Abstract
Objective:
Imaging is essential for diagnosing large-vessel vasculitis (LVV). During diagnostic imaging, assessing disease activity and vascular damage separately is important. Acute-phase findings represent disease activity, while chronic-phase findings represent vascular damage; however, whether the imaging findings are acute or chronic may be unclear. We investigated how vascular lesions change before and after treatment and whether they were acute- or chronic-phase findings.
Methods:
Fifty-one patients with LVV who had undergone contrast-enhanced computed tomography (CT) scans from the neck to the pelvis before treatment and 1-4 months after treatment were recruited. Wall thickening, wall contrast enhancement, stenosis, occlusion, dilation, aneurysm, and calcification were semi-quantitatively assessed in 21 vessels from the common carotid to the common iliac artery.
Results:
Twenty-four patients were diagnosed with Takayasu arteritis (TAK), and 27 with giant cell arteritis (GCA). Wall thickening and wall contrast enhancement improved after the treatment, which was especially significant in the GCA group. No significant differences in stenosis, occlusion, dilation, aneurysm, or calcification were observed before and after treatment. Stenosis and occlusion were more common with TAK, while calcification was more common with GCA.
Conclusion:
Wall thickening and wall contrast enhancement are acute-phase findings (activity), while stenosis, occlusion, dilation, aneurysm, and calcification are chronic-phase findings (damage). The frequencies of these findings differ between TAK and GCA.
Keywords: Contrast-enhanced CT, giant cell arteritis, imaging, large-vessel vasculitis, Takayasu arteritis
Main Points
Imaging findings in LVV are divided into acute and chronic phases.
Acute-phase findings reflect activity, while chronic-phase findings reflect damage.
The frequencies of acute- and chronic-phase findings differ between TAK and GCA.
Introduction
Large-vessel vasculitis (LVV) is a group of diseases that cause vasculitis in the aorta and its primary large branches. Patients with LVV suffer from systemic symptoms, such as fever and malaise, as well as ischemic symptoms induced by vascular stenosis or obstruction in the extremities.1 The Chapel Hill Consensus Conference definition classifies LVV into Takayasu arteritis (TAK) and giant cell arteritis (GCA). According to this classification, a major distinction between TAK and GCA is the age of onset: TAK usually occurs before 50 years of age, while GCA, after 50 years of age.2 In actual clinical practice, LVV is suspected based on symptoms, and various imaging examinations are performed to detect LVV-specific findings and exclude other diseases involving large vessels, such as IgG4-related diseases and infections. New classification criteria for LVV were published by the American College of Rheumatology (ACR) and the European League against Rheumatic Diseases (EULAR) in 2022.3,4
The EULAR Recommendation states that when LVV is clinically suspected, diagnostic imaging should be performed before or immediately after treatment initiation.5,6 Imaging examinations may include ultrasonography, contrast-enhanced computed tomography (CT), contrast-enhanced magnetic resonance imaging (MRI), positron emission tomography (PET), and angiography.7,8 Angiography is the classical imaging modality; however, it is invasive and technically challenging. Thus, the EULAR Recommendation highlights that angiography should not be performed for diagnostic purposes. In diagnostic imaging, it is important to assess disease activity and vascular damage separately.9,10 In other words, disease activity may be expressed as an acute-phase finding, while vascular damage may be expressed as a chronic-phase finding. For activity assessment, fluorodeoxyglucose (FDG) uptake in PET, and vascular wall thickening and contrast enhancement are used. PET is useful for assessing LVV activity. The degree of FDG uptake can be visually and semi-quantitatively assessed.11,12 Thickening and contrast enhancement of the vascular wall often represent reversible lesions. Wall thickening can be assessed by ultrasonography, contrast-enhanced CT, and MRI. Although it is controversial whether wall thickening reflects disease activity or vascular damage, reversible portions are considered to indicate disease activity.10,13-15 Thus, a decrease in wall thickness after treatment indicates an improvement in disease activity. Contrast enhancement of the vascular wall indicates arterial wall inflammation as evidenced by increased blood flow and can be assessed using contrast-enhanced CT or MRI. Thus, contrast enhancement may represent disease activity. A decreased contrast enhancement of the vascular wall after treatment indicates an improvement in disease activity. Meanwhile, PET is unsuitable for damage assessment, because morphological assessment of vessels using this modality is impossible. The advent of PET may appear revolutionary, but unlike other modalities, PET is only useful for activity assessment. Damage assessment must target irreversible vascular lesions, such as stenosis, occlusion, dilatation, aneurysms, and vessel calcification. Repeated assessments are used to observe the rate of progression over time. Stenosis, occlusion, dilatation, and aneurysms of vessels can be assessed using ultrasonography, contrast-enhanced CT, and MRI. However, CT is the only modality that allows for comprehensive assessment of calcification.
Whether the aforementioned lesions (i.e., wall thickening, contrast enhancement of the vascular wall, stenosis, occlusion, dilatation, aneurysm, or calcification) are classified as disease activity (i.e., an acute-phase finding) or vascular damage (i.e., a chronic-phase finding) is based on empirical knowledge from accumulated case reports and case series.16-22 However, we believe that this knowledge has not yet been verified sufficiently. Using contrast-enhanced CT, which allows comprehensive whole-body assessment, we therefore investigated how vascular lesions change before and after treatment, and whether they were acute- or chronic-phase findings.
