tinnitus are substantially steeper than those of people with only noise-induced hearing loss.7 Furthermore, the perceived pitch related to tinnitus typically correlates with the frequencies at which hearing is compromised.7-10 However, this is easier to demonstrate for low frequencies owing to the inherent ambiguity in differentiating high frequencies above 8 kHz.11
Deafferentation of the auditory pathways caused by hearing loss compromises the thalamocortical pathways and causes reactive hyperactivation of the auditory cortex, resulting in fantom sound perception.12 Increased spontaneous activity in the central auditory system can be demonstrated with functional MRI or qEEG in patients with chronic tinnitus. According to Jastreboff,13 the tinnitus sensation becomes for the brain an unrecognizable pattern that cannot be “de-tuned” resulting in continuous limbic and autonomic hyperactivation.
There is no specific treatment for tinnitus; however, several approaches can be used to suppress tinnitus sensation. The 3 most popular methods are auditory, magnetic field, and electrical stimulation.14 Psychotherapy, Tinnitus Retraining Therapy (TRT), and Cognitive Behavioral Therapy (CBT) also play important adjuvant roles.
Some authors have reported that cortical hyperexcitability can be reduced by low-frequency repeated transcranial magnetic stimulation (rTMS). Repeated transcranial magnetic stimulation can temporarily reduce tinnitus in a secure and non-invasive manner, but its effectiveness has not yet been proven.15
Acoustic stimulation increases the activity of the auditory nerve and reduces perceived tinnitus loudness in patients with mild-to-moderate hearing loss. Noise generators and hearing aids are commonly employed for tinnitus therapy. Studies using hearing aids or noise devices have generally shown improvements in tinnitus in approximately half to two-thirds of patients,16 although the cause of the varying degree of success remains unknown. Nonetheless, a crucial factor that has not yet been taken into consideration is that the majority of hearing aids and noise generators have a restricted frequency range and can provide sufficient power up to maximally 5-6 kHz.17 Consequently, the therapeutic benefits are limited in persons with high-pitched tinnitus owing to insufficient acoustic stimulation at high frequencies corresponding to the tinnitus pitch. Therefore, patients may not receive adequate auditory input within a specific high frequency range.9
Electrical stimulation was the earliest form of tinnitus suppression that has been employed in a scientific approach. Grapengiesser utilized a column composed of alternating silver and zinc plates following the pioneering work of Alessandro Volta. He administered an electrical current to the ears of both individuals with normal hearing and those with hearing impairment. Electric current could induce tinnitus in healthy ears and in deaf ears; however, it couldalso sometimes reduce tinnitus in ears that already suffered from tinnitus.14
In patients who cannot be efficiently stimulated by hearing aids or noise generators, electrical stimulation with cochlear implants appears to bethe most effective method for tinnitus suppression.18-20
A comprehensive review of the impact of cochlear implantation on tinnitus in patients with bilateral sensorineural hearing loss was reported by Ramakers et al.21 After cochlear implantation, the overall tinnitus suppression rate ranged from 8% to 45%. Tinnitus decreased in 25-72% of patients, while it remained unchanged in 0-36% of patients. In 0-25% of patients, tinnitus increases after implantation.
According to Kleine Punte et al (2013), the best results of tinnitus suppression with CI were achieved when all electrode contacts of the electrode array were stimulated, and the minimal number of electrode contacts necessary for effective tinnitus suppression was 4. However, not all individuals respond well to full-length electrical cochlear stimulation; thus, it is still important to look for alternative stimulation techniques to reduce tinnitus while employing a CI.22 During an experiment by Kloostra et al,23 the participants were exposed to an electrical pulse sequence using a single CI electrode contact. The location of stimulation in the cochlea, stimulation rate, and stimulation amplitude all changed in different situations. The acute effect of single-electrode stimulation via CI on tinnitus was investigated in that study. Most stimulus conditions resulted in no change in tinnitus, and the effects of single-electrode stimulation on tinnitus differed significantly among the patients.
