Abstract
Background
Little is known about patient outcomes following treatment of malignant pleural effusions (MPE) in the real-world setting.
Research question
We aimed to compare post-procedure all-cause mortality between individuals who received indwelling pleural catheter (IPC) insertion versus chemical pleurodesis for managing MPEs.
Study design and methods
We performed a retrospective population-based study using provincial health administrative data (Ontario, Canada) of adults with a MPE who underwent IPC insertion or chemical pleurodesis between 2015 and 2019. Individuals were followed until death or March 31, 2021. Difference in post-procedure mortality was calculated using inverse probability of treatment weighting (IPTW)-adjusted Cox proportional hazard regression analysis to balance potential confounders at baseline.
Results
We identified 4,790 (77.3%) individuals who received an IPC and 1,407 (22.7%) who had chemical pleurodesis for MPE. IPC insertions are increasing and chemical pleurodesis procedures are decreasing. The majority of IPCs were inserted in outpatients (61%), by pulmonologists (64.2%) and at sites with higher annual IPC volume, while chemical pleurodesis procedures were generally done by thoracic surgeons (74%) and at sites with higher annual pleurodesis volumes. In unadjusted comparison median time from initial cancer diagnosis to intervention was significantly longer in the IPC group (244 days, interquartile range [IQR]:33–903) compared to pleurodesis group (81 days, IQR:10–737; p < 0.0001). Unadjusted median time from index procedure to death was significantly longer in the pleurodesis group (165[IQR:48–457] days vs. 81[IQR:29–256] days, p < 0.0001), however the difference between groups became insignificant after the IPTW was applied (HR 1.27, 95%CI 0.95–1.69). 35% of IPCs were removed prior to death or end of follow-up.
Interpretation
After adjusting for differences in baseline characteristics there was no difference in post-procedure mortality between IPC and chemical pleurodesis groups. In the real world, there are significant differences in the characteristics of patients who receive these two procedures and notable regional practice variation between procedure use. Future research should evaluate these variations in care and their effect on patient outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-03023-6.
Keywords: Pleural Neoplasms, Palliative Medicine, Survival
Introduction
Malignant pleural effusions (MPE) can develop in any advanced stage cancer, including up to 50% of individuals with lung cancer, 65% with disseminated breast cancer and 90% with mesothelioma [1–6]. MPEs are estimated to affect more than 150,000 individuals in the United States each year, based on autopsy case series [2].
International guidelines recommend either indwelling pleural catheter (IPC) insertion or talc pleurodesis as definitive treatment for symptomatic MPEs [7, 8]. Limited data are available on how these treatments are being utilized in clinical practice. Both procedures reduce breathlessness and improve quality of life [9, 10]. Traditionally, chemical pleurodesis requires hospital admission and full lung re-expansion after pleural fluid removal, whereas IPCs can be placed in the outpatient setting and can still be utilized in individuals with a non-expanding lung. IPCs are associated with reduced hospital days and reduced need for repeat ipsilateral interventions due to failed resolution or recurrence of the effusion, but increased infectious complications compared to talc pleurodesis [7–12]. In Ontario (Canada), home nursing services are usually responsible for drainage of IPCs.
Survival with a MPE depends on the underlying cancer type, cancer treatments, and patient factors, but ranges between 1 and 12 months [5, 13–15]. A network meta-analysis of randomized control trials (RCTs) did not find any significant difference in post-procedure survival between IPCs or talc slurry pleurodesis, although there was a low degree of certainty about the findings [12]. These studies have excluded patients with an expected survival of less than 3 months [9, 10]. Given the poor prognosis in individuals with MPE, these RCTs may not have captured how these procedures are being used in the real world.
Our study aimed to: (1) compare post-procedure all-cause mortality (primary); and (2) describe the patterns and patient-, physician- and hospital-characteristics associated with interventions, and (3) compare subsequent pleural procedures between individuals receiving IPC insertion versus chemical pleurodesis for MPEs as secondary. We hypothesized that based on existing literature there would be no difference in post-procedure mortality, but IPCs would be associated with reduced repeat pleural procedures.
Methods
Study design and setting
Using provincial (Ontario, Canada) health administrative data we performed a retrospective cohort study. The use of data in this project was authorized under Sect. 45 of Ontario’s Personal Health Information Protection Act (PHIPA), which does not require review by a Research Ethics Board.
Data sources
Details of publicly funded health services and individual-level characteristics are retained in health administrative databases housed at ICES [16] an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. Details of databases used are described in Additional file 1 and Supplementary Table 1. Datasets were linked using unique encoded identifiers (Supplementary Fig. 1, Additional File 1) and analyzed at ICES following Ontario privacy standards using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC).