Methods
Patients
A total of 166 patients with TAK or GCA were diagnosed at the University of Yamanashi Hospital, Chiba University Hospital, Yamanashi Prefectural Central Hospital, or Shimoshizu Hospital between 2007 and 2019. Of 166 patients, 82 had TAK and 84 had GCA. Of these, 78 patients with TAK and 74 patients with GCA had a history of CT scans. Fifty-one patients underwent contrast-enhanced CT before treatment initiation and at 1-4 months after treatment initiation were included in the study. Regarding activity, patients were evaluated by C-reactive protein (CRP) levels and erythrocyte sedimentation rates (ESR), physician global assessment (PGA) (Active/persistent/Inactive), and Kerr score.23 At the time of CT 1-4 months after treatment initiation, only inactive patients were enrolled. Namely, at the time of after treatment evaluation, all patients had inactive PGA and Kerr score, normal CRP levels, ESR and no symptoms. We then reclassified these patients into TAK and GCA according to the 2022 ACR/EULAR classification criteria and analyzed.3,4
Clinical Assessment
The following patient characteristics were extracted from medical records and retrospectively assessed: age at onset; age at diagnosis; time from onset to diagnosis; pre-treatment CRP levels and ESR; systemic symptoms including fever, arthritis, malaise, neck pain, chest and back pain, reduced visual acuity or visual loss; clinical items of the ACR classification criteria; comorbidities at diagnosis; and initial dose of glucocorticoids.
Imaging Assessment
In total, the following 21 vessels were assessed: the right and left common carotid arteries, right and left vertebral arteries, brachiocephalic artery, right and left subclavian arteries, right and left axillary arteries, ascending aorta, arcuate artery, descending aorta, abdominal aorta, celiac artery, superior mesenteric artery, right and left renal arteries, right and left iliac arteries, and right and left pulmonary arteries. We modified the semi-quantitative scoring method previously reported and assigned points as follows:10
Wall thickening: absent, 0; mild, 1; moderate, 2; and severe, 3
Contrast enhancement of the vascular wall: absent, 0; mild, 1; moderate, 2; and prominent, 3
Stenosis/occlusion: absent, 0; <50% stenosis, 1; ≥50% stenosis, 2; and occlusion, 3
Dilatation/aneurysm: absent, 0; mild, 1; and severe, 2
Calcification: absent, 0; mild, 1; and severe, 2
All imaging assessments were performed by the same radiologist, who was skilled in diagnosing vasculitis. The imaging reviewer was blinded to the clinical features. The combined arteritis damage score (CARDS), a previously reported indicator of vasculitis damage, was also assessed.10,24 Combined arteritis damage score was calculated by applying a numerical weighting to each vascular lesion and adding all the scores of the assessed vessels. The formula is as follows: (number of vessels with <50% stenosis × 0.6) + (number of vessels with ≥50% stenosis × 1.2) + (number of vessels with occlusion × 1.6) + (number of vessels with dilatation or aneurysm × 0.8).
Statistical Analyses
Statistical analyses were performed using SPSS software, version 22.0 J (IBM Japan, Tokyo, Japan). Normally distributed continuous data were summarised using mean and SD and analysed using parametric tests (Student’s t-test). Non-normally distributed data were summarised using the median and interquartile range and analysed using non-parametric tests (Mann–Whitney U-test or Wilcoxon signed-rank test). Categorical data were summarised as percentages and analysed using the chi-square, Fisher’s exact, or McNemar’s tests. Statistical significance was set at P < .05.
Ethics
This study was approved by the Ethics Committee of the University of YYamanashi (reference no. 1493) and was conducted in accordance with the principles of the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. The requirement for written informed consent was waived according to local regulations for retrospective observational studies by the Ethics Committee of the University of Yamanashi. All data were fully anonymised before analysis.
Results
Patient Characteristics
Table 1 shows the baseline patient characteristics, including the clinical course, symptoms, blood test results, and treatments. Of the 51 patients, 24 were diagnosed with TAK and 27 with GCA. Women accounted for 73% of all patients, 75% of patients with TAK, and 70% of patients with GCA. Overall, the mean age at diagnosis was 52 years, 30 years in patients with TAK, and 70 years in patients with GCA. Patients with TAK were younger than those with GCA (P < .0001). The median time from onset to diagnosis was 4 months (7 months in patients with TAK, 3 months in patients with GCA). The time from onset to diagnosis was longer in patients with TAK than in those with GCA (P = .021). The median CRP level at diagnosis was 8.3 mg/L overall (6.4 mg/L in patients with TAK, 8.3 mg/L in patients with GCA). Moreover, the overall median ESR at diagnosis was 72 mm/h (62 mm/h in patients with TAK, 88 mm/h in those with GCA). The ESR was higher in patients with GCA (P = .001). No difference in the prevalence of systemic symptoms between patients with TAK and GCA was observed, while the prevalence of articular symptoms was higher in patients with GCA. Although no difference in the prevalence of neck pain was observed, the prevalence of chest and back pain was higher in patients with TAK. More items of the classification criteria pertaining to ischaemic symptoms in the extremities were identified in patients with TAK, whereas more items suggestive of temporal arteritis were identified in those with GCA. None of the patients with TAK had reduced visual acuity, visual loss, or polymyalgia rheumatica. Meanwhile, none of the patients with GCA had concomitant ulcerative colitis, although its prevalence was as high as 25% in patients with TAK. Glucocorticoids were administered to all patients during the initial treatment. The median initial dose equivalent to prednisolone was higher in patients with TAK than in those with GCA (50 mg/day and 40 mg/day, respectively).
