Abstract
Background
Obesity and adverse lipid profile leads to female infertility. The cardiometabolic index (CMI) is a promising indicator for predicting obesity-related diseases. The correlation between CMI and female infertility merits further investigation.
Methods
The data for this study were acquired from the 2013–2020 National Health and Nutrition Examination Survey (NHANES), with 2333 women enrolled. The cardiometabolic index (CMI) of each participant was calculated as the ratio of triglycerides and high-density lipoprotein cholesterol multiplied by waist-to-height ratio. Weighted multivariate logistic regression models were used to assess the independent correlation between the log-transformed CMI and infertility. Subgroup analyses were carried out to assess the reliability of the findings. Interaction tests were employed to determine whether variables affected infertility by interacting with log CMI.
Results
A total of 2333 participants aged 18–45 years were enrolled, 274 of whom were infertile. Log CMI of the infertility group was significantly higher than that of the non-infertility group (P < 0.001). After adjustment for potential confounders, women with higher CMI were at an increased risk of infertility (OR = 2.411, 95% CI: 1.416–4.112), and this correlation was still consistent in subgroups aged under 35 years (P < 0.001). Furthermore, restricted cubic spline analysis showed a positive non-linear relationship between log CMI and infertility.
Conclusions
Cardiometabolic index levels are positively correlated with increased risk of infertility in American females. Our study demonstrates the predictive capacity of CMI for female infertility.
Keywords: Female infertility, Cardiometabolic index, Obesity, NHANES
Introduction
Infertility is characterized by the inability to conceive after 12 months or more of consistent, unprotected sexual intercourse [1], impacting a onsiderable portion of the population. It has been reported that approximately 12.6% to 17.5% of couples in reproductive age have suffered from infertility globally [2]. As a worldwide public health concern, infertility not only imposes a huge economic burden, but also leads to severe psychological distress and social stigma for individuals.
Infertility can be caused by various factors, such as age, diet, psychological stress, and environmental pollution, among which, obesity has received a considerable attention due to its alarming rise worldwide. A great deal of research has been conducted on the negative association between obesity and reproductive outcomes [3–5]. Typical dyslipidemia in obesity is characterized by an adverse lipid profile, including elevated triglycerides (TG), free fatty acids (FFA), low-density lipoprotein cholesterol (LDL-C), and small dense LDL, as well as reduced high-density lipoprotein cholesterol (HDL-C) levels with impaired HDL function [6–9]. Evidence from human studies indicated that abnormal lipid profiles such as elevated total cholesterol, TG, LDL-C, and reduced HDL-C could lead to diminished fecundability, poorer oocyte quality, and impaired ovarian function [10, 11].
The cardiometabolic index (CMI), calculated by multiplying TG/HDL-C ratio by waist-to-height ratio (WHtR), was initially introduced by Wakabayashi et al. in 2015 [12]. It was developed as an innovative diagnostic tool for identifying diabetes and for assessing the distribution and functional impairments of visceral adipose tissue. A growing number of studies have established a robust correlation between CMI and cardiovascular disease, renal dysfunction, acute pancreatitis as well as adverse metabolic profiles, suggesting that it may serve as a valuable predictor of metabolism-related disorders [13–18]. Considering the positive association between obesity, abnormal lipid profiles, and female infertility, the potential role of CMI in diagnosing female infertility merits further investigation.
Hence, this study aimed to systematically examine the relationship between CMI and female infertility, and to evaluate the predictive efficacy of CMI for diagnosing female infertility.
Materials and methods
Data source
NHANES is a nationwide representative cross-sectional survey to assess and evaluate Americans’ health and nutritional status. Administered by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC), NHANES is based on questionnaires, physical examinations, household interviews, and laboratory testing. Multistage stratified probability sampling is used in the study to ensure a highly representative sample. All participants have provided written informed consent in accordance with the NHANES protocols, which have been approved by the NCHS Research Ethics Review Board. Data used in this study is publicly available at https://www.cdc.gov/nchs/nhanes.