Study population
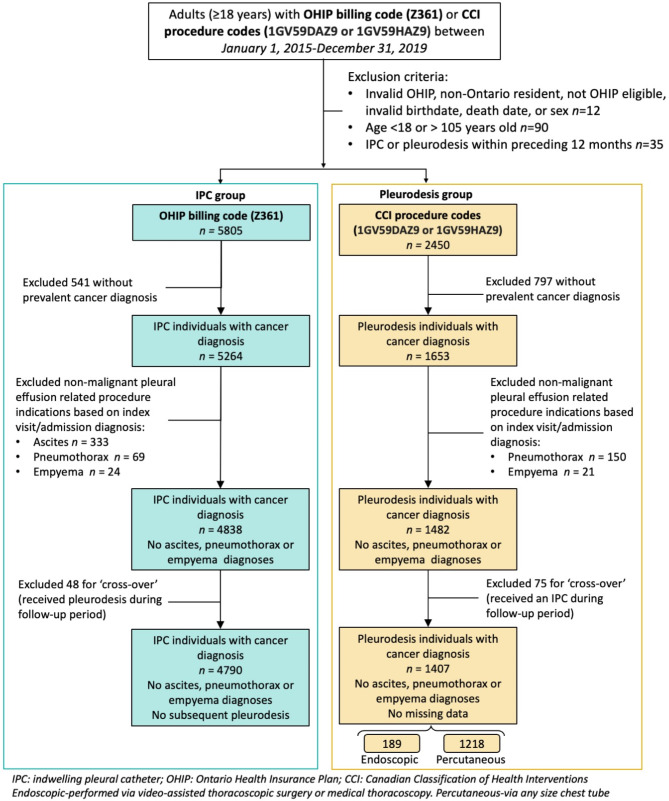
All adults (≥ 18 years) who underwent IPC insertion or chemical pleurodesis between January 1, 2015 to December 31, 2019 in Ontario were included (Fig. 1). The date of the first procedure (IPC insertion or pleurodesis) was the index date. Individuals were followed from index to death, end of follow-up (March 31, 2021), or loss of public health insurance eligibility (whichever occurred first). This timeframe was chosen to ensure individuals had at least 2 years of follow-up after their index procedure. Data on the type of sclerosing agent used, or post drainage lung re-expansion was not available.
Fig. 1.
Flow chart - Creation of study cohorts: Individuals who received an IPC (indwelling pleural catheter) or pleurodesis procedure for a malignant pleural effusion
Individuals were excluded if they: (1) had an IPC or chemical pleurodesis in the year prior to the index date to ensure the index procedure was not a repeat procedure; (2) did not have a prevalent cancer diagnosis (i.e., did not have record in the Ontario Cancer Registry with a diagnosis date prior to or including index date) [17] to exclude those without a MPE; (3) diagnosis code of ascites, pneumothorax, or empyema associated with their procedure visit/admission to exclude alternative locations (e.g. abdominal IPC placement) or non-malignant indications (Fig. 1; Supplementary Table 2, Additional File 1). Individuals who subsequently had codes for the other procedure during the follow-up period (i.e. ‘crossed-over’ from one treatment to the other) were excluded in the primary analysis to limit contamination bias, but were included in a sensitivity analysis where they were assigned to a group based on the earliest procedure performed.
Outcomes
Our primary outcome was adjusted post-procedure mortality. Secondary outcomes included patient-, physician- and hospital-related characteristics associated with either IPC insertion or chemical pleurodesis, rate of IPC removal, and rate of repeat pleural drainage procedures (thoracentesis, chest tube insertion, IPC insertion, chemical pleurodesis).
Baseline characteristics
The following covariates were assessed (1) at the index date: age; sex; rurality; neighbourhood income quintile as a measure of socioeconomic status; most recent cancer diagnosis based on International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) codes (Supplementary Table 3, Additional File 1); days from initial cancer diagnosis until index procedure; frailty [18] and level of comorbidity based on the preceding 2 years of health service utilization, Johns Hopkins’ ACG® System Aggregated Diagnosis Groups (ADG) categories (Version 10.0; https://www.hopkinsacg.org); physician specialty; institution where the procedure was performed; hospital type (community or academic); annual volume of procedures performed at the index institution; (2) prevalent diagnosis of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and renal failure at index date [19, 20]; (3) thoracentesis in the preceding 12 months. Further details on variable definitions available in Supplementary Table 2, Additional File 1.
Analyses
Baseline characteristics were summarised by index procedure using means (standard deviation [SD]), medians (interquartile range [IQR]) or proportions as appropriate. Independent t-test was used to compare means between two groups, Wilcoxon rank-sum test was used to compare medians, and categorical variables were compared using the Chi-square test or Fisher’s exact test where appropriate.
We used inverse probability of treatment weighting (IPTW) to balance the observed baseline characteristics, and estimated treatment effect through the average treatment effect (ATE) in the whole study population (please see Additional File 1 for further details) [21]. The selection of variables included in the IPTW were based on existing literature and clinical expertise; however, it was limited by the availability within ICES databases (Supplementary Table 4, Additional File 1). Induced balance was assessed through weighted standard differences for categorical variables, weighted box-plots for continuous variables, and overall by propensity score density plots (Supplementary Table 4, Supplementary Figs. 2 and 3 in Additional File 1) [21]. We then performed a weighted Cox proportional hazards regression analysis and plotted Kaplan-Meier survival curves based on the weighted cohorts. To assess the possible impact of the COVID-19 pandemic on mortality, we calculated the proportion of individuals in each group alive at the start of the pandemic (March 1, 2020) and compared unweighted mortality in those who had their procedures in 2019 versus pre-2019 (Additional File 1).
