Abstract
Objective
Acute cerebral infarction (ACI) has a high mortality and disability, which brings a heavy burden to the medical and health system. This study aims to discover the clinical prevalence of post-stroke depression (PSD) in patients with ACI, explore the predictive factors leading to this complication, and provide more evidence for better identification of PSD in clinic.
Methods
From April 2021 to April 2023, this retrospective study selected 166 ACI patients as the research subjects, collected clinical symptoms and laboratory indicators at baseline, and observed the prevalence of PSD using the Hamilton depression scale 17 and the diagnostic and statistical manual of mental disorders. Multiple logistic regression analysis was adopted to explore the predictive factors of PSD in patients with ACI.
Results
The total incidence of PSD was 35.54% in 166 patients with ACI. The score of National Institute of Health Stroke Scale (NIHSS), the score of daily life ability scale (ADL), and homocysteine (Hcy) level in the PSD group were higher than non-PSD group (PNIHSS < .001, PADL < .001, PHcy = .001). Multiple logistic regression analysis showed that high Hcy levels, NIHSS scores, and ADL scores were independent risk factors for PSD (PHcy =.038, PNIHSS =.002, PADL <.001). The receiver operating characteristic (ROC) curve showed that areas under curve (AUC) = 0.894, standard errora = 0.025, progressive significanceb <.001, 95% CI = 0.845-0.943, cut-off value = 0.520, sensitivity = 91.60%, specificity = 74.60%, and Hosmer-Lemeshow goodness-of-fit test P = .246, suggesting that ROC curve has a certain clinical predictive efficacy.
Conclusion
The prevalence of early PSD in patients with ACI is relatively high. Homocysteine levels, NIHSS scores and ADL scores may be independent risk factors for PSD, and targeted clinical intervention should be implemented for the above factors.
Keywords: Acute cerebral infarction, depression, predictive factors, prevalence
Main Points
The total incidence of PSD in patients with ACI within 1 week was 35.54%.
High Hcy levels, NIHSS scores, and ADL scores are independent risk factors for PSD in patients with ACI.
ROC model contained 3 characteristics, including NIHSS scores, ADL scores, and Hcy levels. The AUC of the ROC model was 0.894, indicating that the model has certain clinical predictive efficacy.
Introduction
Acute cerebral infarction (ACI) is the most common type of cerebral infarction, accounting for about 60%-80% of all cerebral infarction.1 Acute cerebral infarction is a dynamic process, and there are often ischemic but not fully infarcted brain regions around the irreversible infarcted brain tissue. Depression is a common mental disorder in patients with cerebral infarction,2 mainly manifested as continuous low mood, decreased or absent interest, decreased appetite and activity ability, self-blame, insomnia, and restless sleep, and other related psychological adverse conditions after cerebral infarction.3 Some scholars have considered that depression is caused by brain damage, which disrupts the neural circuits involved in emotional regulation.4 However, due to the prominent problem of cerebral infarction in related patients, people tend to ignore patients’ depression and cover up their depressive symptoms, or consider that the emotional changes of patients are a normal phenomenon caused by the disease, and do not pay enough attention to them, which leads to the failure of timely treatment and control of related problems. In severe cases, patients will have suicidal tendencies and other related behaviors,5 posing a greater threat to their lives. A series of affective disorders caused by post-stroke depression (PSD) can directly affect the rehabilitation of patients’ nerve and limb function, and also lead to their poor mood, experience, and physical dysfunction, which increases the mortality of patients by 3-4 times.6 Difficulty in clinical diagnosis, poor response to antidepressants, high recurrence rate, low awareness rate, and low treatment rate are the problems in this field. Therefore, this study explores the prevalence of PSD in patients with ACI and clarifies the occurrence and development of the disease, which is helpful to understand its clinical characteristics, identify related risk factors, and provide a clinical basis for early intervention of PSD in patients with ACI.