Table 1.
Patient Demographics, Clinical/Laboratory Data at Diagnosis, and Comparison of Takayasu Arteritis (TAK) and Giant Cell Arteritis (GCA)
| Total, n = 51 | TAK, n = 24 | GCA, n = 27 | P | |
|---|---|---|---|---|
| Female, n (%) | 37 (73) | 18 (75) | 19 (70) | .712‡ |
| Age at onset, mean (SD) years | 50 (22.4) | 28 (9.2) | 69 (6.6) | <.0001* |
| Age at diagnosis, mean (SD) years | 52 (21.7) | 30 (10.0) | 70 (6.3) | <.0001* |
| Delay from onset to diagnosis, median (IQR) months | 4 (2-13) | 7 (2-30) | 3 (2-9) | .021† |
| CRP level at diagnosis, median (IQR) mg/l | 8.3 (4.6-12.7) | 6.4 (3.1-12) | 8.3 (6.5-13.2) | .209† |
| ESR at diagnosis, median (IQR) mm/h | 72 (53-103) | 62 (48-71) | 88 (73-116) | .001† |
| Symptoms | ||||
| Fever at diagnosis, no. (%) | 33 (65%) | 16 (67%) | 17 (63%) | .782‡ |
| Arthralgia, myalgia at diagnosis, no. (%) | 11 (22%) | 1 (4%) | 10 (37%) | .004‡ |
| Limb claudication at diagnosis, no. (%) | 12 (24%) | 9 (38%) | 3 (11%) | .027‡ |
| Pulse loss or weakness at diagnosis, no. (%) | 9 (18%) | 6 (25%) | 3 (11%) | .176‡ |
| Blood pressure inequality at diagnosis, no. (%) | 17 (33%) | 12 (50%) | 5 (19%) | .017‡ |
| Bruit at diagnosis, no. (%) | 19 (37%) | 17 (71%) | 2 (7%) | <.0001‡ |
| Carotidynia at diagnosis, no. (%) | 15 (29%) | 10 (42%) | 5 (19%) | .07‡ |
| Chest or back pain at diagnosis, no. (%) | 13 (26%) | 11 (46%) | 2 (7%) | .002‡ |
| Headache at diagnosis, no. (%) | 9 (18%) | 2 (8%) | 7 (26%) | .1‡ |
| Temporal artery abnormality at diagnosis, no. (%) | 9 (18%) | 0 | 9 (33%) | .002‡ |
| Polymyalgia rheumatica at diagnosis, no. (%) | 4 (8%) | 0 | 4 (15%) | .07‡ |
| Positive temporal artery biopsy at diagnosis, no. (%) | 7 (14%) | 0 | 7 (26%) | .008‡ |
| Jaw claudication at diagnosis, no. (%) | 6 (12%) | 0 | 6 (22%) | .016‡ |
| Visual loss, no. (%) | 4 (8%) | 0 | 4 (15%) | .07‡ |
| Complications | ||||
| Ulcerative colitis, no. (%) | 6 (12%) | 6 (25%) | 0 | .007‡ |
| Hypertension, no. (%) | 27 (53%) | 9 (38%) | 18 (67%) | .037‡ |
| Hyperlipidaemia, no. (%) | 20 (40%) | 8 (33%) | 12 (44%) | .417‡ |
| Diabetes mellitus, no. (%) | 11 (22%) | 1 (4%) | 10 (37%) | .004‡ |
| Smoking, no. (%) | 14 (28%) | 6 (25%) | 8 (29%) | .712‡ |
| Treatment | ||||
| Glucocorticoids, no. (%) | 51 (100%) | 24 (100%) | 27 (100%) | 1‡ |
| Initial dose of glucocorticoids, median (IQR) mg/day | 50 (40-50) | 50 (42-55) | 40 (30-40) | <.001† |
The P values are calculated for the differences between TAK and GCA.
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range.
*t-test.
† Mann–Whitney U-test.
‡ chi-square test or Fisher’s exact test with Bonferroni correction.