Cause-specific Cox models were used to estimate cumulative incidence functions for IPC removal with death as a competing risk.
To assess the robustness of our findings we performed sensitivity analyses using alternate weighting approaches including (1) treatment weights and (2) stabilized IPTW, (3) including individuals who ‘crossed-over’ to the alternative intervention, and (4) performed a doubly robust estimation where variables with post-weighting standard difference > 0.1 were included in the regression analysis [22] (further details are available in Additional File 1). Treatment weights are used to estimate the average treatment effect among the treated (ATT) population, where IPC individuals are the treatment group. Weight stabilization was used to lesson the impact of extreme weights.
Results
We identified 4,790 (77.3%) individuals who underwent IPC insertion and 1,407 (22.7%) treated with chemical pleurodesis (Fig. 1; Table 1). Only 13.4% (189) of chemical pleurodesis procedures were performed surgically. Overall, eight individuals (0.1%) were lost to follow-up.
Table 1.
Baseline characteristics for individuals receiving (indwelling pleural catheters) IPCs or pleurodesis (unweighted)
| Characteristics, n (%) | IPC (N = 4790) |
Pleurodesis (N = 1407) |
p-value |
|---|---|---|---|
| Patient characteristics | |||
| Age in years, mean (SD) § | 69.1 (12.9) | 68.9 (11.7) | 0.5732 |
| Female§ | 2722 (56.8) | 681 (48.4) | < 0.0001 |
| Rural§ | 539 (11.3) | 264 (18.8) | < 0.0001 |
| Neighbourhood income quintile§ | 0.2787 | ||
| 1 (lowest) | 991 (20.7) | 297 (21.2) | |
| 2 | 998 (20.9) | 309 (22) | |
| 3 | 997 (20.9) | 273 (19.4) | |
| 4 | 862 (18) | 276 (19.7) | |
| 5 (highest) | 931 (19.5) | 249 (17.7) | |
| Number of thoracentesis in 12 months prior to index date§ | < 0.0001 | ||
| 0 | 1872 (39.1) | 799 (56.8) | |
| 1 | 1585 (33.1) | 357 (25.4) | |
| 2 | 779 (16.3) | 143 (10.2) | |
| ≥ 3 | 554 (11.6) | 108 (7.7) | |
| Chest tube insertion in preceding 12 months† | 916 (19.1) | 362 (25.7) | < 0.0001 |
| Inpatient for index procedure | 1852 (38.7) | 1397 (99.3) | < 0.0001 |
| Days in hospital prior to index procedure, median (IQR) [n = 3249] | 4 (1–9) | 2 (0–6) | < 0.0001 |
| Cancer type§ | < 0.0001 | ||
| Lung | 1889 (39.4) | 641 (45.6) | |
| Breast | 751 (15.7) | 159 (11.3) | |
| Mesothelioma | 178 (3.7) | 153 (10.9) | |
| Other | 1972 (41.2) | 454 (32.3) | |
| Days from cancer diagnosis to index procedure, median (IQR) § | 243.5 (33–903) | 81 (10–737) | < 0.0001 |
| COPD§ | 749 (15.6) | 306 (21.8) | < 0.0001 |
| CHF§ | 679 (14.2) | 219 (15.6) | 0.1964 |
| Renal failure§ | 207 (4.3) | 39 (2.8) | 0.0082 |
| Frailty§ | 762 (15.9) | 180 (12.8) | 0.0041 |
| Aggregated diagnosis groups§ | 0.3350 | ||
| Low comorbidity (0–5) | 106 (2.2) | 28 (2) | |
| Moderate comorbidity (6–9) | 1124 (23.5) | 306 (21.8) | |
| High comorbidity (≥ 10) | 3560 (74.3) | 1073 (76.3) | |
| Physician specialty § | < 0.0001 | ||
| Pulmonology | 3073 (64.2) | 56 (4.2) | |
| Thoracic surgery | 832 (17.4) | 979 (74) | |
| General surgery | 125 (2.6) | 218 (16.5) | |
| Diagnostic radiology | 437 (9.1) | 19 (1.4) | |
| Other | 323 (6.7) | 51 (3.9) | |
| Hospital characteristics | |||
| Hospital type§ | < 0.0001 | ||
| Teaching | 2889 (65.1) | 796 (56.6) | |
| Community and small | 1551 (34.9) | 611 (43.4) | |
| Annual volume of IPCs, median (IQR)‡ § | 62 (23–114) | 7 (1–14) | < 0.0001 |
| Annual volume of pleurodesis, median (IQR)‡ § | 0 (0–6) | 51 (22–100) | < 0.0001 |
| Annual volume of IPCs‡, categorized | < 0.0001 | ||
| Low (0-49.99) | 1677 (37.8) | 1276 (90.7) | |
| High (≥ 50) | 2765 (62.3) | 131 (9.3) | |
| Annual volume of pleurodesis‡, categorized | < 0.0001 | ||
| Low (0-49.99) | 4369 (98.4) | 684 (48.6) | |
| High (≥ 50) | 73 (1.6) | 723 (51.4) |
Results are numbers (column percentages) unless otherwise specified. Estimates may not always sum up to 100% due to missing values. The percentage of missing values ranged between 0.2% for rural status to 5.7% for hospital type
Abbreviations: IPC, indwelling pleural catheter; SD, standard deviation; IQR, interquartile range; COPD, chronic obstructive lung disease; CHF, congestive heart failure
*Based on outpatient visit or hospital admission diagnostic code
†Excluding 3 days prior to index date
‡Number of procedures performed in the calendar year of the index procedure at the institution where the index procedure was performed
§Variables included in IPTW (inverse probability of treatment weighting) analysis (thoracentesis was included as a yes/no variable if had been performed within preceding 12 months)
Patient characteristics (in the unweighted samples)
Patients had a mean age of 69.1 years (SD:12.9) and 68.9 years (SD:11.7) in the IPC and chemical pleurodesis groups, respectively (Table 1). The IPC group had a significantly higher proportion of females (56.8% vs. 48.4%, p < 0.0001), patients who had undergone a previous thoracentesis (60.9% vs. 43.2%, p < 0.0001), and frailty (15.9% v 12.8%, p = 0.0041) compared to the chemical pleurodesis group. Median days from initial cancer diagnosis to index procedure was significantly longer for the IPC group (244 vs. 81 days, p < 0.0001). The most common malignancy was lung, followed by breast cancer. A full listing of assigned cancer types is available in Supplementary Table 5, Additional File 1. Multiple cancer diagnoses were found in 21% of individuals.