Methods
Research Design
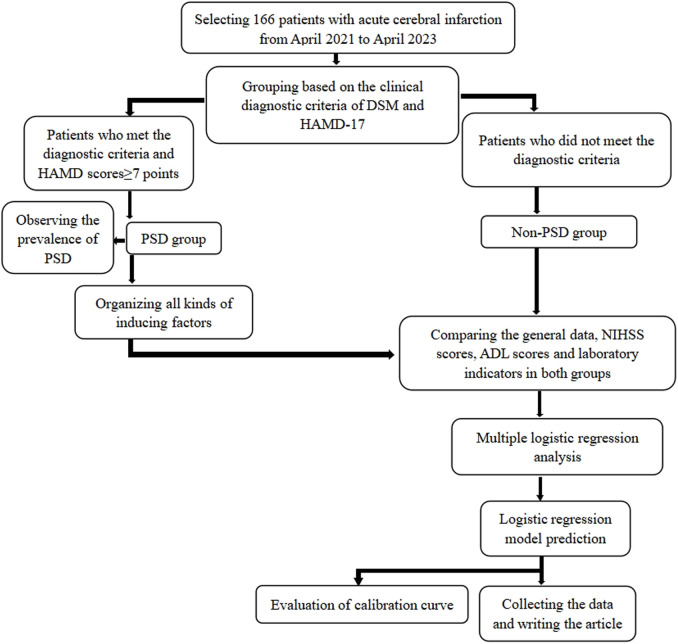
The clinical symptoms and laboratory indicators of patients at baseline were collected for a retrospective study. The technical route was shown in Figure 1.
Figure 1.
Technical route. This figure was the technical route of this study, involving the grouping and analysis process. ADL, Activity of Daily Living Scale; DSM, Diagnostic and Statistical Manual; HAMD-17, Hamilton Depression Scale 17; NIHSS, National Institute of Health Stroke Scale; PSD, Post-stroke Depression.
Inclusion and Exclusion Criteria
This study conforms to the principles of the Declaration of Helsinki (2013).7
Inclusion Criteria
(1) Patients met the diagnostic criteria in the Guidelines for Acute Ischemic Stroke Treatment8 and were confirmed by imaging examination. (2) Patients had stable conditions and clear consciousness. (3) Patients were <80 years old.
Exclusion Criteria
(1) Patients had a family history of mental illness or had a clear history of depression or organic mental illness. (2) Patients had severe liver and kidney dysfunction and cardiopulmonary dysfunction. (3) Patients had malignant tumors and other organ and system diseases.
Questionnaire Survey
The characteristics of the participants were recorded, including age, gender, education levels (illiterate, primary school, and junior high school and above), marital status (married, divorced, and widowed), inhabiting information (living alone or living with others), previous history of stroke, risk factors for cerebrovascular disease (hypertension, diabetes mellitus, smoking history, and drinking history), size and location of the infarction, and admission time.
Questionnaires were completed by 3 uniformly trained investigators, who conducted on-site questionnaire surveys, completed, and collected the questionnaires on the spot.
Quality Control
The researchers set up a group to design the questionnaire based on previous literature and clinical experience, and they developed a unified and standardized questionnaire guidance language. The questionnaire was filled out in the form of a question-and-answer and reviewed by 2 researchers. Researchers should not use tendentious language to interfere with the collection of normal information during the investigation process. After the investigation, 10% of the participants were re-examined in the form of question-and-answer by telephone. A coincidence rate of more than 85% was considered qualified to ensure the reliability of the collected data.
Definition of Key Variables
Diagnostic Criteria of Post-Stroke Depression
This study used the Diagnostic and Statistical Manual of mental disorders (DSM)9 as the main diagnostic criteria and the Hamilton Depression Scale 17 (HAMD-17)10 as an auxiliary diagnostic tool for determining PSD in patients with ACI. The Hamilton Depression Scale 17 mainly evaluated 7 factors, including anxiety/somatization, weight, cognitive disorders, blocking, sleep disorders, feeling of despair, and circadian changes. A higher score indicated that patients had more severe depression. The diagnostic criteria were divided into normal conditions (HAMD score < 7 points), mild depression (7 points ≤ HAMD score <17 points), moderate depression (17 points ≤ HAMD score <24 points), and severe depression (HAMD score ≥ 24 points). In this study, patients who were diagnosed by DSM and had a HAMD score of ≥7 points were included in the PSD group.