Aran and Cazals24 discovered that round-window stimulation could fully suppress tinnitus in 60% of patients, while promontory stimulation had the same effect in only 25% of patients. Self-reported total tinnitus suppression was observed in 4 out of 6 patients using round window stimulation (RWS) and in 1 out of 6 patients using promontory stimulation according to the study.25 In another study employing promontory stimulation, 4 out of 7 patients reported total tinnitus suppression, while 2 out of 7 patients reported a decrease in tinnitus.26
Poels et al investigated the predictive value of trial RWS for successful tinnitus suppression with cochlear implants. According to their study from 2021, during the RWS, there was no tinnitus suppression in 14 patients (41%), moderate suppression in 3 patients (9%), and total suppression in 13 patients (38%). Twelve patients (35%) showed short residual inhibition, whereas 22 patients (65%) did not. Thirteen individuals who showed total tinnitus suppression during RWS received CI. After implantation, 7 patients (54%) reported total tinnitus suppression with the Speech Processor (SP) turned on, 3 patients (23%) reported virtually complete suppression, and 3 patients (23%) reported partial suppression. The degree of tinnitus suppression with a cochlear implant was precisely as expected by the RWS in 11 of 13 implanted patients (85%). In 2 other patients, stimulation with a cochlear implant produced sub-total/moderate tinnitus suppression.27
Acoustic stimulation with classical hearing aids/noise generators or electrical stimulation with cochlear implants can effectively suppress tinnitus in selected groups of patients. However, a large group of patients with high-pitched tinnitus only presented with high-frequency hearing loss. In such patients, acoustic suppression of tinnitus is impossible because of the abovementioned bandwidth limits of classical hearing aids. These patients were also excluded from cochlear implantation because of good (normal) hearing at low and midrange frequencies and a significant risk for the loss of residual hearing after implantation. The ideal solution for these patients would be electrical stimulation delivered to an extracochlear electrode placed in the round window niche with galvanic contact with cochlear fluids. This type of stimulation could allow for efficient stimulation of high frequencies and simultaneously guarantee the preservation of residual hearing.
The objective of this study was to evaluate whether stimulation of a single electrode positioned in the vicinity of the round window would effectively suppress tinnitus sensations.
Methods
Subjects
This study involved 10 patients with unilateral deafness who underwent cochlear implantation on the deaf side (5 females and 5 males). Subjects 1-5 (range 3-16 years after implantation) were Nucleus recipients, subject 1 is a CI user with a CI532 implant, and subjects P02-P05 have CI24RE Contour Advance implants (Cochlear Ltd., Sydney, Australia). Subjects 6-10 (within 1-5 years after implantation) were implanted with Mi12xx Series, FLEX28 (MED-EL, Innsbruck, Austria). Table 1 provides a description of the patient demographic data.
Table 1.
Demographics of the Patients
| Subject | Implanted Ear | Age at CI-Surgery (Years) | Duration of CI Use (Years) | Gender | Implant Type | FI Contralateral Ear (dB) |
Etiology of Hearing Loss |
|---|---|---|---|---|---|---|---|
| P01 | Left | 68 | 3 | M | CI532 | 83 | Chronic Otitis Media |
| P02 | Right | 66 | 13 | F | CI24RE CA | 85 | Unknown |
| P03 | Left | 77 | 9 | M | CI24RE CA | 40 | Unknown |
| P04 | Right | 65 | 16 | M | CI24RE CA | 90 | Ménière’s disease |
| P05 | Right | 57 | 8 | F | CI24RE CA | 13 | Head Trauma |
| P06 | Left | 54 | 1 | F | Mi12xx, FLEX28 | 30 | Ménière’s disease |
| P07 | Left | 57 | 4 | F | Mi12xx, FLEX28 | 3.3 | Ménière’s disease |
| P08 | Right | 66 | 2 | F | Mi12xx, FLEX28 | 42 | Trauma |
| P09 | Right | 49 | 1 | M | Mi12xx, FLEX28 | 53 | Chronic Otitis Media |
| P10 | Right | 56 | 5 | M | Mi12xx, FLEX28 | 52 | Unknown |
Duration of CI (cochlear implantation) use is considered according to the investigation date.
F, female; FI, Fletcher index; M, male.
The Fletcher index is shown in Table 1 shows that P01, P02, and P04 had severe hearing loss in the contralateral ear. Participants P05 and P07 on the other hand demonstrate a normal air conduction threshold in the contralateral ear within the frequency range of 125 Hz to 4 kHz. Participants P03 and P06 displayed mild contralateral hearing loss. However, the hearing threshold for P03 decreased dramatically at higher frequencies (1-8 kHz). Participants P08 and P09 fell into the category of mild-to-moderate hearing loss. Participant P10 experienced moderate contralateral hearing loss in the middle frequency while retaining normal hearing at low and high frequencies (Table 1).
Adult patients with unilateral or bilateral impaired hearing were included if they had at least 6 months of CI experience. To meet the inclusion criteria, subjects had to suffer from tinnitus when their implant was inactive and showed considerable tinnitus reduction when the implant was switched on. Candidates with fluctuating tinnitus were excluded if tinnitus was absent on the day of examination. Dominating contralateral tinnitus was another exclusion criterion because in such cases the evaluation of tinnitus suppression in the implanted ear was expected to be unreliable.