After applying weights the standard difference for many baseline variables improved (Supplementary Table 4, Additional File 1), with improved overlap of the overall density score plots (Supplementary Fig. 3, Additional File 1). Individuals with missing data in any baseline variable were excluded from the weighted Cox regression analysis (7.2% of overall; IPC = 358, chemical pleurodesis = 87, Supplementary Table 6, Additional File 1). Those with missing data in the IPC group had less mesothelioma and “other cancers”, in the chemical pleurodesis group they had less mesothelioma and more “other cancers”. Individuals with missing data in the IPC group also had undergone more thoracenteses prior to their index procedure, and those in the chemical pleurodesis group had less COPD.
All-cause mortality
In the unweighted sample, during the first 12-months post-procedure 3,726 (77.8%) in the IPC group and 841 (59.8%) in the chemical pleurodesis group died. By the end of follow-up these numbers increased to 4,533 (94.6%) and 1,221 (86.8%) respectively. Median time from index procedure to death was significantly shorter in the IPC group (81 [IQR:29–256] days vs. 165 [IQR:48–457] days, p < 0.0001). There was no significant difference in median time from initial cancer diagnosis to death between groups (IPC: 486 [IQR:168–1210] days, chemical pleurodesis: 469 [IQR:171–1206] days; p = 0.9878). Crude (unweighted) mortality rate per 1000-person years in the IPC group was 1,329 versus 698 in the chemical pleurodesis group.
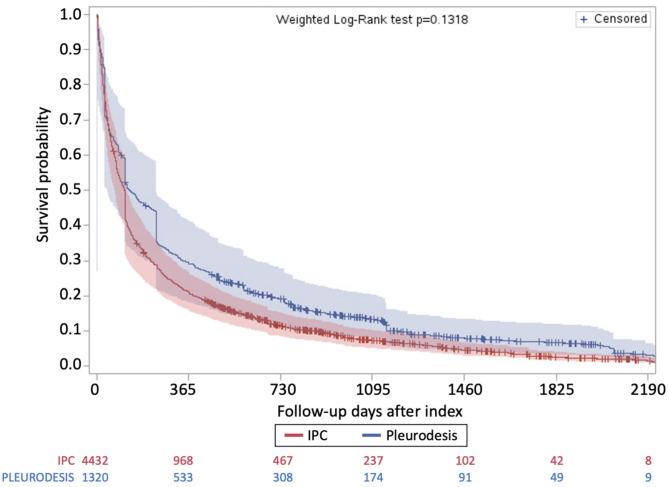
Weighted Cox regression revealed no significant difference in adjusted all cause-mortality for individuals with IPC compared to chemical pleurodesis (HR 1.27, 95%CI 0.95–1.69). Weighted Kaplan-Meier survival curves from time of index procedure are presented in Fig. 2. After weighting, mean follow-up time was shorter in the IPC group compared to chemical pleurodesis group (253 days vs. 349 days; standard difference = 0.22). Only 593 (10%) of the overall cohort were still alive at the start of the COVID-19 pandemic (Additional File 1 Appendix 1).
Fig. 2.
Kaplan-Meier survival curves after index procedure on IPT weighted sample
Secondary outcomes
Procedure trends
The number of IPC insertions increased across the 5-years (from 910 in 2015 to 994 in 2019), while chemical pleurodesis procedures decreased in more recent years (from 320 in 2015 to 228 in 2019; Supplementary Fig. 4, Additional File 1). All except one regional health authority performed more IPC insertions than chemical pleurodesis procedures, with significant variation in the ratio between procedures across regional health authorities (Supplementary Fig. 5, Additional File 1). The majority (61%) of IPCs were placed in the outpatient setting, this did not significantly change over the 5-year interval. Most procedures were performed at teaching hospitals with pulmonologists inserting the majority of IPCs and surgeons performing the majority of chemical pleurodesis procedures (Table 1). Institutions where individuals received IPCs inserted a median of 62 (IQR:23–114) IPCs annually compared to a median of zero (IQR:0–6) chemical pleurodesis procedures. This trend was reversed for institutions where individuals had undergone chemical pleurodesis.