Study population
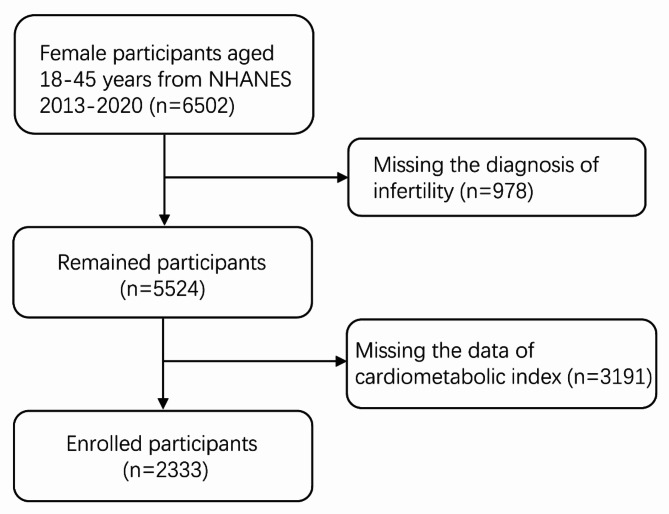
The present study incorporated NHANES data from 2013-2014, 2015-2016, 2017-2018 and 2019-2020. Women aged 18-45 years were enrolled (n = 6502); after the exclusion of missing data of CMI (n = 3191) or diagnosis of infertility (n = 978), 2333 participants were finally included in the analysis (Fig. 1).
Fig. 1.
The flow chart of study participants
Assessment of infertility
The infertility was derived from the responses to the Reproductive Health Questionnaire (questionnaire: RHQ074). Participants were assumed to be infertile if they responded positively to the survey question, “Have you ever attempted to become pregnant for at least a year without becoming pregnant?” [19].
Assessment of cardiometabolic index
The TG/HDL-C ratio was calculated by dividing the serum concentration of TG (mg/dL) by HDL-C (mg/dL), which was obtained from the database. Additional information about laboratory examinations is available at https://www.cdc.gov/nchs/nhanes. WHtR was obtained by dividing waist circumference (WC, cm) by height (cm). CMI was computed as TG/HDL-C×WHtR using the formula in previously published research [12].
Assessment of covariates of interest
Our study considered the following variables that may influence the relationship between CMI and infertility: age (years), race (non-Hispanic White/non-Hispanic Black/Mexican-American/other race), marital status (married or cohabiting/widowed or divorced or separated/other), education level (below high school/ high school/above high school), smoking status (never/former/current), drinking status (never/former/mild/moderate/heavy), hypertension (no/yes) and diabetes (no/impaired glucose tolerance (IGT)/yes). The complete measurement procedures for these variables are available at https://www.cdc.gov/nchs/nhanes.
Statistical analysis
All statistical analyses were carried out in accordance with the recommendations of the CDC using the appropriate NHANES sampling weights.
The participants were divided into two groups based on their infertility status. Continuous variables were presented as mean ± standard deviation, whereas categorical variables are expressed as percentages. To lessen data skewness, control outlier effects, and enhance the interpretation of association results, the CMI was log-transformed. An unweighted chi-squared test was used for categorical data, and an unweighted Student’s t-test or Mann-Whitney U-test was employed for continuous variables to evaluate differences between the two groups. Using the log-transformed CMI data as continuous variables and quartiles, respectively, unweighted multivariate logistic regression models were used to evaluate the independent relationship between infertility and the log-transformed CMI. No covariate adjustment was made in the crude model. In Model 1, age, race, marital status and education were adjusted. In addition to the covariates in Model 1, Model 2 was further adjusted for smoking status, drinking status, hypertension and diabetes. The study employed a restricted cubic spline analysis to examine the potential linear correlation between the log-transformed CMI and infertility. Subgroup analyses were carried out to evaluate the reliability of the findings. To find out if variables affected infertility by interacting with the log-transformed CMI, interaction tests were employed.
All statistical analyses were conducted using R Version 4.3.1 (http://www.R-project.org, The R Foundation). P < 0.05 (two-tailed) was considered statistically significance.
