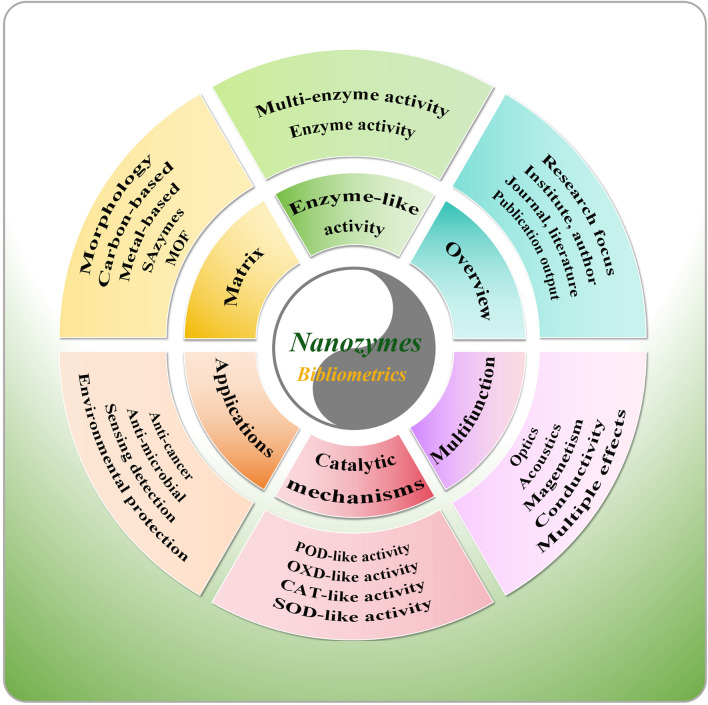
Abstract
As novel multifunctional materials that merge enzyme-like capabilities with the distinctive traits of nanomaterials, nanozymes have made significant strides in interdisciplinary research areas spanning materials science, bioscience, and beyond. This article, for the first time, employed bibliometric methods to conduct an in-depth statistical analysis of the global nanozymes research and demonstrate research progress, hotspots and trends. Drawing on data from the Web of Science Core Collection database, we comprehensively retrieved the publications from 2004 to 2024. The burgeoning interest in nanozymes research across various nations indicated a growing and widespread trend. This article further systematically elaborated the enzyme-like activities, matrix, multifunctional properties, catalytic mechanisms and various applications of nanozymes, and the field encounters challenges. Despite notable progress, and requires deeper exploration guide the future research directions. This field harbors broad potential for future developments, promising to impact various aspects of technology and society.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-02907-5.
Keywords: Nanozymes, Research trend, Bibliometrics, Enzyme-like activity, Matrix, Multifunctional properties, Catalytic mechanism, Applications
Background
Enzymes play a crucial role in many fields such as micro physiological process and industrial production as the efficient catalyst [1, 2]. Nevertheless, natural enzymes and genetically modified enzymes generally have intrinsic disadvantages such as high cost, poor stability, harsh storage conditions and low usage efficiency, which severely restrict their large-scale application. The exploration of artificial enzymes with high biocatalysis activity and controllable performance thus has been the goal of assiduous efforts in the field of enzymology [3, 4].
The advancement of nanotechnology has provided a significant boost to the emerging field of artificial enzymes, particularly nanozymes [5, 6]. Possessing both specific catalytic activity of enzymes and unique physicochemical property of nanomaterials, nanozymes exhibit many obvious advantages in high catalytic activity, adjustability, mild reaction conditions, good stability, unique multi-enzyme activity compared with traditional artificial mimetic enzymes [7]. Due to the interdisciplinary nature of nanozymes, accurately defining the meaning of the term is not always obvious. Therefore, some controversies over the appropriateness of the definition of the new term “nanozyme”, reflected the continuous deepening understanding of the catalytic characteristics of nanomaterials in this field [8–10]. It has also strongly inspired more and more researcher to deeply think and explore this field from different perspectives in order to clarifying some scientific critical issues [11–16].
As a result, nanozymes are increasingly attracting the attention of the global scientific and technological community. Since the term was first introduced in 2004 [17], it has generated thousands of research outputs in a rapidly evolving interdisciplinary field. Nanozymes were thus announced by International Union of Pure and Applied Chemistry (IUPAC) as one of the 2022 top ten emerging chemistry technologies [18].
Considering the rapid development of nanozymes research, it is very essential to comprehensively understand the knowledge domain and accurately predict the emerging trends of nanozymes by analyzing the fundamental information and exploring the changing trends in nanozymes research. However, although numerous reviews on nanozymes have been reported, there are few reports on nanozymes-related studies from the viewpoint of quantitative analysis to the best of our knowledge.
Herein, this article aims to comprehensively evaluated the status and global trends of the nanozymes research by reviewing the scientific papers published in the period from 2004 to 2024 using bibliometrics analysis method. The temporal development of publication output and citation metrics, geographical and institutional distributions, academic journals, authorship patterns, research hotspots and so on with significant contribution to nanozymes were investigated thoroughly. Based on the statistical analysis of this information, the art-of-state and progress of nanozymes-related research, encompassing enzyme-like activity, matrix morphology, multifunctional properties, catalytic mechanisms and applications were thoroughly reviewed. The challenges faced by the nanozymes research and the future perspectives were also discussed and summarized. It is expected that this article would deepen the understanding of this promising field from the scientometric view, and facilitate the development of more advanced nanoplatforms nanozyme-based to push forward the frontier of nanozymes-related research.
Methodology
Sources of data and search strategy
All data were retrieved and downloaded from the Web of Science Core Collection database (WOS, www.webofknowledge.com).
Inclusion criteria: (1) The searching terms were set as: TS = (“nano*zyme*” OR ((“enzyme-mim*” OR “enzyme-like”) AND “nano*”)); (2) The document type was “article” or “review”; (3) The publication time was from Jan. 2004 to Sep. 2024.
Exclusion criteria: (1) Letters or news or meeting abstract or proceedings papers or editorial material or book chapter; (2) Documents not officially published; (3) Withdrawn or repeated publications; (4) Documents that cannot provide the basic information required for bibliometric analysis.
Data analysis
The basic information in the records, such as article title, author, publication year, abstract, keywords, and citation frequency, were firstly extracted and classified. The latest impact factors (IF) and quartile of a journal category (JCR) were searched from the official websites of WOS. Microsoft Excel (2021.15.56), VOSviewer (www.vosviewer.com), CiteSpace (www. citespace.podia.com) were used to perform data statistics and visualization analysis. Among them, Microsoft Excel was used for managing, screening and ranking documents. VOSviewer (version 1.6.19) was used to create network visualization maps to analyze the collaborative relationships between countries/regions, institutions, journals and authors. CiteSpace (version 6.3 R1) was employed for keywords analysis and dual-map overlays, revealing the associations between keywords and identifying research trends and hotspots.
Results and discussion
Overview of nanozymes
Publication output
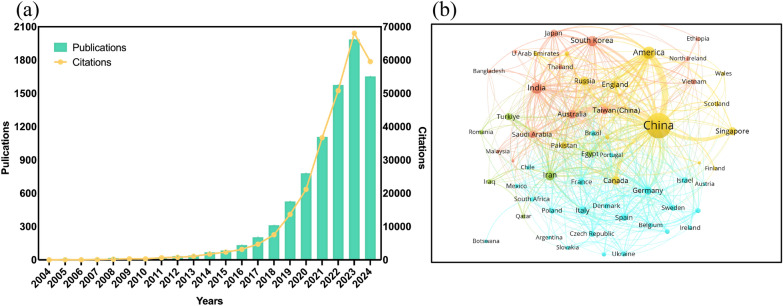
The number of annual publications from 2004 to Sep. 2024 is shown in Fig. 1a. A total of 8587 publications including 7664 research articles and 923 reviews were identified. Although some sources indicated the existence of approximately 13,000 papers related to nanozymes [19], this article mainly collected 8587 records from WOS. The main reasons for the differences in data primarily included such as different databases and different search strategies. Especially, selecting a suitable database is crucial for ensuring the comprehensiveness and scientific validity of the collected data. WOS database, as an internationally recognized database reflecting the level of scientific research, was selected as the source for data retrieval in order to ensure the authority of the original documents. Due to the fact that we only searched the WOS database in this study, it inevitably caused the omission of original literature. Consequently, papers published not indexed by the WOS database or do not adhere to specific inclusion and exclusion criteria are beyond the scope of this study. The total of citations for these publications were 273,321, with an average of 31.83 citations per article. The term of “nanozymes” was first appeared in 2004, when Scrimin et al. discovered Au nanoparticles (NPs) modified with triazacyclononane/Zn2+ had the ability to mimic Rnase [17]. The article, widely recognized as the pioneering study of nanozymes research, was reported by Yan’ s group in 2007 [5]. This study was the first time to discover the intrinsic peroxidase (POD)-like activity of ferromagnetic NPs, which breaks the traditional concept that inorganic materials have biological inertness. The number of publications on nanozymes slowly increased in the decade after the introduction of nanozymes, mainly due to the exploratory stage. It has made significant breakthroughs after 2017. As of Sep. 2024, the annual publication output has reached 1653 articles, with an average of 183 articles per month. Compared to the monthly average of 165 articles in 2023, it is expected that the total number of articles for 2024 will achieve a new breakthrough.
Fig. 1.
Research progress in the field of nanozymes. a Trends of annual publications and cations. b Collaboration network of countries/regions
A total of 92 countries/regions worldwide have conducted in the nanozyme field, including 33 European countries, 36 Asian countries, 11 African countries, 10 American countries, and 2 Oceanian countries, indicating that nanozymes are attracting widespread attention. Among the top 20 countries/regions in publication volume and citation (Fig. S1, in Supporting information), China had the highest publication with 6733 papers and 213,103 citations, indicating that the dominant position, and played a crucial role in this field. The countries/regions with over 100 papers were America (535, 28,521), India (428, 13,385), South Korea (264, 7918), Iran (200, 4307), Australia (157, 7473), Canada (131, 7509), Russia (122, 3297), Germany (107, 4098), Taiwan of China (102, 4099), Italy (101, 3547), Singapore (101, 6316) in order. It should be mentioned that in terms of citation frequency per article, China lagged behind some countries/regions. To further explore any cooperation relationship between countries/regions, we used VOSviewer software to perform a cooccurrence clustering analysis. The size of the nodes represents the number of publications in a country/region, and the thickness of the connections between nodes represents the strength of collaboration between countries/regions. Distinct clusters, indicated by color variations, represent cohesiveness within each cluster, and similar colors indicate closer collaboration between them. As shown in Fig. 1b, China the highest strength of connection among of them, reflecting the closest collaboration with other countries/regions. Particularly, China and America, Canada, and Singapore, formed a yellow collaboration cluster. European countries such as Germany, Spain, Italy, and France formed the blue cluster. In addition to, South Korea, India, Australia, Japan, and others formed the red cluster, and Iran, Turkiye, and Egypt formed the green cluster. Moreover, there were close cooperative relationships between different clusters. The result indicates more and more countries/regions are beginning to engage in research related to nanozymes. This spreading trend has injected many new vitalities into the research of nanozymes.
Institution and author
A total of 3714 institutions worldwide have conducted research on nanozyme. The top 20 academic institutions in term of publication volume are all from China (Fig. S2, in Supporting information). Collaboration network of institutions (Fig. S3, in Supporting information).The institution with the highest publication volume was the Chinese Academy of Sciences with 909 papers followed by University of Chinese Academy of Sciences, Jilin University, University of Science and Technology of China, Nanjing University. A total of 28,514 authors have published papers in the field, and there are 1311 core authors with more than 7 publications according to Price's Law. Collaboration network of authors (Fig. S4, in Supporting information) showed that H Wei, J W Liu, X G Qu, J S Ren, K L Fan, X Y Yan, L Z Gao and others have published over 35 papers. The authors also formed different collaboration clusters led by H Wei, X G Qu, X Y Yan, C Zhu, and others.
Journal and literature
Studies on nanozyme are distributed in 686 journals from over 99 publishers. Among the top 10 journals (Table S1, in Supporting information), ACS Applied Materials and Interfaces has published 314 studies with the highest number of publications. The reason for this is that the journal mainly focuses on interdisciplinary science of chemistry, materials science, engineering, physics, and biology, and aims to develop new materials and their specific applications, very suitable for publishing research related to nanozyme. Other journals are more focused on research of biosensing and biochemical detection, closely related to the properties and applications of nanozymes. The dual-map overlays of journals (Fig. S5, in Supporting Information) showed that research published in physics, materials, chemistry journals were mainly cited by research published in chemistry, materials, physics/environmental, toxicology, nutrition/molecular, biology, genetics journals, revealing the interdisciplinary characteristics of nanozymes research.
Among top 10 cited literatures (Table 1) with more than 800 citated times per article, the highest cited article was published in Nature Nanotechnology in 2007 from Yan's group with more than 5203 citated times as a pioneering study in the field of nanozyme. Most of the other papers were reviews, mainly including Wei et al. published in Chemical Society Reviews in 2019 [20], Wang et al. published in Chemical Society Reviews in 2013 [6], Qu et al. published in Chemical Reviews in 2019 [21]. These literatures contributed to researchers’ understanding of the status of nanozymes research and provide direction for promoting breakthroughs in the field.
Table 1.
Top 10 literatures cited
| Rank | Corresponding Author | Year | Title | Journal | IF* | Citations |
|---|---|---|---|---|---|---|
| 1 |
S Perrett, X Y Yan |
2007 | Intrinsic peroxidase-like activity of ferromagnetic nanoparticles | Nature nanotechnology | 38.1 | 5203 |
| 2 | H Wei | 2019 | Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II) | Chemical society reviews | 40.4 | 3089 |
| 3 |
H Wei, E K Wang |
2013 | Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes | Chemical society reviews | 40.4 | 2077 |
| 4 | X G Qu | 2019 | Nanozymes: classification, catalytic mechanisms, activity regulation, and applications | Chemical Reviews | 51.4 | 2049 |
| 5 |
H Wei, E K Wang |
2008 | Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics and Their Applications in H2O2 and Glucose Detection | Analytical chemistry | 6.7 | 1259 |
| 6 |
H Peng, X Y Yan, W B Cai |
2019 | Nanozyme: new horizons for responsive biomedical applications | Chemical society reviews | 40.4 | 1152 |
| 7 |
Y Chen, J L Shi |
2017 | Tumor-selective catalytic nanomedicine by nanocatalyst delivery | Nature communications | 14.7 | 1075 |
| 8 | X G Qu | 2014 | Catalytically active nanomaterials: a promising candidate for artificial enzymes | Accounts of chemical research | 16.4 | 997 |
| 9 |
M M Liang, X Y Yan |
2019 | Nanozymes: from new concepts, mechanisms, and standards to applications | Accounts of chemical research | 16.4 | 970 |
| 10 | X G Qu | 2014 | Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications | NPG Asia materials | 8.6 | 833 |
Research focus
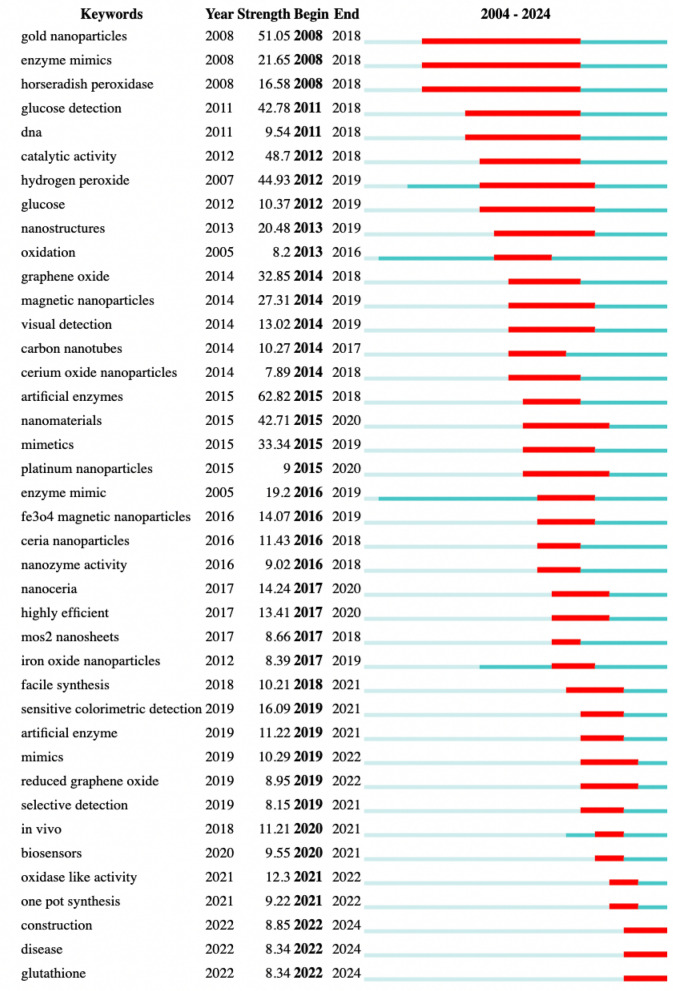
By detecting keyword prominence, one can understand the development changes such as research hotspots, trends, and cutting-edge dynamics within a certain time frame on nanozymes research. The top 40 keywords with the strongest citation bursts on nanozymes research was shown in Fig. 2, with the red line indicating the time period of keyword bursts. The keyword “artificial enzymes” indicated the highest citation bursts with strengths of 62.82 (2015–2018). Other keywords showcasing substantial citation bursts include “gold nanoparticles” (S/51.05) “catalytic activity” (S/48.7), “hydrogen peroxide” (S/44.93), “glucose detection” (S/42.78) and “nanomaterials” (S/42.71). In terms of burst duration, “gold nanoparticles”, “enzyme mimics” and “horseradish peroxidase” sustained the burst status for an impressive 10 years, spanning 2008–2018. Notably, the current evolving trends in the field were exemplified by recently bursting keywords, encompassing terms like “biosensors”, “one pot synthesis”, “construction” and “disease”. Furthermore, the burst analysis provides temporal insights for nanozymes research. The research on nanozymes mainly focused on fundamental studies including discovery of nanozymes, exploration their enzyme-like activities and catalytic mechanisms (e.g., “gold nanoparticles”, “catalytic activity”, “nanomaterials”) until around 2018. It gradually expanded into the field of design and applications after 2018 (e.g., “construction”, and “in vivo” “sensitive colorimetric detection”). The citation burst time for keywords such as “construction”, “disease” and “glutathione”, started from 2022 and continued up to the present, indicating the application in medicine including diagnosis and therapeutics have become research hotspot.
Fig. 2.
Top 40 keywords with the strongest citation bursts
Based on the evaluation of the high-frequently keywords and cluster analysis of keywords and discipline (Fig. S7, in Supporting Information), the trends in the main directions of nanozymes primarily include the new activities and new materials of nanozymes, design strategies, catalytic mechanism, biomedical applications, etc. These results indicated that the unique characteristics and high catalytic efficiency of nanozymes have enormous potential in various disciplinary fields.
Enzyme-like activity of nanozymes
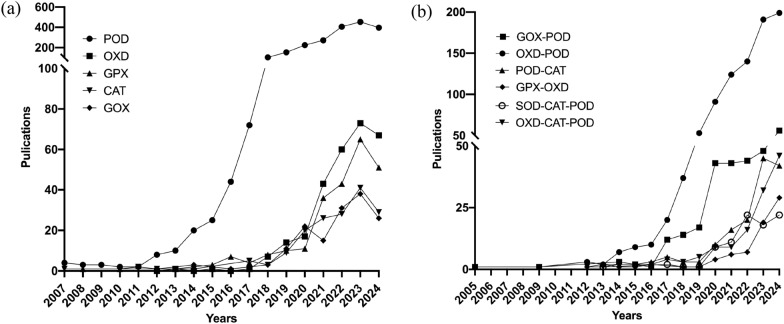
To date, nanozymes have been discovered to include oxidoreductase-, hydrolase-, lyase-, isomerase-, transferase- and ligase-like six major catalytic classes, exhibiting diversified catalytic activities with more than 30 different single-enzyme-like types (Table 2). Among them, POD-like nanozyme, being the earliest discovered, are the most abundant with about 2202 research papers. The enzyme-like activities that have published over 100 papers are POD, oxidase (OXD), glutathione POD (GPX), catalase (CAT), glucose OXD (GOX) (Fig. 3a). Unlike natural enzymes, nanozymes exhibit broad substrate adaptability. POD-like nanozymes can mimic the activities of various enzymes such as POD, GPX, lipid POD and ascorbate POD. And OXD-like nanozymes mainly possess the activities of OXD, GOX, cysteine OXD (COX), ascorbate OXD (AOX) and urate OXD (UOX or UOD). Although diverse enzymatic activities have been discovered such as carbonic anhydrase and topoisomerase I, the current nanozymes mainly mimic the enzymatic activity of oxidoreductase system, participating in intracellular redox reactions and modulating biological processes such as oxidative stress.
Table 2.