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Sint-Augustinus Hospital Antwerp (approval number: B2021099000005, July 09, 2021).
Stimulation Paradigms
All the sound preprocessing algorithms of the sound processor were switched off. Only the most basal electrode contact was stimulated (all the other contacts were deactivated). Two types of stimulation patterns were used.
A pulse train with a constant stimulation rate and amplitude was delivered to the basal single-electrode contact.
The Nucleus recipients received constant stimulation, while the L34 sound processor was connected to the laptop via programming POD. The stimuli were generated by the MATLAB-based research software NIC (Nucleus Implant Communicator, Cochlear Ltd., Sydney, Australia) Version 3.0.
The Mi12xx recipients were exposed to a series of constant stimuli via the standard clinical software (Maestro System Software Version 9.0, MED-EL, Innsbruck, Austria), while the Diagnostic Interface Box II connected the Sonnet SP to the implant, and a Fine Structure Processing (FSP) sound coding strategy was selected to gain access to modifying the current level for the patients. The T-level- and C-levels were equal to present constant stimulation. Stimulation levels above and below the T-level were presented.
A modulated pulse train was derived from the speech input by restricting the sound processor to a single-band output of electrical pulses, as opposed to the typical multi-band output distributed over the electrode array. The frequency band delivered to the basal most electrode contact was broadened to its maximum range (from ≅500 to 8500 Hz) throughout programming.
The modulated stimuli were delivered to the Nucleus recipients with the N6 SP connected to the laptop by POD, and the standard clinical software Custom Sound Version 6.1 generated the stimuli with the ACE strategy.
The hardware and software were the same as the constant stimuli for the Mi12xx recipients, while the T-level setting was varied owing to the participants’ auditory perception.
Both low and high pulse rates were used in this study. For constant stimulation, the variation in the low pulse rates was between 100 and 300 pps and ≥900 pps for the high pulse rates. The modulated stimuli were presented at a pulse rate in the range of 200-300 pps for low pulse rates and ≥900 pps for high pulse rates.
The amplitudes of the stimulation varied between the threshold level and the most comfortable level (MCL), however in patients implanted with Mi12xx also sub-threshold stimulation was applied). Subthreshold stimulation had not been evaluated in Nucleus patients because tinnitus suppression with subthreshold stimuli was discovered only during the course of the study, and subthreshold stimulation was introduced in the amended protocol applied only to Mi12xx patients.
The electrode contacts that are available for stimulation are numbered 1-22 in the basal-to-apical direction for the Nucleus electrode array and in the apical-to-basal direction from E01 to E12 for the FLEX28 electrode array.