Results
Basic characteristics of the included participants
Table 1 presents the baseline characteristics of participants selected from NHANES 2013 to 2020, stratified by their fertility status. The analysis included 274 participants with infertility, which accounted for 11.74% of women aged 18-45 years. The average age of infertile women was 33.91 ± 7.15 years, while the non-infertility group consisted of 2059 participants with a mean age of 31.03 ± 8.28 years (P < 0.001). Additionally, race, marital status, hypertension, smoking and drinking status exhibited significant differences between the non-infertile and infertile groups (all P < 0.05), whereas educational level and diabetes did not demonstrate statistical differences (P > 0.05). Furthermore, we found that women with self-reported infertility had a higher log-transformed CMI, averaging -0.90 ± 0.79, compared to -0.97 ± 0.27 among non-infertile women (P < 0.05).
Table 1.
Baseline characteristics of participants
| Variables | Total (n = 2333) |
Non-Infertility (n = 2059) |
Infertility (n = 274) |
P |
|---|---|---|---|---|
| Age, years, mean (SD) | 31.37 (8.21) | 31.03 (8.28) | 33.91 (7.15) | < 0.001* |
| Age | < 0.001* | |||
| < 35 years | 1426 (61.12) | 1289 (62.60) | 137 (50.00) | |
| ≥ 35 years | 907 (38.88) | 770 (37.40) | 137 (50.00) | |
| Race | 0.033* | |||
| Non-Hispanic White | 748 (32.06) | 639 (31.03) | 109 (39.78) | |
| Non-Hispanic Black | 551 (23.62) | 492 (23.90) | 59 (21.53) | |
| Mexican-American | 378 (16.20) | 337 (16.37) | 41 (14.96) | |
| Other Race | 656 (28.12) | 591 (28.70) | 65 (23.72) | |
| Marital status | < 0.001* | |||
| Married/Cohabiting | 1228 (52.64) | 1024 (49.73) | 204 (74.45) | |
| Widowed/Divorced/Separated | 218 (9.34) | 190 (9.23) | 28 (10.22) | |
| Other | 887 (38.02) | 845 (41.04) | 42 (15.33) | |
| Education level | 0.128 | |||
| Below high school | 444 (19.03) | 396 (19.23) | 48 (17.52) | |
| High school | 482 (20.66) | 436 (21.18) | 46 (16.79) | |
| Above high school | 1407 (60.31) | 1227 (59.59) | 180 (65.69) | |
| Smoking status | 0.002* | |||
| Never | 1689 (72.40) | 1515 (73.58) | 174 (63.50) | |
| Former | 267 (11.44) | 223 (10.83) | 44 (16.06) | |
| Current | 377 (16.16) | 321 (15.59) | 56 (20.44) | |
| Drinking status | 0.021* | |||
| Never | 530 (22.72) | 483 (23.46) | 47 (17.15) | |
| Former | 79 (3.39) | 66 (3.21) | 13 (4.74) | |
| Mild | 580 (24.86) | 507 (24.62) | 73 (26.64) | |
| Moderate | 568 (24.35) | 510 (24.77) | 58 (21.17) | |
| Heavy | 576 (24.69) | 493 (23.94) | 83 (30.29) | |
| Hypertension | < 0.001* | |||
| No | 1967 (84.31) | 1764 (85.67) | 203 (74.09) | |
| Yes | 366 (15.69) | 295 (14.33) | 71 (25.91) | |
| Diabetes | 0.201 | |||
| No | 1929 (82.68) | 1705 (82.81) | 224 (81.75) | |
| IGT | 215 (9.22) | 194 (9.42) | 21 (7.66) | |
| Yes | 189 (8.10) | 160 (7.77) | 29 (10.58) | |