IPC removal
Over one-quarter (1,677) of individuals in the IPC group had their catheter removed during the study period. Median time to removal was 70 days (IQR:38–120). Supplementary Fig. 6 in Additional File 1 shows the predicted cumulative incidence function curves for the cause-specific time-to-IPC removal analysis with death as a competing risk.
Repeat pleural drainage procedures (unweighted groups)
Repeat pleural drainage procedures were performed at a median 33 days (IQR 14–97) after the index procedure in 1179 (24.6%) of IPC individuals compared to 15 days (IQR 2–89) in and 264 (18.8%) individuals from the pleurodesis group (p < 0.0001). The number and type of repeat procedures performed within the first 12 months following the index procedure is presented in Table 2. We were unable to identify if a subsequent pleural procedure was ipsilateral or contralateral to the index procedure. In the IPC group, there was a higher proportion of females, particularly with breast and ovarian cancer, who required repeat procedures compared to those who had received chemical pleurodesis and needed repeat interventions.
Table 2.
Repeat pleural procedures within 12 months of index (indwelling pleural catheter) IPC insertion or pleurodesis procedure in unweighted groups
| Characteristics | IPC, n(%) (N = 4790) |
Pleurodesis, n(%) (N = 1407) |
p-value |
|---|---|---|---|
| Thoracentesis | 712 (14.9) | 119 (8.5) | < 0.0001 |
| Chest tube insertion | 307 (6.4) | 149 (10.6) | < 0.0001 |
| IPC insertion | 387 (8.1) | -* | |
| Pleurodesis | -* | 112 (8) |
Abbreviations: IPC, indwelling pleural catheter
*Patients excluded to prevent cross-over (48 from IPC group, 75 from pleurodesis group)
Sensitivity analyses
Results from all sensitivity analyses were consistent with the findings of the primary analyses except the doubly robust estimation which showed significantly higher mortality in the IPC group (HR 1.29, 95%CI 1.05–1.58).
Discussion
In our large population-based study comparing individuals who received IPC insertion to chemical pleurodesis for malignant pleural effusions, no significant difference in post-procedure all-cause mortality was found after balancing baseline characteristics. There were significant differences across several variables at baseline. IPCs were inserted significantly later following a cancer diagnosis compared to chemical pleurodesis procedures, and in more frail individuals. In general, IPCs were more commonly used, however there was significant practice variation regionally, at the hospital-level, and by physician specialty. Despite this, the majority of IPCs were still inserted in the outpatient setting.
Post-procedure time-to-death is similar to a previous study of health administrative data in the US which found the median survival from first thoracentesis for MPE was 88 days (IQR 26–320) [14]. The significantly shorter time from index procedure until death (in the unweighted comparison) in our IPC group compared to chemical pleurodesis group may be explained by the longer time from initial cancer diagnosis to index procedure, as there was no significant difference from time of initial cancer diagnosis until death between groups. Guidelines recommend definitive management for a MPE after an initial thoracentesis if it is symptomatic and recurrent [7, 8]. A previous study of administrative data in the US found that only 24% of individuals received guideline consistent care for MPE [14]. The longer time from cancer diagnosis to index procedure in the IPC group may be partially explained by a higher proportion of IPC patients receiving repeated thoracenteses prior to their index procedure, guideline inconsistent care, compared to chemical pleurodesis patients (27.5% vs. 18.1%). There was also a higher proportion of frail individuals in the IPC group. This may be due to more frail individuals being less likely to be offered chemical pleurodesis due to the potential side effects, or a delay in referral for IPC treatment, during which time they become frail, an independent risk factor for mortality [23].
In our study, 50.2% of IPC patients and 31.5% of chemical pleurodesis patients died before 90 days suggesting there is a significant discrepancy between individuals eligible for inclusion in most RCTs (expected survival more than 3 months) compared to those receiving these interventions in the real-world. In previous RCTs and a network meta-analysis comparing these procedures, no significant survival difference has been found, regardless of whether individuals with expected survival of less than 3 months were excluded [9–12]. A retrospective cohort study previously found improved survival from time of index procedure and from time of first effusion in the IPC group, however is limited by the non-contemporaneous time periods during which the procedures were used [24]. Our sensitivity analyses all showed consistent findings with our main analysis, except the doubly robust method which showed IPC individuals had higher mortality, which should be further investigated in future studies. We did not analyze cause-specific mortality (such as death resulting from post-procedure complications) due to the potential risk of misclassification bias and the delay in the availability of cause-of-death information within health administrative data [25].
Our study also revealed the drastic practice change which has occurred over the last two decades with increasing IPC use. Between 2015 and 2019 we found IPCs were used to manage MPEs nearly four times more frequently than chemical pleurodesis. This is the opposite found in an earlier observational study from Australia and Spain, where chemical pleurodesis was used twice as often as IPC insertion for MPEs between 2007 and 2013 [26]. Our results likely represent an ongoing trend noted from earlier US data, where the proportion of IPCs for definitive treatment of MPEs increased from 15% in 2007 to 28% in 2011, whereas chemical pleurodesis rates declined between 2009 and 2013 [14, 27]. The steady level of IPC insertion seen in our study is similar to a multicentre study showing a plateau in IPC use after 2012 [28].