Procedures
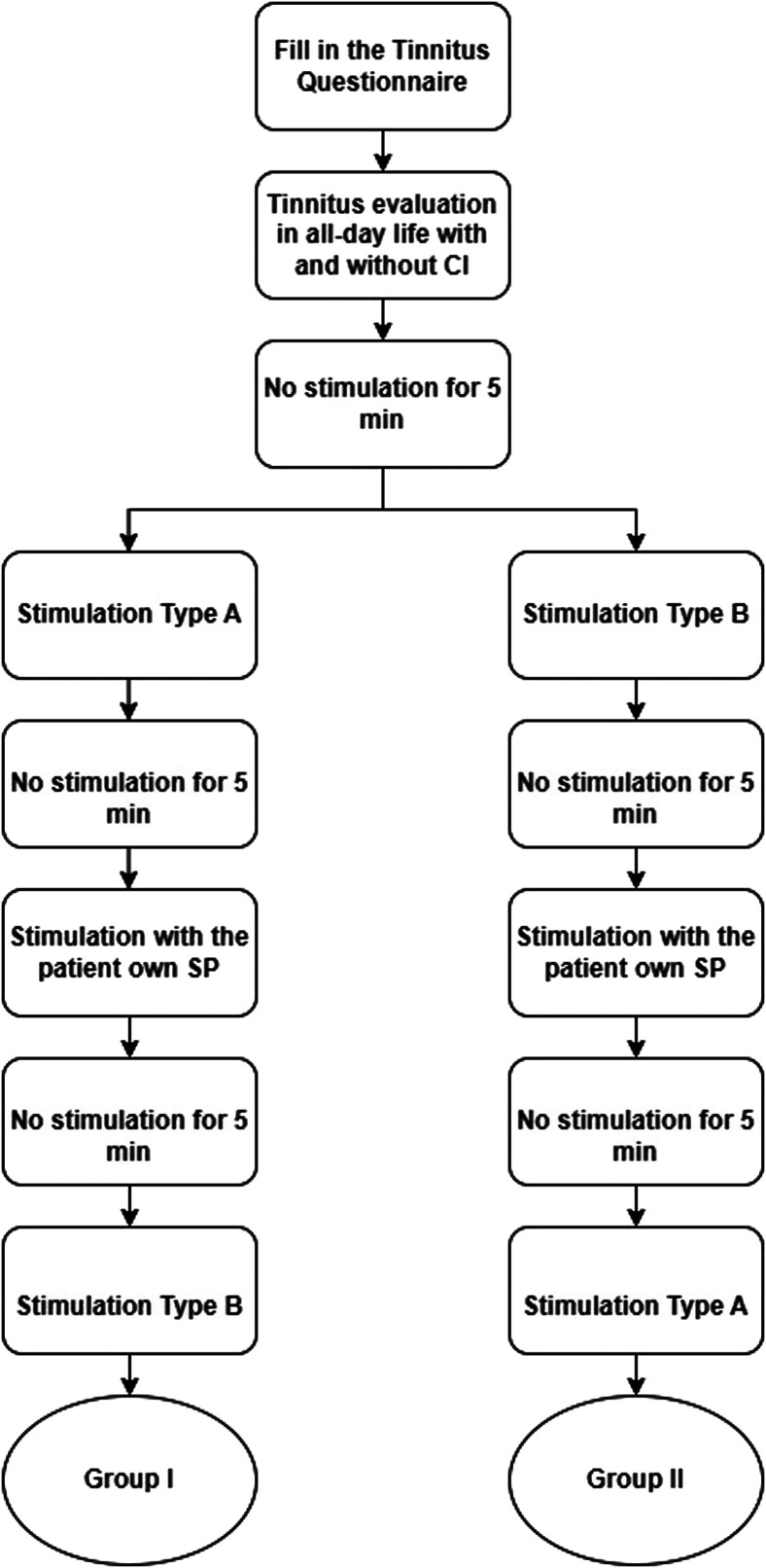
After written consent for participation in the study was obtained, the participants completed the tinnitus questionnaire (TQ). Tinnitus perception was assessed by the Visual Analog Scale (VAS) at the beginning of the experiment with the SP switched on and off. The experiment continued by establishing the MCL for the defined stimulation pattern, defined as rating 6- on a 10-point rating scale. By delivering pulse patterns to the most basal electrode contact that was active in the patient’s current programming MAP while tracking the respondents’ perception of loudness on a 10-point rating scale, electric loudness profiles were created. At least a 5-minute rest, or more, if necessary, to return to the baseline tinnitus loudness, was applied between each type of stimulation. At the most basal active electrode contact, stimulation types A and B were provided randomly during the first stimulation session. The patient’s own SP programs for CI hearing restoration were applied between the stimulation sessions. During the second session, the other stimulation type (A or B) was provided. A flowchart of the experimental procedure is shown in Figure 1.
Figure 1.
The flowchart of the experiment procedure while stimulation type A is a constant stimulation and type B is a modulated stimulation. The electrical stimulation commenced either with stimulation type A or B and followed by evaluation of tinnitus suppression with the patients’ own SP programming MAP. The process continued with the other stimulation type.
The current level started from the MCL and was eventually reduced to the lowest level allowing for tinnitus suppression. For Nucleus patients lowering of the current levels stopped at the T-level of the programming MAP and for Mi12xx also stimulus amplitudes below T-level were presented. At each current level, electrical stimulation was applied for 1 minute before the subject evaluated the perceived loudness level of tinnitus using the VAS score. The test stimulations and acute tinnitus evaluations were performed during a single visit with a duration of approximately 2 hours.
Results
The VAS scores for each subject and stimulation condition are presented in Table 2. The mean VAS score of tinnitus suppression with inactive CI was 7.25 and with active CI was 3.05. Participants P03, P04, and P06 had fluctuating tinnitus during the day without CI and P09 has it with CI active and inactive CI. The comfortable level for the P01-P05 is between 145 and 184 (CL) and it is between 7.5 and 17.47 (qu) for P06-P10. The comfortable level and threshold level are expressed in Current Levels (CL) for Nucleus and in charge units (qu) for the Mi12xx patients referring to their programming maps. The levels determined during the experiment and the preferred stimulation type (mentioned as A or B) varied from one subject to another. Whenever the participants’ VAS score was equal for both stimuli, stimulation with a lower rate was chosen as preferable.
Table 2.