| Log CMI, mean (SD) | –0.96 (0.27) | –0.97 (0.27) | –0.90 (0.26) | < 0.001* |
| Log CMI | < 0.001* | |||
| Q1 | 586 (25.12) | 546 (26.52) | 40 (14.60) | |
| Q2 | 584 (25.03) | 513 (24.92) | 71 (25.91) | |
| Q3 | 580 (24.86) | 499 (24.24) | 81 (29.56) | |
| Q4 | 583 (24.99) | 501 (24.33) | 82 (29.93) |
Data are shown as number (%) unless otherwise indicated
*Statistically significant (P < 0.05)
Log CMI quartile range: Q1, − 1.799, − 1.153; Q2, − 1.154, − 0.984; Q3, − 0.985, − 0.785; Q4, − 0.786, 0.318
SD, standard deviation; IGT, impaired glucose tolerance; CMI, cardiometabolic index; Q, quartile
Correlation between CMI and prevalence of infertility
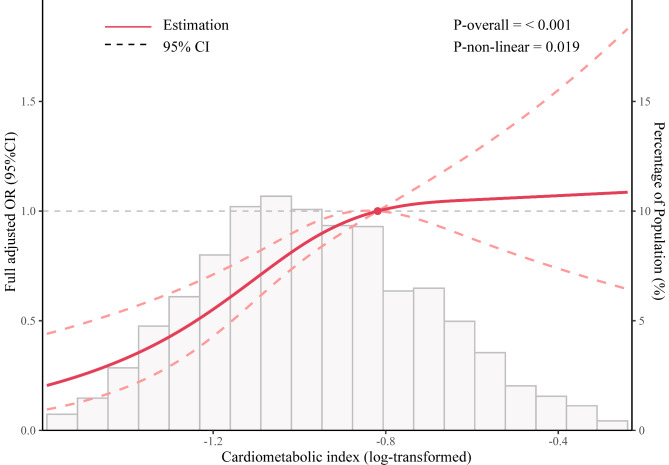
Table 2 shows the correlation between log CMI and the risk of infertility. In the crude model, the odds ratio (OR) was 2.859 (95% CI: 1.806-4.528), indicating a significant positive correlation between log CMI and infertility. Model 1, which adjusted for age, race, marital status, and education, also showed a positive correlation (OR = 2.680, 95% CI: 1.647-4.365). In addition, in Model 2, after further adjustment for smoking status, drinking status, hypertension, and diabetes, a positive correlation between the log CMI and infertility was still observed (OR = 2.411, 95% CI: 1.416-4.112). To achieve a more comprehensive understanding of the correlation between CMI and infertility, log CMI was categorized into quartiles. Based on Model 2, the OR between the highest quartile (Q4) and the lowest quartile (Q1) was 1.843 (95% CI: 1.205-2.848), suggesting a positive correlation between higher CMI levels and infertility. As demonstrated by the results of restricted cubic spline analysis presented in Fig. 2, we observed a positive non-linear relationship between infertility and log CMI (P for trend < 0.05).
Table 2.
Logistic regression analysis on the association between log CMI and infertility
| Log CMI | Crude model | Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |||
| Continuous | 2.859 (1.806–4.528) | < 0.001* | 2.680 (1.647–4.365) | < 0.001* | 2.411 (1.416–4.112) | 0.001* | ||
| Categories | ||||||||
| Q1 | Ref. | Ref. | Ref. | |||||
| Q2 | 1.889 (1.265–2.857) | 0.002* | 1.820 (1.217–2.756) | 0.004* | 1.790 (1.197–2.707) | 0.005* | ||
| Q3 | 2.216 (1.498–3.327) | < 0.001* | 2.045 (1.375–3.083) | < 0.001* | 1.937 (1.298–2.929) | 0.001* | ||
| Q4 | 2.234 (1.511–3.353) | < 0.001* | 2.053 (1.371–3.117) | < 0.001* | 1.843 (1.205–2.848) | 0.005* | ||
| P for trend | < 0.001* | < 0.001* | 0.008* | |||||
* Statistically significant (P < 0.05)
aModel 1was adjusted for age, race, marital status, and education
bModel 2 was adjusted for age, race, marital status, education, smoking status, drinking status, hypertension and diabetes
CMI, cardiometabolic index; OR, odds ratio; CI, confidence interval; Q, quartile
Fig. 2.