We found significant practice variation by specialty, with pulmonologists more likely to place IPCs and surgeons more likely to treat with chemical pleurodesis. This practice variation is consistent with surveys revealing that the majority of interventional pulmonologists favoured IPCs as the primary intervention while thoracic surgeons preferred chemical pleurodesis [29, 30]. One-quarter of pulmonologists previously reported referring to thoracic surgeons for chemical pleurodesis, potentially contributing to the procedure imbalances between specialities [31]. Consistent with our data showing an increased proportion of females in the IPC group, it has previously been found that women are less likely to be referred to thoracic surgery for definitive management of their MPEs, even when gynecologic and breast cancer diagnoses were excluded [32]. Future research is required to better understand what is driving these disparities in care, such as local resource availability and the beliefs of referring physicians.
Individuals tended to receive the treatment that was performed more frequently at the hospital where their index procedure occurred. We also found practice variation at the level of regional health authorities, with some regions performing similar numbers of IPC and chemical pleurodesis procedures, while others performed many more IPC insertions, and only one performing more chemical pleurodesis procedures. In some regions IPC insertion may be limited due to lack of physician or home nursing expertise to facilitate insertions and home drainage. Expanding training may allow all patients to have more equitable access to these treatments. Although guidelines recommend patient characteristics and preferences be taken into account for deciding between interventions, our results suggest that the choice of procedure may be influenced more by the specialty of the treating physician, and referral and practice patterns within the region [7, 8].
The number of individuals with missing data excluded from our survival analysis was predominantly driven by missing institutional level data (hospital type and annual procedure volume) in the IPC group receiving outpatient procedures. The majority of baseline characteristics were not significantly different between those with missing data and the analyzed groups. Although more individuals who were excluded from the IPC group had undergone previous thoracenteses, their time from cancer diagnosis to index procedure was not significantly different, suggesting that the procedures were not necessarily performed later in the disease course, and thus not likely to affect the mortality outcomes. Excluded individuals, had less mesothelioma and other cancers in the IPC group, and less mesothelioma but more other cancers in the chemical pleurodesis group. Although there were statistically significant differences in cancer types between excluded and included individuals, these differences were still small and not felt to be clinically significant.
We did not include cancer therapies received by patients at the time of their index procedure due to the difficulty in categorizing these for such a diverse group of cancers. Prognostic scores (e.g. LENT, PROMISE) have been developed for patients with MPE, in addition to having limitations, the components of these scores were not available for the majority of patients and therefore we were unable to include them [13, 15, 33].
Strengths of our study include the use of large databases to identify individuals treated in a variety of real-world clinical settings and practices, a perspective not previously captured in studies utilizing administrative data and not always available in clinical trials [14, 27, 34]. Previous population-based studies have assessed individuals with MPE, but lacked codes to identify IPC insertions and only evaluated inpatients [27, 34]. Individuals receiving IPCs represented 77.3% of our cohort with 61% of those procedures performed in the outpatient setting. Another study evaluating characteristics associated with ‘guideline consistent care’ in MPE patients between 2007 and 2011 was limited to Medicare patients older than 65, with IPCs only accounting for up to 28% of definitive procedures [14]. Individuals under 65 represented 36% of our overall cohort and 36% of IPC insertions. Given the variation in healthcare systems and practice patterns between countries, the real-world data from Ontario can provide valuable insights for other countries with similar health structures.
Limitations of our study include potential misclassification bias and unmeasured confounding (e.g., systemic anti-cancer treatments received in post-procedure period, re-expanding lung, sclerosing agent used, concurrent diagnostic biopsies) inherently related to using administrative data. Due to the absence of imaging data, we were not able to evaluate the presence of non-expanding lung, which would typically exclude individuals from receiving chemical pleurodesis, or determine when the MPE first developed. We therefore used patients’ initial cancer diagnosis date as a time reference. This may explain the prolonged time from cancer diagnosis to index procedure in some individuals who initially had early-stage disease, before developing recurrence or progression to advanced-stage disease, which ultimately lead to their MPE (Supplementary Fig. 7, Additional File 1). Further, the specific type of sclerosing agent used for chemical pleurodesis was not documented in the health administrative data. A recent network meta-analysis found no significant differences in pleurodesis failure rates between talc slurry and talc poudrage, but did indicate that talc slurry may have fewer failures compared to bleomycin and doxycycline [12]. Furthermore, it remains unclear whether the surgeries performed for chemical pleurodesis were also used for diagnostic purposes. However, the time from cancer diagnosis to the index procedure was not significantly different between patients who underwent surgical chemical pleurodesis and those who received the procedure percutaneously. The absence of a shorter interval for the surgical group may suggest that fewer procedures were performed for diagnostic purposes. We are unable to determine if repeat pleural procedures were ipsilateral or contralateral to the index procedure. However, for the individuals who had repeat procedures, there were higher proportions of individuals with malignancies that are more likely to cause bilateral effusions (e.g. breast and gynecologic cancers), which may require contralateral pleural drainage.