The Patients’ Responses During the Experiment for Each Phase
| Patient No. |
CI Inactive |
CI Active | Stimulus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contact | C-Level | Best | Constant (Type A) | Modulated (Type B) | |||||||||
| Lowest VAS | T- level |
Current Level | Pulse rate |
Lowest VAS | T- level |
Current Level | Pulse Rate |
||||||
| P01 | 7 | 5 | E01 | 160 | – | 7 | – | – | – | 5 | 112 | 163 | 900 |
| P02 | 7 | 4 | E01 | 184 | A | 0 | <145 | 145 | 100 | 0 | 135 | 170 | 900 |
| P03 | 6 > 4 | 2 | E01 | 145 | B | 2 | <135 | 135 | 200 | 0 | 119 | 135 | 900 |
| P04 | 6 | 3 | E02 | 153 | B | 0 | <150 | 150 | 200 | 0 | <125 | 125 | 250 |
| P05 | 5 | 0 | E01 | 158 | A | 0 | <125 | 125 | 900 | 0 | 111 | 130 | 900 |
| P06 | 10 > 9 | 7 | E09 | 15.98 | A | 0 | 13.6 | 8.66 | 256 | 0 | 8.41 | 2.23 | 1600 |
| P07 | 7.5 | 0 | E12 | 17.47 | B | 3 | 4.13 | 3.00 | 1200 | 3 | 10.00 | 6.00 | 200 |
| P08 | 6 | 4.5 | E11 | 11.01 | B | 5 | 6.00 | 8.00 | 200 | 0 | 10.00 | 6.00 | 200 |
| P09 | 10 > 9 | 6 > 4 | E12 | 7.5 | A | 1 | 8.30 | 4.00 | 200 | 1.5 | 8.0 | 6.00 | 200 |
| P10 | 10 | 0 | E12 | 17.46 | A | 6 | 7.26 | 14.64 | 1200 | 8 | 13.0 | 6.00 | 1200 |
VAS: Visual Analogue Scale from 0 (the lowest tinnitus loudness) to 10 (the highest tinnitus loudness), the lower VAS shows the better result; C-level: comfortable level at the most basal active electrode; T-level: the threshold level at the most basal active electrode. The lowest VAS indicates the effectiveness of the stimulation in suppressing tinnitus; current level: the stimulation amplitude (the unit is current level [CL] for P01-P05 while it is current unit [qu] for P06-P10); pulse rate: the frequency of stimulation while the unit is pps (pulse per second); electrode: the electrode contact that received the stimulation. The most efficient condition mentioned in the “best” column.
The pulse rate for constant stimulation was 100-1200 pps, and the range was 200-1200 pps for modulated stimulation. The subthreshold current levels were also presented to patients Flex28 implants, in these patientsfull tinnitus suppression with current amplitudes under the thresholds for auditory perception could beobtained.
In the first patient (P01), only limited variations of the stimulus levels and repetition rates were used. It is possible that a better result could have been obtained if the same variation in settings is performed as for other subjects. Modulated and constant stimulations were able to fully suppressed tinnitus in P02. For P03, modulated stimuli suppressed tinnitus completely, while constant stimulation resulted in a minimum VAS score of 2. The electrode contact E02 was stimulated during the experiment in the case of P04 because E01 was not active. However, both modulated and constant stimulation resulted in complete suppression of tinnitus. Participant P05 predominantly used the CI for its tinnitus suppression effect; therefore, the VAS score was 0, while the CI was active. In this patient, tinnitus could be fully suppressed using constant stimulation at the most basal contact E01 and the modulated stimulation type. Modulated stimulation at E01 and E02 simultaneously was more effective as tinnitus was not perceived in the current setting.
For P06, E09 was stimulated because the last 3 electrodes on the basal side of the cochlea were locally disabled due to pain and excessive noise (E11, E12) and poor sound quality (E10) as perceived by this patient. Participant P07 reported complete tinnitus suppression with its own stimulation map; however, the single-electrode contact stimulation still allowed mild tinnitus. Tinnitus could be fully suppressed with modulated stimuli by stimulation of E11 and with constant stimuli by stimulation of E06 for P08. In comparison, at P09, almost complete tinnitus suppression was achieved by constant stimuli, and the VAS score remained 1.5 for modulated stimulation. Participant P10 could not distinguish between the presented stimulus and tinnitus; therefore, tinnitus was not completely suppressed. The best VAS score in this patient was 6, with constant stimulation.
A conclusion on the “best” pattern and pulse rate was based on the lowest VAS score, pulse rate, and current level, respectively; however, this clearly varied from patient to patient.