National Institutes of Health Stroke Scale
National Institutes of Health Stroke Scale was the most commonly used scale to evaluate the degree of neurological deficits, including 11 items such as visual field, gaze, consciousness level, movement, sensation, ataxia, facial paralysis, dysarthria, language, and neglect.11 The score range was 0-45 points. The higher the score, the more serious the neurological deficit.
Activity of Daily Living
Activity of Daily Living Scale (ADL)12 consisted of 14 items, including the Physical Self-Maintenance Scale (6 items) and the Instrumental Activity of Daily Living Scale (8 items). The scale adopted the 4-level scoring method (1-4 points). A total score <16 points was normal, and a score ≥16 points indicated functional decline in different degrees of living ability, with the highest score of 56 points. The higher the score, the worse the ability for daily living.
Laboratory Indicators
Fasting venous blood samples were taken from patients within 24 h after admission to detect serum prealbumin, homocysteine (Hcy), fibrinogen, and blood lipid (total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol). The HITACHI 7180 automatic biochemical analyzer (Hitachi Limited, Tokyo, Japan), matching reagent (enzyme cycling assay), and calibration were applied. Detection method13 adopted reagent preparation. The reagents were all liquid and could be directly prepared for application.
Statistical Analysis
Statistical analysis was conducted using SPSS v25.0 (IBM SPSS Corp.; Armonk, NY, USA). Categorical variables were expressed as frequencies and percentages, and the χ 2 test was used to compare rates between groups. The normal distribution test of continuous variables was conducted using the Shapiro–Wilk method. Data that did not conform to a normal distribution were shown as median (Q1-Q3) and tested by the Mann–Whitney U-test. The variables with P < .05 in univariate analysis were input into the multiple logistic regression model, and then variables (Hcy levels, NIHSS scores, and ADL scores) were added to the multiple logistic regression. The predicted probability value of the regression model was used as an inspection variable to draw the receiver operating characteristic (ROC) curve. The maximum Youden index was used to determine the critical value, and odds ratio (OR) and 95% confidence interval (CI) were calculated. When P < .05, the difference was statistically significant. Figures 1 and 3 were drawn using Microsoft Office Word v2016 and Microsoft Excel (Microsoft, Seattle, WA, USA), respectively.
Figure 3.
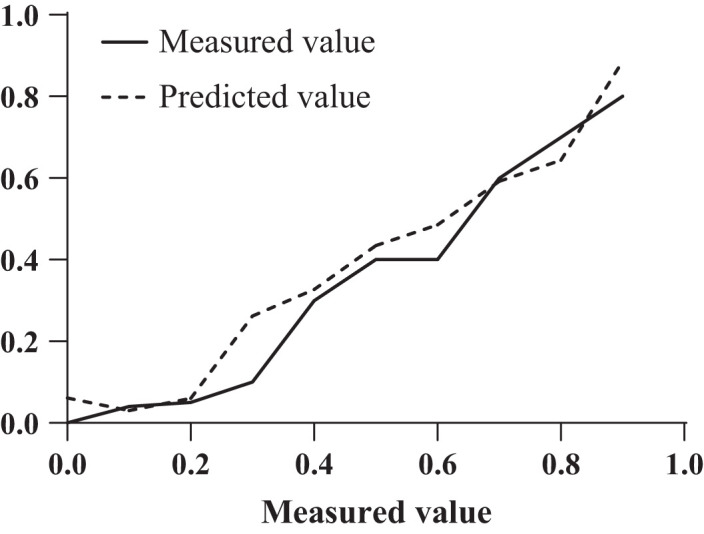
Evaluation of calibration curve. This is the calibration curve of the logistic regression model prediction.
Results
Baseline Data
According to the diagnosis results of PSD, the patients were divided into a PSD group and a non-PSD group. By comparing the general data of the 2 groups, it was found that both groups had no significant difference in age, gender, education levels, and marital status (P > .05) (Table 1).