Changes in the Vascular Lesions Before and After Treatment
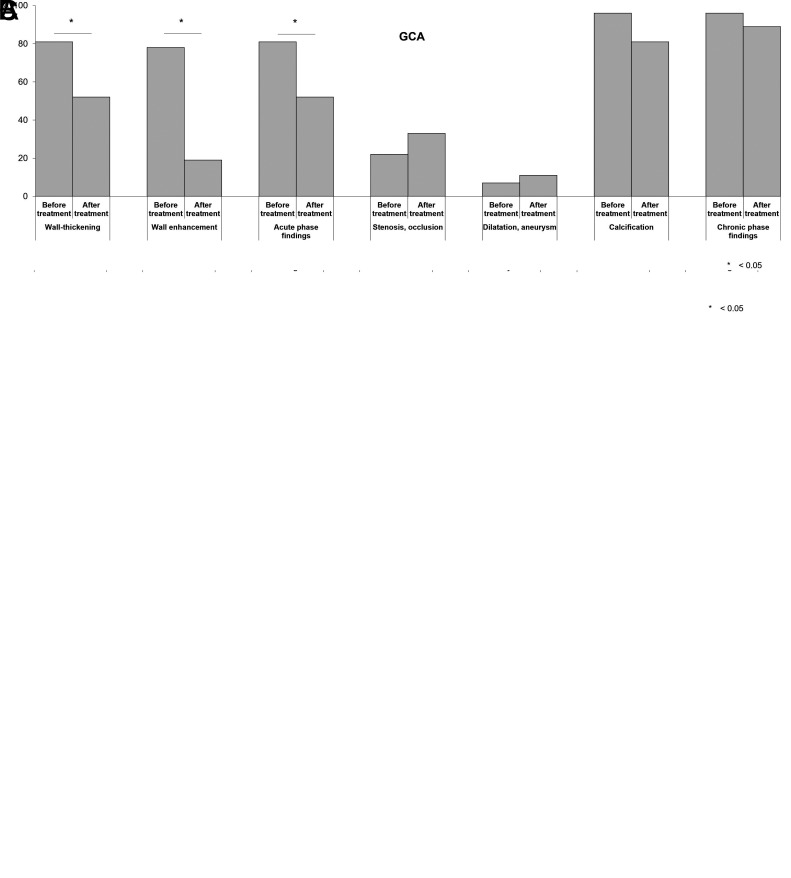
Table 2 shows the changes in vascular lesions before and after treatment. The values presented are the median sums of the semi-quantitatively assessed scores for all 21 vessels. In all patients with TAK or GCA, wall thickening and contrast enhancement of the vascular wall significantly improved after treatment compared with those before treatment. In contrast, no significant changes in stenosis, occlusion, dilatation, aneurysm, or calcification before and after treatment were observed in all patients. The prevalence of vascular lesions per patient before and after treatment (i.e., presence or absence of vascular lesions in any vessel) was also similar between the groups (Figure 1). Furthermore, no significant between-group differences were observed when these features were assessed separately for each vessel (data not shown).
Table 2.
Changes in Vascular Lesions Before and After Treatment of Takayasu Arteritis and Giant Cell Arteritis
| Before Treatment | After Treatment | P | |
|---|---|---|---|
| Wall thickening | |||
| Total, n = 51, median (IQR) | 6 (4-13) | 2 (0-5) | <.0001 |
| TAK, n = 24, median (IQR) | 8 (5-12.5) | 4 (2-7) | .01 |
| GCA, n = 27, median (IQR) | 5 (3-15) | 1 (0-3) | <.0001 |
| Wall enhancement | |||
| Total, n = 51, median (IQR) | 4 (2-8) | 0 (0-2) | <.0001 |
| TAK, n = 24, median (IQR) | 3 (2-8) | 1 (0-4) | .005 |
| GCA, n = 27, median (IQR) | 5 (1-9) | 0 (0-0) | <.0001 |
| Acute-phase findings | |||
| Total, n = 51, median (IQR) | 11 (6-20) | 2 (0-8) | <.0001 |
| TAK, n = 24, median (IQR) | 11.5 (6-20) | 6.5 (2-10.5) | .003 |
| GCA, n = 27, median (IQR) | 9 (4-25) | 1 (0-4) | <.0001 |
| Stenosis, occlusion | |||
| Total, n = 51, median (IQR) | 0 (0-2) | 0 (0-2) | .475 |
| TAK, n = 24, median (IQR) | 1 (0-2.7) | 2 (0-3) | .661 |
| GCA, n = 27, median (IQR) | 0 (0-0) | 0 (0-1) | .499 |
| Dilatation, aneurysm | |||
| Total, n = 51, median (IQR) | 0 (0-0) | 0 (0-0) | .167 |
| TAK, n = 24, median (IQR) | 0 (0-0) | 0 (0-0) | .334 |
| GCA, n = 27, median (IQR) | 0 (0-0) | 0 (0-0) | .317 |
| Calcification | |||
| Total, n = 51, median (IQR) | 1 (0-5) | 0 (0-5) | .115 |
| TAK, n = 24, median (IQR) | 0 (0-0) | 0 (0-0) | .891 |
| GCA, n = 27, median (IQR) | 4 (2-7) | 4 (2-7) | .066 |
| Chronic-phase findings | |||
| Total, n = 51, median (IQR) | 3 (1-6) | 4 (2-6) | .965 |
| TAK, n = 24, median (IQR) | 1.5 (0-3.7) | 2 (0-4) | .325 |
| GCA, n = 27, median (IQR) | 5 (3-9) | 6 (4-9) | .34 |
Data are analyzed using the Wilcoxon signed-rank test. Acute-phase findings include a combination of wall thickening and contrast enhancement of the vascular wall. Chronic-phase findings include a combination of stenosis, occlusion, dilatation, aneurysm, and calcification. The values are the median sums of the semi-quantitatively assessed scores for all 21 vessels.
GCA, giant cell arteritis; IQR, interquartile range; TAK, Takayasu arteritis.
Figure 1.
A, B, C. Prevalence of vascular lesions in all patients before and after treatment. The frequencies of wall thickening, wall contrast enhancement, stenosis, occlusion, dilatation, aneurysm, and calcification at any vessel in (A) the entire group, (B) patients with TAK, and (C) patients with GCA are shown. GCA, giant cell arteritis; TAK, Takayasu arteritis. Data are analysed using McNemar’s test. Acute-phase findings include a combination of wall thickening and contrast enhancement of the vascular wall. Chronic-phase findings include a combination of stenosis, occlusion, dilatation, aneurysm, and calcification. The P values are calculated for pre- and post-treatment differences.