Restricted cubic spline of odds ratio and 95% confidence interval (shaded area) for the association between log cardiometabolic index and infertility
Subgroup analysis
To further determine the robustness of the correlation between log CMI and infertility, subgroup analysis was conducted. As presented in Table 3, the results from the interaction analysis indicated that age significantly influences the correlation between log CMI and infertility (P for interaction < 0.05), while neither hypertension nor diabetes showed a modifying effect (P for interaction > 0.05). The subgroup analysis results showed that participants who were either under 35 years old (OR = 6.847; 95% CI: 3.115-15.255; P < 0.001) did not have hypertension (OR = 2.634; 95% CI: 1.416-4.909; P = 0.002), or did not have diabetes (OR = 2.799; 95% CI: 1.523-5.157; P = 0.001) exhibited a consistent positive correlation between log CMI and infertility, highlighting the robustness of this relationship. However, elevated log CMI did not increase the risk of infertility in individuals aged ≥ 35 or in those with hypertension or diabetes (including IGT).
Table 3.
The results of subgroup analyses and interaction analyses
| Variables | OR | 95% CI | P | P for interaction |
|---|---|---|---|---|
| Age | < 0.001* | |||
| < 35 years | 6.847 | 3.115–15.255 | < 0.001* | |
| ≥ 35 years | 0.857 | 0.388–1.878 | 0.700 | |
| Hypertension | 0.402 | |||
| No | 2.634 | 1.416–4.909 | 0.002* | |
| Yes | 2.116 | 0.634–7.196 | 0.225 | |
| Diabetes | 0.351 | |||
| No | 2.799 | 1.523–5.157 | 0.001* | |
| IGT | 0.288 | 0.029–2.542 | 0.270 | |
| Yes | 2.684 | 0.397–19.365 | 0.316 |
*Statistically significant (P < 0.05)
Adjusted for age, hypertension and diabetes. Stratified variables were not adjusted in the subgroup analysis
Discussion
This cross-sectional research conducted a thorough investigation into the association between log CI and infertility in non-institutionalized American women, indicating that participants with higher log CMI had an increased risk of infertility. Subgroup analysis and interaction tests further confirmed the strength of the correlation between log CMI and infertility. Based on our research, CMI has the potential to serve as a valuable predictor of infertility risk.
This study represents the initial attempt to evaluate the association between CMI and female infertility directly. Obesity poses a substantial worldwide public health concern, leading to a multitude of detrimental health consequences. According to the 2016 population statistics from the World Health Organization (WHO), 40% of women were classified as overweight and 15% were classified as obese, and the pronounced adverse outcomes associated with obesity among women require special attention [20]. Obesity has been linked to numerous negative impacts on female fertility [4, 21]. Ovulatory dysfunction is more common in obese women due to disruptions in the hypothalamic-pituitary-ovarian (HPO) axis [22]. Additionally, obese women with polycystic ovarian syndrome (PCOS) tend to experience more severe metabolic and reproductive symptoms [23, 24]. Obesity can also affect the development of oocyte and preimplantation embryo [25, 26]. Excess free fatty acids may cause negative impacts on reproductive tissues, resulting in chronic inflammation and cellular dysfunction [27]. Additionally, the endometrium is vulnerable to obesity, as manifested by compromised stromal decidualization processes among obese female individuals [5].