In total, the mean best VAS score, regardless of the delivered stimulation pattern, was 1.5. During the experiment, 8 out of 10 subjects reported total suppression or substantial reduction in tinnitus loudness. Complete suppression of tinnitus during the experiment was observed in 6 patients. Among them, patients with Flex28 implants, who were stimulated at sub-threshold levels, reported no auditory percepts at all, as their tinnitus was fully suppressed and the stimulation was under the perception threshold. Two other participants (P07 and P09) indicated substantial reduction in tinnitus. In patient (P01) the tinnitus was not fully suppressed by stimulation at the most basal contact (VAS score 5), but the result was exactly the same as with the CI. In patient (P10), tinnitus was completely suppressed by full-band stimulation with CI (VAS score 0), while the best VAS score during the experiment was 6.
In 8 out of 10 patients, the stimulation at the most basal contacts resulted in at least the same level of tinnitus suppression as the full-band stimulation with CI.
None of the patients experienced an increase in tinnitus loudness during the test.
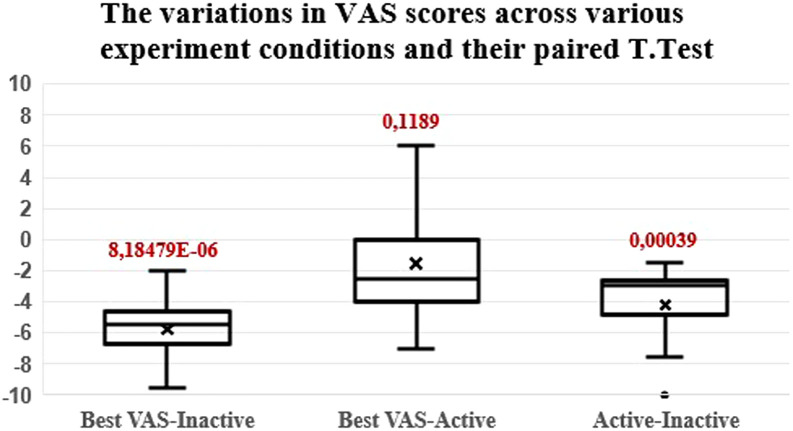
The mean VAS score drops from 7.25 without stimulation to 3.05 with the patients’ programming MAP active and to 1.5 with the best scoring stimulus of the experiment. This means an average reduction of −5.75 and −1.55, respectively. Boxplots of tinnitus reduction are presented in Figure 2. A paired t-test proved that tinnitus suppression was statistically significant both in the case of normal CI use (P < .001) and in the case of the best stimulation type at the most basal active electrode (P < .001). Figure 2. also shows a boxplot for the best VAS score of the experimental stimulation types versus the VAS score when the daily programming MAP was active. The mean and median values were negative (mean: −1.55 and median: −2.5), meaning that, on average, stimulation at the most basal active electrode would be more favorable for tinnitus than daily CI use. However, significance could not be shown (P = .1189) with the limited number of participants in this study.
Figure 2.
The variations in VAS scores across various experiment conditions. First box: maximum VAS irrespective of the experimental stimulation pattern; minus CI inactive VAS. Second box: maximum VAS minus CI active VAS, independent of experimental stimulation pattern. Third box: CI active VAS minus CI inactive VAS. VAS, Visual Analog Scale.
Discussion
The objective of this study was to test whether tinnitus suppression could be achieved by electrical stimulation at an electrode contact near the Round Window. When compared to the suppression obtained with the standard CI stimulation MAP, tinnitus suppression was more or at least equally effective in 8 of 10 cases with stimulation at a single basal-most contact.
Our study shows contradictory results to the study of Kleine Punte et al,18 where no effect on tinnitus was observed when only the basal 4-electrode contacts (8-10 mm deep in the cochlea) were stimulated neither on VAS nor psycho-acoustically. They concluded that in individuals with single-sided deafness (SSD) and severe ipsilateral tinnitus, full cochlear stimulation was necessary for the successful use of CI for tinnitus therapy. According to their study, it was not possible to alleviate tinnitus by electrical stimulation of the round window.
However, our results provide hope for patients with intractable tinnitus who cannot be helpedby hearing aids/noise generators or cochlear implants. In these patients, electrical stimulation delivered by an extracochlear electrode placed at the round window could alleviate tinnitus without the risk of deterioration of residual hearing. The possibility of effective tinnitus suppression with subthreshold stimulation would additionally benefit this group of patients.