Table 1.
Comparison of Basic Data Between 2 Groups
| Items | PSD Group (n = 59) | Non-PSD Group (n = 107) | P | |
|---|---|---|---|---|
| Age [years, median (Q1-Q3)] | 65.00 (60.00, 70.00) | 65.00 (60.00, 69.00) | .748 | |
| Gender | Male | 32 (54.24) | 56 (52.34) | .814 |
| Female | 27 (45.76) | 51 (47.66) | ||
| Education levels | Illiterate | 7 (11.86) | 11 (10.28) | .945 |
| Primary school | 26 (44.07) | 49 (45.79) | ||
| Junior high school and above | 26 (44.07) | 47 (43.93) | ||
| Marital status | Married | 37 (62.71) | 69 (64.49) | .971 |
| Divorced | 16 (27.12) | 28 (26.17) | ||
| Widowed | 6 (10.17) | 10 (9.35) | ||
| Degree of depression | Mild depression | 31 (18.67%) | / | / |
| Moderate depression | 19 (11.45%) | / | ||
| Severe depression | 9 (5.42%) | / | ||
| Inhabiting information | Living with others | 39 (66.10) | 75 (70.09) | .596 |
| Living alone | 20 (33.90) | 32 (29.91) | ||
| Previous history of stroke | No | 48 (81.36) | 86 (80.37) | .878 |
| Yes | 11 (18.64) | 21 (19.63) | ||
| History of hypertension | No | 32 (54.24) | 61 (57.01) | .731 |
| Yes | 27 (45.76) | 46 (42.99) | ||
| Diabetes mellitus history | No | 39 (66.10) | 74 (69.16) | .686 |
| Yes | 20 (33.90) | 33 (30.84) | ||
| Smoking history | No | 43 (72.88) | 81 (75.70) | .689 |
| Yes | 16 (27.12) | 26 (24.30) | ||
| Drinking history | No | 42 (71.19) | 74 (69.16) | .785 |
| Yes | 17 (28.81) | 33 (30.84) | ||
| Size of infarction | Small infarction | 19 (32.20) | 42 (39.25) | .657 |
| Middle infraction | 34 (57.63) | 56 (52.34) | ||
| Big infarction | 6 (10.17) | 9 (8.41) | ||
| Location of infarction | Internal carotid artery stenosis | 16 (27.12) | 39 (36.45) | .222 |
| Intracranial artery stenosis | 43 (72.88) | 68 (63.55) | ||
| Admission time (h) | 9.00 (5.00,12.50) | 11.00 (6.00,14.00) | .108 | |
PSD, Post-stroke Depression.
Comparison of National Institute of Health Stroke Scale and Activity of Daily Living Scale Scores Between 2 Groups
The NIHSS and ADL scores in the PSD group were significantly higher than those in the non-PSD group (P < .001) (Table 2).
Table 2.
Comparison of National Institute of Health Stroke Scale (NIHSS) and Activity of Daily Living (ADL) Scale Scores Between 2 Groups [points, median (Q1-Q3)]
| Indicators | PSD Group (n = 59) | Non-PSD Group (n = 107) | P |
|---|---|---|---|
| NIHSS | 30.00 (25.00, 35.00) | 24.00 (18.00, 31.00) | <.001 |
| ADL | 32.00 (28.00, 35.00) | 25.00 (21.00, 29.00) | <.001 |
Comparison of Laboratory Indicators Between 2 Groups
PSD group had significantly higher Hcy levels than non-PSD group (P < .05). Both groups had no significant differences in prealbumin, fibrinogen, total cholesterol, and other indicators (P > .05) (Table 3).
Table 3.