Comparison of Findings Between Patients with TAK and those with GCA
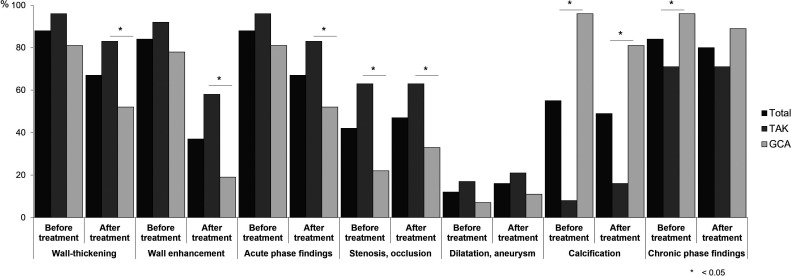
Table 3 compares the total scores between patients with TAK and those with GCA for the 21 semi-quantitatively assessed vessels. Although no between-group differences in the wall thickening or contrast enhancement scores before treatment were observed, the scores after treatment were significantly higher in patients with TAK than in those with GCA. The pre- and post-treatment scores for stenosis and occlusion were significantly higher in patients with TAK than in patients with GCA. Moreover, the pre- and post-treatment calcification scores were higher in patients with GCA than in those with TAK. Similarly, the prevalence of vascular lesions per patient before and after treatment was higher in patients with GCA than in patients with TAK (Supplementary Figure 1).
Table 3.
Comparison of the Total Scores between Takayasu Arteritis and Giant Cell Arteritis for 21 Vessels
| Total, n = 51 | TAK, n = 24 | GCA, n = 27 | P | |
|---|---|---|---|---|
| Wall thickening before treatment, median (IQR) | 6 (4-13) | 8 (5-12.5) | 5 (3-15) | .289 |
| Wall thickening after treatment, median (IQR) | 2 (0-5) | 4 (2-7) | 1 (0-3) | .003 |
| Wall enhancement before treatment, median (IQR) | 4 (2-8) | 3 (2-8) | 5 (1-9) | .609 |
| Wall enhancement after treatment, median (IQR) | 0 (0-2) | 1 (0-4) | 0 (0-0) | .005 |
| Total of acute-phase findings before treatment, median (IQR) | 11 (6-20) | 11.5 (6-20) | 9 (4-25) | .610 |
| Total of acute-phase findings after treatment, median (IQR) | 2 (0-8) | 6.5 (2-10.5) | 1 (0-4) | .003 |
| Stenosis, occlusion before treatment, median (IQR) | 0 (0-2) | 1 (0-2.7) | 0 (0-0) | .004 |
| Stenosis, occlusion after treatment, median (IQR) | 0 (0-2) | 2 (0-3) | 0 (0-1) | .009 |
| Dilatation, aneurysm before treatment, median (IQR) | 0 (0-0) | 0 (0-0) | 0 (0-0) | .320 |
| Dilatation, aneurysm after treatment, median (IQR) | 0 (0-0) | 0 (0-0) | 0 (0-0) | .395 |
| Calcification before treatment, median (IQR) | 1 (0-5) | 0 (0-0) | 4 (2-7) | <.0001 |
| Calcification after treatment, median (IQR) | 0 (0-5) | 0 (0-0) | 4 (2-7) | <.0001 |
| Total of chronic-phase findings before treatment, median (IQR) | 3 (1-6) | 1.5 (0-3.7) | 5 (3-9) | <.0001 |
| Total of chronic-phase findings after treatment, median (IQR) | 4 (2-6) | 2 (0-4) | 6 (4-9) | .001 |
Data were analysed using the Mann–Whitney U-test. The P values are calculated for the differences between TAK and GCA. Acute-phase findings include a combination of wall thickening and contrast enhancement of the vascular wall. Chronic-phase findings include a combination of stenosis, occlusion, dilatation, aneurysm, and calcification.
GCA, giant cell arteritis; IQR, interquartile range; TAK, Takayasu arteritis.
Supplementary Figure 1.
Prevalence of vascular lesions in the TAK and GCA groups before and after treatment. The frequencies of wall thickening, wall contrast enhancement, stenosis, occlusion, dilatation, aneurysm, and calcification at any vessels in the TAK and GCA groups before and after treatment are shown. TAK, Takayasu arteritis; GCA, giant cell arteritis. Data are analyzed using the chi-square and Fisher’s exact tests. Acute-phase findings include a combination of wall thickening and contrast enhancement of the vascular wall. Chronic-phase findings include a combination of stenosis, occlusion, dilatation, aneurysm, and calcification. The P values are calculated for the differences between the TAK and GCA groups.
Changes in the Comparison of CARDS
Pre-treatment CARDS was higher in patients with TAK than in patients with GCA (Table 4). Damage progression did not significantly differ between the TAK and GCA groups pre- and post-treatment (P = .815 and P = .327, respectively; Supplementary Table 1).
Table 4.
Comparison of Combined Arteritis Damage Scores Between Takayasu Arteritis and Giant Cell Arteritis
| Total, n = 51 | TAK, n = 24 | GCA, n = 27 | P | |
|---|---|---|---|---|
| CARDS before treatment, median (IQR) | 0 (0-1.6) | 1.2 (0.3-1.9) | 0 (0-0.3) | .001 |
| CARDS after treatment, median (IQR) | 0.6 (0-1.6) | 1.2 (0-2.2) | 0 (0-1.2) | .018 |
Data were analysed using the Mann–Whitney U-test. The P values are calculated for the differences between TAK and GCA. CARDS is computed as follows: (number of vessels with <50% stenosis × 0.6) + (number of vessels with ≥50% stenosis × 1.2) + (number of vessels with occlusion × 1.6) + (number of vessels with dilatation or aneurysm × 0.8).