Types of catalytic activity of nanozymes
| Classification | Enzyme-like type | Catalytic reaction | Enzyme type | Research articles | Representative nanozymes | Application |
|---|---|---|---|---|---|---|
| Oxidoreductase | Peroxidases-like | H2O2 + 2A-H H2O + 2A [22] | Peroxidase | 2202 |
Au nanoclusters [23], Graphene oxide [24], Fe-MOF [25], Non-heme Fe SAzymes [26] |
Detection, Detection, Detection, Tumor therapy |
| Glutathione peroxidase | 243 |
MIL-47(V) [27], GO-Se nanocomposite [28] |
Anti-inflammation, Anti-oxidant stress |
|||
| Lipid peroxidase | 32 |
IONzymes [29], HMPB@Lip [30] |
Anti-virus, Anti-cancer |
|||
| NADH peroxidase | 11 | Cu2+-GO NPs [31] | Detection | |||
| Ascorbate peroxidase | 6 | Cu SAs/CN [32], | Anti-oxidant damage | |||
| Haloperoxidase | 6 | Ce-MOF [33] | Detection | |||
| Oxidase-like |
Ared + O2 + H2O Aox + H2O2 Ared + O2 Aox + H2O Ared + O2 Aox + 2O2−·[6] |
Oxidase | 283 |
OV-Mn3O4 [34], Ce-MOF [35] |
Detection, Detection |
|
| Glucose oxidase | 153 |
Au NPs [36], Au-MOF [37] |
Detection, Detection, |
|||
| Cysteine oxidase | 24 |
CuO NPs [38], Mn-UMOF [39] |
Detection, Detection |
|||
| Laccase | 20 |
Cu-CDs [40], DNA-Cu [41] |
Detection, Detection |
|||
| Amino acid oxidase | 14 | Pt/SiO2 [42] | Selective catalysts | |||
| Ascorbate oxidase | 11 | Au@Pt [43] | Detection | |||
| Sulfite oxidase | 9 | Carbon dot [44] | Bioimaging detection and ameliorating acute lung injury | |||
| Urate oxidase | 45 | MVSM [45] | Treatment of gouty arthritis | |||
| Nitric oxide synthase | 11 | Graphene-hemein [46] | Anti-thrombotic | |||
| NADH oxidase | 6 | MNGR [47] | Treatment of the metabolic flux anomaly in obesity | |||
| Cytochrome c oxidase | 15 | Cu2O [48] | Catalytic oxidation of biomacromolecules | |||
| Reductase-like | NO3− + H2O + 2e− NO2− + 2OH− [49] | Reductase | 27 | CdS-Pt [49] | Alternative to native enzymes in the photoactivated reduction | |
| Catalase-like | H2O2 + H2O2 2H2O + O2 [22] | Catalase | 167 |
Co-N3PS Sazyme [50], α-Fe2O3 [51] |
To mimic natural enzymes, Wound healing |
|
| Superoxide dismutase -like | 2O−·2 + 2H+ H2O2 + O2 [6] | Superoxide dismutase | 88 |
MnO [52], CeO2 [53] |
Detection, Sensor |
|
| Hydrolase | Hydrolase-like | A-B + H2O A-OH + A-H | Phosphatase | 56 |
VE CeO2 [54], GO [55] |
Degradation nerve agents, Degradation nerve agents |
| Protease | 18 |
MOF-808 [56], CQDs/Cu2O [57] |
Lysozyme hydrolysis, Protein hydrolysis |
|||
| Hydrolase | 28 |
Minimal metallo-nanozymes [58], DAFB-DCTP COF [59] |
Catalytic prodrug conversion, Detection |
|||
| Esterase | 38 | Pt NPs [60] | Immunosensor | |||
| Nuclease | 24 | Cu-doped carbon spheres [61] | Anti-bacterial | |||
| Urease | 2 | CeO2 − x [62] | Environmental governance | |||
| Glucuronidase | 1 | Cu NPs [63] | Catalytic prodrug conversion | |||
| Lyase | CO2 + H2O HCO3− + H+ [64] | Lyase | 19 | ZIF-8 [64] | Alternative to hydratase | |
| Transferase | A·X + B A + B·X | Transferase | 6 | ZnPc-CeNZ [65] | Detection | |
| Isomerase | A-B -A | Topoisomerase I | 4 | Chiral carbon dots [66] | Chiral selective catalysis | |
| Ligase | A + B A·B | Ligase | 2 | MSNs [67] | Successive product amplification |
Fig. 3.
Trends in the publication of research articles with over 100 papers on a catalytic activity types of nanozymes and b multi-enzyme activity of nanozymes
Unlike other artificial enzymes, nanozymes also exhibit diverse multi-enzyme-like activity. More than 20 different kinds of multi-enzyme-like nanozymes, including bi-, tri-, tetra-and penta-enzyme-like activities, have been discovered to date (Table 3).
Table 3.
Types of multi-enzyme-like activity of nanozymes
| Multi-enzyme-like type | Enzyme type | Number of publications | Representative nanozymes | Application |
|---|---|---|---|---|
| Bi-enzyme | OXD-POD | 1040 |
MnNS [68], Co/2Fe MOF [69] |
Detection, Detection |
| GOX-POD | 326 |
g-C3N4 [70], DMSN-Au-Fe3O4 NPs [71] |
Detection, Anti-tumor |
|
| POD-CAT | 190 |
Fe3O4 NPs [72], Co3O4 nanoplates [73] |
Diminishing cytotoxicity, Detection |
|
| GPX-OXD | 20 |
CuGQD/PdNPs@Psi [74], Mo/Fe@CuO2 [75] |
Anti-cancer, Anti-bacterial |
|
| SOD-CAT | 102 |
Pt/Co-SA-NSG [76], CeO2 NPs [77] |
Treatment of osteoarthritis, Treatment of ischemia–reperfusion injury |
|
| OXD-CAT | 68 |
DOX@CuMn-Dazymes [78], Co-SAs@NC [79] |
Treatment of tumor, Anti-cancer |
|
| COX-POD | 5 |
Cu-BTC [80], U66-PV-Pep@AuNP [81] |
Detection, Detection |
|
| AAO-POD | 7 | CuO NPs/AA [82] | Anti-bacterial | |
| POD-LAC | 4 | Cu FMA [83] | Detection | |
| Protease-SOD | 1 | AuNPs@POMD-8pep-6pep NPs [84], | Treatment of Alzheimer’s disease | |
| UOX-CAT | 5 | Pt/CeO2 [85] | Treatment of acute gout | |
| GPX-CAT | 2 | Sn Fe2O4 [86] | Anti-cancer | |
| Tri–enzyme | OXD-CAT-POD | 159 |
Co3O4/CoFe2O4 HNCs [87], Ir-N-5 SA [88] |
Environmental governance, Anti-cancer |
| SOD-CAT-POD | 105 |
Au/CeO2 [89], NLRP3 [90] |
Detection, Anti-inflammation |
|
| SOD-CAT-UOX | 2 | ARP-PtNC [91] | Treatment of gout and hyperuricemia | |
| Tetra-enzyme | OXD-POD- SOD-CAT | 57 |
polymer-coated/CeO2 [92], hNiPt@CoNC [93] |
Anti-oxidant, Detection |
| OXD-POD-CAT-GPX | 15 | CuO NP-POM [94] | Detection | |
| POD-CAT-SOD-AOX | 4 | Cu NCs [95] | Detection | |
| Laccase-POD-OXD-CAT | 5 | Co1.5Mn1.5O4 [96] | Detection | |
| Penta-enzyme | POD-OXD- UOD-SOD-CAT | 1 | Pero-nanozysome [97] |
Treatment of hyperuricemia and ischemic stroke, |
| SOD-POD-LOX-GPX-NDH | 1 | TADI-COF-Fc [98] | Anti-cancer |
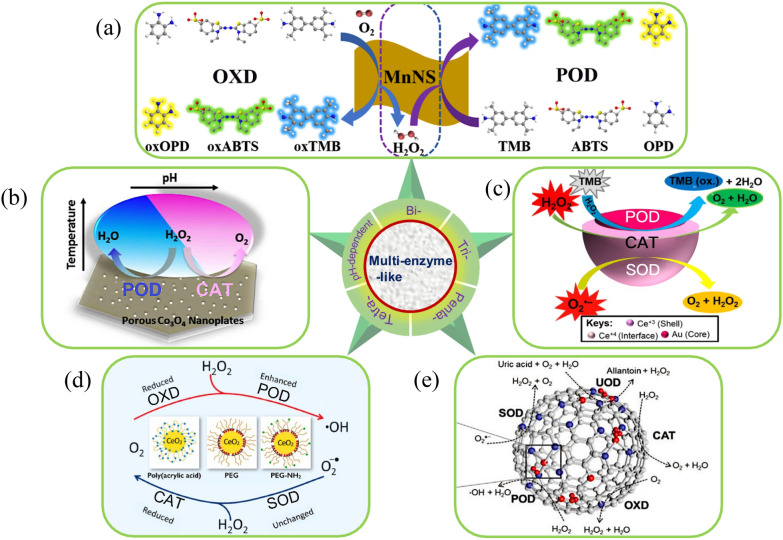
Gu et al. for the first time, revealed the pH-dependent multi-enzyme activity of nanozymes [72]. Iron oxide NPs were found to catalyzed H2O2 to produce OH· under acidic conditions (pH 4.8), exhibiting POD-like activity. It also catalyzed H2O2 to O2 under neutral and alkaline conditions, exhibiting CAT-like activity.
OXD-POD-like nanozyme is the most extensively studied class among nanozymes with multi-enzyme-like activity (Fig. 3b). The typical feature of OXD-POD nanozyme was the ability to combine the ability of OXD to reduce O2 to H2O2 with the ability of POD to consume H2O2 to produce HO·, forming a cascade catalytic system (Fig. 4a). The types of nanozymes with OXD-POD bi-enzyme-like activity mainly included GOX-POD, COX-POD, AAO-POD, and LAC-POD.
Fig. 4.
Various types of multi-enzyme-like activity nanozymes. a OXD-POD-like activities of 2D MnO2 nanozyme [68]. b pH-dependent POD-like and CAT-like activities of Co3O4 nanozyme [73]. c POD-CAT-SOD-like activities of Au/CeO2 nanozyme [89]. d POD-CAT-XOD-SOD-like activities of polymer-coated/CeO2 nanozymes [92]. e SOD-CAT-UOD-POD-OXD-like activities of pero-nanozysome [97]
Dong et al. also found that Co3O4 nanoplates simultaneously possessed POD-CAT-like activity [73]. As shown in Fig. 4b, these two kinds of enzyme-like could be switched by pH. And temperature and the concentrations of Co3O4 had a significance effect on the switch pH and the dual-enzyme-like catalytic ability.
Nanozymes with tri-enzyme-like activity could formed two cascade catalytic reactions. For example, OXD-CAT-POD-like nanozymes, can form OXD-POD and CAT-OXD two kind of cascade catalytic systems. The nanozymes utilized its POD- CAT-like activity to catalyze the generation of HO· and O2. O2 was further utilized by its OXD-like activity to generate O2−·. The HO· and O2−· generated by the catalytic cascade system synergistically may induce tumor cell death for tumor therapy. Singh et al. prepared Au core and CeO2 shell NPs (Au/CeO2 CSNPs) through a process of heterogeneous nucleation and growth, which possessed excellent POD-CAT-SOD-like activity (Fig. 4c) [89]. A significant enzyme-like activity of this core–shell nanoparticle was conserved at extreme conditions (pH (2–11, temperatures, up to 90 °C), clearly the superiority over natural enzymes, which opened new directions for development of sensors platform in multiple biosensing applications.
In addition, an increasing number of nanozymes with tetra- or penta-enzyme-like activity have also been discovered. Gao et al. designed a pero-nanozysome, in which atomic Fe clusters were embedded in an atomically dispersed Fe–N4–C matrix [97]. The pero-nanozysome (Fig. 4e), exhibited stable and penta-enzyme-like activities: SOD, CAT, POD, OXD, and UOD. Through employing the tandem different kinds of enzyme-like activities, the nanozyme showed therapeutic effect in treating hyperuricemia and protecting neurons from free radicals invasion during ischemic stroke, promising candidate to design artificial peroxisome performing in vivo functions.
It should be pointed out that these kinds of multi-enzyme-like activity of nanozymes originate from the oxidoreductase enzyme system. And most of them are still based on POD-like as the central enzyme. Nonetheless, they can be assembled into cascading reaction systems (Fig. S6, in Supporting Information), enabling them to be self-sufficient in generating intermediate products and establishing closed-loop reactions, significantly enhancing reaction efficiency and presenting a novel strategy for achieving highly sensitive detection and synergistic therapeutic applications.
Matrix of nanozymes
The catalytic activities of nanozymes are primarily dependent on the inherent properties of the materials. Thousands of types of nanomaterials, ranging from metals to non-metals, inorganic to organic, and the nanoscale to the single-atom scale, have been designed and explored to enzyme-like catalytic properties to date. In terms of materials matrix, nanozymes mainly include metal-based, carbon-based, metal–organic frameworks (MOF) and single-atom nanozymes (SAzyme) (Fig. 5, Table S3, in Supporting Information).
Fig. 5.
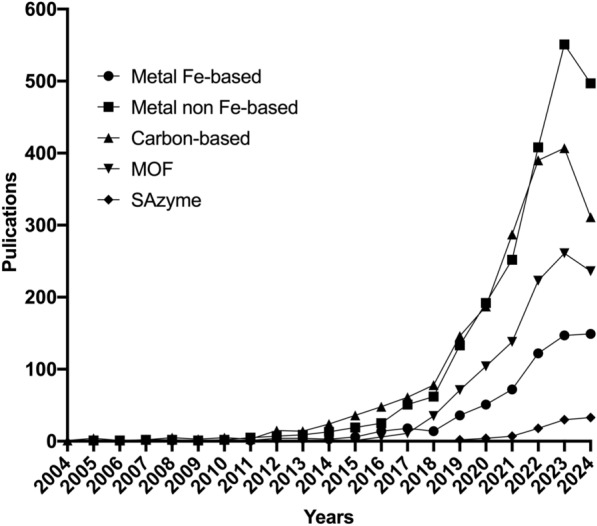
Trends in the publication and distribution of matrix of nanozymes
Metal-based nanozymes
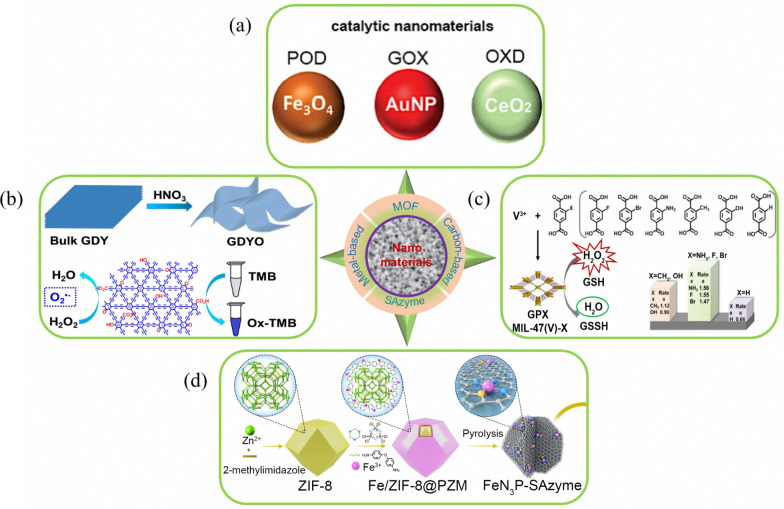
Among matrix of nanozymes, metal-based nanozymes are the most abundant with about 2877 research papers. Metal-based nanozymes may be divided to Fe-based and non-Fe-based. Fe-based nanozyme, discovered for the first time [5], mainly include iron oxide NPs, iron chalcogenides, iron phosphates, prussian blue and its cyanometallate structural analogues [72, 99]. Non-Fe-based nanozymes mainly include noble metal nanomaterials (e.g., Au, Ag, Pt, Ir and their multimetallic nanomaterials) [23, 25, 49, 51] and other non-iron metallic nanomaterials (e.g. Cu, V, Mn, Ce, Cd oxides and sulfides nanomaterials) (Fig. 6a). The hybrid nanomaterials of metal-based and functional modification have become the most extensively researched category for nanozymes recently, due to their flexible and controllable properties in terms of structure, morphology, and oxidation state.
Fig. 6.
Various types of nanozyme materials. a Metal-based nanozymes [10]. b Graphdiyne oxide (GDYO) nanozymes [100]. c MIL-47(V)-X MOF nanozymes [27]. d FeN3P-SAzyme [11]
Zhao et al. synthesized SnFe2O4 (SFO) nanozyme with CAT-GPX-like activity by hydrothermal method [86]. After modified with poly(styrene)-block-poly(ethylene glycol)(pS-PEG), its hydrophilicity and biocompatibility were further enhanced. Yang et al. fabricated a Fe@Fe3O4@Cu2 − xS (MNPs) nanozyme [101]. After introduced β-lapachone (La) to assemble, the LaMNPs could boost the production of ROS and upregulate the level of H2O2 by the released La in an acidic tumor micro-environment (TME). After injected intravenously into mice model, LaMNPs could efficiently inhibited the tumor growth.
The composition and morphologies of metals have a significant impact on the activity and variety of nanozymes. Compared to single component of metal, multi-metallic nanozymes typically exhibit improved catalytic performance due to the synergistic effect and electronic effect. It is one of the important directions in metal-based nanozymes research through reasonable regulation the proportion of metal components and structures to enhancing catalytic performance [102, 103].
Cai et al. designed a precise control of Au-Pt bimetallic structures in three representative structural configurations, including segregated, alloy, and core–shell structures for catalyzing the glucose cascade reaction as nanozymes [104]. These bimetallic aerogels demonstrate improved POD- and GOX-like activities compared to their monometallic counterparts. Notably, the segregated Au-Pt aerogel showed optimal catalytic activity, which was 2.80 and 3.35 times higher than that of the alloy and core–shell variants, respectively. This enhanced activity was mainly attributed to the synergistic effect induced by the high-density Au-Pt interface boundaries within the segregated structure, altered electronic structures, and favorable aerogel structures. which fostered greater substrate affinity and superior catalytic efficiency. This work provided a platform for the investigation of the structure–property relationship of bimetallic materials. Qu et al. developed a MnO2@PtCo nanozymes by self-assembly to initiate intracellular biochemical reactions against hypoxic tumors [105]. Among them, PtCo NPs with OXD-like activity could catalyze the oxidation reaction cascades to induce intracellular oxidative damage. Meanwhile, MnO2 component with the intrinsic CAT-like activity could induce the decomposition of H2O2 into O2 rapidly and efficiently, which not only surmounted the intrinsic hypoxic environment of tumors but, importantly, makes the therapeutic system independent on O2. By the cooperation between the oxidative activity of PtCo and the supply O2 ability of MnO2, the nanozymes could effectively alleviate hypoxic condition and generating ROS efficiently in hypoxic tumors, thereby resulting in remarkable therapeutic outcome specificity for tumor. Zhu et al. constructed a trace amount of Bi-doped core–shell Pd@Pt mesoporous nanospheres (Pd@PtBi2) [106]. Atomic-level composition regulation not only effectively prevented the aggregation of doped metal atoms but also the strong interaction between the ultra-low concentration of doped metals and the metal carrier at the atomic level can significantly modified the electronic structure of the active sites, thereby specifically enhancing their catalytic activity. With the incorporation of trace Bi, there was a more than 4-times enhancement in the POD-like performance of Pd@PtBi2 compared to that of Pd@Pt. In addition, the OXD-CAT-like activity of Pd@PtBi2 was not significantly enhanced, preventing interference from dissolved O2 in the tested liquid samples and unwanted consumption of the substrate H2O2.
Carbon-based nanozymes
Carbon-based nanozymes, first reported in 2010 [107], including fullerene, carbon nanotube, graphene, carbon dots, graphdiyne and their doped derivatives etc., have widely applied as nanozymes in sensing, therapy, and catalysis for their facial preparation, low cost, and high stability.
Qu et al. discovered the intrinsic POD-like activity of graphene oxide and used for glucose detection [24]. Mao et al. demonstrated GDYO, as shown in Fig. 6b, with efficient POD-like activity [100]. The catalytic reaction followed first order reaction kinetics. The POD-like activity of GDYO mainly originated from the generation of O2-· from the H2O2 decomposition, and the oxygen-containing groups of GDYO played vital roles in the activity of mimicking POD, which served as active sites for absorbing H2O2 and breaking O–H bond to HO2.and H+.
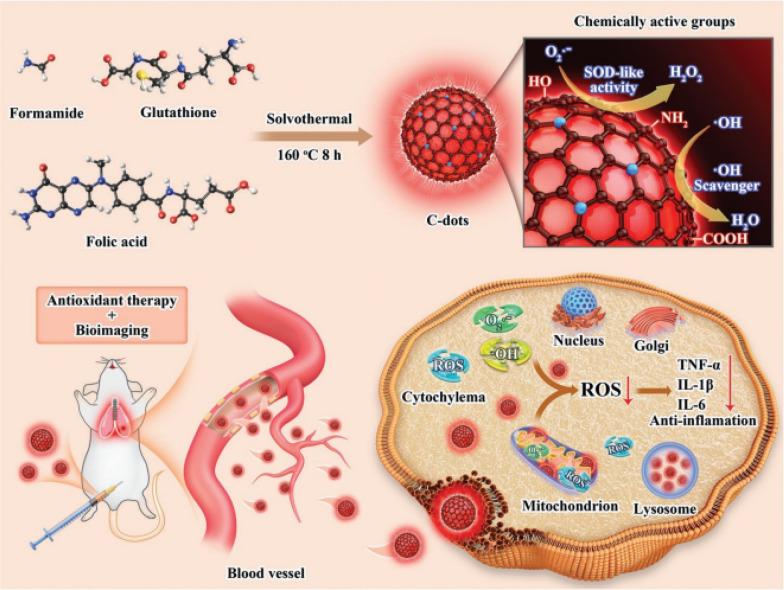
Fan et al. prepared a carbon dots (C-dot) with high SOD-like activity by a solvothermal method (Fig. 7) [44]. Through passivation of functional groups, the catalytic mechanism of C-dots was investigated and proposed. Firstly, the superoxide was anchored on the surface of the C-dots through the H bond with the carboxyl group, hydroxyl, and amine groups. Then, the electron-deficient structure on the C-dots obtained one electron from the superoxide anion, producing oxygen and reduced-state C-dots. Due to the large π-system (C = C/C = N) on the C-dots, a p-π conjugate was formed with the obtained electron, and thereby stabilizing the intermediate product (reduce-state C-dots). Subsequently, another superoxide obtained an electron from the reduced state C-dots, resulting in the formation of H2O2, while the structure of C-dots returned to their original state. This research revealed that the carboxyl, hydroxyl, and amino groups on the C-dots were related to the SOD-like activity. Through H bond, these surface functional groups bound with superoxide radicals to promote the electron transfer and then accelerated the dismutation of superoxide radicals. In addition, C-dots exhibited red fluorescence with emission wavelength of 683 nm and absolute quantum yield of ≈14%. C-dot nanozyme could also effectively enter the cells, accumulate at mitochondria, and protect living cells from oxidative damage by scavenging ROS and reducing the levels of pro-inflammatory factors, which provided great potential in biological imaging and management of ROS-related diseases.
Fig. 7.