Conclusion
In 8 of the 10 patients included in the study, we were able to obtain complete or almost complete tinnitus suppression with electrical stimulation at only one most basal electrode contact. It was also possible to completely suppress tinnitus with sub-threshold stimulation amplitudes. Therefore, round-window stimulation with a single electrode may be a potential treatment for tinnitus in patients with significant residual hearing. The long-term effects of this therapeutic method should be confirmed in future studies.
Funding Statement
The authors declare that this study has received no financial support.
Footnotes
Ethics Committee Approval: Ethics committee approval was received from the Ethics Committee of Sint-Augustinus Hospital Antwerp (approval number: B2021099000005, July 9, 2021).
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer-Review: Externally peer-reviewed.
Author Contributions: Concept – A.Z., M.L., J.V.D., G.D.G., E.O.; Design – A.Z., M.L., K.K.; Supervision – A.Z.; Resources – K.K., M.L.; Materials – K.K., M.L.; Data Collection and/or Processing – K.K., M.L., V.V.K., A.Z.; Analysis and/or Interpretation – K.K., V.V.K.; Literature Search – K.K., V.V.K.; Writing Manuscript – K.K., M.L., V.V.K., A.Z.; Critical Review – A.Z., M.L., J.V.D., G.D.G., E.O.
Acknowledgment: We would like to thank CI recipients for agreeing to have their data collected for this study. The authors wish to thank audiologists Liesbeth De Coninck, Tinne Theunen, and Annelies Vermeiren (ENT department, GZA Sint-Augustinus Antwerp) for their dedication and audiological support during the study.
Presented at Conferences: Sixteenth European symposium on pediatric cochlear implantation, May 31-June 3, 2023, De Doelen, Rotterdam, the Netherlands. Royal Belgian Society of Oto-Rhino-Laryngology, Head and Neck Surgery, Spring Symposium, March 16, 2024, Brussels, Belgium. South Africa Cochlear Implant group, May 17-May 19, 2024, Sandton Johannesburg, South Africa.
Declaration of Interests: The authors declare that they have no known competing financial interests that could have appeared to influence the work reported in this paper. However, K.K. works part-time as a research consultant at Cochlear Technology Centre, Belgium, which may be perceived as a potential conflict of interest, though it did not influence the outcomes of this study.
References
- 1. Baguley D, McFerran D, Hall D. Tinnitus. In: Lancet. Elsevier B.V. 2013;382(9904):1600 1607. ( 10.1016/S0140-6736(13)60142-7) [DOI] [PubMed] [Google Scholar]
- 2. Kim HJ, Lee HJ, An SY, et al. Analysis of the prevalence and associated risk factors of Tinnitus in adults. PLOS ONE. 2015;10(5):e0127578. ( 10.1371/journal.pone.0127578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarach CM, Lugo A, Scala M, et al. Global prevalence and incidence of tinnitus: a systematic review and meta-analysis. JAMA Neurol. 2022;79(9):888 900. ( 10.1001/jamaneurol.2022.2189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bauer CA. Tinnitus. N Engl J Med Solomon CG, ed. 2018;378(13):1224 1231. ( 10.1056/NEJMcp1506631) [DOI] [PubMed] [Google Scholar]
- 5. Henton A, Tzounopoulos T. What’s the buzz? The neuroscience and the treatment of tinnitus. Physiol Rev. 2021;101(4):1609 1632. ( 10.1152/physrev.00029.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisz N, Hartmann T, Dohrmann K, Schlee W, Norena A. High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear Res. 2006;222(1-2):108 114. ( 10.1016/j.heares.2006.09.003) [DOI] [PubMed] [Google Scholar]
- 7. König O, Schaette R, Kempter R, Gross M. Course of hearing loss and occurrence of tinnitus. Hear Res. 2006;221(1-2):59 64. ( 10.1016/j.heares.2006.07.007) [DOI] [PubMed] [Google Scholar]
- 8. Roberts LE, Moffat G, Baumann M, Ward LM, Bosnyak DJ. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J Assoc Res Otolaryngol. 2008;9(4):417 435. ( 10.1007/s10162-008-0136-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schaette R, König O, Hornig D, Gross M, Kempter R. Acoustic stimulation treatments against tinnitus could be most effective when tinnitus pitch is within the stimulated frequency range. Hear Res. 2010;269(1-2):95 101. ( 10.1016/j.heares.2010.06.022) [DOI] [PubMed] [Google Scholar]