Most studies on the relationship between female infertility and obesity are based on body mass index (BMI, in kg/m2) and use the ranges of 18.5-24.9 for normal weight, 25-29.9 for overweight, and ≥ 30 for obesity. Although BMI is a conventional and economical way to assess obesity, it lacks the ability to accurately reflect fat mass and fails to provide insights into fat distribution. Hence, relying solely on BMI for obesity assessment is inadequate [28]. Recently, researchers have proposed various indicators for the scientific evaluation of obesity, which can provide a more precise depiction of fat distribution and are considered to be more scientifically rigorous than BMI. Numerous studies have shown that visceral adiposity index (VAI) [29], weight-adjusted waist circumference index (WWI) [30], WC [31, 32] and waist-hip ratio (WHR) [33] were associated with an increased risk of infertility. As a novel indicator, CMI involves anthropometric and biochemical parameters, and shows a robust correlation with abnormal lipid profiles as well as metabolism-related disorders, such as atherosclerosis, hypertension, ischemic stroke and left ventricular dilation [15]. Metabolic diseases and infertility are complicated processes, with abnomalities in lipid metabolism potentially having a significant impact on follicular growth, egg maturation, and hormone release [34, 35]. Studies have shown that CMI is higher in PCOS patients compared to control subjects, and is positively associated with insulin resistance in these patients [36], which may lead to ovulation disorders and infertility [37, 38]. Numerous investigations conducted on animals have verified that dyslipidemia can reduce female reproduction capacity [39–42]. Combined with the findings of our study, CMI serves not only as an early indicator of metabolic dysfunction, but also as a predictor of infertility risk, reinforcing the importance of daily health maintenance.
Based on the NHANES database, our study for the first time elucidates the direct correlation and shows the non-linear relationship between log CMI and female infertility risk through a cross-sectional study. However, there are some limitations of our study. First, our study is aimed on women aged 18–45 years in the United States; whether the findings can be generalized to populations outside of this age range and location requires futher investigation. Secondly, the database may lack information on family history of infertility and other reproductive disorders, such as tubal obstruction or PCOS, which can also contribute to infertility. Furthermore, additional data, such as sex hormone levels, should be included to enhance the understanding of the predictive potential of CMI for infertility risk.
Conclusion
According to our research, a high level of CMI is positively correlated with an increased risk of female infertility. CMI plays a predictive role in assess metabolic and reproductive problems in women. Nevertheless, further extensive prospective investigations are required to support the findings of this study.
Author contributions
YZ performed the statistical analysis and drafted the manuscript. WS collected data and performed further methodology. YL reviewed and revised the manuscript. NQ conceptualized and organized the research. HH conceptualized and supervised the research; reviewed and revised the manuscript. All authors reviewed the manuscript and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (82088102, 82301814, 82201882), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-064), Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), Key Discipline Construction Project (2023–2025) of Three-Year Initiative Plan for Strengthening Public Health System Construction in Shanghai (GWVI-11.1-35), Shanghai Clinical Research Center for Gynecological Diseases (22MC1940200), Shanghai Urogenital System Diseases Research Center (2022ZZ01012) and Shanghai Frontiers Science Research Center of Reproduction and Development.
Data Availability
Data used in this study is publicly available at https://www.cdc.gov/nchs/nhanes.
Declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All data obtained from NHANES were reviewed and approved by National Center for Health Statistics (NCHS) Ethics Review Board and all participants agreed on the survey and signed written consent. The NHANES was conducted in compliance with local laws and institutional guidelines. Since NHANES is a publicly accessible database, no additional ethical approvals are required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yiran Zhao and Weihui Shi contributed equally to this work.
Contributor Information
Ningxin Qin, Email: qinningxin@163.com.
Hefeng Huang, Email: huanghefg@hotmail.com.