Schematic illustration of red-emissive C-dots with high SOD-like activity for ameliorating ALI and bioimaging [44]
Notably, the enzyme-like activity of carbon-based nanozymes can be further enhanced by doping such as Fe and N in carbon-based nanomaterials. Wang et al. prepared N doped Q-graphene (N-QG) nanozyme by doping heteroatom N2 into QG at ambient temperature based on a plasma treatment strategy [108]. N-QG could possess a higher intensity ratio of D band to G band (ID/IG) than QG, due to the formation of surface defects by the plasma-assisted N doping and etching. Therefore, its catalytic activity was significantly enhanced, nearly fivefold higher than that of QG.
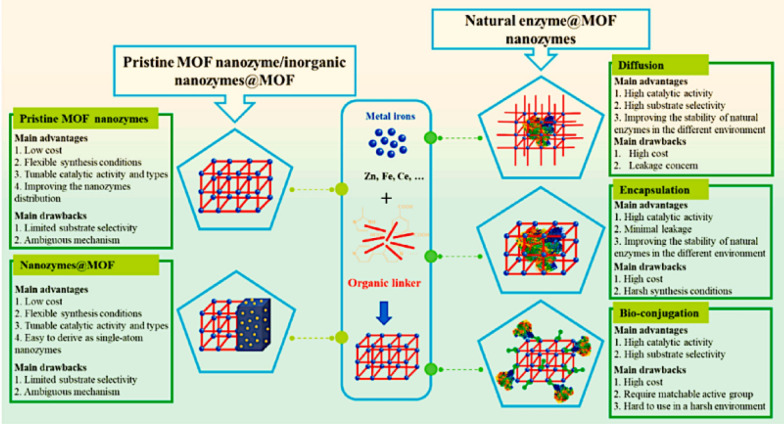
MOFs-based nanozymes
MOFs, as a novel and unique class of hybrid organic–inorganic porous crystalline materials, were first discovered to possess nanozyme activity in 2017 [109]. Their porous structure and multiple channels can facilitate small molecule substrates to enter and fully contact with the active sites, which are also beneficial to transport and diffusion of products. The high uniform sizes and pore shapes of MOFs are important to size-selective catalytic reaction, which can effectively control the size of the participating molecules. In addition, the diverse organic ligands and metal-based nodes, as well as multifunctional modifications [110, 111], can enhance their biocompatibility and biodegradability, and functionality and so on. These characteristics enable MOFs to exhibit diverse enzyme-like properties in such as biosensing, biocatalysis, and biomedical fields.
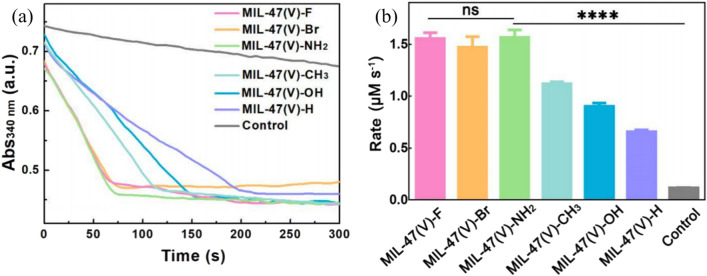
The performance of MOFs nanozymes depends on the design and preparation strategy. Wei et al. developed a ligand engineering strategy to modulate the GPX-like activity of a MOFs nanozymes (Fig. 6c) [27]. With different substituted ligands, the GPY-like activities of MIL-47(V)-X MOFs were rationally regulated. As shown in Fig. 8, each of the MIL-47(V)-X MOFs exhibited GPX-like activity, with MIL-47(V)-NH2, MIL-47(V)-F, and MIL-47(V)-Br having the highest activities among these isostructural MOFs. Of these, MIL-47(V)-NH2 showed a slightly higher activity than the other two, proving that high-performance nanozymes can be rationally designed. Chen et al. presented the main synthetic strategies for MOFs-based nanozymes as well as summarized some key advantages/disadvantages of each strategy (Fig. 9) [112].
Fig. 8.
a Time evolution of absorbance at 340 nm for monitoring the GPX-like catalytic activities of MIL-47(V)-X MOFs. b Comparison of GPX-like activities of MIL-47(V)-X MOFs. X = F, Br, NH2, CH3, OH, H [27]
Fig. 9.
Schematic representation of the main synthetic strategies for MOFs-based nanozymes [112]
SAzymes
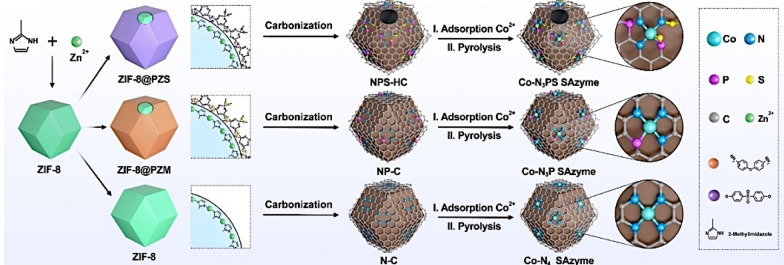
SAzymes, first reported in 2019 [114], emerge as a novel high performance nanozyme and have attracted extensive study interests. For traditional nanozymes, their activity mainly comes from a very few active sites on the surface of the nanomaterial, such as unsaturated coordinated atoms at edges and defect sites. These sites are difficult to distinguish and control quantitatively, which not only makes the catalytic mechanism extremely complex but also results in their activity being far inferior to that of natural enzymes. When nanoparticles are reduced to the atomic level, they can exhibit geometric and electronic effects that are significantly different from those of nanoparticles. They not only have the highest atomic utilization rate but also have a simple atomic structure, showing clear coordination structures, active sites, catalytic mechanisms, and superior catalytic performance. As a class of single-atom nanomaterials with enzyme-like activity, SAzymes, featuring the metal atoms independently dispersed onto the supports, successfully met the challenges, exhibit extremely high enzyme-like activity. The unique characteristics of the SAzymes lead to maximum atom utilization rates compared to nanoparticles and attribute itself the defined geometric and electronic structures.
The property of SAzymes mainly is related to the type of central atoms and it’s coordination environment, with M-N-nC emerging as the main structure among various types of SAzymes prepared. M represents the metal center. The metal atom is the core of catalytic activity, and its type (e.g., Fe, Cu, Co) and oxidation state can significantly affect the catalytic performance of SAzymes. N represents nitrogen coordination. Nitrogen atoms, as coordinating atoms, form coordination bonds with the metal center, stabilizing the metal center and modulating its electronic density and geometric structure. nC represents the carbon matrix. Carbon materials serve as a carrier, provide a stable platform to anchor the metal center and coordinating atoms, and can also affect the stability and accessibility of SAzymes through their porous structure and surface properties. The coordination environment in the M-N-nC structure is also crucial for the activity and selectivity of SAzymes. Changes in the coordination number and geometric structure can regulate the catalytic activity of the metal center. Therefore, the high property of SAzymes can be achieved by precisely adjusting the atomic active center and spatial configuration [11, 12, 15, 88, 115–117].
Li et al. designed and synthesized an engineered FeN3P-centred SAzyme (FeN3P-SAzyme) by controlling the electronic structure of the single-atom Fe active center through the precise coordination of phosphorus and nitrogen (Fig. 6d) [11]. The FeN3P-SAzyme, with well-defined geometric and electronic structures, displayed superior POD-like activity. Wang et al. developed an ordered structure-oriented coordination design strategy for rationally engineering SAzymes and established a correlation between the structure and enzyme-like performance (Fig. 10) [116]. Co-N3PS SAzyme were constructed by precise controlling over the atomic configuration of the active centers of Co SAzyme to enable the rational regulation of their enzyme-like performance guided by theorical calculations. This SAzyme was proven to process an excellent CAT-like activity, exceeding the representative controls of Co-based SAzymes with different atomic configurations. These studies demonstrated that precise control over the active centers of SAzymes is an efficient strategy to mimic the highly evolved active sites of natural enzymes.
Fig. 10.
Scheme of the synthesis of the Co-based SAzymes by the ordered structure oriented coordination design strategy [116]
In additional to one metal as active centers, SAzymes with dual active sites have been recently emerged and shown to improve the catalytic performance [118, 119]. Zhao et al. designed a paradigm of synergy and “division of labor” bimetallic dual active sites single-atom catalyst combined with ROS circulation and parallel catalytic therapy for efficient tumor therapy. Both Fe and Co atoms were isolated in the N-doped carbon material with a sharp-angled dodecahedron in a monodisperse state to form an independent bimetallic non-alloy structured atom pair (FeCo-DIA/NC). FeCo-DIA/NC synergistically initiated both POD-, OXD- and CAT-like reactions, directly and simultaneously catalyzing H2O2 and O2-· into ROS. The above ROS are further converted into H2O2 in the acid TME, forming a “ROS Cycle” for highly efficient tumor inhibition. Overall, with the diverse transition metals with different electronic structures that could potentially serve as the SAzymes’ active centers, there are substantial potentials for the SAzymes to replace nature enzymes.
Besides the representative nanomaterials mentioned above, various types of hybrid nanomaterials such as carbon metal hybrid materials, high-entropy alloy, covalent organic framework (COF), have been prepared and exhibited diverse and excellent enzyme-like activities [120–123].
Morphology
It's worth noting that even nanozymes of the same material can exhibit varying enzyme activities and catalytic types due to differences in their morphology, valence state, and so on [20, 116]. By regulating these parameters, the performance of nanozymes can be further optimized.
Yan’s group discovered the enzyme-like activity of Fe3O4 NPs changed with different sizes [5]. Fe3O4 NPs with a particle size of 30 nm exhibited the highest POD-like property compared to those of larger particles exhibiting the size-dependent POD-like activity (Fig. 11).
Fig. 11.
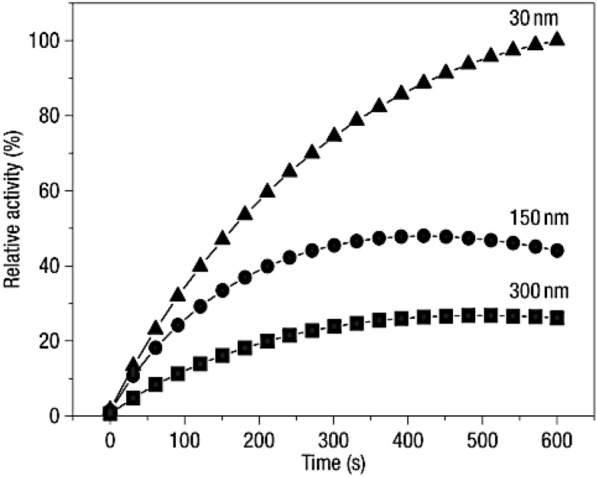
The size dependent POD-like activity of the Fe3O4 MNPs [5]
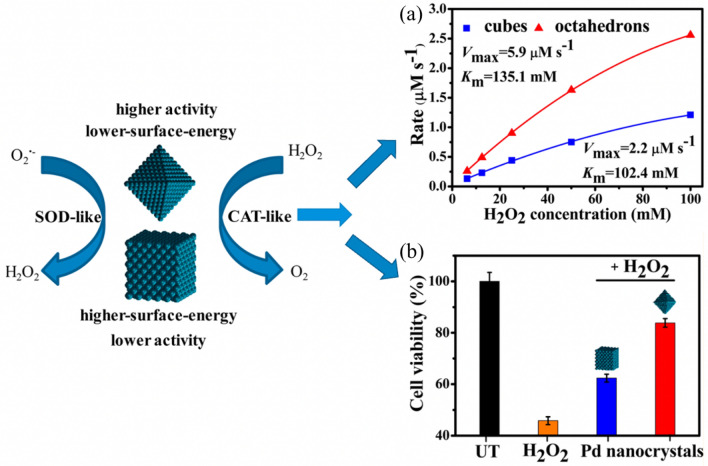
It revealed that the smaller the size of the nanozymes, the larger the specific surface area, and the more active sites it has, resulting in higher catalytic activity. Pathak et al. further studied the relationship between the POD-like property of Fe3O4 NPs and their morphology [124]. Due to the difference of surface energy facets of two NPs, the POD-like activity of Fe3O4 NPs with truncated octahedron-shaped exhibited was higher than that of spherical Fe3O4. Mugesh et al. prepared MnFe2O4 nanocrystals with POD-like activity [125]. The result showed that the morphology of the MnFe2O4 could be tune by optimizing the reaction conditions with co-precipitation method, and its OXD-like activity was highly morphology-dependent. Chen et al. fabricated two Pd nanomaterials: nano-cubes and octahedrons [126]. Pd octahedrons with the lower surface energy (111)-faceted had greater intrinsic antioxidant SOD-CAT-like activity than Pd nanocubes with the higher surface energy(100)-faceted (Fig. 12). Cell experiments in vitro also indicated that those Pd octahedrons were more effective than Pd nanocubes at scavenging ROS. The theoretical calculation was consistent with the experimental data, confirming the influence of nanomaterials morphology on enzyme-like activity.
Fig. 12.
a Different morphology of Pd nanocrystals with enzyme-like property. b Different morphology of Pd nanocrystals for cytoprotection [126]
Multifunctional properties of nanozymes
In addition to their enzyme-like activity, nanozymes possess the unique physical and chemical properties of nanomaterials such as optical, electricity, magnetic, thermal, sound etc. Therefore, effectively integrating the catalytic activity and physicochemical properties of nanozymes, not only enhance their enzyme activity but also facilitates many unique new functions, expanding the applications of nanozymes (Fig. 13).
Fig. 13.
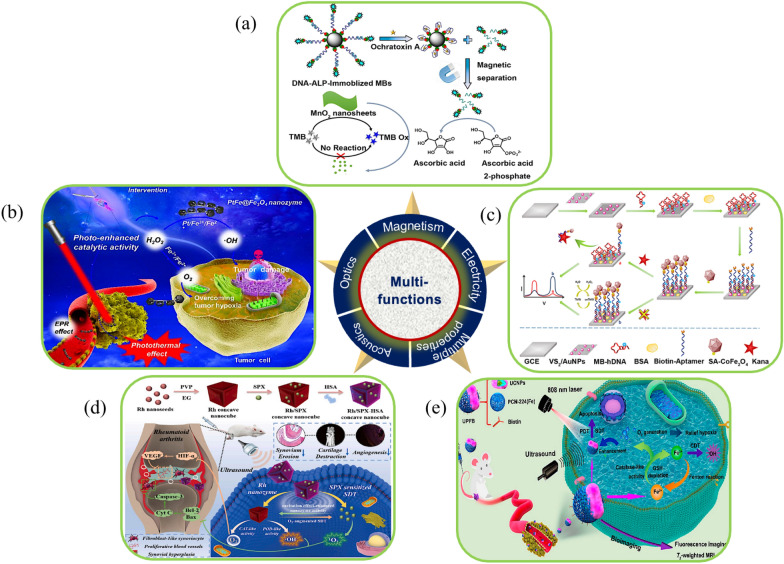
Illustration of functional characteristics of nanozymes. a Nanozyme-based cascade colorimetric aptasensor for detection of ochratoxin A using magnetic separation [127]. b Illustration of the photo-enhanced tumor catalytic therapy for PtFe@Fe3O4 nanozyme [128]. c Illustration of electrochemical biosensor for ultrasensitive kanamycin detection with VS2/AuNPs nanocomposites and CoFe2O4 nanozyme [141]. d Mutual-reinforcing sonodynamic therapy against RAs based on sparffoxacin sonosensitizer doped concave-cubic rhodium nanozyme [130]. e Illustration of Janus nanocomposite architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy combined with precisely guided imaging [131]
Magnetism
Magnetism is a distinctive property of metal-based materials such as Fe3O4, MnO2 and Co3O4 NPs. Magnetic properties of nanozymes can enable the separation recycle, and enrichment of samples or nanozymes by applying an external magnetic field thereby reducing the matrix interference in the system and improving cyclic utilization of nanozymes. Magnetic nanozymes are also used as contrast agents for magnetic resonance imaging (MRI) for real-time imaging of cells or tissues in vivo, improving diagnostic accuracy [127, 132–134]. Moreover, magnetic nanozymes not only can be deliver targeted and aggregate to tumor or other lesion areas, but also can generate thermal effects and are used for targeted hyperthermia of lesion areas under the action of an external magnetic field. Through targeted delivery, imaging, and thermal effects, synergistic diagnosis and treatment of diseases can be achieved using magnetic nanozymes.
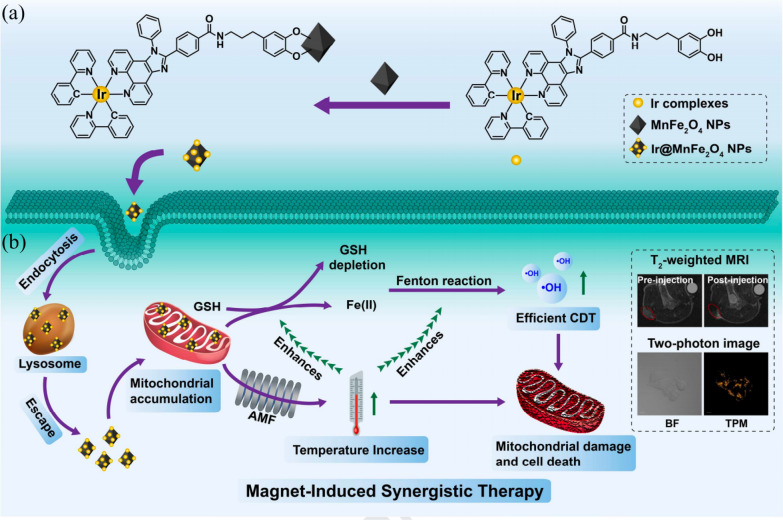
He et al. designed a colorimetric aptasensor for the ochratoxin A (OTA) assay (Fig. 13a) [127]. The biotin-labelled OTA aptamer was immobilized onto streptavidin magnetic bead through the biotin-streptavidin reaction. With OTA binding to its aptamer, the structural switching of aptamer results into the release of the alkaline phosphatase-labelled oligonucleotide, which was partially complimentary to the aptamer. The cascade reaction was initiated through the enzymatic conversion of ascorbic acid-2-phosphate into ascorbic acid after the magnetic separation. The generated ascorbic acid thus reduced MnO2 to Mn2+, accordingly disrupting the OXD-like activity of MnO2. Ultimately, 3, 3’,5,5’-tetramethylbenzidine (TMB) cannot be oxidized by Mn2+ to produce the blue color product (TMB Ox). The color of system faded from blue to colorless with the increasing amount of OTA. By taking advantages of cascade reaction signals amplification and magnetic separation to reduce matrix interference, this colorimetric sensor significantly improved detection sensitivity. Lai et al. synthesized a polydopamine (PDA)-mediated magnetic bimetallic nanozyme (Fe3O4@PDA@Pd/Pt) with POD-like activity [132]. The magnetic property of Fe3O4@PDA@Pd/Pt enabled effective magnetic enrichment of targets, thereby reducing the matrix interference in the sample. Using the nanozymes as a probe in immunochromatographic assay, human chorionic gonadotropin, and Escherichia coli O157:H7 were successfully detected to be as low as 0.0094 mIU/mL in human blood serum and 9 × 101 CFU/mL in the milk sample, respectively. Chen et al. synthesized Fe-coordinated carbon nanozyme dots (Fe-CDs) via hydrothermal reaction [134]. In addition to be applied in detecting glucose of serum due to the POD-like activity, The Fe-CDs as also exhibited good magnetism properties with a magnetization value of 0.33 emu g−1 with paramagnetism. Fe-CDs was further evaluated in vivo with U87MG tumor-bearing mice as T2-MRI contrast agents for tumor imaging. A negative signal intensity in the tumor region was observed. The signal decrease was most obvious at 1 h postinjection and recovered to the prescan level at 4 h postinjection, indicating that Fe-CDs could work as a T2-MRI contrast agent for observation tumors. Chao et al. developed a mitochondria-targeting magnetothermogenic nanozyme (Ir@MnFe2O4 NPs) for highly efficient cancer therapy (Fig. 14) [135]. An iridium (III) complex (Ir) acted as a mitochondria-targeting agent on the surface of MnFe2O4 NPs. On exposure to an alternating magnetic field (AMF), the Ir@MnFe2O4 NPs induce a localized increase in temperature causing mitochondrial damage (MHT effect). Meanwhile glutathione (GSH) reduced Fe(III) to Fe(II) on the NPs surface, which in turn catalyzed the conversion of H2O2 to cytotoxic HO. (CDT). The depletion of GSH increased CDT efficacy, while the localized increase in temperature increased the rate of conversion of both Fe(III) to Fe(II) and H2O2 to HO. further enhancing the CDT effect. In addition, the disruption of cellular redox homeostasis due to CDT, led to greater sensitivity of the cell towards MHT. This nanoplatform integrated these excellent therapeutic properties to enable the precise and effective treatment of cancer.
Fig. 14.
a The synthesis of Ir@MnFe2O4 NPs. b Schematic representation of Ir@MnFe2O4 NPs as mitochondria-targeting magnetothermogenic nanozymes for magnet induced synergistic therapy [135]
Koo et al. designed catalytic antimicrobial robots (CARs) that precisely, efficiently, and controllably killed, degraded, and removed biofilms based on iron oxide NPs [136]. The iron oxide NPs of CARs with dual catalytic-magnetic functionality can generate free radical to kill bacterial and break down the biofilm exopolysaccharide matrix, and remove the fragmented biofilm debris to prevent the regeneration of broken biofilm fragments via magnetic field–driven robotic assemblies. These “kill-degrade-and-remove” CARs systems may fight persistent biofilm infections and mitigate biofouling of medical devices and diverse surfaces.
Optical properties
Most of nanomaterials also have excellent optical properties, especially in terms of fluorescence and near-infrared (NIR) light. The fluorescence properties of nanomaterials, typified by Au, Pt, C, can be widely used in the fields of imaging and detection [129, 137, 138]. The NIR photothermal properties of nanomaterials can enable the conversion of light energy into heat, typified by such as Au, MoS2 NPs. The introduction of the NIR laser to nanozymes not only induce photothermal effects but also enhances their catalytic activities. The synergistic application of multiple functions of enzymes is considered as a promising strategy for anti-tumor and anti-bacterial therapy [128, 139, 140].