Comparison of Laboratory Indicators Between 2 Groups
| Indicators | PSD Group (n = 59) | Non-PSD Group (n = 107) | P |
|---|---|---|---|
| Prealbumin [mg/L, median (Q1-Q3)] | 212.50 (188.92, 247.28) | 227.22 (202.01, 259.15) | .084 |
| Hcy [μmol/L,median (Q1-Q3)] | 23.88 (13.40, 32.46) | 15.22 (9.87, 22.80) | .001 |
| Fibrinogen [g/L, median (Q1-Q3)] | 2.41 (1.66, 3.17) | 2.53 (1.87, 3.09) | .696 |
| Total cholesterol [mmol/L, median (Q1-Q3)] | 4.25 (3.73, 4.75) | 4.32 (3.66, 5.00) | .406 |
| Triglyceride [mmol/L, median (Q1-Q3)] | 1.69 (0.95, 2.41) | 1.94 (1.19, 2.75) | .120 |
| High-density lipoprotein cholesterol [mmol/L, median (Q1-Q3)] | 1.10 (0.73, 1.36) | 1.03 (0.73, 1.37) | .626 |
| Low-density lipoprotein cholesterol [mmol/L, median (Q1-Q3)] | 3.04 (1.98, 3.63) | 2.57 (1.94, 3.27) | .110 |
Multiple Logistic Regression Analysis
Multiple logistic regression analysis was conducted using Hcy levels, NIHSS scores, and ADL scores as independent variables, and the presence or absence of PSD as the dependent variable. The results showed that Hcy levels, NIHSS scores, and ADL scores were independent risk factors for PSD in patients with ACI (P < .05) (Table 4).
Table 4.
Multiple Logistic Regression Analysis
| Factors | β | Standard Error | Wald | OR | P | 95% CI |
|---|---|---|---|---|---|---|
| Hcy | −0.051 | 0.022 | 5.417 | 0.950 | .020 | 0.910-0.992 |
| NIHSS | −0.127 | 0.037 | 11.936 | 0.881 | .001 | 0.820-0.947 |
| ADL | −0.329 | 0.058 | 31.826 | 0.720 | < .001 | 0.642-0.807 |
Notes: P < .001 in Omnibus test of model coefficients. OR, odds ratio; CI, confidence interval.
Logistic Regression Model Predication
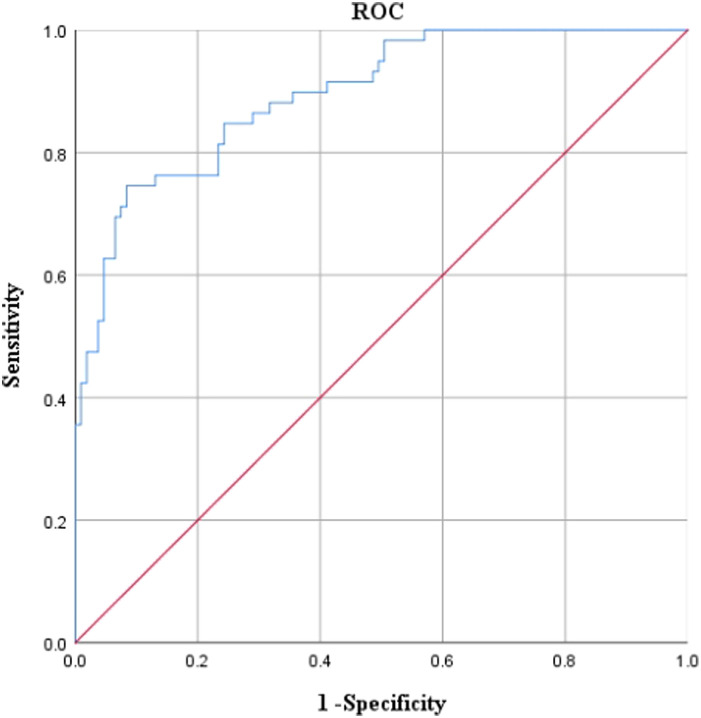
ROC curve was drawn with the presence or absence of PSD as the state variable and the predicted probability value of the regression model as the inspection variable. The results showed that the AUC = 0.894, standard errora = 0.025, progressive significanceb < 0.001, 95% CI = 0.845-0.943, cut-off value = 0.520, sensitivity = 91.60%, and specificity = 74.60%, suggesting that the predictive efficacy of the logistic regression model has certain clinical diagnostic efficacy (Figure 2).