References
- 1.Carson SA, Kallen AN. Diagnosis and management of infertility. JAMA. 2021;326(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox CM, Thoma ME, Tchangalova N, Mburu G, Bornstein MJ, Johnson CL, Kiarie J. Infertility prevalence and the methods of estimation from 1990 to 2021: a systematic review and meta-analysis. Hum Reprod Open 2022, 2022(4). [DOI] [PMC free article] [PubMed]
- 3.Armstrong A, Berger M, Al-Safi Z. Obesity and reproduction. Curr Opin Obstet Gynecol. 2022;34(4):184–9. [DOI] [PubMed] [Google Scholar]
- 4.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. 2017;107(4):840–7. [DOI] [PubMed] [Google Scholar]
- 5.Yang T, Zhao J, Liu F, Li Y. Lipid metabolism and endometrial receptivity. Hum Reprod Update. 2022;28(6):858–89. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang J, Wang S, Wang Y, Hu R, Wu Y. Association between triglyceride glucose index and infertility in Reproductive-aged women: a cross-sectional study. Int J Women’s Health. 2024;16:937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stadler JT, Marsche G. Obesity-related changes in high-density lipoprotein metabolism and function. Int J Mol Sci. 2020;21(23):8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klop B, Elte J, Cabezas M. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vekic J, Stefanovic A, Zeljkovic A. Obesity and dyslipidemia: a review of current evidence. Curr Obes Rep. 2023;12(3):207–22. [DOI] [PubMed] [Google Scholar]
- 10.Pugh SJ, Schisterman EF, Browne RW, Lynch AM, Mumford SL, Perkins NJ, Silver R, Sjaarda L, Stanford JB, Wactawski-Wende J, et al. Preconception maternal lipoprotein levels in relation to fecundability. Hum Reprod. 2017;32(5):1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller WL, Auchus RJ. The Molecular Biology, Biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi I, Daimon T. The cardiometabolic index as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin Chim Acta. 2015;438:274–8. [DOI] [PubMed] [Google Scholar]
- 13.Lazzer S, D’Alleva M, Isola M, De Martino M, Caroli D, Bondesan A, Marra A, Sartorio A. Cardiometabolic Index (CMI) and visceral Adiposity Index (VAI) highlight a higher risk of metabolic syndrome in women with severe obesity. J Clin Med. 2023;12(9):3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Q, Ren Q, Du L, Chen S, Wu S, Zhang B, Wang B. Cardiometabolic Index (CMI), lipid Accumulation products (LAP), Waist triglyceride Index (WTI) and the risk of acute pancreatitis: a prospective study in adults of North China. Lipids Health Dis 2023, 22(1). [DOI] [PMC free article] [PubMed]
- 15.Miao M, Deng X, Wang Z, Jiang D, Lai S, Yu S, Yan L. Cardiometabolic index is associated with urinary albumin excretion and renal function in aged person over 60: data from NHANES 2011–2018. Int J Cardiol. 2023;384:76–81. [DOI] [PubMed] [Google Scholar]
- 16.Ye R, Zhang X, Zhang Z, Wang S, Liu L, Jia S, Yang X, Liu X, Chen X. Association of cardiometabolic and triglyceride-glucose index with left ventricular diastolic function in asymptomatic individuals. Nutr Metabolism Cardiovasc Dis 2024. [DOI] [PubMed]
- 17.Shi W-R, Wang H-Y, Chen S, Guo X-F, Li Z, Sun Y-X. Estimate of prevalent diabetes from cardiometabolic index in general Chinese population: a community-based study. Lipids Health Dis 2018, 17(1). [DOI] [PMC free article] [PubMed]
- 18.Song J, Li Y, Zhu J, Liang J, Xue S, Zhu Z. Non-linear associations of cardiometabolic index with insulin resistance, impaired fasting glucose, and type 2 diabetes among US adults: a cross-sectional study. Front Endocrinol 2024, 15. [DOI] [PMC free article] [PubMed]
- 19.Dick MLB. Self-reported difficulty in conceiving as a measure of infertility. Hum Reprod. 2003;18(12):2711–7. [DOI] [PubMed] [Google Scholar]
- 20.Organization WH. WHO consultation to adapt influenza sentinel surveillance systems to include COVID-19 virological surveillance: virtual meeting, 6–8 October 2020. World Health Organization; 2022.