Liu et al. constructed PtFe@Fe3O4 nanozyme with both POD-CAT-like activities and NIR photothermal property by assembling multiple uniform Fe3O4 NPs on the surface of the PtFe NRs (Fig. 13b) [128]. Its catalytic activity was significantly enhanced under the NIR laser irradiation. This nanozyme could effectively overcome tumor hypoxia and kill tumor cells with inhibition rate of 99.8% for deep pancreatic cancer combining the photothermal effect. The possible catalytic mechanism was as following. During the catalytic process of PtFe@Fe3O4 nanozyme, Fe3O4|PtFe@Fe3O4 could accept electrons from H2O2 and constantly transport electrons to Pt|PtFe@Fe3O4 as an electronic pump. The electron-rich state of Pt| PtFe@Fe3O4 was thus formed, and H2O2 can easily receive electrons from the surface of Pt| PtFe@Fe3O4 and produced HO· under acidic condition (Formula 1). Meanwhile, electron flowed from Fe3O4|PtFe@Fe3O4 to PtFe|PtFe@Fe3O4 and the exposed Fe3+ stimulated the occurrence of the reaction (Formula 2). Then, Fe3+ reacted with O2−. to generate O2 (Formula 3).
| 1 |
| 2 |
| 3 |
In addition, under the irradiation of the NIR laser, the laser could promote the generation of high-energy electrons in PtFe|PtFe@Fe3O4, and accelerate the excitation of electron–hole, leading to enhance POD-CAT-like activity.
Song et al. designed Au NPs doped Fe-based MOF (GIM) nanozymes [140]. GIM not could protect the GOX-like activity of Au NPs with the satisfactory shield capability, but also possess excellent photothermal conversion ability and unique NIR-enhanced GOX-like activity, which displayed NIR-enhanced cascade nanozyme against hypoxic tumors in vivo.
Conductivity
Nanomaterials, such as Ag, Au, Pt, carbon dots, etc., exhibit excellent conductivity. This characteristic allows them to catalyze the generation of electrons and expedite electron transfer, thereby enhancing the sensitivity of detection as electrochemical biosensors [70, 141, 142].
Cui et al. designed an electrochemical ratiometric aptasensor for Kana quantification based on the signal amplification elements of planar VS2/Au NPs nanocomposites and CoFe2O4 nanozyme (Fig. 13c) [141]. Incorporating VS2/AuNPs nanocomposites with excellent conductivity and high specific surface area, and streptavidin-functionalized CoFe2O4 nanozyme with superior POD-like catalytic activity, the electrochemical signal of the aptasensor revealed a linear relation with the logarithmic concentration of Kana from 1 pM to 1 μM, with the limit of detection (LOD) down to 0.5 pM. This aptasensor can be used for real samples detection and for diverse targets detection by easy replacement of the matched aptamer. Exosomal miRNAs are important and reliable biomarkers for early diagnosis of tumors. Ding et al. designed an electrochemical strategy for ultrasensitive detection of exosomal miRNA based on the cascade primer exchange reaction (PER) with MOF@Pt@MOF nanozyme [142]. The biosensor showed high sensitivity with LOD 0.29 fM and high specificity that can distinguish homologous miRNAs with single base mismatch. It had broad potential in the early screening of tumors.
Acoustics
Some nanomaterials, such as PtCu3 nanocages, Fe3O4 NPs, showed excellent properties of sonosensitizer to generate highly toxic ROS when exposed to ultrasound (US). Moreover, US has the advantage of high penetration up to ≈10 cm away from the skin compared to the limited penetration depth of light, showing great potential to the deep-seated tumors therapy. The developed sonodynamic therapy (SDT) based on nanozyme has emerged as a newly arisen noninvasive therapeutic modality for such as anti-tumor and anti-bacterial infections [130, 143–145].
Li et al. developed a sonosensitizer spafloxacin (SPX) doped and human serum albumin (HSA) loaded concave-cubic rhodium (Rh) nanozyme (Rh/SPX-HSA) platform for the treatment of rheumatoid arthritis (RA) (Fig. 13d) [130]. After accumulating in the inflamed joints, SPX was released from the Rh NPs under US irradiation. concave-cubic Rh NPs could generate O2 and HO⋅simultaneously for its inherent CAT-POD-like activity. The generated O2 in situ can inhibit the expression of HIF-1α, thereby alleviating hypoxia and achieving anti-angiogenesis in the joints. Then, sonosensitizer SPX triggered by US could induce overproduction of O2 in fibroblast-like synoviocyte (FLS). Combined with HO⋅generated from Rh NPs, the elevated levels of ROS in RA could activate the mitochondrial caspases cascade pathway, promoting apoptosis in FLS, and ultimately inhibiting synovial hyperplasia and cartilage destruction. Moreover, the US effect could enhance the catalytic activity of Rh NPs, facilitating the diffusion of H2O2 and increasing the interaction between H2O2 and Rh NPs, thus accelerating the generation of ROS. In return, Rh-induced self-supply of O2 further enabled the interaction of SPX and O2, leading to the production of high-efficiency O2, which also boosted the efficiency of SDT. This novel nanocomposite integrating Rh nanozyme and SPX sonosensitizer provided potentials for highly potent RA therapy. Given the ineffective accumulation of sonosensitizers at disease sites, the hypoxic microenvironment during SDT, and the rapid depletion of O2, all of which significantly hinder the therapeutic efficacy of sonodynamic therapy, Zheng et al. developed a Pd@Pt-T790 nanotherapeutic platform by modifying the Pd@Pt nanoplates with organic sonosensitizer meso-tetra(4-carboxyphenyl)porphine (T790) via a PEG linker [144]. As a US-switched nanozyme system, Pd@Pt could control generation of catalytic O2 and sonosensitizer-mediated ROS during US activation, thereby alleviating hypoxia-associated barrier and augmenting SDT against deep-seated bacterial infection. This nanozyme system was successfully applied to eradicate methicillin-resistant Staphylococcus aureus induced myositis, and its progress was non-invasively monitored using photoacoustic and MRI. Some metal nanomaterials, especially noble metal nanomaterials (e.g. Au, Ag, Pt, Ir) also show excellent photoelectric performance such as surface enhanced Raman scattering and surface plasma resonance, which can achieve real time and highly sensitive detection [146, 147].
Multiple effects
In addition to theses, some nanozymes exhibit multiple physical and chemical properties to achieve such as synergistic chemodynamic therapy (CDT), photodynamic therapy (PDT) and photothermal sonodynamic therapy significantly enriching the functional applications of nanozymes [131, 148–150].
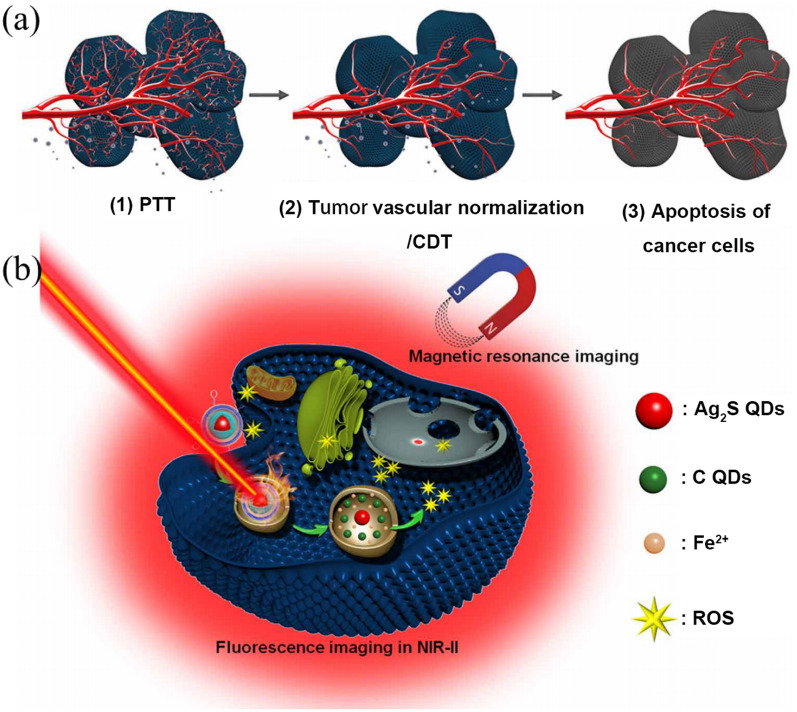
Lin et al. designed and constructed a US driven SDT and NIR mediated PDT strategy by fabricating a biocatalytic Janus nanocomposite (denoted as UPFB composed of (NaYF4:20%Yb, 1%Tm@NaYF4:10%Yb@NaNdF4) and porphyrin-based MOFs [PCN-224(Fe)] (Fig. 13e) [131]. UPFB can be synchronously activated by 808 nm laser and US. Due to the long distance between UCNPs and porphyrin-based MOFs, the energy transfer between the two kinds of NPs was inefficient with only NIR laser was used. ROS generation from UPFB was sequentially increased with the assistance of US driven SDT. Additionally, the high CAT-like activity of Fe3+ ions coordinated in the UPFB can not only catalyzed H2O2 to O2 for relieving hypoxia in the TME, but also consume excess intracellular GSH to reduce the impact on the generated ROS. Both these processes greatly enhanced the ROS generation, thereby causing oxidative damage tumor cells and to induce them death. Additionally, Fe2+ reduced from Fe3+ can consume intracellular H2O2 to generate HO· under acidic conditions, inducing apoptosis of tumor cells. The UPFB also showed good biocompatibility and targeting ability, which can rapidly enter many types of cancer cells (e.g., HeLa cells, U14 cells, 4T1 cells) after modified with ethanediamine. Moreover, UPFB can be used as a better contrast agent for fluorescence and T2 in vivo-Weighted MRI due to porphyrin ligands and Fe2+. These imaging methods could directly reveal the accumulation, process distribution and metabolism of UPFB at the tumor site. Combined with precise guided imaging, GSH depletion, and targeted CAT-like ability, UPFB can significantly enhance its synergistic therapeutic effect in cancer treatment. Hou et al. designed a coreshell Ag2S@Fe2CDSPEPEGiRGD nanozyme system by coating a tumor-homing penetration peptide–modified distearoyl phosphoethanolamine-PEG-iRGD peptide (DSPE-PEG-iRGD) on the surface of Ag2S@Fe2C NPs for theragnostic of breast cancer with a synergetic enhancement strategy (Fig. 15) [151]. This nanozyme exhibited excellent photothermal properties. When laser irradiation was applied to Ag2S@Fe2C-DSPE-PEG-iRGD–injected mice, the local temperature of the tumor site rapidly increased from 37° to 54.7 °C within 5 min, demonstrating the superior targeting capability, which was consistent with the above results of bioimaging. The nanozyme also generate ROS efficiently under the stimulation of TME. Additionally, it possessed remarkable imaging performance for both fluorescence imaging in the second NIR region and MIR for visualization tracing in vivo. A significant therapeutic effect was proved by the treatment of 4T1 breast cancer–bearing mice in vivo. The tumor of harvested mice injected with the nanozyme and bevacizumab under the laser irradiation (808 nm, 0.3 W cm−2) was completely eradicated after treatment about 30 days. And an obvious damage was evidenced to the tumor cells of mice by cell necrosis and apoptosis, which provided a new therapeutic strategy by the cooperation between catalysis of imaging-guided nanozyme and tumor vascular normalization for intensive combination therapy of breast cancer.
Fig. 15.
Scheme of the combination therapeutic strategy between visualization nanozyme and tumor vascular normalization for breast cancer. a Schematic illustration of combination therapeutic strategy. b Schematic diagram of biochemical process for multifunctional Ag2S@Fe2C-DSPE-PEG-iRGD in breast cancer cell. PTT, photothermal therapy [151]
Catalytic mechanisms of nanozymes
Exploring the catalytic mechanisms of nanozymes can provide support for understanding their mechanism of action and the design of high-performance nanozymes. This section mainly summarized the progress in the catalytic mechanisms of several typical enzyme-like activities.
POD-like activity
As the earliest confirmed enzyme-like activity, POD-like activity has been discovered in various nanomaterials including metal-based, and carbon-based nanozymes. Similar with natural POD, the catalytic pathway can be divided into ROS production and electron transfer process. H2O2, one of the substrates, first adsorbed onto the surface of nanomaterial. The O–O bond of H2O2 was then cleaved into two HO., which interacted through electron exchange to be stabilized by the nanomaterial. The generated OH· further oxidized the other substrate, leading to the formation of intermediate species, producing a typical color. Nanozymes such as iron metal-based nanozymes (e.g., iron oxide NPs), carbon-based nanozymes (e.g., graphene, and graphene) all followed the ping-pong mechanism and Michaelis–Menten kinetics, similar with Fenton-like reactions.
Qu et al. further explored the catalytic mechanism of GQDs with POD-like activity. They found that the − C = O and − OCO − groups could serve as the catalytic activity site and substrate-binding site, respectively. The modification of these groups might effectively improve the catalytic activity [152].
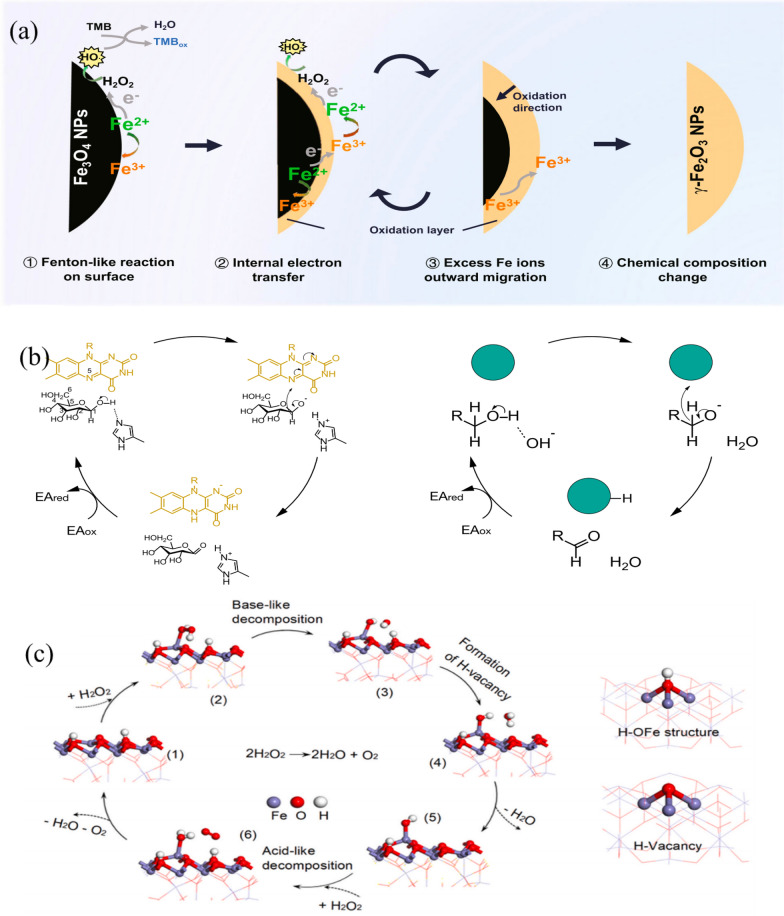
It is generally speculated that since catalysis primarily occurs on the surface or at the interface of nanomaterial, only the surface active sites play a decisive role in the enzyme-like property of nanozymes. Zhang et al. first revealed the consumption of surface active sites and the migration of internal electrons and Fe3+during the POD-activity of Fe3O4 nanozymes using cyclic catalysis experiments, proving the significant contribution of internal components of nanozymes to catalytic reactions [153].
As shown in Fig. 16a. the catalytic mechanism of Fe3O4 NPs can be summarized as follows: (1) Fenton-like reaction on the surface. H2O2 first adsorbed on the surface of NPs accepted electrons from the surface Fe2+, and then dissociated into highly active HO· to oxidize the substrates. The surface Fe2+ was oxidized to Fe3+. (2) Internal electrons transfer. The adjacent Fe2+ inside the surface transferred its electron to the surface Fe3+ via the Fe2+-O-Fe3+ chain, retrieving the surface Fe2+ and providing the dynamics for the sustained catalytic reaction. (3) Excess Fe ions outward migration. With the in situ oxidation of internal Fe2+, to maintain electroneutrality, the excess Fe3+ in the lattice had to migrate outward to the surface, leaving cation vacancies. (4) Chemical composition change. With the continuous POD-like catalytic reaction, Fe3O4 NPs were oxidized from the surface to the interior and finally transformed into γ-Fe2O3 NPs. This enzyme-like reaction-triggered oxidation process of Fe3O4 NPs was thought to be analogous to the conventional low-temperature air oxidation of magnetite, in which iron ion migration was probably a rate-limiting step.
Fig. 16.
a Schematic diagram of the catalytic mechanism of the POD-like activity for Fe3O4 NPs. The catalytic POD-like reaction of Fe3O4 NPs occurs along with internal electron transfer and excess Fe ions migration. After prolonged catalysis, Fe3O4 NPs suffer the phase transformation to γ-Fe2O3 NPs with depletable POD-like activity [153]. b Mechanism of glucose oxidation catalyzed by GOX (left) and noble metal NPs (right), respectively [159]. c Proposed mechanism of CAT-like catalysis of ferrihydrite [161]
Some nanozymes with POD-like activity especially noble-metal nanozymes, such as Au, and Pt NPs, did not follow Fenton reaction mechanism. These nanozymes did not undergo a change in the valence state of metal elements in catalytic reactions, but instead exerted their catalytic effects through the adsorption, activation, and electron transfer of catalytic substrates on their surfaces. The substrate could be oxidized directly without producing OH· [154, 155].
OXD-like activity
Some nanomaterials (e.g., CuO, CeO2 and Au NPs) have the ability of oxidizing organic substrates (electron donors, e.g., Ared) with O2 ((electron accepter) via three main pathways to generate H2O H2O2 and 2O2·− ((Formula 4–6), exhibiting OXD-like catalytic functions, as shown in Table 2.
Their catalytic activity strongly depends on parameters such as temperature, pH and substrates, and also shows typical Michaelis–Menten behavior. The catalytical pathway of nanozymes with OXD-like activity includes electron transfer and reactive species generation. In electron transfer process, nanozymes act as the recipient and transfer of electrons. For the Au NPs with OXD-like activity, it was speculated that the hydrated glucose anion was first adsorbed onto the Au surface to form electron rich gold species, subsequently activating dissolved oxygen by a nucleophilic attack and generating a dioxo Au intermediate to transform electrons from glucose to dioxygen [45, 156]. For reactive species generation, free radicals originate from either oxygen molecules or substrates. Some reports identified that free radicals came from activated dissolved oxygen. Nanozymes with large specific surface areas could adsorb and activate massive dissolved oxygen to produce ROS intermediates, which were responsible for substrate oxidation [157, 158].
Dong et al. recently studied in detail the process of glucose oxidation catalyzed by Au NPs using ABTS+. instead of O2 as an electron acceptor, and proposed a possible GOX-like mechanism (Fig. 16b) [159]. As for GOX, glucose was first activated by a close His residue (as a Brønsted base) to remove the C1 hydroxyl proton to form an intermediate. The removal of a hydroxyl proton from glucose facilitated hydride transfer from the C1 of glucose to the isoalloxazine ring of flavin adenine dinucleotide (FAD). Then, a direct hydride transfer occurred from the C1 position in glucose to the N5 position in FAD to produce FADH−. FADH− was very sensitive to air and quickly oxidized by O2 to produce H2O2. The reaction path of Au NPs catalysis was the same as that of natural enzyme, except that OH− was used as a Brønsted base to abstract H+ from glucose. Other noble metal NPs with OXD-like activity had the same catalytic process, except that O2 was preferable to be reduced to water. In addition, noble metal NPs can catalyze electron transfer from glucose to other electron acceptors [159].
CAT-like activity
Many nanomaterials show CAT-like activity, which can catalyze H2O2 to H2O and O2. The catalytic process of CAT-like mainly involves adsorption activation and redox reactions. For metal-based nanozymes, their catalytic mechanism is closely related to radical chain reactions. Pre-adsorbed OH* (* denotes species adsorbed on the metal surface) plays a key role on the nanozymes surface, which can cause H2O2 to tend to undergo acidic decomposition. Then the decomposed H reacts with the pre-adsorbed OH* to produce HO2* and H2O*. Subsequently, the generated H2O* transfers H to another H2O2*, leaving O2* and converting H2O2* into H2O* and OH* [47, 160].
The CAT-like activity is closely associated with environmental pH, nanomaterials morphology, structure, and surface valence. Iron oxide NPs exhibited the POD-like activity under acidic conditions (pH 4.8), but exhibited CAT-like activity under neutral and alkaline conditions. The catalytic mechanism was speculated to the Fenton-based reactions under acidic conditions [72]:
| 4 |
| 5 |
| 6 |
While at higher pH, the reaction rate of Formula (4) was higher, leading to overproduction of FeOOH2+ and HO2·, the latter can then be ionized into O2− (Formula 7). The generated HO2·/O2− reacted with HO· to O2 (Formula 8).
| 7 |
| 8 |
Fan et al. found that the CAT-like activity of ferrihydrite was superior to other iron oxide nanomaterials, and the Fe-OH groups may have a dominating effect on the CAT-like catalytic activity of ferrihydrite. Both experiments and theoretical calculations (structure–activity fitting and density functional analysis) revealed that the abundant surface iron-associated hydroxyl groups dominantly affected the CAT-like activity of ferrihydrites. The mechanism of CAT-like catalysis of ferrihydrite was presented as Fig. 16c. There were 5 steps from the adsorption of the first H2O2 to the formation of O2, including 4 stable states and 1 transition state in the proposed reaction pathway. After an H2O2 molecule was adsorbed on H-terminated ferrihydrite, the iron-bound OH groups (H-OFe) on the surface of ferrihydrite first broke the O–O bond of an H2O2 molecule through base-like decomposition. One of the dissociated OH group bound to H-OFe forming a H2O* molecule and a H-Vacancy, and another dissociated OH group bound to the 3-coordinated iron atom forming HO* group. After the escape of H2O*, there was a OH* group and a H-Vacancy left with CAT activity in catalytic center, which can easily combine with H atoms obtained from the acid-like decomposition of H2O2. When the second H2O2 molecule was close to the catalytic center, a H2O*, a O2*, and a surface H-OFe group were generated as products. This study revealed the structure–activity relationship of ferrihydrite CAT-like activity through experiments and theoretical calculations, providing a good platform for comprehensively exploring the catalytic mechanism of nanozymes.