CARDS, Combined Arteritis Damage Score; GCA, giant cell arteritis; IQR, interquartile range; TAK, Takayasu arteritis.
Supplementary Table 1.
Changes in Combined Arteritis Damage Scores Before and After Treatment for the 21 Vessels
| CARDS before treatment, median (IQR) | CARDS after treatment, median (IQR) | p value | |
|---|---|---|---|
| Total, n = 51 | 0 (0-1.6) | 0.6 (0-1.6) | 0.443 |
| TAK, n = 24 | 1.2 (0.3-1.9) | 1.2 (0-2.2) | 0.815 |
| GCA, n = 27 | 0 (0-0.3) | 0 (0-1.2) | 0.327 |
CARDS, Combined Arteritis Damage Score; IQR, interquartile range; TAK, Takayasu arteritis; GCA, giant cell arteritis. Data are analyzed using the Mann–Whitney U-test. CARDS is computed as follows: (number of vessels with <50% stenosis × 0.6) + (number of vessels with ≥50% stenosis × 1.2) + (number of vessels with occlusion × 1.6) + (number of vessels with dilatation or aneurysm × 0.8).
Discussion
In this study, contrast-enhanced CT images taken before and after treatment were used to comprehensively assess changes in vascular lesions in LVV in terms of wall thickening, contrast enhancement of the vascular wall, stenosis, dilatation, aneurysm, and calcification. We then assessed changes in the vascular lesions after treatment. We assumed that vascular lesion improvement after treatment represented disease activity (i.e., acute-phase findings), and that poor lesion improvement represented vascular damage (i.e., chronic-phase findings). Our results demonstrated that wall thickening and contrast enhancement of the vascular wall improved after treatment in patients with TAK and those with GCA. However, there were no improvements in stenosis, occlusion, or calcification after treatment. Dilatation and aneurysm were difficult to assess because of their low prevalence in our cohort.25,26 When wall thickening and contrast enhancement of the vascular wall were regarded as acute-phase findings, the total pre- and post-treatment scores were significantly different, whereas when stenosis, occlusion, dilatation, aneurysm, and calcification were regarded as chronic-phase findings, the total pre- and post-treatment scores did not significantly differ (Table 2). Therefore, while the significance of vascular lesions has not been previously clarified, our study revealed that wall thickening and contrast enhancement of the vascular wall are acute-phase findings indicating disease activity, and that stenosis, occlusion, dilatation, aneurysm, and calcification are chronic-phase findings indicating vascular damage.
However, while the reversible portion of wall thickening represents disease activity, residual irreversible wall thickening may be a chronic finding indicative of vascular damage. Tso et al reported that wall thickening may not correspond to contrast enhancement of the vascular wall on contrast-enhanced MRI.13 This suggests that wall thickening may represent both disease activity and vascular damage. Assessment of wall thickening in combination with contrast enhancement of the vascular wall may help distinguish between disease activity and vascular damage. In other words, poorly contrast-enhanced wall thickening persisting after the initial treatment is likely to represent vascular damage, while contrast-enhanced wall thickening is likely to represent an active lesion. Therefore, focusing on only the degree of improvement in wall thickening without taking into consideration the contrast enhancement of the vascular wall may result in unnecessary and excessive treatment.
We also found that the prevalence of wall thickening per patient did not decrease significantly in patients with TAK (Figure 1). The improvement in wall thickening and contrast enhancement after treatment was smaller in patients with TAK than in patients with GCA (Table 3, Supplementary Figure 1), despite the initial dose of glucocorticoids being higher in patients with TAK than in patients with GCA. This suggests that TAK may be more resistant to the initial treatment than GCA. The pre-treatment prevalence of stenosis and occlusion was high in patients with TAK (Table 3, Figure 1 and Supplementary Figure 1). In TAK, vessels are likely to be damaged at the time of diagnosis. This may be related to the greater delay in the diagnosis of TAK in comparison with GCA.10,27 Giant cell arteritis is relatively easy to diagnose when symptoms of temporal arteritis or polymyalgia rheumatica occur in combination with arthralgia. In contrast, the initial symptoms of TAK (e.g., fever and malaise) are often non-specific, making TAK difficult to diagnose.9 By the time the ACR classification criteria are applicable, imaging examinations may already show damaged lesions. Another hypothesis is that TAK progresses from an acute to a chronic phase faster than GCA.28 The acute- and chronic-phase findings in TAK and GCA were clearly different, supporting the theory that TAK and GCA are distinct diseases.29-34
Finally, CARDS, an imaging indicator of LVV-associated damage, significantly differed between the TAK and GCA groups before treatment (Table 4). In early-stage TAK, vascular damage may develop rapidly, which is consistent with the findings of an original report on CARDS showing that vascular damage is more severe in TAK than in GCA.10 Furthermore, no differences in CARDS were observed between the TAK and GCA groups before and after treatment. This is likely because the assessment was performed only during the short remission induction period of this study. Differences may be detectable with a multi-year, long-term follow-up.
This study has some limitations. First, the sample size was small, as the study included only patients who had undergone contrast-enhanced CT before and 1-4 months after treatment initiation. Giant cell arteritis with temporal arteritis and TAK with poor imaging findings may not be re-examined within a few months, increasing the likelihood of a selection bias. However, the collection of imaging data within the specified period and from multiple institutions may be a strength of this study. Second, only contrast-enhanced CT was used in this study. Simultaneous assessment of contrast-enhanced CT and PET images may be useful in confirming that wall thickening and contrast enhancement of the vascular wall represent disease activity. Regarding damage assessment, it may be more appropriate to evaluate the accumulation of vascular damage before treatment and during a multi-year follow-up.