- 21.Klenov VE, Jungheim ES. Obesity and reproductive function. Curr Opin Obst Gynecol. 2014;26(6):455–60. [DOI] [PubMed] [Google Scholar]
- 22.Jungheim ES, Moley KH. Current knowledge of obesity’s effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol. 2010;203(6):525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran LJ, Norman RJ, Teede HJ. Metabolic risk in PCOS: phenotype and adiposity impact. Trends Endocrinol Metab. 2015;26(3):136–43. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Wu H, Sun S, Zhao R, Deng Y, Zeng S, Chen J. Effect of high Fat Diet on Disease Development of polycystic ovary syndrome and lifestyle intervention strategies. Nutrients. 2023;15(9):2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghasemi-Tehrani H, Askari G, Allameh FZ, Vajdi M, Amiri Khosroshahi R, Talebi S, Ziaei R, Ghavami A, Askari F. Healthy eating index and risk of diminished ovarian reserve: a case–control study. Sci Rep 2024, 14(1). [DOI] [PMC free article] [PubMed]
- 26.Wołodko K, Castillo-Fernandez J, Kelsey G, Galvão A. Revisiting the Impact of Local Leptin Signaling in Folliculogenesis and Oocyte Maturation in obese mothers. Int J Mol Sci. 2021;22(8):4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumford SL, Chavarro JE, Zhang C, Perkins NJ, Sjaarda LA, Pollack AZ, Schliep KC, Michels KA, Zarek SM, Plowden TC, et al. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr. 2016;103(3):868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caballero B. Humans against obesity: who Will Win? Adv Nutr. 2019;10(suppl1):S4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang J, Wang Y, Wang S, Hu R, Wu Y. Association between visceral adiposity index and infertility in reproductive-aged women in the United States. Sci Rep. 2024;14(1):14230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen Z, Li X. Association between weight-adjusted-waist index and female infertility: a population-based study. Front Endocrinol (Lausanne). 2023;14:1175394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin YH, Zhou SY, Lu DF, Chen XP, Liu B, Lu S, Han XD, Wu AH. Higher waist circumference is associated with increased likelihood of female infertility: NHANES 2017–2020 results. Front Endocrinol (Lausanne). 2023;14:1216413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke J, Feng Y, Chen Z. Association between waist circumference and female infertility in the United States. PLoS ONE. 2023;18(12):e0295360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai J, Li X, Liu Z, Liao Y, Xiao Z, Wei Y, Cao Y. Association between Waist-Hip Ratio and Female Infertility in the United States: data from National Health and Nutrition Examination Survey 2017–2020. Obes Facts 2024:1–14. [DOI] [PMC free article] [PubMed]
- 34.Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018;16(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautam D, Purandare N, Maxwell CV, Rosser ML, O’Brien P, Mocanu E, McKeown C, Malhotra J, McAuliffe FM. The challenges of obesity for fertility: a FIGO literature review. Int J Gynaecol Obstet. 2023;160(Suppl 1Suppl 1):50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Y, Li M, Li X, Tang Q, Li X, Ji X, Tian W, Zhang H. Characteristics of different obesity metabolic indexes and their correlation with Insulin Resistance in patients with polycystic ovary syndrome. Reprod Sci 2024. [DOI] [PubMed]
- 37.Lei R, Chen S, Li W. Advances in the study of the correlation between insulin resistance and infertility. Front Endocrinol (Lausanne). 2024;15:1288326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fica S, Albu A, Constantin M, Dobri GA. Insulin resistance and fertility in polycystic ovary syndrome. J Med Life. 2008;1(4):415–22. [PMC free article] [PubMed] [Google Scholar]
- 39.Miettinen HE, Rayburn H, Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest. 2001;108(11):1717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yesilaltay A, Dokshin GA, Busso D, Wang L, Galiani D, Chavarria T, Vasile E, Quilaqueo L, Orellana JA, Walzer D, et al. Excess cholesterol induces mouse egg activation and may cause female infertility. Proc Natl Acad Sci U S A. 2014;111(46):E4972–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Berardino C, Peserico A, Capacchietti G, Zappacosta A, Bernabò N, Russo V, Mauro A, El Khatib M, Gonnella F, Konstantinidou F et al. High-Fat Diet and female fertility across Lifespan: a comparative lesson from Mammal models. Nutrients 2022, 14(20). [DOI] [PMC free article] [PubMed]
- 42.Hou YJ, Zhu CC, Duan X, Liu HL, Wang Q, Sun SC. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016;6:18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study is publicly available at https://www.cdc.gov/nchs/nhanes.