SOD-like activity
Nanomaterials (e.g., Pd, MnO2, CeO2) have been discovered to exhibit excellent SOD-like activity, and considered as promising alternatives to SOD. The SOD-like activity nanozymes depends on such as pH, the structure and surface ions of nanomaterials, and the kinetics follows a ping-pong mechanism. When nanozymes possess multi-enzyme-like activity, the SOD-like activity can be made the dominant activity by adjusting these parameters [162].
CeO2 NPs are currently the most used with SOD-like activity. The activity is mainly derived from the convertible valence state (Ce3+ and Ce4+) and oxygen vacancies. Due to the change of the valence of Ce3+ and Ce4+ (Formula 9, 10), CeO2 can form oxygen vacancies or defects in the lattice structure by losing oxygen or electrons.
| 9 |
| 10 |
The defects compensated oxygen vacancies by oxygen vacancies are enriched at the surface of NPs. The superoxide adsorbed at the oxygen vacancies further reacts with protons, leading to the leave of H2O2 from the surface [163].
Other metals (e.g., Pt, Mn, Ni, Co, Fe) and their oxides, sulfides also showed SOD-like activity. The catalytic process includes the protonation of O2−· and the adsorption and rearrangement of HO2· on the metal surface. And the catalytic process can be described that O2−· can easily capture protons in water, forming HO2· and OH−. The HO2· adsorption on the surface of metals is a highly exothermic process that may occur, which the potential energy distribution for rearrangement of HO2·on surfaces is very low. This indicates that once HO2· is adsorbed on the surface, it can easily transform into O2* and H2O2*, which then into O2 and H2O2 [164].
Gao et al. used density functional theory (DFT) calculations to study the thermodynamics and kinetics of the catalytic processes of nanozymes with SOD-like activity [165]. They developed and verified a new energy level principle and adsorption energy principle for the SOD-like activity of nanomaterials on the basis of their electronic band structures and surface adsorption energies, respectively. The energy level principle revealed the critical role of the intermediate frontier molecular orbital (iFMO), which was defined as the FMO of the nanomaterials with energy located in between φ1 and φ2, where φ1 and φ2 were the potentials of the half-reactions of O2−· dismutation, in transferring electrons for catalysis. The adsorption energy principle quantitatively described the competition between the target catalytic reaction and possible side reactions for O2−· on the catalysts. The ability of the principles to predict the SOD-like activities of MOF were also verified by experiments, which provided not only and in-depth insight into the mechanisms of the SOD-like activity of NMs but also a general guide for the computational design and screening of SOD-like nanomaterials.
Applications of nanozymes
With the continuous expansion of catalytic types and the sustained enhancement of catalytic activity, research on the applications of nanozymes are increasingly broad and profound. Based on keyword analysis, research on the applications of nanozymes primarily focuses on the field of chemical detection, biomedicine, and ecological environment, mainly including sensing detection, anti-tumor, anti-microbials, environmental protection (Fig. 17, 18).
Fig. 17.
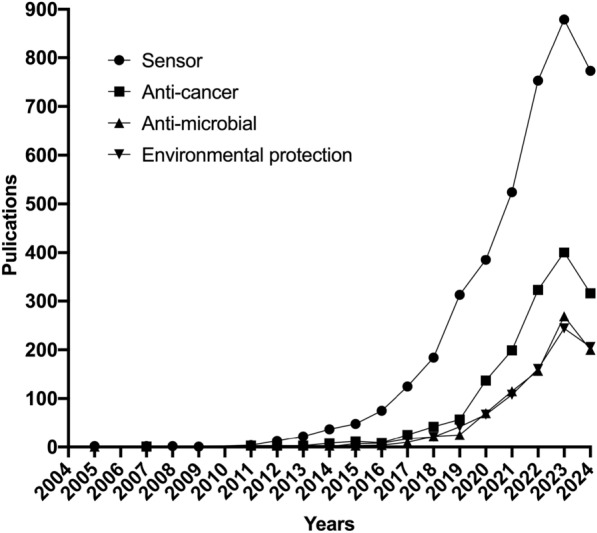
Trends in the publication and distribution of applications of nanozymes
Fig. 18.
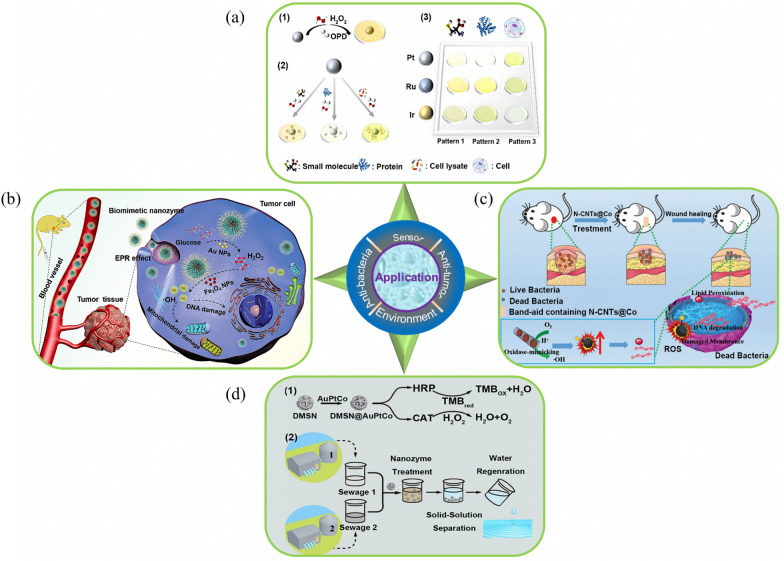
Illustration of the application of nanozymes. a Nanozyme sensor arrays for detecting versatile analytes by Pt, Ru, and Ir nanozymes [168]. ((1) Catalytic oxidation of ophenylenediamine (OPD) in the presence of POD-like nanozymes; (2) Catalytic oxidation of OPD in the presence of nanozyme with small molecules, proteins, and cells. (3) Pattern-based recognition of small molecules, proteins, and cells)). b Illustration of “toxic-drug-free” noncatalytic tumor therapy by biomimetic inorganic nanomedicine-triggered cascade catalytic reaction [71]. c Illustration of bamboo-like nanozyme based on N-doped carbon nanotubes encapsulating cobalt nanoparticles for wound anti-bacterial [169]. d Illustration of the DMSN@AuPtCo nanozyme for water decontamination [167]. ((1) General procedure for the preparation of DMSN@AuPtCo possessing HRP and CAT like activities; (2) Treating two kinds of wastewater together with the nanozyme))
Sensing detection
Sensing detection with about 4142 research papers, are the most widely used application fields of nanozyme. A variety of sensing technologies and methods, ranging from static detection to real-time dynamic monitoring, spectrum to visualization and imaging, in vitro to in vivo, single mode to multimodal detection and so on, have been developed. And the detection targets range from small molecule (e.g., H2O2, glucose, lactate, dopamine, urea, ascorbic acid, acetylcholine, phosphates, cholesterol, metal ions), nucleic acid, protein (e.g., acetylcholinesterase, HSA, Cyt, EGFR, human thrombin), virus (e.g., Ebola, hepatitis E virus), bacteria (e.g., vibrio cholera, sticholysin I, sulfate reducing bacteria, Escherichia col) to cell (e.g., A2780, Hep-G2, MCF-7, Hela, 4T1). These methods have practically applied to biochemical detection, diseases diagnostics, infectious disease control, environmental monitoring, and food safety [5, 21, 166–168].
Yan’ s group reported the first the versatile applications of ferromagnetic NPs with POD-like activity in sensing detection including medicine, biotechnology and environmental chemistry [5]. Shen et al. fabricated a Pd/GDYO nanocomposite with excellent POD-like activity [123]. Based on the shielding effect of GSH on the sensor system, a colorimetric detection method for GSH was developed with a wide linear range from 0.1 μM to 40 μM and a LOD of 0.1 M. The method was also successfully applied for fast and accurate detection of GSH in injection powder drugs. Wang et al. constructed a nanozyme sensor array for ratiometric fluorescence detection of exosomal proteins by using aptamer-modified C3N4 nanosheets together with a solvent-mediated signal amplification strategy [166]. The LOD for exosome was 2.5 × 103 particles/mL. In addition, the accurate identification of cancer can be achieved by machine learning algorithms to analyze the difference of exosomal proteins from different patients’ blood, which was a promising tool in the field of cancer diagnosis. At the level of cell detection, Wang et al. synthesized nanoflower-shaped Au nanostructures for the colorimetric assay of cancer cells [167]. Upon incorporation of 808 nm laser irradiation, high sensitivity and selectivity for the cancer cell assay were achieved with the lowest detection level of 10 cells/mL. Wei et al. constructed a sensor array based on POD-like Pt, Ru, and Ir nanozymes for detecting versatile analytes from small molecules to proteins and cells (Fig. 18a) [168]. Six biothiols, nine proteins and five cancer cells were successfully discriminated. The practical application of the nanozyme sensor arrays was successfully applied in discrimination of biothiols in serum and proteins in human urine.
Anti-cancer therapy
Nanozymes have also been explored for treating various diseases. Especially, anti-cancer (anti-tumor) therapy has emerged as the second most widely explored field for the application of nanozymes. On the one hand, nanozyme can serve as therapeutic agents, directly killing tumor cells or inhibiting tumor growth through their enzyme-like activity. It can also improve the TME through catalysis and regulation of the ROS system such as catalyze the production of O2 to alleviate the hypoxic environment of tumors and assist traditional tumor therapy.
On the other hand, based on the unique physicochemical properties of nanomaterials, they can combine various therapeutic approaches, including chemotherapy, radiotherapy, photothermal therapy, photodynamic therapy, SDT, and immunotherapy, to synergistically enhance anti-tumor therapeutic effects. More than 10 types of cancer (e.g., lung cancer, liver cancer, gastric cancer, colon cancer, breast cancer, pancreatic cancer, esophageal cancer) based on in vitro cell and in vivo animal experiments have been studied to date [26, 71, 86, 98, 121, 128, 145, 151, 170–172].
Dong et al. designed a multifunctional TADI-COF-Fc nanozyme with excellent SOD-POD-LOX-GPX-NDH-like activities. Combination with the radiotherapy and multi- enzyme-like activities, this nanozyme exerted a high degree of lipid peroxidation and ferroptosis, ultimately resulting in increased cancer cell death and tumor inhibition [98]. Hou et al. developed a synergetic enhancement strategy through the combination between nanozyme and tumor vascular normalization to destruct tumors, which showed remarkable therapeutic effect for breast cancer [151]. Shi et al. constructed a nanozyme-based nanoplatform for tumor-specific therapy with marked therapeutic efficacy based on DMSN-Au-Fe3O4 composite with bi-enzyme-like activity (Fig. 18b) [71]. The AuNPs in DMSN-Au-Fe3O4 with GOX-like activity specifically could catalyze β-D-glucose oxidation into gluconic acid and H2O2 under aerobic conditions, while the coloaded Fe3O4-based with POD-like activity could catalyze the produced H2O2 to HO., which substantially triggered the death of tumor cell. Both the in vitro cell-level and in vivo tumor-bearing mice xenograft experiments demonstrated the efficient tumor therapy on killing the cancer cells and suppressing the tumor growth with high as 69.08% of inhibition rate. Lu et al. f developed an efficient redox Fe-MoOv nanozyme by leveraging a defect engineering strategy, which could induce substantial disruption of redox and metabolism homeostasis in the tumor region via enzyme-mimicking cascade reactions under NIR-II laser irradiation, thus significantly augmenting therapeutic effects [171]. Zhao et al. designed SFO nanozyme for synergistic cancer therapy [86]. SFO nanozyme with GPX-CAT-like activities could produce HO. in situ for CDT and the consumption of GSH to relieve antioxidant capability of the tumors in the TME. Meanwhile, the nanozyme catalyzed H2O2 to produce O2 for improving the tumor hypoxia and facilitating better PDT. Furthermore, SFO exhibited a prominent phototherapeutic effect with excellent enhanced photothermal conversion efficiency (η = 42.3%) and highly toxic free radical (HO. and O2-.) production performance when exposed to 808 nm laser. Integrated with multiple treatment modalities, computed tomography, and MRI properties, and persistent modulation of TME, this nanozyme exhibited excellent tumor theragnostic performance. There was no apparent inhibitory effect on the tumor growth with saline or NIR laser irradiation alone (Fig. 19b). When SFO or SFO/Vc combined with NIR lase irradiation alone, partial tumor growth was inhibited. Once SFO plus NIR laser irradiation on mice with magnetic field assistance, tumor growth was significantly inhibited and eliminated. The toxicity evaluation of the nanozyme showed that the body weights of the mice in each group did not significantly decrease during the treatment period, indicating a negligible toxicity of SFO (Fig. 19c). It also can be found the difference in the tumor mass was consistent with the relative tumor volume growth curves after the mice were euthanized and the tumors from various treatment groups were collected (Fig. 19d).The photographs of excised tumors from mice in each group (Fig. 19e) showed that the SFO had excellent treatment efficacy. Hematoxylin and eosin (H&E) staining of tumor sections from the tumor-bearing mice in different treatment groups (Fig. 19f) showed the tumors of mice injected with SFO were completely eradicated by exposure to 808 nm laser plus magnetic field assistance at the end of treatment administrated, compared to the other group with mild damage or small necrotic areas. And the tumor tissue showed significant atrophy and damage, manifested as cell necrosis and apoptosis, which confirmed that SFO possessed highly efficient anti-tumor capacity.
Fig. 19.
In vivo synergistic anti-cancer efficacy with SFO nanozyme. a Schematic illustration of the in vivo anticancer treatment procedure on tumor-bearing mice. b, c Relative tumor volume growth curves and body weight changes of mice after various treatments during the therapy (***p < 0.001, **p < 0.01, or *p < 0.05). d Average weights of tumors collected from different treatment groups. e Representative photographs of excised tumors and corresponding mice collected from different treatment groups. f H&E staining of tumor sections from the tumor-bearing mice in different treatment groups. The yellow and red arrows represent ffbroblast and inffammatory cells. Scale bar: 100 µm [86]
Nanozymes are also utilized in the treatment of other diseases such as neurological disorders (e.g., Parkinson's, Alzheimer's disease), acute gout, ischemia–reperfusion injury and brain injury [77, 84, 97, 173–175]. It should be noted that current diseases therapy based nanozyme is limited to the laboratory stage, targeting cellular and experimental animal models, with no clinical reports as of yet.
Anti-microbial
Nanozymes can also mimic the catalytic killing mode of the innate immune defense system against infectious pathogens, and are widely used in treatment of various infections caused by such as bacteria viral, and fungal pathogens. Nanozymes exert their action on anti-bacterial through two primary mechanisms. One is interact with bacterial cells, leading to direct damage to the cell membrane or wall. The other involves the generation of ROS, which can oxidative damage cell membranes, proteins, and nucleic acids, leading to the elimination of pathogenic microorganisms [14, 113, 169, 176–178].
The drug-resistance and poor treatment efficacy are a typical challenge faced by anti-microbial therapy. Zhang et al. prepared N-doped carbon nanotubes encapsulating cobalt nanoparticles (N-CNTs@Co) that could catalyze O2 to produce ROS [169]. The bacterial membrane was damaged by the attack of ROS, which induced the peroxidation of lipid and the degradation of DNA, eventually causing bacterial death (Fig. 18c). Moreover, N-CNTs@Co did not cause the bacteria to develop resistance. After 20 days of antibacterial experiment, two representative bacteria, Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) still did not develop adaptability. Wang et al. designed and synthesized CuL/PHI nanozyme through adjusting the spatial positioning of Cu single atom site [178]. Its photo-catalytic antibacterial activity against methicillin-resistant Staphylococcus aureus was ≈100%, similar to anti-bacterial effects as antibiotics. The experiments of healing ability of skin wound infection indicted that the nanozyme had good wounds healing. Moreover, it had no obvious bio-toxicity. Zhao et al. constructed a p-Cu nanozyme by modifying the surface of Cu nanozymes with polyethyleneimine, which exhibited excellent anti-fungal activity against F. solani, significantly superior to commonly used clinical anti-fungal drugs (voriconazole and natamycin) [179]. Gao et al. designed a peptide-assembled nanozyme with excellent antifungal property through de novo strategy and peptide assembly [180]. The heptapeptide of IHIHICI can form an ordered nanotube structure by self-assembly (Ni–H-7), which not only readily bind with mannan in the outer wall and disrupt membrane integrity of Candida albicans (C. albicans), but also acted as phospholipase C (PLC)-, and POD-like activitives. The assembled Ni–H-7 nanozyme could kill > 90% C. albicans within 10 min on disinfection pad through cascade synergistic antifungal actions including outer mannan docking, wall disruption, lipid peroxidation and subsequent ferroptotic death (Fig. 20a).
Fig. 20.
a Schematic diagram of multi-antifungal actions model of Ni-IH-7 nanozyme [180]. b Schematic of Ag-TiO2 SAN with anti-SARS-CoV2 activity [181]
Based on the unique advantage of nanozymes in anti-microbial therapy, Gao et al. first proposed the concept of “nanozybiotics” to define antimicrobial nanozymes, which will be a promising new generation of anti-microbial candidates [183, 184]. The exploration of nanozymes in anti-viral therapy has also attracted growing attention due to the frequent occurrence of a pandemic such as SARS-CoV2 in recent years. It can address the great challenges faced by natural enzyme-based therapies, such as high cost and storage difficulties. Nie et al. designed a novel TiO2 supported Ag atoms nanozyme (Ag-TiO2 SAN) for resisting and clearing SARS-CoV2 [181]. The Ag-TiO2 SAN had a high absorption ability for spike 1 (S1) protein of SARS-CoV2, which could effectively inhibit the interaction between receptor binding domain of S1 protein and its receptor hACE2. Once binding to the virus, the SAN/virus complex was phagocytosed by macrophages and colocalized with lysosomes. Ag-TiO2 SAN with high POD-like activity could produce high level of ROS, and lysosome further accelerate oxygen reduction reaction process, thereby leading to significantly eliminate the virus, and protect host cells from infections (Fig. 20b). In addition, gram-scale SANs can be produced via an energy- and cost-efficient approach, providing new insight for employing anti-SARS-CoV2 therapy. Qin et al. designed and synthesized a cold-adapted nMnBTC nanozyme, which not only exhibited excellent OXD-like activity at 0 °C, but also retained almost no loss of activity even when the temperature increased to 45 °C [182]. Based on the robust performance of nMnBTC, they developed a low-temperature antiviral strategy to inactivate influenza virus H1N1 even at − 20 °C, paving a novel way for biomedical application in cryogenic fields.
Environmental protection
The development of efficient, stable, and cost-effective catalysts for the detection and degradation of pollutants in environment holds significant importance for environmental protection and human health. Nanozymes have been demonstrated substantial potential in environmental protection such as pollutant detection and remediation. In the field of environmental protection, nanozymes are applied in the following areas. Firstly, they can be utilized for the sensitive, rapid, and visual detection or monitoring of metal ions (e.g., Pb, Hg, Cu, Cr, Al, As) [185–192], pesticides (organophosphorus), antibiotic (e.g., kanamycin, penicillin, streptomycin), neurotoxins (sarin) and other organic pollutants [193–198] in water based on the color changes induced by the direct or indirect interaction between nanozymes and substrates. Secondly, they also can be employed for the efficient catalytic degradation of environmental pollutants (e.g., phenol, aniline, Rhodamine B, methylene blue, 2,4-DP, tetracycline, and xylenol orange) [199–209] based on their enzyme-mimetic catalytic activity and highly effective pollutant adsorption capacity.
He et al. prepared CH-Cu nanozyme with laccase-like activity for the degradation and detection of phenolic pollutants [199]. Compared with laccase, CH-Cu nanozymes exhibited higher degradation efficacy chlorophenols and bisphenols in addition to the relatively low cost, high catalytic activity, excellent stability, recyclability, and substrate versatility. Yu et al. synthesized an artificial-natural bi-enzyme by grafting a Pt nanozyme onto the body of natural laccase [207]. The enzymatic activities of artificial-natural bienzyme with and without NIR irradiation were, respectively, 46.2 and 29.5% higher than that of free laccase. Moreover, the reversible catalytic activity of the coupled enzyme could be manipulated with and without a near-infrared light at 808 nm. Compared with laccase, the degradation rates of methylene blue catalyzed by the nanozyme were significantly higher. Yin et al. synthesized Os-citrate nanozyme, which possesses complex dual-valent osmium(0/IV) characteristics and exhibited excellent peroxidase-like specific activity [208]. Based on the efficient production of HO., this Os-citrate nanozyme could be a highly efficient and stable catalysts for the degradation of six phenolic pollutants. The maximum degradation efficiency of six phenolic pollutants can reach 100% within 30 min. This is the first report on the application of Os-based peroxidase-like nanozymes in highly efficient degradation of organic pollutants. Qu et al. constructed a DMSN@AuPtCo consisting of Au/Pt/Co tri-metal as a water purifier for decontaminating organic wastewater [209]. Au/Pt/Co tri-metal clusters were confined in a silica NPs scaffold (Fig. 18d), the nanozyme showed excellent POD-CAT-like activities. and high stability and well-conditioned tolerance. Besides, the nanozyme has a huge advantage of treating different sewages in one system, which could effectively remove organic pollutants from organic wastewater and completely remove the excess H2O2 in sewage without using extra resources and energy. This integrated processing mode avoided the secondary pollution and greatly improved environmental protection efficiency and safety.
In addition to the mentioned above, nanozymes are also utilized in biocatalytic transformations, enzymatic biofuel cells, energy conversion and so on [209–214]. Zhu et al. designed a novel photo-switch for enzymatic biofuel cells based on the photo-oxidation of TMB in the cathode catalyzed by C-dots nanozymes [210]. After the 20 min of blue light irradiation, the current of the enzymatic biofuel cells equipped with the photo-switch approximately increased from 2.3 to 9 μA, and the power density increased from 2.8 to 35 μW cm−2, exceeding over the previous reported performance. Zhou et al. designed a heme-based nanozyme as electrolyte additive to scavenge superoxide radicals in the Li-O2 battery system [213]. The nanozyme can serve as a bifunctional catalyst for discharge and charge by coordinating with superoxide intermediates, acting as a molecular shuttle of superoxide species and electrons between cathodes and products. Therefore, the Li-O2 batteries exhibited high discharge capacity, lower charge polarization and excellent cycling stability in the presence of the nanozyme additive.