In this study, we evaluated vascular lesions and confirmed their characteristics in LVV. Specifically, wall thickening and contrast enhancement of the vascular wall are likely to represent disease activity (i.e., acute-phase findings), while stenosis, occlusion, dilatation, aneurysm, and calcification are likely to represent vascular damage (i.e., chronic-phase findings). These features can be used to clinically differentiate between TAK and GCA.
Funding Statement
The authors declare that this study received no financial support.
Footnotes
Ethics Committee Approval: This study was approved by the Ethics Committee of University of Yamanashi (Approval No: 1493; Date: 28th/Jul/2016).
Informed Consent: The requirement for written informed consent was waived according to local regulations for retrospective observational studies by the Ethics Committee of the University of Yamanashi.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – D.N., T.S.; Design – D.N., T.S.; Supervision – S.F., H.O., H.N.; Resources – D.N., S.F.; Materials – D.N., T.S.; Data Collection and/or Processing – T.S., K.K.,Y.K, S.H., K.H., T.K., C.A., T.S.; Analysis and/or Interpretation – D.N., T.S.; Literature Search – D.N., T.S.; Writing – D.N.; Critical Review – S.F., H.O., H.N.
Declaration of Interests: The authors have no conflicts of interest to declare.
Supplementary Materials
References
- 1. Saadoun D, Vautier M, Cacoub P. Medium- and large-vessel vasculitis. Circulation. 2021;143(3):267 282. ( 10.1161/CIRCULATIONAHA.120.046657) [DOI] [PubMed] [Google Scholar]
- 2. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1 11. ( 10.1002/art.37715) [DOI] [PubMed] [Google Scholar]
- 3. Grayson PC, Ponte C, Suppiah R, et al. 2022 American College of Rheumatology/EULAR classification criteria for Takayasu arteritis. Ann Rheum Dis. 2022;81(12):1654 1660. ( 10.1136/ard-2022-223482) [DOI] [PubMed] [Google Scholar]
- 4. Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR classification criteria for giant cell arteritis. Ann Rheum Dis. 2022;81(12):1647 1653. ( 10.1136/ard-2022-223480) [DOI] [PubMed] [Google Scholar]
- 5. Bardi M, Diamantopoulos AP. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice summary. Radiol Med. 2019;124(10):965 972. ( 10.1007/s11547-019-01058-0) [DOI] [PubMed] [Google Scholar]
- 6. Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636 643. ( 10.1136/annrheumdis-2017-212649) [DOI] [PubMed] [Google Scholar]
- 7. Keser G, Aksu K. Diagnosis and differential diagnosis of large-vessel vasculitides. Rheumatol Int. 2019;39(2):169 185. ( 10.1007/s00296-018-4157-3) [DOI] [PubMed] [Google Scholar]
- 8. Schäfer VS, Jin L, Schmidt WA. Imaging for diagnosis, monitoring, and outcome prediction of large vessel vasculitides. Curr Rheumatol Rep. 2020;22(11):76. ( 10.1007/s11926-020-00955-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakagomi D, Jayne D. Outcome assessment in Takayasu arteritis. Rheumatol (Oxf Engl). 2016;55(7):1159 1171. ( 10.1093/rheumatology/kev366) [DOI] [PubMed] [Google Scholar]
- 10. Nakagomi D, Cousins C, Sznajd J, et al. Development of a score for assessment of radiologic damage in large-vessel vasculitis (Combined Arteritis Damage Score, CARDS). Clin Exp Rheumatol. 2017;35(1)(suppl 103):139 145. [PubMed] [Google Scholar]
- 11. van der Geest KSM, Treglia G, Glaudemans AWJM, et al. Diagnostic value of [18F]FDG-PET/CT for treatment monitoring in large vessel vasculitis: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48(12):3886 3902. ( 10.1007/s00259-021-05362-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dashora HR, Rosenblum JS, Quinn KA, et al. Comparing semiquantitative and qualitative methods of vascular (18)F-FDG PET activity measurement in large-vessel vasculitis. J Nucl Med. 2022;63(2):280 286. ( 10.2967/jnumed.121.262326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tso E, Flamm SD, White RD, Schvartzman PR, Mascha E, Hoffman GS. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum. 2002;46(6):1634 1642. ( 10.1002/art.10251) [DOI] [PubMed] [Google Scholar]
- 14. Hommada M, Mekinian A, Brillet PY, et al. Aortitis in giant cell arteritis: diagnosis with FDG PET/CT and agreement with CT angiography. Autoimmun Rev. 2017;16(11):1131 1137. ( 10.1016/j.autrev.2017.09.008) [DOI] [PubMed] [Google Scholar]
- 15. Olthof SC, Krumm P, Henes J, et al. Imaging giant cell arteritis and Aortitis in contrast enhanced 18F-FDG PET/CT: which imaging score correlates best with laboratory inflammation markers? Eur J Radiol. 2018;99:94 102. ( 10.1016/j.ejrad.2017.12.021) [DOI] [PubMed] [Google Scholar]
- 16. Sharma S, Sharma S, Taneja K, Gupta AK, Rajani M. Morphologic mural changes in the aorta revealed by CT in patients with nonspecific aortoarteritis (Takayasu’s arteritis). AJR Am J Roentgenol. 1996;167(5):1321 1325. ( 10.2214/ajr.167.5.8911205) [DOI] [PubMed] [Google Scholar]