Johns Hopkins ADG and frailty flag were used as surrogates for performance status, which was not available. These have previously been found to accurately predict one-year mortality [35]. Due to the lack of a validated definition of MPE, previous studies of administrative data have defined MPE using inpatient diagnostic codes, however in order to capture the over 60% of individuals who received their IPC as an outpatient, we used a conservative definition to identify procedures performed for MPEs [27, 34, 36]. In general, patient characteristics in our cohort are similar by baseline characteristics to those from other population-based studies of patients with MPEs [14, 27, 34, 36].
Conclusion
This large population-based study found no significant difference in post-procedure all-cause mortality following IPC insertion versus chemical pleurodesis after adjusting for differences in baseline characteristics. IPCs were inserted later in a disease course compared to chemical pleurodesis procedures. There were significant differences in patient-, physician- and hospital-level characteristics between those who receive IPC compared to chemical pleurodesis for treatment of MPEs, suggesting heterogeneity of health care delivery for these individuals in the real-world setting. The choice of definitive treatment for MPE may be dependent on specialty referral patterns and physician treatment practices. Our findings support further research into what is causing these discrepancies in care and how we can ensure all patients have access to timely guideline-driven care in diverse health care settings.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Meltem Tuna, Anan Eddeen, and Roshanak Mahdavi from ICES, Ottawa (Ontario. Canada), for their assistance with the cohort creation and analysis questions.
Abbreviations
- ADG
aggregated diagnosis groups
- ATE
average treatment effect
- ATT
average treatment effect among the treated
- CHF
congestive heart failure
- COPD
chronic obstructive pulmonary disease
- IPC
indwelling pleural catheter
- IPTW
inverse probability of treatment weighting
- IQR
interquartile range
- MPE
malignant pleural effusions
- RCT
randomized control trial
- SD
standard deviation
Author contributions
CK takes responsibility for (is the guarantor of) the content of the manuscript. All co-authors (CK, KT, KA, SA, TK) were involved in: study conception and design, interpretation of the data, critically revising the manuscript for accuracy and important intellectual content, and final approval of published version. CK also had full access to all data in the study, performed the analyses and takes responsibility for the integrity of the data and the accuracy of the data analysis. CK wrote the first manuscript draft.
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from: TOHAMO Innovation Fund grant and The Ottawa Hospital Department of Medicine Academic Scholarship to support Dr. Kwok in completing this study as a part of her thesis for a Master’s program. Dr. Kwok has received a CIHR-ICRH Travel Award - General pool (Funding Reference Number: AW1–187094) to attend the 2023 ATS conference in Washington DC (May 2023) and present preliminary results of this study. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from ©Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by: Ontario MOH, Ontario Health (OH), and Canadian Institute for Health Information (CIHI). The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Data availability
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Declarations
Ethics Approval
The use of the provincial (Ontario, Canada) health administrative data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA), which does not require review by a Research Ethics Board. Details of publicly funded health services and individual-level characteristics are retained in health administrative databases housed at ICES an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iyer NP, Reddy CB, Wahidi MM, Lewis SZ, Diekemper RL, Feller-Kopman D, et al. <ArticleTitle Language=“En”>Indwelling pleural catheter versus pleurodesis for malignant pleural effusions. A systematic review and Meta-analysis. Ann Am Thorac Soc. 2019;16(1):124–31. [DOI] [PubMed] [Google Scholar]
- 2.Management of Malignant Pleural Effusions. Am J Respir Crit Care Med. 2000;162(5):1987–2001. [DOI] [PubMed] [Google Scholar]
- 3.van Zandwijk N, Clarke C, Henderson D, Musk AW, Fong K, Nowak A, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis. 2013;5(6):E254–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgensztern D, Waqar S, Subramanian J, Trinkaus K, Govindan R. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7(10):1485–9. [DOI] [PubMed] [Google Scholar]
- 5.Porcel JM, Gasol A, Bielsa S, Civit C, Light RW, Salud A. Clinical features and survival of lung cancer patients with pleural effusions. Respirology. 2015;20(4):654–9. [DOI] [PubMed] [Google Scholar]
- 6.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175–80. [DOI] [PubMed] [Google Scholar]
- 7.Feller-Kopman DJ, Reddy CB, DeCamp MM, Diekemper RL, Gould MK, Henry T, et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(7):839–49. [DOI] [PubMed] [Google Scholar]
- 8.Bibby AC, Dorn P, Psallidas I, Porcel JM, Janssen J, Froudarakis M et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J 2018;52(1). [DOI] [PubMed]