Conclusion and outlook
In this article, we conducted a thorough bibliometric analysis of scientific publications in the field of nanozymes research, spanning from 2004 to 2024 for the first time. The research progress, hotspots trends and applications in nanozymes research were systematically mapped and reviewed. It is expected to provide reference for a deeper understanding of its future development.
While significant breakthroughs on nanozymes have been made in recent years, the field still faces several challenges that need to be addressed.
(1) Efficient synthesis of nanozymes
Although more than thousands of nanozymes have been developed, the current methods for their design and synthesis often rely on traditional empirical and trial-and-error approaches, which were no longer sufficient to meet the requirements of high performance nanozymes with the diversification and complexity of its applications. Therefore, transforming nanozymes from current empirical science to a science based on theory and solid foundations is key to solving one of the problems. Considering the rapid development of technologies such as big data analytics, theoretical computing, machine learning, and artificial intelligence, as well as their intersection and integration with nanozymes research, it is suggested to build a database for the relationship between the structure of nanozymes and the catalytic performance, and use rational design tools to predict the activity of nanozymes. Efficient synthesis and preparation of nanozymes based on theoretical guidance will provide new approaches for addressing the problem [215–217].
(2) Precise elucidation of the catalytic mechanism of nanozymes
The catalytic mechanism of nanozymes is currently not thoroughly understood. The catalytic activity of nanozymes is influenced by various factors, including material size, shape, composition, surface chemistry, and functional groups. Elucidating the catalytic mechanism and understanding these factors how to affect catalytic activity accurately are crucial for optimizing the performance of nanozymes and expanding their applications. By utilizing techniques including experimental instruments such as spectroscopy and microscopy, as well as theoretical methods such as DFT, computing and data-driven approaches, the reaction pathways, intermediates, rate-determining reaction energy barrier, adsorption energy on the nanozyme surface, and other intrinsic parameters steps involved in nanozyme catalysis process, should be investigated, which enables a deeper insights into the catalytic mechanism of nanozymes at the atomic and molecular level.
(3) Improvement of the specificity of nanozymes
Unlike the high specificity of enzymes, nanozymes have a broader substrate spectrum. However, their general lack of selectivity makes them challenging to apply in fields that require catalytic specificity. Inspired by natural enzymes, learning from nature will be one of the promising methods to improve the high specificity of nanozymes. Using natural enzymes (e.g. iron porphyrin) as models and incorporating certain recognition mechanisms, various experimental methods, including surface modification, size and shape control, structural engineering, catalytic domain functionalization, and theoretical simulation, such as constructing molecularly imprinted polymers that mimic protein like (or adapter like) binding bags and selective substrate bags [218], are employed to finely modulate the interaction of substrates with nanozymes,, which will be one of the promising methods to improve the high specificity of nanozymes.
(4) Exploration of nanozymes from laboratory research to market application
Although nanozymes have showed great potential in the application of biochemical sensing detection, medicine and health, environmental monitoring, and pollutant treatment at present, how to truly gain recognition and penetrate the market to create significant social benefits, is still a bottleneck currently faced. The transition of nanozymes from laboratory research to market-oriented applications is a multifaceted process. To address this issue, it is suggested to focus on the following aspects that encompasses several key studies. Firstly, based on fundamental research outcomes, to develop scalable manufacturing processes for nanozymes production to ensure consistency and reproducibility of the products. Secondly, to enhance the functionality of nanozymes, such as improving targeting capabilities, increasing biocompatibility, or boosting stability. Thirdly, to establish production, testing, and usage standards for nanozyme products to ensure compliance with industry and international norms. These systematic approaches ensure that nanozymes are not only scientifically sound but also safe, effective, and of reliable quality for various market applications.
(5) Biosafety evaluation of nanozymes
The physical and chemical properties of nanozymes, such as size, shape, surface modification, and chemical composition, have a significant impact on their biocompatibility and potential toxicity. These characteristics can affect the distribution, metabolism, and excretion of nanozymes in organisms, as well as their interactions with biomolecules. So far, nanozymes have been widely used in the laboratory stage for in vivo treatment. In addition to further developing novel functional nanozymes with good biocompatibility (e.g., PepNzymes) [219], the biosafety evaluation of nanozymes is also a critical aspect, ensuring that these nanomaterials are safe for both human health and the environment. the biosafety evaluation typically will focus on several aspects such as cytotoxicity, genotoxicity, immunotoxicity, in vivo toxicity, environmental impact, long-time exposure effects, degradation and persistence. These comprehensive assessment approaches help to minimize risks and maximize the benefits of using nanozymes in biomedicine, environmental protection, and other industries.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- AAO
Amino acid oxidase
- AOX
Ascorbate oxidase
- CAT
Catalase
- C-dot
Carbon dot
- CDT
Chemodynamic therapy
- COF
Covalent organic framework
- COX
Cysteine oxidase
- CYS
Cysteine
- Cyt
Cytochrome
- DMSN
Dendritic mesoporous silica nanosphere
- DNA
DeoxyriboNucleic Acid
- EGFR
Epidermal growth factor receptor
- Fc
Ferrocenyl
- Fe-CDs
Fe-coordinated carbon nanozyme dots
- FLS
Fibroblast-like synoviocyte
- GDYO
Graphdiyne oxide
- GIM
Au NPs doped Fe-based Metal–Organic Frameworks
- GOX
Glucose oxidase
- GPX
Glutathione peroxidase
- GSH
Glutathione
- H2O2
Hydrogen peroxide
- HSA
Human serum albumin
- LAC
Laccases
- LaMNPs
β-Lapachone Fe@Fe3O4@Cu2-xS
- LOD
Limit of detection
- LOX
Lipoxygenase
- MNPs
Fe@Fe3O4@Cu2-xS
- MOF
Metal–organic frameworks
- NIR
Near-infrared
- NPs
Nanoparticles
- N-QG
N doped Q-graphene
- OPD
Ophenylenediamine
- OTA
Ochratoxin A
- OXD
Oxidase
- PDA
Polydopamine
- PDT
Photodynamic therapy
- PER
Primer exchange reaction
- PEG
Polyethylene glycol
- POD
Peroxidase
- pS
Poly styrene
- PTT
Photothermal therapy
- RA
Rheumatoid arthritis
- Rh
Rhodium
- RNase
Ribonuclease
- ROS
Reactive oxygen species
- SAzyme
Single-atom nanozymes
- SDT
Sonodynamic therapy
- SFO
SnFe2O4
- SOD
Superoxide dismutase
- SPX
Sonosensitizer spafloxacin
- TMB
3,3’,5,5’-Tetramethylbenzidine
- TME
Tumor microenvironment
- UCNPs
Upconversion nanoparticles
- UOX/UOD
Urate oxidase
- UPFB
Upconversion nanoparticles PCN-224(Fe) Biotin
- US
Ultrasound
- WOS
Web of science
Author contributions
Z.F., A.Z., J.Y. and G.S. designed this study; Z.F., Y.G., and Y.Z. screened and extracted the data; Z.F., Y.G., and M.J. conducted and visualized the data analysis; Z.F., and Y.G. wrote this manuscript. J.Y., A.Z. and G.S. reviewed the manuscript’s intellectual content. All authors read and approved the final manuscript.
Funding
The work was supported by the National Natural Science Foundation of China (No. 82374161, 22174156, 21974148), the foundation of Key Laboratory of Ethnomedicine (Minzu University of China), Ministry of Education (No. KLEM-ZZ202302).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zihan Feng and Yuexin Guo have contributed equally to this study.
Contributor Information
Aiqin Zhang, Email: zhangaiqin582@163.com.
Junfa Yin, Email: jfyin@rcees.ac.cn.
Gangyi Shen, Email: shengy@muc.edu.cn.
References
- 1.Nelson A. Catalytic machinery of enzymes expanded. Nature. 2019;570:172–3. [DOI] [PubMed] [Google Scholar]
- 2.Shoda S, Uyama H, Kadokawa J, Kimura S, Kobayashi S. Enzymes as green catalysts for precision macromolecular synthesis. Chem Rev. 2016;116:2307–413. [DOI] [PubMed] [Google Scholar]
- 3.Breslow R, Overman LE. An “artificial enzyme” combining a metal catalytic group and a hydrophobic binding cavity. J Am Chem Soc. 1970;92:1075–7. [DOI] [PubMed] [Google Scholar]
- 4.Shen J, Chen J, Qian Y, Wang X, Wang D, Pan H, Wang Y. Atomic engineering of single-atom nanozymes for biomedical applications. Adv Mater. 2024;36: e2313406. [DOI] [PubMed] [Google Scholar]
- 5.Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–83. [DOI] [PubMed] [Google Scholar]
- 6.Wei H, Wang EK. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060–93. [DOI] [PubMed] [Google Scholar]
- 7.Liang M, Yan X. Nanozymes: from new concepts, mechanisms, and standards to applications. Acc Chem Res. 2019;52:2190–200. [DOI] [PubMed] [Google Scholar]
- 8.Scott S, Zhao HM, Dey A, Gunnoe TB. Nano-apples and orange-zymes. Acs. Catalysis. 2020;10:14315–7. [Google Scholar]
- 9.Wei H, Gao LZ, Fan KL, Liu JW, He JY, Qu XG, Dong SJ, Wang EK, Yan XY. Nanozymes: A clear definition with fuzzy edges. Nano Today. 2021;40;101269. [Google Scholar]
- 10.Zandieh M, Liu JW. Nanozymes: definition, activity, and mechanisms. Adv Mater. 2024;36:2211041. [DOI] [PubMed] [Google Scholar]
- 11.Ji SF, Jiang B, Hao HG, Chen YJ, Dong JC, Mao Y, Zhang ZD, Gao R, Chen WX, Zhang RF, et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat Catal. 2021;4:407–17. [Google Scholar]
- 12.Chen Y, Wang P, Hao H, Hong J, Li H, Ji S, Li A, Gao R, Dong J, Han X, Liang M, Wang D, Li Y. Thermal atomization of platinum nanoparticles into single atoms: an effective strategy for engineering high-performance nanozymes. J Am Chem Soc. 2021;143:18643–51. [DOI] [PubMed] [Google Scholar]
- 13.Guo Z, Hong J, Song N, Liang M. Single-atom nanozymes: from precisely engineering to extensive applications. Acc Mater Res. 2024;5:347–57. [Google Scholar]
- 14.Song N, Yu Y, Zhang Y, Wang Z, Guo Z, Zhang J, Zhang C, Liang M. Bioinspired hierarchical self-assembled nanozyme for efficient antibacterial treatment. Adv Mater. 2024;36: e2210455. [DOI] [PubMed] [Google Scholar]
- 15.Jiang B, Guo Z, Liang M. Recent progress in single-atom nanozymes research. Nano Res. 2023;16:1878–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan X, Cai W. Nanozyme: new horizons for responsive biomedical applications. Chem Soc Rev. 2019;48:3683–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manea F, Houillon FB, Pasquato L, Scrimin P. Nanozymes: gold-nanoparticle-based transphosphorylation catalysts. Angew Chem Int Ed Engl. 2004;43:6165–9. [DOI] [PubMed] [Google Scholar]
- 18.Gomollón-Bel F, García-Martínez J. Emerging chemistry technologies for a better world. Nat Chem. 2022;14:113–4. [DOI] [PubMed] [Google Scholar]
- 19.Fan KL, Gao LZ, Wei H, Jiang B, Wang DJ, Zhang RF, He JY, Meng XQ, Wang ZR, Fan HZ, et al. Nanozymes. Progress in Chemistry. 2023;35:1–87. [Google Scholar]
- 20.Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev. 2019;48:1004–76. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Ren J, Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119:4357–412. [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Fan K, Yan X. Iron oxide nanozyme: a multifunctional enzyme mimetic for biomedical applications. Theranostics. 2017;7:3207–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Q, Chen S, Huang H, Zhang L, Wang L, Liu F, Chen J, Zeng Y, Chu PK. Colorimetric and ultra-sensitive fluorescence resonance energy transfer determination of H2O2 and glucose by multi-functional Au nanoclusters. Analyst. 2014;139:1498–503. [DOI] [PubMed] [Google Scholar]
- 24.Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010;22:2206–10. [DOI] [PubMed] [Google Scholar]
- 25.Guo R, Xue L, Cai G, Qi W, Liu Y, Lin J. Fe-MIL-88NH2 Metal-organic framework nanocubes decorated with pt nanoparticles for the detection of salmonella. ACS Appl Nano Mater. 2021;4:5115–22. [Google Scholar]
- 26.Yang Q, Liu J, Cai W, Liang X, Zhuang Z, Liao T, Zhang F, Hu W, Liu P, Fan S, et al. Non-heme iron single-atom nanozymes as peroxidase mimics for tumor catalytic therapy. Nano Lett. 2023;23:8585–92. [DOI] [PubMed] [Google Scholar]
- 27.Wu JJX, Yu YJ, Cheng Y, Cheng CQ, Zhang YH, Jiang B, Zhao XZ, Miao LY, Wei H. Ligand-dependent activity engineering of glutathione peroxidase-mimicking MIL-47(V) metal-organic framework nanozyme for therapy. Angew Chem-Int Edition. 2021;60:1227–34. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Liu C, Pu F, Liu Z, Ren J, Qu X. A GO-Se nanocomposite as an antioxidant nanozyme for cytoprotection. Chem Commun (Camb). 2017;53:3082–5. [DOI] [PubMed] [Google Scholar]
- 29.Qin T, Ma R, Yin Y, Miao X, Chen S, Fan K, Xi J, Liu Q, Gu Y, Yin Y, et al. Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics. 2019;9:6920–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing L, Liu XY, Zhou TJ, Wan X, Wang Y, Jiang HL. Photothermal nanozyme-ignited fenton reaction-independent ferroptosis for breast cancer therapy. J Control Release. 2021;339:14–26. [DOI] [PubMed] [Google Scholar]
- 31.Wang S, Cazelles R, Liao WC, Vázquez-González M, Zoabi A, Abu-Reziq R, Willner I. Mimicking horseradish peroxidase and nadh peroxidase by heterogeneous Cu-modified graphene oxide nanoparticles. Nano Lett. 2017;17:2043–8. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Zou H, Yan B, Wu XJ, Cao WW, Qian YH, Zheng L, Yang GW. Atomically dispersed cu nanozyme with intensive ascorbate peroxidase mimic activity capable of alleviating ROS-mediated oxidation damage. Adv Sci. 2022;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Y, Liang L, Ye F, Zhao S. Ce-MOF with intrinsic haloperoxidase-like activity for ratiometric colorimetric detection of hydrogen peroxide. Biosensors. 2021;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu WH, Chen J, Kong LS, Zhu F, Feng ZY, Zhan JH. Oxygen vacancies modulation MnO nanozyme with enhanced oxidase-mimicking performance for L-cysteine detection. Sens Actuators B-Chem. 2021;333:129560. [Google Scholar]
- 35.Xiong YH, Chen SH, Ye FG, Su LJ, Zhang C, Shen SF, Zhao SL. Synthesis of a mixed valence state Ce-MOF as an oxidase mimetic for the colorimetric detection of biothiols. Chem Commun. 2015;51:4635–8. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Han TQ, Wei Q, Zhang SS. Efficient enhancement of electrochemiluminescence from cadmium sulfide quantum dots by glucose oxidase mimicking gold nanoparticles for highly sensitive assay of methyltransferase activity. Anal Chem. 2016;88:2976–83. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Zhao MT, Han SK, Lai ZC, Yang J, Tan CL, Ma QL, Lu QP, Chen JZ, Zhang X, et al. Growth of Au nanoparticles on 2D metalloporphyrinic metal-organic framework nanosheets used as biomimetic catalysts for cascade reactions. Adv Mater. 2017;29:32. [DOI] [PubMed] [Google Scholar]
- 38.Hu AL, Deng HH, Zheng XQ, Wu YY, Lin XL, Liu AL, Xia XH, Peng HP, Chen W, Hong GL. Self-cascade reaction catalyzed by CuO nanoparticle-based dual-functional enzyme mimics. Biosens Bioelectron. 2017;97:21–5. [DOI] [PubMed] [Google Scholar]
- 39.Kaushal N, Kumari A, Singh AK, Bijalwan K, Saha A, Indr A. Oxidase-like nanozyme activity of manganese metal organic framework nanosheets for colorimetric and fluorescence sensing of ICysteine. ACS Appl Nano Mater. 2023;6:8036–45. [Google Scholar]
- 40.Ren XL, Liu J, Ren J, Tang FQ, Meng XW. One-pot synthesis of active copper-containing carbon dots with laccase-like activities. Nanoscale. 2015;7:19641–6. [DOI] [PubMed] [Google Scholar]
- 41.Tran TD, Nguyen PT, Le TN, Kim MI. DNA-copper hybrid nanoflowers as efficient laccase mimics for colorimetric detection of phenolic compounds in paper microfluidic devices. Biosens Bioelectron. 2021;182:8. [DOI] [PubMed] [Google Scholar]
- 42.Lancien A, Heuson E, Dumeignil F, Itabaiana I, Froidevaux R, Wojcieszak R. Pt nanoparticles with enhanced deaminase-like activity: example of oxidative deamination of 5-hydroxymethylfurfurylamine and glutamic acid. Chemnanomat. 2022;8:6. [Google Scholar]
- 43.Liu JB, Hu XN, Hou S, Wen T, Liu WQ, Zhu X, Yin JJ, Wu XC. Au@Pt core/shell nanorods with peroxidase- and ascorbate oxidase-like activities for improved detection of glucose. Sens Actuators B-Chem. 2012;166:708–14. [Google Scholar]
- 44.Liu C, Fan WB, Cheng WX, Gu YP, Chen YM, Zhou WH, Yu XF, Chen MH, Zhu MR, Fan KL, Luo QY. Red emissive carbon dot superoxide dismutase nanozyme for bioimaging and ameliorating acute lung injury. Adv Func Mater. 2023;33:12. [Google Scholar]
- 45.Parmekar MV, Salker AV. Highly tuned cobalt-doped MnO nanozyme as remarkably efficient uricase mimic. Appl Nanosci. 2020;10:317–28. [Google Scholar]
- 46.Xue T, Peng B, Xue M, Zhong X, Chiu CY, Yang S, Qu Y, Ruan L, Jiang S, Dubin S, et al. Integration of molecular and enzymatic catalysts on graphene for biomimetic generation of antithrombotic species. Nat Commun. 2014;5:3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Li J, Zitolo A, Gao M, Jiang J, Geng X, Xie Q, Wu D, Zheng H, Cai X, et al. Doped graphene to mimic the bacterial NADH oxidase for one-step NAD(+) supplementation in mammals. J Am Chem Soc. 2023;145:3108–20. [DOI] [PubMed] [Google Scholar]
- 48.Chen M, Wang ZH, Shu JX, Jiang XH, Wang W, Shi ZH, Lin YW. Mimicking a natural enzyme system: cytochrome oxidase-like activity of CuO nanoparticles by receiving electrons from cytochrome. Inorg Chem. 2017;56:9400–3. [DOI] [PubMed] [Google Scholar]
- 49.Slocik JM, Govorov AO, Naik RR. Photoactivated biotemplated nanoparticles as an enzyme mimic. Angew Chem Int Ed Engl. 2008;47:5335–9. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Jiang B, Hao H, Li H, Qiu C, Liang X, Qu Q, Zhang Z, Gao R, Duan D, et al. Atomic-level regulation of cobalt single-atom nanozymes: engineering high-efficiency catalase mimics. Angew Chem Int Ed Engl. 2023;62: e202301879. [DOI] [PubMed] [Google Scholar]
- 51.Hu MH, Korschelt K, Daniel P, Landfester K, Tremel W, Bannwarth MB. Fibrous nanozyme dressings with catalase-like activity for HO reduction to promote wound healing. ACS Appl Mater Interfaces. 2017;9:38024–31. [DOI] [PubMed] [Google Scholar]