Figure 2.
Receiver operating characteristic curve of post-stroke depression predicted by logistic regression model. This figure was the ROC (Receiver operating characteristic) curve of logistic regression model prediction. The abscissa represented 1 − specificity, and the ordinate represented sensitivity.
Evaluation of Calibration Curve
The Hosmer–Lemeshow goodness of fit test showed a P-value of .246, which was significantly greater than .05, indicating that the model was well calibrated, as shown in Figure 3.
Discussion
Acute cerebral infarction caused by many risk factors not only leads to physical dysfunction in patients, but also seriously affects their cognitive and psychological status. Post-stroke depression is a common complication of mental disorders in patients with cerebral infarction.14 There may be a two-way relationship between cerebral infarction and depression. Cerebral infarction increases the risk of PSD, and depression is also an important factor leading to an increase in the incidence of cerebral infarction.15 The clinical manifestations of PSD after cerebral infarction are different from those of severe depression. Besides depression, they include cognitive impairment, apathy, loss of interest, loss of appetite, sleep disorders, and even self-mutilation and suicidal tendencies.16,17 Therefore, early prevention and treatment of PSD after cerebral infarction are crucial. The data from this study showed that among 166 patients with ACI, 59 patients had PSD, with a total incidence of 35.54%, which was consistent with the results of most previous studies.18,19 According to the data, there were 31 cases (18.67%) with mild depression, 19 cases (11.45%) with moderate depression, and 9 cases (5.42%) with severe depression in 59 patients with PSD, suggesting that the incidence of moderate and mild PSD was higher.
The etiology of PSD is still unclear. At present, there are many risk factors that are considered to affect the incidence of PSD,20 such as gender, personal history of mental illness (especially history of severe depression and anxiety), family history, diabetes mellitus, low education level, social status, etc. However, due to the small number of research subjects and scope of research, this study did not find that age, gender, and education levels were related to the incidence of PSD, which still needs to be confirmed by large sample studies. Schöttke Henning et al.21 have found that patients with more severe neurological deficits are more likely to develop PSD. This study revealed that the PSD group had significantly higher NIHSS scores than the non-PSD group, indicating a certain relationship in the occurrence between neurological deficits and PSD. High NIHSS scores were significantly correlated with the occurrence of PSD, suggesting that patients with more severe neurological deficits were more likely to develop PSD, which was consistent with previous studies.22 The reason may be that patients with cerebral infarction are accompanied by neurological deficits, and the symptoms vary from person to person, most commonly manifested as impaired upper limb use and walking. A higher NIHSS score indicates more severe symptom impairment, and the more limited the patient’s ability of daily living, the higher the possibility of PSD. Neurological deficits are accompanied by a decrease in the ability of daily living, and patients with a rapid decline in the quality of life may experience a significant increase in depression.23 The data of the study showed that the PSD group had remarkably higher ADL scores than the non-PSD group, and the high ADL score was an independent risk factor for PSD in patients with ACI (P < .05), indicating that the lower the ability of daily life, the more likely one is to develop PSD. Due to physical activity disorders, some patients with cerebral infarction have limited mobility and disrupted life patterns and need to adapt to the new daily state, thereby generating depression symptoms. Therefore, improving patients’ ability of daily living is of positive significance in alleviating negative emotions and promoting recovery from disease.
In addition to the above research results, the study also found that the PSD group had remarkably higher Hcy levels than non-PSD group. The reason is that Hcy has a direct toxic effect on blood vessels, and abnormal methylation metabolism of serum Hcy reduces the production of S-adenosylmethionine with antidepressant effects, thereby affecting the production and metabolism of monoamine neurotransmitters,24 and leading to the occurrence of depression. A previous study by Liu Haiyan et al25 on the correlation between serum Hcy and depression after stroke found that the risk of depression in stroke patients is significantly correlated with serum Hcy levels, which is consistent with the results of this study. Serum lipid indicators (such as total cholesterol, triglycerides, and high-density lipoprotein cholesterol) are risk factors for cardiovascular and cerebrovascular diseases. However, the data from this study did not show that total cholesterol, triglycerides, high-density lipoprotein cholesterol, and other indicators have an impact on PSD, so more large-scale studies are still needed to confirm the impact of lipid indicators on PSD and its related mechanisms.