- 10. Norena A, Micheyl C, Chéry-Croze S, Collet L. Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol Neurootol. 2002;7(6):358 369. ( 10.1159/000066156) [DOI] [PubMed] [Google Scholar]
- 11. Bourez PH, Fournier P, Noreña AJ. The difference in poststimulus suppression between residual inhibition and forward masking. Prog Brain Res. 2021;262:23 56. ( 10.1016/bs.pbr.2020.08.010) [DOI] [PubMed] [Google Scholar]
- 12. Møller AR. Similarities between chronic pain and tinnitus. Am J Otol. 1997;18(5):577 585. [PubMed] [Google Scholar]
- 13. Jastreboff PJ. Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neurosci Res. 1990;8(4):221 254. ( 10.1016/0168-0102(90)90031-9) [DOI] [PubMed] [Google Scholar]
- 14. Mahmoudian S, Lenarz M, Esser KH, et al. Alterations in early auditory evoked potentials and brainstem transmission time associated with tinnitus residual inhibition induced by auditory electrical stimulation. Int Tinnitus J. 2013;18(1):63 74. ( 10.5935/0946-5448.20130009) [DOI] [PubMed] [Google Scholar]
- 15. Peter N, Kleinjung T. Neuromodulation for tinnitus treatment: an overview of invasive and non-invasive techniques. J Zhejiang Univ Sci B. 2019;20(2):116 130. ( 10.1631/jzus.B1700117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boecking B, Rausch L, Psatha S, et al. Hearing therapy improves tinnitus-related distress in mildly distressed patients with chronic tinnitus and mild-to-moderate hearing loss: a randomized-controlled cross-over design. J Clin Med. 2022;11(7):1764. ( 10.3390/jcm11071764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore BCJ. Cochlear Hearing Loss: Physiological, Psychological and Technical Issues. John Wiley & Sons; Chichester: , UK; 2007. [Google Scholar]
- 18. Punte AK, De Ridder D, Van De Heyning P. On the necessity of full length electrical cochlear stimulation to suppress severe tinnitus in single-sided deafness. Hear Res. 2013;295:24 29. ( 10.1016/j.heares.2012.08.003) [DOI] [PubMed] [Google Scholar]
- 19. Arts RAGJ, George ELJ, Janssen M, Griessner A, Zierhofer C, Stokroos RJ. Tinnitus suppression by intracochlear electrical stimulation in single sided deafness - A prospective clinical trial: follow-up. PLOS ONE. 2016;11(4):e0153131. ( 10.1371/journal.pone.0153131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mertens G, Van Rompaey V, Van de Heyning P. Electric-acoustic stimulation suppresses tinnitus in a subject with high-frequency single-sided deafness. Cochlear Implants Int. 2018;19(5):292 296. ( 10.1080/14670100.2018.1473940) [DOI] [PubMed] [Google Scholar]
- 21. Ramakers GGJ, van Zon A, Stegeman I, Grolman W. The effect of cochlear implantation on tinnitus in patients with bilateral hearing loss: A systematic review. Laryngoscope. 2015;125(11):2584 2592. ( 10.1002/lary.25370) [DOI] [PubMed] [Google Scholar]
- 22. Arts RAGJ, George ELJ, Griessner A, Zierhofer C, Stokroos RJ. Tinnitus suppression by intracochlear electrical stimulation in single-sided deafness: A prospective clinical trial - Part i. Audiol Neurootol. 2015;20(5):294 313. ( 10.1159/000381936) [DOI] [PubMed] [Google Scholar]
- 23. Kloostra FJJ, De Kleine E, Free RH, Hofman R, Van Dijk P. Changes in tinnitus by cochlear implantation: A parametric study of the effect of single-electrode stimulation. Audiol Neurootol. 2021;26(3):140 148. ( 10.1159/000509202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aran JM, Cazals Y. Electrical suppression of tinnitus. Ciba Found Symp. 1981;85:217 231. ( 10.1002/9780470720677.ch13) [DOI] [PubMed] [Google Scholar]
- 25. Cazals Y, Negrevergne M, Aran JM. Electrical stimulation of the cochlea in man: hearing induction and tinnitus suppression. J Am Audiol Soc. 1978;3(5):209 213. [PubMed] [Google Scholar]
- 26. Assouly KKS, Dullaart MJ, Stokroos RJ, van Dijk B, Stegeman I, Smit AL. Systematic review on intra-and extracochlear electrical stimulation for tinnitus. Brain Sci. 2021;11(11):1394. ( 10.3390/brainsci11111394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poels L, Zarowski A, Leblans M, Vanspauwen R, van Dinther J, Offeciers E. Prognostic value of trial round window stimulation for selection of candidates for cochlear implantation as treatment for tinnitus. J Clin Med. 2021;10(17):3793. ( 10.3390/jcm10173793) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a