- 52.Ragg R, Schilmann AM, Korschelt K, Wieseotte C, Kluenker M, Viel M, Völker L, Preiss S, Herzberger J, Frey H, et al. Intrinsic superoxide dismutase activity of MnO nanoparticles enhances the magnetic resonance imaging contrast. J Mater Chem B. 2016;4:7423–8. [DOI] [PubMed] [Google Scholar]
- 53.Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Commun. 2007;10:1056–8. [DOI] [PubMed] [Google Scholar]
- 54.Vernekar AA, Das T, Mugesh G. Vacancy-engineered nanoceria: enzyme mimetic hotspots for the degradation of nerve agents. Angew Chem Int Ed Engl. 2016;55:1412–6. [DOI] [PubMed] [Google Scholar]
- 55.Ma X, Zhang L, Xia M, Li S, Zhang X, Zhang Y. Mimicking the active sites of organophosphorus hydrolase on the backbone of graphene oxide to destroy nerve agent simulants. ACS Appl Mater Interfaces. 2017;9:21089–93. [DOI] [PubMed] [Google Scholar]
- 56.Ly HGT, Fu GX, Kondinski A, Bueken B, De Vos D, Parac-Vogt TN. Superactivity of MOF-808 toward peptide bond hydrolysis. J Am Chem Soc. 2018;140:6325–35. [DOI] [PubMed] [Google Scholar]
- 57.Li B, Chen DM, Nie MF, Wang JQ, Li YZ, Yang YP. Carbon Dots/CuO composite with intrinsic high protease-like activity for hydrolysis of proteins under physiological conditions. Particle Particle Syst Characterizat. 2018;35:11. [Google Scholar]
- 58.Han JJ, Zou QL, Su WW, Yan XH. Minimal metallo-nanozymes constructed through amino acid coordinated self-assembly for hydrolase-like catalysis. Chem Eng J. 2020;394:124987. [Google Scholar]
- 59.Xiao SJ, Yuan MY, Shi YD, Wang MP, Li HH, Zhang L, Qiu JD. Construction of covalent organic framework nanozymes with photo-enhanced hydrolase activities for colorimetric sensing of organophosphorus nerve agents. Anal Chim Acta. 2023;1278: 341706. [DOI] [PubMed] [Google Scholar]
- 60.Nandhakumar P, Kim G, Park S, Kim S, Kim S, Park JK, Lee NS, Yoon YH, Yang H. Metal nanozyme with ester hydrolysis activity in the presence of ammonia-borane and its use in a sensitive immunosensor. Angew Chem Int Ed Engl. 2020;59:22419–22. [DOI] [PubMed] [Google Scholar]
- 61.Xi JQ, An LF, Wei G, Huang YL, Li DD, Fan L, Gao LZ. Photolysis of methicillin-resistant using Cu-doped carbon spheres. Biomaterials Science. 2020;8:6225–34. [DOI] [PubMed] [Google Scholar]
- 62.Korschelt K, Schwidetzky R, Pfitzner F, Strugatchi J, Schilling C, von der Au M, Kirchhoff K, Panthöfer M, Lieberwirth I, Tahir MN, et al. CeO nanorods with intrinsic urease-like activity. Nanoscale. 2018;10:13074–82. [DOI] [PubMed] [Google Scholar]
- 63.Walther R, van den Akker W, Fruergaard AS, Zelikin AN. Nanozymes and glucuronides: glucuronidase, esterase, and/or transferase activity. Small. 2020;16:7. [DOI] [PubMed] [Google Scholar]
- 64.Chen JX, Huang L, Wang QQ, Wu WW, Zhang H, Fang YX, Dong SJ. Bio-inspired nanozyme: a hydratase mimic in a zeolitic imidazolate framework. Nanoscale. 2019;11:5960–6. [DOI] [PubMed] [Google Scholar]
- 65.Wu H, Xia F, Zhang L, Fang C, Lee J, Gong L, Gao J, Ling D, Li F. A ROS-sensitive nanozyme-augmented photoacoustic nanoprobe for early diagnosis and therapy of acute liver failure. Adv Mater. 2022;34: e2108348. [DOI] [PubMed] [Google Scholar]
- 66.Li F, Li S, Guo XC, Dong YH, Yao C, Liu YP, Song YG, Tan XL, Gao LZ, Yang DY. Chiral carbon dots mimicking topoisomerase i to mediate the topological rearrangement of supercoiled dna enantioselectively. Angew Chem Int Ed Engl. 2020;59:11087–92. [DOI] [PubMed] [Google Scholar]
- 67.He X, Luo Q, Guo Z, Li Y, Liu Z. Construction of DNA ligase-mimicking nanozymes via molecular imprinting. J Mater Chem B. 2022;10:6716–23. [DOI] [PubMed] [Google Scholar]
- 68.Wu JH, Yang QT, Li Q, Li HY, Li F. Two-dimensional MnO nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of HO and color. Anal Chem. 2021;93:4084–91. [DOI] [PubMed] [Google Scholar]
- 69.Yang HG, Yang RT, Zhang P, Qin YM, Chen T, Ye FG. A bimetallic (Co/2Fe) metal-organic framework with oxidase and peroxidase mimicking activity for colorimetric detection of hydrogen peroxide. Microchim Acta. 2017;184:4629–35. [Google Scholar]
- 70.Zhang P, Sun D, Cho A, Weon S, Lee S, Lee J, Han JW, Kim DP, Choi W. Modified carbon nitride nanozyme as bifunctional glucose oxidase-peroxidase for metal-free bioinspired cascade photocatalysis. Nature Commu. 2019;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao SS, Lin H, Zhang HX, Yao HL, Chen Y, Shi JL. Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv Sci. 2019;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen ZW, Yin JJ, Zhou YT, Zhang Y, Song L, Song MJ, Hu SL, Gu N. Dual enzyme-like activities of iron oxide nanoparticles and their implication for diminishing cytotoxicity. ACS Nano. 2012;6:4001–12. [DOI] [PubMed] [Google Scholar]
- 73.Wang QQ, Chen JX, Zhang H, Wu WW, Zhang ZQ, Dong SJ. Porous CoO nanoplates with pH-switchable peroxidase- and catalase-like activity. Nanoscale. 2018;10:19140–6. [DOI] [PubMed] [Google Scholar]
- 74.Zhao JW, Duan W, Liu XY, Xi FN, Wu JM. Microneedle patch integrated with porous silicon confined dual nanozymes for synergistic and hyperthermia-enhanced nanocatalytic ferroptosis treatment of melanoma. Adv Func Mater. 2023;33:13. [Google Scholar]
- 75.Xiao F, Yang D, Xun C, Li H, Li Q, Zhong Z, Wei D, Yang Y. H2O2 self-supplying Mo/Fe@CuO2 nanozyme with NIR light enhanced catalytic activity and photothermal synergistic antibacterial application. Appl Surf Sci. 2024;645:158862. [Google Scholar]
- 76.Yang X, Xiang J, Su W, Guo J, Deng J, Tang L, Li G, Liang Y, Zheng L, He M. Modulating Pt nanozyme by using isolated cobalt atoms to enhance catalytic activity for alleviating osteoarthritis. Nano Today. 2023;49:101809. [Google Scholar]
- 77.Ni D, Wei H, Chen W, Bao Q, Rosenkrans ZT, Barnhart TE, Ferreira CA, Wang Y, Yao H, Sun T, Jiang D, Li S, Cao T, Liu Z, Engle JW, Hu P, Lan X, Cai W. Ceria nanoparticles meet hepatic ischemia-reperfusion injury: the perfect imperfection. Adv Mater. 2019;40:1902956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li K, Miao Y, Song K, He S, Zhang G, Waterhouse GIN, Guan S, Zhou S. Collaborative CuMn diatomic nanozyme to boost nanocatalytic/mild photothermal/chemo-therapy through overcoming therapeutic resistance. Chem Eng J. 2023;471: 144693. [Google Scholar]
- 79.Morajkar R, Fatrekar AP, Vernekar A. A single-atom nanozyme cascade for selective tumor-microenvironment-responsive nanocatalytic therapy. ChemMedChem. 2023;18: e202200585. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, Chen J, Wang Y, Li J. Metal site and size-controlled BTC-Based MOF as cysteine oxidase mimic for self-cascade detection of cysteine and Hg(2). J Phys Chem B. 2023;127:9513–9. [DOI] [PubMed] [Google Scholar]
- 81.Tian L, Cheng C, Zhao Z, Liu W, Qi L. Enhancing the catalytic performance of MOF-polymer@AuNP-based nanozymes for colorimetric detection of serum L-cysteine. Analyst. 2023;148:3785–90. [DOI] [PubMed] [Google Scholar]
- 82.Zhuang QQ, Deng Q, He SB, Chen QQ, Peng HP, Deng HH, Xia XH, Chen W. Bifunctional cupric oxide nanoparticle-catalyzed self-cascade oxidation reactions of ascorbic acid for bacterial killing and wound disinfection. Composites Part B-Eng. 2021;222:9. [Google Scholar]
- 83.Ying MH, Yang GZ, Xu YJ, Ye HL, Lin X, Lu Y, Pan HB, Bai Y, Du M. Copper fumarate with high-bifunctional nanozyme activities at different pH values for glucose and epinephrine colorimetric detection in human serum. Analyst. 2021;147:40–7. [DOI] [PubMed] [Google Scholar]
- 84.Gao N, Dong K, Zhao AD, Sun HJ, Wang Y, Ren JS, Qu XG. Polyoxometalate-based nanozyme: design of a multifunctional enzyme for multi-faceted treatment of Alzheimer’s disease. Nano Res. 2016;9:1079–90. [Google Scholar]
- 85.Lin AQ, Sun ZY, Xu XQ, Zhao S, Li JW, Sun H, Wang Q, Jiang Q, Wei H, Shi DQ. Self-cascade uricase/catalase mimics alleviate acute gout. Nano Lett. 2022;22:508–16. [DOI] [PubMed] [Google Scholar]
- 86.Feng LL, Liu B, Xie R, Wang DD, Qian C, Zhou WQ, Liu JW, Jana D, Yang PP, Zhao YL. An Ultrasmall SnFe2O4 Nanozyme with endogenous oxygen generation and glutathione depletion for synergistic cancer therapy. Adv Func Mater. 2021;31:14. [Google Scholar]
- 87.Jiang S, Su G, Wu J, Song C, Lu Z, Wu C, Wang Y, Wang P, He M, Zhao Y, et al. Co3O4/CoFe2O4 hollow nanocube multifunctional nanozyme with oxygen vacancies for deep-learning-assisted smartphone biosensing and organic pollutant degradation. ACS Appl Mater Interfaces. 2023;15:11787–801. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Wang B, Zhu JJ, Xu XN, Zhou B, Yang Y. Single-atom nanozyme with asymmetric electron distribution for tumor catalytic therapy by disrupting tumor redox and energy metabolism homeostasis. Adv Mater. 2023;35:10. [DOI] [PubMed] [Google Scholar]
- 89.Bhagat S, Srikanth Vallabani NV, Shutthanandan V, Bowden M, Karakoti AS, Singh S. Gold core/ceria shell-based redox active nanozyme mimicking the biological multienzyme complex phenomenon. J Colloid Interface Sci. 2018;513:831–42. [DOI] [PubMed] [Google Scholar]
- 90.Chen ZY, Chen P, Zhu YY, Qian JY, Huang XW, Zhang W, Zhang H, Mo Q, Lu YT, Zhang YJ. 2D Cobalt Oxyhydroxide Nanozymes Inhibit Inflammation by Targeting the NLRP3 Inflammasome. Adv Func Mater. 2023;33:13. [Google Scholar]
- 91.Liu Y, Qin Y, Zhang Q, Zou W, Jin L, Guo R. Arginine-rich peptide/platinum hybrid colloid nanoparticle cluster: a single nanozyme mimicking multi-enzymatic cascade systems in peroxisome. J Colloid Interface Sci. 2021;600:37–48. [DOI] [PubMed] [Google Scholar]
- 92.Baldim V, Yadav N, Bia N, Graillot A, Loubat C, Singh S, Karakoti AS, Berret JF. Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Appl Mater Interfaces. 2020;12:42056–66. [DOI] [PubMed] [Google Scholar]
- 93.Zhang T, Lu N, Wang C, Jiang H, Zhang M, Zhang R, Zhong Y, Xing D. Artificial peroxisome hNiPt@Co-NC with Tetra-enzyme Activities for colorimetric glutathione sensing. ACS Appl Mater Interfaces. 2023;15:46738–46. [DOI] [PubMed] [Google Scholar]
- 94.Xu YX, Li PP, Hu XJ, Chen HY, Tang Y, Zhu Y, Zhu XH, Zhang YY, Liu ML, Yao SZ. Polyoxometalate nanostructures decorated with cuo nanoparticles for sensing ascorbic acid and Fe Ions. Acs Applied Nano Materials. 2021;4:8302–13. [Google Scholar]
- 95.Liu CY, Cai YY, Wang J, Liu X, Ren H, Yan L, Zhang YJ, Yang SQ, Guo J, Liu AH. Facile preparation of homogeneous copper nanoclusters exhibiting excellent tetraenzyme mimetic activities for colorimetric glutathione sensing and fluorimetric ascorbic acid sensing. ACS Appl Mater Interfaces. 2020;12:42521–30. [DOI] [PubMed] [Google Scholar]
- 96.Liu XX, Yang J, Cheng J, Xu Y, Chen W, Li YC. Facile preparation of four-in-one nanozyme catalytic platform and the application in selective detection of catechol and hydroquinone. Sens Actuat B-Chem. 2021;337:129763. [Google Scholar]
- 97.Xi JQ, Zhang RF, Wang LM, Xu W, Liang Q, Li JY, Jiang J, Yang YL, Yan XY, Fan KL, Gao LZ. A nanozyme-based artificial peroxisome ameliorates hyperuricemia and ischemic stroke. Adv Func Mater. 2021;31:13. [Google Scholar]
- 98.Zhou LL, Guan Q, Zhou W, Kan JL, Teng K, Hu M, Dong YB. A Multifunctional covalent organic framework nanozyme for promoting ferroptotic radiotherapy against esophageal cancer. ACS Nano. 2023;17:20445–61. [DOI] [PubMed] [Google Scholar]
- 99.Huang CY, Liu ZM, Chen MZ, Du L, Liu CP, Wang ST, Zheng YF, Liu W. Tumor-derived biomimetic nanozyme with immune evasion ability for synergistically enhanced low dose radiotherapy. J Nanobiotechnol. 2021;19:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ma W, Xue Y, Guo S, Jiang Y, Wu F, Yu P, Mao L. Graphdiyne oxide: a new carbon nanozyme. Chem Commun. 2020;56:5115–8. [DOI] [PubMed] [Google Scholar]
- 101.Li X, Zhao C, Deng G, Liu W, Shao J, Zhou Z, Liu F, Yang H, Yang S. Nanozyme-augmented tumor catalytic therapy by self-supplied H2O2 generation. ACS Appl Bio Mater. 2020;3:1769–78. [DOI] [PubMed] [Google Scholar]
- 102.He W, Wu X, Liu J, Hu X, Zhang K, Hou S, Zhou W, Xie S. Design of AgM bimetallic alloy nanostructures (M = Au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem Mater. 2010;22:2988–94. [Google Scholar]
- 103.Xia X, Zhang J, Lu N, Kim MJ, Ghale K, Xu Y, McKenzie E, Liu J, Ye H. Pd-Ir core-shell nanocubes: A type of highly efficient and versatile peroxidase mimic. ACS Nano. 2015;9:9994–10004. [DOI] [PubMed] [Google Scholar]
- 104.Wang C, Wang L, Nallathambi V, Liu Y, Kresse J, Hübner R, Reichenberger S, Gault B, Zhan J, Eychmüller A, Cai B. Structural regulation of Au-Pt bimetallic aerogels for catalyzing the glucose cascade reaction. Adv Mater. 2024:36:2405200. [DOI] [PubMed] [Google Scholar]
- 105.Wang Z, Zhang Y, Ju E, Liu Z, Cao F, Chen Z, Ren J, Qu X. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat Commun. 2018;9:3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lei M, Ding X, Liu J, Tang Y, Chen H, Zhou Y, Zhu C, Yan H. Trace amount of bi-doped core-shell Pd@Pt mesoporous nanospheres with specifically enhanced peroxidase-like activity enable sensitive and accurate detection of acetylcholinesterase and organophosphorus nerve agents. Anal Chem. 2024;96:6072–8. [DOI] [PubMed] [Google Scholar]
- 107.Song Y, Wang X, Zhao C, Qu K, Ren J, Qu X. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chemistry. 2010;16:3617–21. [DOI] [PubMed] [Google Scholar]
- 108.Li S, Zhao XT, Gang RT, Cao BQ, Wang H. Doping nitrogen into Q-graphene by plasma treatment toward peroxidase mimics with enhanced catalysis. Anal Chem. 2020;92:5152–7. [DOI] [PubMed] [Google Scholar]
- 109.Zhu W, Yang Y, Jin Q, Chao Y, Tian L, Liu J, Dong Z, Liu Z. Two-dimensional metal-organic-framework as a unique theranostic nano-platform for nuclear imaging and chemo-photodynamic cancer therapy. Nano Res. 2019;12:6. [Google Scholar]
- 110.Jiang SK, Wang J, Zhu Z, Shan S, Mao YL, Zhang X, Pei XB, Huang C, Wan QB. The synthesis of nano bio-MOF-1 with a systematic evaluation on the biosafety and biocompatibility. Microporous Mesoporous Mater. 2022;334:16. [Google Scholar]
- 111.Vodyashkin A, Sergorodceva A, Kezimana P, Morozova M, Nikolskaya E, Mollaeva M, Yabbarov N, Sokol M, Chirkina M, Butusov L, Timofeev A. Synthesis and activation of pH-sensitive metal-organic framework Sr(BDC)∞ for oral drug delivery. Dalton Trans. 2024;53:1048–57. [DOI] [PubMed] [Google Scholar]
- 112.Ali A, Ovais M, Zhou H, Rui Y, Chen C. Tailoring metal-organic frameworks-based nanozymes for bacterial theranostics. Biomaterials. 2021;275: 120951. [DOI] [PubMed] [Google Scholar]
- 113.Hou JJ, Xianyu YL. Tailoring the surface and composition of nanozymes for enhanced bacterial binding and antibacterial activity. Small. 2023;19:27. [DOI] [PubMed] [Google Scholar]
- 114.Huang L, Chen JX, Gan LF, Wang J, Dong SJ. Single-atom nanozymes. Sci Adv. 2019;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao C, Xiong C, Liu XK, Qiao M, Li ZJ, Yuan TW, Wang J, Qu YT, Wang XQ, Zhou FY, et al. Unraveling the enzyme-like activity of heterogeneous single atom catalyst. Chem Commun. 2019;55:2285–8. [DOI] [PubMed] [Google Scholar]
- 116.Chen Y, Gao R, Ji S, Li H, Tang K, Jiang P, Hu H, Zhang Z, Hao H, Qu Q, et al. Atomic-level modulation of electronic density at cobalt single-atom sites derived from metal-organic frameworks: enhanced oxygen reduction performance. Angew Chem Int Ed Engl. 2021;60:3212–21. [DOI] [PubMed] [Google Scholar]
- 117.Chu CH, Huang DH, Gupta S, Weon S, Niu JF, Stavitski E, Muhich C, Kim JH. Neighboring Pd single atoms surpass isolated single atoms for selective hydrodehalogenation catalysis. Nat Commun. 2021;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao MY, Yang RG, Wei YR, Su JJ, Wang XA, Zhang N, Sun PC, Chen DL, Zhao YX. Dual isolated bimetal single-atom catalysts for tumor ROS cycle and parallel catalytic therapy. Nano Today. 2022;44:15. [Google Scholar]
- 119.Ma CB, Xu YP, Wu LX, Wang Q, Zheng JJ, Ren GX, Wang XY, Gao XF, Zhou M, Wang M, Wei H. Guided synthesis of a Mo/Zn dual single-atom nanozyme with synergistic effect and peroxidase-like activity. Angew Chem Int Ed Engl. 2022;61:25. [DOI] [PubMed] [Google Scholar]
- 120.Gao SS, Lin H, Zhang HX, Yao HL, Chen Y, Shi JL. Nanocatalytic tumor therapy by biomimetic dual inorganic nanozyme-catalyzed cascade reaction. Adv Sci. 2019;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ai YJ, He MQ, Sun H, Jia XM, Wu L, Zhang XY, Sun HB, Liang QL. Ultra-small high-entropy alloy nanoparticles: efficient nanozyme for enhancing tumor photothermal therapy. Adv Mater. 2023;35:13. [DOI] [PubMed] [Google Scholar]
- 122.Guo YJ, Deng L, Li J, Guo SJ, Wang EK, Dong SJ. Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano. 2011;5:1282–90. [DOI] [PubMed] [Google Scholar]
- 123.Lan WF, Hu RF, Huang DR, Dong X, Shen GY, Chang S, Dai DS. Palladium nanoparticles/graphdiyne oxide nanocomposite with excellent peroxidase-like activity and its application for glutathione detection. Chem Res Chin Univ. 2022;38:529–34. [Google Scholar]
- 124.Puvvada N, Panigrahi PK, Mandal D, Pathak A. Shape dependent peroxidase mimetic activity towards oxidation of pyrogallol by H2O2. RSC Adv. 2012;2:3270–3. [Google Scholar]
- 125.Vernekar AA, Das T, Ghosh S, Mugesh G. A Remarkably efficient MnFe2O4 -based oxidase nanozyme. Chem Asian J. 2016;11:72–6. [DOI] [PubMed] [Google Scholar]
- 126.Ge C, Fang G, Shen X, Chong Y, Wamer WG, Gao X, Chai Z, Chen C, Yin JJ. Facet energy versus enzyme-like activities: the unexpected protection of palladium nanocrystals against oxidative damage. ACS Nano. 2016;10:10436–45. [DOI] [PubMed] [Google Scholar]
- 127.Tian F, Zhou J, Jiao B, He Y. A nanozyme-based cascade colorimetric aptasensor for amplified detection of ochratoxin A. Nanoscale. 2019;11:9547–55. [DOI] [PubMed] [Google Scholar]
- 128.Li SS, Shang L, Xu BL, Wang SH, Gu K, Wu QY, Sun Y, Zhang QH, Yang HL, Zhang FR, et al. A nanozyme with photo-enhanced dual enzyme-like activities for deep pancreatic cancer therapy. Angew Chem Int Ed Engl. 2019;58:12624–31. [DOI] [PubMed] [Google Scholar]
- 129.Li WJ, Liu C, Liu D, Liu SD, You TY. Ratiometric fluorescent sensing of mercury (II) ion based on the Pt nanozyme-triggered fluorescence resonance energy transfer between Si quantum dots and 2,3-diaminophenazine. Sens Actuat a-Physical. 2021;331:8. [Google Scholar]
- 130.Li W, Song YL, Liang XY, Zhou Y, Xu M, Lu Q, Wang XX, Li N. Mutual-reinforcing sonodynamic therapy against Rheumatoid Arthritis based on sparfloxacin sonosensitizer doped concave-cubic rhodium nanozyme. Biomaterials. 2021;276:12. [DOI] [PubMed] [Google Scholar]
- 131.Wang Z, Liu B, Sun QQ, Feng LL, He F, Yang PAP, Gai SL, Quan ZW, Lin J. Upconverted metal-organic framework janus architecture for near-infrared and ultrasound co-enhanced high performance tumor therapy. ACS Nano. 2021;15:12342–57. [DOI] [PubMed] [Google Scholar]
- 132.Lai X, Zhang G, Zeng L, Xiao X, Peng J, Guo P, Zhang W, Lai W. Synthesis of PDA-mediated magnetic bimetallic nanozyme and its application in immunochromatographic assay. ACS Appl Mater Interfaces. 2021;13:1413–23. [DOI] [PubMed] [Google Scholar]