- 17. Prieto-González S, García-Martínez A, Tavera-Bahillo I, et al. Effect of glucocorticoid treatment on computed tomography angiography detected large-vessel inflammation in giant-cell arteritis. A prospective, longitudinal study. Med (Baltim). 2015;94(5):e486. ( 10.1097/MD.0000000000000486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Berthod PE, Aho-Glélé S, Ornetti P, et al. CT analysis of the aorta in giant-cell arteritis: a case-control study. Eur Radiol. 2018;28(9):3676 3684. ( 10.1007/s00330-018-5311-8) [DOI] [PubMed] [Google Scholar]
- 19. Spira D, Xenitidis T, Henes J, Horger M. MRI parametric monitoring of biological therapies in primary large vessel vasculitides: a pilot study. Br J Radiol. 2016;89(1058):20150892. ( 10.1259/bjr.20150892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang N, Pan L, Liu J, et al. Comparison of different thoracic aortic wall characteristics for assessment of disease activity in Takayasu arteritis: a quantitative study with 3.0 T magnetic resonance imaging. Rev Cardiovasc Med. 2022;23(3):92. ( 10.31083/j.rcm2303092) [DOI] [PubMed] [Google Scholar]
- 21. Chen B, Wang X, Yin W, et al. Assessment of disease activity in Takayasu arteritis: a quantitative study with computed tomography angiography. Int J Cardiol. 2019;289:144 149. ( 10.1016/j.ijcard.2019.04.086) [DOI] [PubMed] [Google Scholar]
- 22. Prieto-González S, Arguis P, García-Martínez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012;71(7):1170 1176. ( 10.1136/annrheumdis-2011-200865) [DOI] [PubMed] [Google Scholar]
- 23. Kerr GS, Hallahan CW, Giordano J, et al. Takayasu arteritis. Ann Intern Med. 1994;120(11):919 929. ( 10.7326/0003-4819-120-11-199406010-00004) [DOI] [PubMed] [Google Scholar]
- 24. Nakaoka Y, Yanagawa M, Hata A, et al. Vascular imaging of patients with refractory Takayasu arteritis treated with tocilizumab: post hoc analysis of a randomized controlled trial. Rheumatol (Oxf Engl). 2022;61(6):2360 2368. ( 10.1093/rheumatology/keab684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jud P, Verheyen N, Dejaco C, et al. Prevalence and prognostic factors for aortic dilatation in giant cell arteritis - a longitudinal study. Semin Arthritis Rheum. 2021;51(4):911 918. ( 10.1016/j.semarthrit.2020.11.003) [DOI] [PubMed] [Google Scholar]
- 26. García-Martínez A, Hernández-Rodríguez J, Arguis P, et al. Development of aortic aneurysm/dilatation during the followup of patients with giant cell arteritis: a cross-sectional screening of fifty-four prospectively followed patients. Arthritis Rheum. 2008;59(3):422 430. ( 10.1002/art.23315) [DOI] [PubMed] [Google Scholar]
- 27. Furuta S, Cousins C, Chaudhry A, Jayne D. Clinical features and radiological findings in large vessel vasculitis: are Takayasu arteritis and giant cell arteritis 2 different diseases or a single entity? J Rheumatol. 2015;42(2):300 308. ( 10.3899/jrheum.140562) [DOI] [PubMed] [Google Scholar]
- 28. Misra R. Takayasu arteritis: a distinct syndrome of large vessel vasculitis: a view point by late Professor Paul Bacon. Int J Rheum Dis. 2019;22(suppl 1):49 52. ( 10.1111/1756-185X.13383) [DOI] [PubMed] [Google Scholar]
- 29. Koster MJ, Warrington KJ. Classification of large vessel vasculitis: can we separate giant cell arteritis from Takayasu arteritis? Presse Med. 2017;46(7-8 Pt 2):e205 e213. ( 10.1016/j.lpm.2016.11.032) [DOI] [PubMed] [Google Scholar]
- 30. Gribbons KB, Ponte C, Carette S, et al. Patterns of arterial disease in Takayasu arteritis and giant cell arteritis. Arthritis Care Res (Hoboken). 2020;72(11):1615 1624. ( 10.1002/acr.24055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoshifuji H. Pathophysiology of large vessel vasculitis and utility of interleukin-6 inhibition therapy. Mod Rheumatol. 2019;29(2):287 293. ( 10.1080/14397595.2018.1546358) [DOI] [PubMed] [Google Scholar]
- 32. Kurata A, Saito A, Hashimoto H, et al. Difference in immunohistochemical characteristics between Takayasu arteritis and giant cell arteritis: it may be better to distinguish them in the same age. Mod Rheumatol. 2019;29(6):992 1001. ( 10.1080/14397595.2019.1570999) [DOI] [PubMed] [Google Scholar]
- 33. Robinette ML, Rao DA, Monach PA. The immunopathology of giant cell arteritis across disease spectra. Front Immunol. 2021;12:623716. ( 10.3389/fimmu.2021.623716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsumoto K, Suzuki K, Yoshida H, Magi M, Kaneko Y, Takeuchi T. Longitudinal monitoring of circulating immune cell phenotypes in large vessel vasculitis. Autoimmun Rev. 2022;21(10):103160. ( 10.1016/j.autrev.2022.103160) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 Content of this journal is licensed under a
Content of this journal is licensed under a