Conclusion
In summary, through research and analysis, it was found that high Hcy levels, NIHSS scores, and ADL scores were independent risk factors for PSD in patients with ACI. The ROC curve contained 3 characteristics of NIHSS scores, ADL scores, and Hcy levels, and it showed that AUC = 0.894, standard errora = 0.025, progressive significanceb <0.001, 95% CI = 0.845-0.943, cut-off value = 0.520, sensitivity = 91.60%, specificity = 74.60%, and Hosmer-Lemeshow goodness-of-fit test P = .246, indicating that the ROC curve has certain clinical predictive efficacy. However, the sample size collected in this study was relatively small, the evaluation time was selected within 5-7 days after admission, and some scales used in the study may have certain errors. Therefore, it is necessary to further increase the sample size and explore more studies.
Availability of Data and Materials
The corresponding author will provide the data that underpin the study’s conclusions with a reasonable application.
Funding Statement
The authors declare that this study received no financial support.
Footnotes
Ethics Committee Approval: This study has been approved by the Medical Ethics Committee of Tianjin Huanhu Hospital (Approval number: 2021031B).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – F.Y., P.Z.; Design – F.Y., P.Z.; Supervision – F.Y., P.Z.; Resources – F.Y., P.Z.; Materials – F.Y., P.Z.; Data collection and/or processing – F.Y., P.Z.; Analysis and/or interpretation – F.Y., P.Z.; Literature search – F.Y., P.Z.; Writing – F.Y., P.Z.; Critical review – F.Y., P.Z.
Acknowledgment: N/A.
Declaration of Interests: The authors have no conflicts of interest to declare.
References
- 1. Sveinsson ÓÁ, Kjartansson Ó, Valdimarsson EM. Cerebral ischemia/infarction - diagnosis and treatment. Laeknabladid. 2014;100(7-8):393 401. ( 10.17992/lbl.2014.0708.553) [DOI] [PubMed] [Google Scholar]
- 2. Schöttke H, Giabbiconi CM. Post-stroke depression and post-stroke anxiety: prevalence and predictors. Int Psychogeriatr. 2015;27(11):1805 1812. ( 10.1017/S1041610215000988) [DOI] [PubMed] [Google Scholar]
- 3. Cai W,Mueller C,Yi-Jing Li, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102 109. ( 10.1016/j.arr.2019.01.013) [DOI] [PubMed] [Google Scholar]
- 4. van Mierlo ML, van Heugten CM, Post MW, de Kort PL, Visser-Meily JM. Psychological factors determine depressive symptomatology after stroke. Arch Phys Med Rehabil. 2015;96(6):1064 1070. ( 10.1016/j.apmr.2015.01.022) [DOI] [PubMed] [Google Scholar]
- 5. Zhou L, Wang T, Yu Y, et al. The etiology of poststroke-depression: a hypothesis involving HPA axis. Biomed Pharmacother. 2022;151:113146. ( 10.1016/j.biopha.2022.113146) [DOI] [PubMed] [Google Scholar]
- 6. Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: A 2020 updated review. Gen Hosp Psychiatry. 2020;66:70 80. ( 10.1016/j.genhosppsych.2020.06.011) [DOI] [PubMed] [Google Scholar]
- 7. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191 2194. ( 10.1001/jama.2013.281053) [DOI] [PubMed] [Google Scholar]
- 8. Qiu S, Xu Y. Guidelines for acute ischemic stroke treatment. Neurosci Bull. 2020;36(10):1229 1232. ( 10.1007/s12264-020-00534-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Battle DE. Diagnostic and statistical manual of mental disorders (DSM). CoDAS. 2013;25(2):191 192. ( 10.1590/s2317-17822013000200017) [DOI] [PubMed] [Google Scholar]
- 10. Rosenberg LI. The ham-D is not Hamilton's depression scale. Psychopharmacol Bull. 2022;52(2):117 153. [PMC free article] [PubMed] [Google Scholar]
- 11. Alemseged F, Rocco A, Arba F, et al. Posterior National Institutes of Health stroke scale improves prognostic accuracy in posterior circulation stroke. Stroke. 2022;53(4):1247 1255. ( 10.1161/STROKEAHA.120.034019) [DOI] [PubMed] [Google Scholar]