- 9.Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383–9. [DOI] [PubMed] [Google Scholar]
- 10.Thomas R, Fysh ETH, Smith NA, Lee P, Kwan BCH, Yap E, et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA. 2017;318(19):1903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boshuizen RC, Noort Vvd, Burgers JA, Herder GJM, Hashemi SMS, Hiltermann TJN, et al. A randomized controlled trial comparing indwelling pleural catheters with talc pleurodesis (NVALT-14). Lung Cancer. 2017;108:9–14. [DOI] [PubMed] [Google Scholar]
- 12.Dipper A, Bhatnagar R, Maskell N, Jones HE, Preston NJ, Clive A. Interventions for the management of malignant pleural effusions: An updated network meta-analysis. Eur Respir Rev. 2021;30(160):210025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, et al. Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax. 2014;69(12):1098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ost DE, Grosu HB, Niu J, Zhao H, Giordano SH. Quality Gaps and Comparative Effectiveness of Management Strategies for Recurrent Malignant Pleural Effusions. Chest 2018;153(2):438–452. [DOI] [PMC free article] [PubMed]
- 15.Psallidas I, Kanellakis NI, Gerry S, Thezenas ML, Charles PD, Samsonova A, et al. Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol. 2018;19(7):930–9. [DOI] [PubMed] [Google Scholar]
- 16.ICES [Internet]. ICES. [cited 2024 Sep 5]; https://www.ices.on.ca/our-organization/
- 17.McLaughlin JR, Kreiger N, Marrett LD, Holowaty EJ. Cancer incidence registration and trends in Ontario. Eur J Cancer. 1991;27(11):1520–4. [DOI] [PubMed] [Google Scholar]
- 18.Sternberg SA, Bentur N, Abrams C, Spalter T, Karpati T, Lemberger J, et al. Identifying frail older people using predictive modeling. Am J Manag Care. 2012;18(10):e392–397. [PubMed] [Google Scholar]
- 19.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD. 2009;6(5):388–94. [DOI] [PubMed] [Google Scholar]
- 20.Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160–6. [PubMed] [Google Scholar]
- 21.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly Robust Estimation of Causal Effects. Am J Epidemiol. 2011;173(7):761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–75. [DOI] [PubMed] [Google Scholar]
- 24.Srour N, Amjadi K, Forster AJ, Aaron SD. Management of Malignant Pleural Effusions with Indwelling Pleural Catheters or Talc Pleurodesis. Can Respir J. 2013;20(2):106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai RJ, Levin R, Lin KJ, Patorno E. Bias Implications of Outcome Misclassification in Observational Studies Evaluating Association Between Treatments and All-Cause or Cardiovascular Mortality Using Administrative Claims. Journal of the American Heart Association: Cardiovascular and Cerebrovascular Disease [Internet] 2020 [cited 2024 Sep 5];9(17). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7660765/ [DOI] [PMC free article] [PubMed]
- 26.Fysh ETH, Bielsa S, Budgeon CA, Read CA, Porcel JM, Maskell NA, et al. Predictors of Clinical Use of Pleurodesis and/or Indwelling Pleural Catheter Therapy for Malignant Pleural Effusion. Chest. 2015;147(6):1629–34. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee K, Goyal A, Kakkera K, Meena N. Etiology of Malignant Pleural Effusion and Utilization of Diagnostic and Therapeutic Procedures: A Nationwide Analysis. J Bronchol Interv Pulmonol. 2017;24(1):e10–2. [DOI] [PubMed] [Google Scholar]
- 28.Wilshire CL, Chang S-C, Gilbert CR, Akulian JA, AlSarraj MK, Asciak R, et al. Temporal Trends in Tunneled Pleural Catheter Utilization in Patients With Malignancy: A Multicenter Review. Chest. 2021;159(6):2483–7. [DOI] [PubMed] [Google Scholar]
- 29.Raman T, Mcclelland S, Bartter T, Meena N. Current practice in management of exudative pleural effusions-A survey of American Association of Bronchology and Interventional Pulmonology (AABIP). J Thorac Dis. 2018;10(6):3874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scarci M, Bedetti B, Caruana E, Bertolaccini L, Brunelli A, Papagiannopoulos K, et al. Current practices in the management of malignant pleural effusions: A survey among members of the European Society of Thoracic Surgeons. Interact Cardiovasc Thorac Surg. 2017;24(3):414–7. [DOI] [PubMed] [Google Scholar]
- 31.Lee YCG, Baumann MH, Maskell NA, Waterer GW, Eaton TE, Davies RJO, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries: survey of pulmonologists. Chest. 2003;124(6):2229–38. [DOI] [PubMed] [Google Scholar]
- 32.Foote DC, Burke CR, Pandian B, Banks S, Haug KL, Hipp M, et al. Gender Disparity in Referral for Definitive Care of Malignant Pleural Effusions. J Surg Res. 2019;244:409–16. [DOI] [PubMed] [Google Scholar]
- 33.Dipper A, Maskell N. Prognostication in malignant pleural effusion: One size does not fit all. Respirology. 2020;25(12):1229–30. [DOI] [PubMed] [Google Scholar]
- 34.Fortin M, Taghizadeh N, Tremblay A. Procedures performed during hospitalizations for malignant pleural effusions: Data from the 2012 national inpatient sample. Respiration. 2018;95(4):228–34. [DOI] [PubMed] [Google Scholar]
- 35.Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49(10):932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell MA, Dhaliwal I, Mulpuru S, Amjadi K, Chee A. Early Readmission to Hospital in Patients With Cancer With Malignant Pleural Effusions: Analysis of the Nationwide Readmissions Database. Chest. 2020;157(2):435–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.