- 12. Jin G,Qing G,Liting H, et al. Impaired activity of daily living status of the older adults and its influencing factors: A cross-sectional study. Int J Environ Res Public Health. 2022;19: undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu J, Wang L, Fan K, et al. The association between systemic inflammatory markers and post-stroke depression: A prospective stroke cohort. Clin Interv Aging. 2021;16:1231 1239. ( 10.2147/CIA.S314131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Monika S,Katrin W,Jonas N, et al. Health-related quality of life, anxiety and depression up to 12 months post-stroke: influence of sex, age, stroke severity and atrial fibrillation - A longitudinal subanalysis of the Find-AF trial. J Psychosom Res. 2021;142:110353. [DOI] [PubMed] [Google Scholar]
- 15. Ezema CI, Akusoba PC, Nweke MC, Uchewoke CU, Agono J, Usoro G. Influence of post-stroke depression on functional independence in activities of daily living. Ethiop J Health Sci. 2019;29(1):841 846. ( 10.4314/ejhs.v29i1.5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shuai S,Zhifang L,Qinghui X, et al. An updated review on prediction and preventive treatment of post-stroke depression. Expert Rev Neurother. 2023, undefined: 1-19. [DOI] [PubMed] [Google Scholar]
- 17. Pedersen A, Almkvist E, Holmegaard L, et al. Fatigue 7 years post-stroke: predictors and correlated features. Acta Neurol Scand. 2022;146(3):295 303. ( 10.1111/ane.13665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhanina MY, Druzhkova TA, Yakovlev AA, et al. Development of post-stroke cognitive and depressive disturbances: associations with neurohumoral indices. Curr Issues Mol Biol. 2022;44(12):6290 6305. ( 10.3390/cimb44120429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schöttke H, Gerke L, Düsing R, Möllmann A. Post-stroke depression and functional impairments - A 3-year prospective study. Compr Psychiatry. 2020;99:152171. ( 10.1016/j.comppsych.2020.152171) [DOI] [PubMed] [Google Scholar]
- 20. Luo S, Zhang W, Mao R, et al. Establishment and verification of a nomogram model for predicting the risk of post-stroke depression. PeerJ. 2023;11:e14822. ( 10.7717/peerj.14822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiriţă AL, Gheorman V, Bondari D, Rogoveanu I. Current understanding of the neurobiology of major depressive disorder. Rom J Morphol Embryol. 2015;56(2)(suppl):651 658. [PubMed] [Google Scholar]
- 22. Li J, Yang L, Lv R, Kuang J, Zhou K, Xu M. Mediating effect of post-stroke depression between activities of daily living and health-related quality of life: meta-analytic structural equation modeling. Qual Life Res. 2023;32(2):331 338. ( 10.1007/s11136-022-03225-9) [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Zou H, Peng M, Chen Y. Association between homocysteine levels in acute stroke and poststroke depression: A systematic review and meta-analysis. Brain Behav. 2022;12(6):e2626. ( 10.1002/brb3.2626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiu X, Wang H, Lan Y, et al. Blood biomarkers of post-stroke depression after minor stroke at three months in males and females. BMC Psychiatry. 2022;22(1):162. ( 10.1186/s12888-022-03805-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu H, Pu J, Zhou Q, Yang L, Bai D. Peripheral blood and urine metabolites and biological functions in post-stroke depression. Metab Brain Dis. 2022;37(5):1557 1568. ( 10.1007/s11011-022-00984-9) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author will provide the data that underpin the study’s conclusions with a reasonable application.



 Content of this journal is licensed under a
Content of this journal is licensed under a