- 133.Yin ZB, Ji Q, Wu D, Li ZW, Fan MF, Zhang HX, Zhao XL, Wu AG, Cheng L, Zeng LY. HO-responsive gold nanoclusters @ Mesoporous silica @ manganese dioxide nanozyme for “Off/On” modulation and enhancement of magnetic resonance imaging and photodynamic therapy. ACS Appl Mater Interfaces. 2021;13:14928–37. [DOI] [PubMed] [Google Scholar]
- 134.Qin R, Feng Y, Ding D, Chen L, Li S, Deng H, Chen S, Han Z, Sun W, Chen H. Fe-coordinated carbon nanozyme dots as peroxidase-like nanozymes and magnetic resonance imaging contrast agents. ACS Appl Bio Mater. 2021;4:5520–8. [DOI] [PubMed] [Google Scholar]
- 135.Shen J, Rees TW, Zhou Z, Yang S, Ji L, Chao H. A mitochondria-targeting magnetothermogenic nanozyme for magnet-induced synergistic cancer therapy. Biomaterials. 2020;251: 120079. [DOI] [PubMed] [Google Scholar]
- 136.Hwang G, Paula AJ, Hunter EE, Liu Y, Babeer A, Karabucak B, Stebe K, Kumar V, Steager E, Koo H. Catalytic antimicrobial robots for biofilm eradication. Sci Robot. 2019;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lu D, Jiang H, Zhang GY, Luo Q, Zhao Q, Shi XB. An In Situ generated prussian blue nanoparticle-mediated multimode nanozyme-linked immunosorbent assay for the detection of aflatoxin B1. ACS Appl Mater Interfaces. 2021;13:25738–47. [DOI] [PubMed] [Google Scholar]
- 138.Wang XY, Qin L, Lin MJ, Xing H, Wei H. Fluorescent graphitic carbon nitride-based nanozymes with peroxidase-like activities for ratiometric biosensing. Anal Chem. 2019;91:10648–56. [DOI] [PubMed] [Google Scholar]
- 139.Mu J, He LC, Fan WP, Tang W, Wang ZT, Jiang C, Zhang DY, Liu YJ, Deng HZ, Zou JH, et al. Cascade reactions catalyzed by planar metal-organic framework hybrid architecture for combined cancer therapy. Small. 2020;16:8. [DOI] [PubMed] [Google Scholar]
- 140.Liu XL, Pan YC, Yang JJ, Gao YF, Huang T, Luan XW, Wang YZ, Song YJ. Gold nanoparticles doped metal-organic frameworks as near-infrared light-enhanced cascade nanozyme against hypoxic tumors. Nano Res. 2020;13:653–60. [Google Scholar]
- 141.Tian L, Zhang Y, Wang LB, Geng QJ, Liu DX, Duan LL, Wang YH, Cui JS. Ratiometric dual signal-enhancing-based electrochemical biosensor for ultrasensitive kanamycin detection. ACS Appl Mater Interfaces. 2020;12:52713–20. [DOI] [PubMed] [Google Scholar]
- 142.Li XY, Li XM, Li DD, Zhao M, Wu HP, Shen B, Liu P, Ding SJ. Electrochemical biosensor for ultrasensitive exosomal miRNA analysis by cascade primer exchange reaction and MOF@Pt@MOF nanozyme. Biosens Bioelectron. 2020;168:7. [DOI] [PubMed] [Google Scholar]
- 143.Chen M, Deng G, He Y, Li XL, Liu W, Wang W, Zhou ZG, Yang H, Yang SP. Ultrasound-enhanced generation of reactive oxygen species for MRI-guided tumor therapy by the Fe@FeO-based peroxidase-mimicking nanozyme. ACS Appl Bio Mater. 2020;3:639–47. [DOI] [PubMed] [Google Scholar]
- 144.Sun D, Pang X, Cheng Y, Ming J, Xiang SJ, Zhang C, Lv P, Chu CC, Chen XL, Liu G, Zheng NF. Ultrasound-switchable nanozyme augments sonodynamic therapy against multidrug-resistant bacterial infection. ACS Nano. 2020;14:2063–76. [DOI] [PubMed] [Google Scholar]
- 145.Zhong XY, Wang XW, Cheng L, Tang YA, Zhan GT, Gong F, Zhang R, Hu J, Liu Z, Yang XL. GSH-Depleted PtCu3 Nanocages for Chemodynamic- Enhanced Sonodynamic Cancer Therapy. Adv Functional Mater. 2020;30:4. [Google Scholar]
- 146.Hu YH, Cheng HJ, Zhao XZ, Wu JJ, Muhammad F, Lin SC, He J, Zhou LQ, Zhang CP, Deng Y, et al. Surface-enhanced raman scattering active gold nanoparticles with enzyme-mimicking activities for measuring glucose and lactate in living tissues. ACS Nano. 2017;11:5558–66. [DOI] [PubMed] [Google Scholar]
- 147.Zheng XX, Liu Q, Jing C, Li Y, Li D, Luo WJ, Wen YQ, He Y, Huang Q, Long YT, Fan CH. Catalytic gold nanoparticles for nanoplasmonic detection of DNA hybridization. Angewandte Chemie-International Edition. 2011;50:11994–8. [DOI] [PubMed] [Google Scholar]
- 148.Jana D, Wang D, Bindra AK, Guo Y, Liu J, Zhao Y. Ultrasmall alloy nanozyme for ultrasound- and near-infrared light-promoted tumor ablation. ACS Nano. 2021;15:7774–82. [DOI] [PubMed] [Google Scholar]
- 149.Liang S, Deng XR, Chang Y, Sun CQ, Shao S, Xie ZX, Xiao X, Ma PA, Zhang HY, Cheng ZY, Lin J. Intelligent Hollow Pt-CuS Janus architecture for synergistic catalysis-enhanced sonodynamic and photothermal cancer therapy. Nano Lett. 2019;19:4134–45. [DOI] [PubMed] [Google Scholar]
- 150.Zhao Y, Wang S, Ding Y, Zhang Z, Huang T, Zhang Y, Wan X, Wang ZL, Li L. Piezotronic effect-augmented Cu2–xO-BaTiO3 sonosensitizers for multifunctional cancer dynamic therapy. ACS Nano. 2022;16:9304–16. [DOI] [PubMed] [Google Scholar]
- 151.Wang ZY, Li ZY, Sun ZL, Wang SR, Ali Z, Zhu SH, Liu S, Ren QS, Sheng FG, Wang BD, Hou YL. Visualization nanozyme based on tumor microenvironment “unlocking” for intensive combination therapy of breast cancer. Sci Adv. 2020;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun H, Zhao A, Gao N, Li K, Ren J, Qu X. Deciphering a nanocarbon-based artificial peroxidase: chemical identification of the catalytically active and substrate-binding sites on graphene quantum dots. Angew Chem Int Ed Engl. 2015;54:7176–80. [DOI] [PubMed] [Google Scholar]
- 153.Dong H, Du W, Dong J, Che R, Kong F, Cheng W, Ma M, Gu N, Zhang Y. Depletable peroxidase-like activity of Fe3O4 nanozymes accompanied with separate migration of electrons and iron ions. Nat Commun. 2022;13:5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang C, Shi Y, Dan YY, Nie XG, Li J, Xia XH. Enhanced peroxidase-like performance of gold nanoparticles by hot electrons. Chemistry. 2017;23:6717–23. [DOI] [PubMed] [Google Scholar]
- 155.Chen TM, Wu XJ, Wang JX, Yang GW. WSe(2) few layers with enzyme mimic activity for high-sensitive and high-selective visual detection of glucose. Nanoscale. 2017;9:11806–13. [DOI] [PubMed] [Google Scholar]
- 156.Comotti M, Dellapina C, Falletta E, Rossi M. Aerobic oxidation of glucose with gold catalyst: hydrogen peroxide as intermediate and reagent. Adv Synth Catal. 2006;348:313–6. [Google Scholar]
- 157.Li SQ, Liu XD, Chai HX, Huang YM. Recent advances in the construction and analytical applications of metal-organic frameworks-based nanozymes. Trac-Trends in Analytical Chem. 2018;105:391–403. [Google Scholar]
- 158.Grundner S, Markovits MA, Li G, Tromp M, Pidko EA, Hensen EJ, Jentys A, Sanchez-Sanchez M, Lercher JA. Single-site trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol. Nat Commun. 2015;6:7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen J, Ma Q, Li M, Chao D, Huang L, Wu W, Fang Y, Dong S. Glucose-oxidase like catalytic mechanism of noble metal nanozymes. Nat Commun. 2021;12:3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li JN, Liu WQ, Wu XC, Gao XF. Mechanism of pH-switchable peroxidase and catalase-like activities of gold, silver, platinum and palladium. Biomaterials. 2015;48:37–44. [DOI] [PubMed] [Google Scholar]
- 161.Zhang RF, Chen L, Liang Q, Xi JQ, Zhao HQ, Jin YL, Gao XF, Yan XY, Gao LZ, Fan KL. Unveiling the active sites on ferrihydrite with apparent catalase-like activity for potentiating radiotherapy. Nano Today. 2021;41:15. [Google Scholar]
- 162.Zhao HQ, Zhang RF, Yan XY, Fan KL. Superoxide dismutase nanozymes: an emerging star for anti-oxidation. J Mater Chem B. 2021;9:6939–57. [DOI] [PubMed] [Google Scholar]
- 163.Korsvik C, Patil S, Seal S, Self WT. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem Comm. 2007;10:1056–8. [DOI] [PubMed] [Google Scholar]
- 164.Shen X, Liu W, Gao X, Lu Z, Wu X, Gao X. Mechanisms of oxidase and superoxide dismutation-like activities of gold, silver, platinum, and palladium, and their alloys: a general way to the activation of molecular oxygen. J Am Chem Soc. 2015;137:15882–91. [DOI] [PubMed] [Google Scholar]
- 165.Wang Z, Wu J, Zheng JJ, Shen X, Yan L, Wei H, Gao X, Zhao Y. Accelerated discovery of superoxide-dismutase nanozymes via high-throughput computational screening. Nat Commun. 2021;12:6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu MX, Zhang H, Zhang XW, Chen S, Yu YL, Wang JH. Nanozyme sensor array plus solvent-mediated signal amplification strategy for ultrasensitive ratiometric fluorescence detection of exosomal proteins and cancer identification. Anal Chem. 2021;93:9002–10. [DOI] [PubMed] [Google Scholar]
- 167.Wei Z, Yu Y, Hu S, Yi X, Wang J. Bifunctional Diblock DNA-mediated synthesis of nanoflower-shaped photothermal nanozymes for a highly sensitive colorimetric assay of cancer cells. ACS Appl Mater Interfaces. 2021;13:16801–11. [DOI] [PubMed] [Google Scholar]
- 168.Wang XY, Qin L, Zhou M, Lou ZP, Wei H. Nanozyme sensor arrays for detecting versatile analytes from small molecules to proteins and cells. Anal Chem. 2018;90:11696–702. [DOI] [PubMed] [Google Scholar]
- 169.He SY, Huang JQ, Zhang Q, Zhao W, Xu ZA, Zhang W. Bamboo-like nanozyme based on nitrogen-doped carbon nanotubes encapsulating cobalt nanoparticles for wound antibacterial applications. Adv Functional Mater. 2021;31:41. [Google Scholar]
- 170.Ding SS, He L, Bian XW, Tian G. Metal-organic frameworks-based nanozymes for combined cancer therapy. Nano Today. 2020;35:25. [Google Scholar]
- 171.Yu B, Wang W, Sun WB, Jiang CH, Lu LH. Defect engineering enables synergistic action of enzyme-mimicking active centers for high-efficiency tumor therapy. J Am Chem Soc. 2021;143:8855–65. [DOI] [PubMed] [Google Scholar]
- 172.Chong G, Zang J, Han Y, Su R, Weeranoppanant N, Dong H, Li Y. Bioengineering of nano metal-organic frameworks for cancer immunotherapy. Nano Res. 2021;14:1244–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guan YJ, Li M, Dong K, Gao N, Ren JS, Zheng YC, Qu XG. Ceria/POMs hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials. 2016;98:92–102. [DOI] [PubMed] [Google Scholar]
- 174.Hao CL, Qu AH, Xu LG, Sun MZ, Zhang HY, Xu CL, Kuang H. Chiral Molecule-mediated Porous CuO nanoparticle clusters with antioxidation activity for ameliorating Parkinson’s disease. J Am Chem Soc. 2019;141:1091–9. [DOI] [PubMed] [Google Scholar]
- 175.Zhang SF, Liu Y, Sun S, Wang JY, Li QF, Yan RJ, Gao YL, Liu HL, Liu SJ, Hao WT, et al. Catalytic patch with redox Cr/CeO nanozyme of noninvasive intervention for brain trauma. Theranostics. 2021;11:2806–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu X, Yan Z, Zhang Y, Liu Z, Sun Y, Ren J, Qu X. Two-dimensional metal-organic framework/enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano. 2019;13:5222–30. [DOI] [PubMed] [Google Scholar]
- 177.Xi JQ, Wei G, An LF, Xu ZB, Xu ZL, Fan L, Gao LZ. Copper/carbon hybrid nanozyme: tuning catalytic activity by the copper state for antibacterial therapy. Nano Lett. 2019;19:7645–54. [DOI] [PubMed] [Google Scholar]
- 178.Ou H, Qian Y, Yuan L, Li H, Zhang L, Chen S, Zhou M, Yang G, Wang D, Wang Y. Spatial position regulation of Cu single atom site realizes efficient nanozyme photocatalytic bactericidal activity. Adv Mater. 2023;35: e2305077. [DOI] [PubMed] [Google Scholar]
- 179.Han L, Geng X, Zhang J, Sun S, Li J, Zhao M. Cu-based nanozymes effectively inhibit proliferation of fungal pathogens by catalyzing ROS yield. Mater Lett. 2024;355: 135509. [Google Scholar]
- 180.Yuan Y, Chen L, Song K, Cheng M, Fang L, Kong L, Yu L, Wang R, Fu Z, Sun M, et al. Stable peptide-assembled nanozyme mimicking dual antifungal actions. Nat Commun. 2024;15:5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang D, Zhang B, Ding H, Liu D, Xiang J, Gao XJ, Chen X, Li Z, Yang L, Duan H, et al. TiO2 supported single Ag atoms nanozyme for elimination of SARS-CoV2. Nano Today. 2021;40:101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Y, Tian Q, Wang H, Ma R, Han R, Wang Y, Ge H, Ren Y, Yang R, Yang H, et al. A manganese-based metal-organic framework as a cold-adapted nanozyme. Adv Mater. 2024;36: e2206421. [DOI] [PubMed] [Google Scholar]
- 183.Zhou C, Wang Q, Jiang J, Gao L. Nanozybiotics: nanozyme-based antibacterials against bacterial resistance. Antibiotics. 2022;11:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou C, Wang Q, Cao H, Jiang J, Gao L. Nanozybiotics: advancing antimicrobial strategies through biomimetic mechanisms. Adv Mater. 2024;36: e2403362. [DOI] [PubMed] [Google Scholar]
- 185.Han KN, Choi JS, Kwon J. Gold nanozyme-based paper chip for colorimetric detection of mercury ions. Scientific Reports. 2017;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shi W, He MQ, Li WT, Wei X, Bui B, Chen ML, Chen W. Cu-based metal-organic framework nanoparticles for sensing Cr(VI) Ions. Acs Appl Nano Mater. 2021;4:802–10. [Google Scholar]
- 187.Sun K, Li SY, Chen HL, Huang QG, Si YB. MnO nanozyme induced the chromogenic reactions of ABTS and TMB to visual detection of Fe and Pb ions in water. Int J Environ Anal Chem. 2019;99:501–14. [Google Scholar]
- 188.Sun K, Liu QZ, Zhu R, Liu Q, Li SY, Si YB, Huang QG. Oxidase-like catalytic performance of nano-MnO and its potential application for metal ions detection in water. Int J Analytical Chem. 2019;2019:5416963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tian X, Liao H, Wang M, Feng LY, Fu WS, Hu LZ. Highly sensitive chemiluminescent sensing of intracellular Al based on the phosphatase mimetic activity of cerium oxide nanoparticles. Biosens Bioelectronics. 2020;152:112027. [DOI] [PubMed] [Google Scholar]
- 190.Unnikrishnan B, Lien CW, Huang CC. Nanozyme based detection of heavy metal ions and its challenges: a minireview (Retracted Article). J Analysis Testing. 2019;3:206–18. [Google Scholar]
- 191.Xu XC, Luo ZJ, Ye K, Zou XB, Niu XH, Pan JM. One-pot construction of acid phosphatase and hemin loaded multifunctional metal-organic framework nanosheets for ratiometric fluorescent arsenate sensing. J Hazard Mater. 2021;412:8. [DOI] [PubMed] [Google Scholar]
- 192.Yang ZP, Liu YQ, Liu Y, Wang YY, Rao HB, Liu Y, Yin JJ, Yue GZ, Wu CM, Li H, et al. Preparation of porous uranium oxide hollow nanospheres with peroxidase mimicking activity: application to the colorimetric determination of tin(II). Microchimica Acta. 2019;186:1–8. [DOI] [PubMed] [Google Scholar]
- 193.Chen L, Zhu X, Tang J, Ouyang XL, Liao YB, Lu YT, Wang JJ, Wei ZM, Xi BD, Tang L. Porous Fe/CeO nanozyme-based hydrogel colorimetric platform for on-site detection of kanamycin. Acs Es&T Water. 2023;3:2318–27. [Google Scholar]
- 194.Li SH, Ma XH, Pang CH, Wang MY, Yin GH, Xu Z, Li JP, Luo JH. Novel chloramphenicol sensor based on aggregation-induced electrochemiluminescence and nanozyme amplification. Biosens Bioelectron. 2021;176:7. [DOI] [PubMed] [Google Scholar]
- 195.Efremenko EN, Lyagin IV, Klyachko NL, Bronich T, Zavyalova NV, Jiang YH, Kabanov AV. A simple and highly effective catalytic nanozyme scavenger for organophosphorus neurotoxins. J Control Release. 2017;247:175–81. [DOI] [PubMed] [Google Scholar]
- 196.Weerathunge P, Ramanathan R, Shukla R, Sharma TK, Bansal V. Aptamer-controlled reversible inhibition of gold nanozyme activity for pesticide sensing. Anal Chem. 2014;86:11937–41. [DOI] [PubMed] [Google Scholar]
- 197.Wei DL, Zhang XY, Chen B, Zeng K. Using bimetallic Au@Pt nanozymes as a visual tag and as an enzyme mimic in enhanced sensitive lateral-flow immunoassays: Application for the detection of streptomycin. Anal Chim Acta. 2020;1126:106–13. [DOI] [PubMed] [Google Scholar]
- 198.Zhang C, Hu JN, Wu XX, Shi JY, Hammock BD. Development of the Au@Pt-labeled nanobody lateral-flow nanozyme immunoassay for visual detection of 3-phenoxybenzoic acid in milk and lake water. Acs Agricultural Sci Technol. 2022;2:573–9. [Google Scholar]
- 199.Wang JH, Huang RL, Qi W, Su RX, Binks BP, He ZM. Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants. Appl Catalysis B-Environ. 2019;254:452–62. [Google Scholar]
- 200.Borthakur P, Boruah PK, Das P, Das MR. CuS nanoparticles decorated MoS sheets as an efficient nanozyme for selective detection and photocatalytic degradation of hydroquinone in water. New J Chem. 2021;45:8714–27. [Google Scholar]
- 201.Chen QM, Zhang XD, Li SQ, Tan JK, Xu CJ, Huang YM. MOF-derived CoO@Co-Fe oxide double-shelled nanocages as multi- functional specific peroxidase-like nanozyme catalysts for chemo/biosensing and dye degradation. Chem Eng J. 2020;395:125130. [Google Scholar]
- 202.Le PG. Research progress and prospects of nanozyme-based glucose biofuel cells. Nanomaterials. 2021;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li SQ, Hou YJ, Chen QM, Zhang XD, Cao HY, Huang YM. Promoting Active Sites in MOF-derived homobimetallic hollow nanocages as a high-performance multifunctional nanozyme catalyst for biosensing and organic pollutant degradation. ACS Appl Mater Interfaces. 2020;12:2581–90. [DOI] [PubMed] [Google Scholar]
- 204.Niu HY. Dizhang, Meng ZF, Cai YQ: Fast defluorination and removal of norfloxacin by alginate/Fe@FeO core/shell structured nanoparticles. J Hazard Mater. 2012;227:195–203. [DOI] [PubMed] [Google Scholar]
- 205.O’Mara PB, Wilde P, Benedetti TM, Andronescu C, Cheong S, Gooding JJ, Tilley RD, Schuhmann W. Cascade reactions in nanozymes: spatially separated active sites inside Ag-Core-Porous-Cu-shell nanoparticles for multistep carbon dioxide reduction to higher organic molecules. J Am Chem Soc. 2019;141:14093–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang HK, Wu X, Su L, Ma YM, Graham NJD, Yu WZ. The Fe-N-C oxidase-like nanozyme used for catalytic oxidation of NOM in surface water. Water Res. 2020;171:13. [DOI] [PubMed] [Google Scholar]
- 207.Zheng GL, Cui YF, Zhou Y, Jiang Z, Wang Q, Zhou M, Wang P, Yu YY. Photoenzymatic activity of artificial-natural bienzyme applied in biodegradation of methylene blue and accelerating polymerization of dopamine. ACS Appl Mater Interfaces. 2021;13:56191–204. [DOI] [PubMed] [Google Scholar]
- 208.Tang C, Fang TJ, Chen SK, Zhang DP, Yin JF, Wang HL. Citrate-functionalized osmium nanoparticles with peroxidase-like specific activity for highly efficient degradation of phenolic pollutants. Chem Eng J. 2023;464:10. [Google Scholar]
- 209.Wang FM, Zhang Y, Liu ZW, Ren JS, Qu XG. A mesoporous encapsulated nanozyme for decontaminating two kinds of wastewater and avoiding secondary pollution. Nanoscale. 2020;12:14465–71. [DOI] [PubMed] [Google Scholar]
- 210.Li ZH, Kang ZP, Zhu ZG. A photo-switch for enzymatic biofuel cells based on the photo-oxidization of electron acceptor in cathode by C-dots nanozyme. Chem Eng J. 2022;428:9. [Google Scholar]
- 211.Wang X, Xu YC, Cheng N, Wang XX, Huang KL, Luo YB. Recent advances in nucleic acid modulation for functional nanozyme. Catalysts. 2021;11:24. [Google Scholar]
- 212.Zhang XL, Li GL, Chen G, Wu D, Wu YN, James TD. Enzyme Mimics for engineered biomimetic cascade nanoreactors: mechanism, applications, and prospects. Adv Func Mater. 2021;31:43. [Google Scholar]
- 213.Mu XW, Liu YF, Zhang XP, Wei H, He P, Zhou HS. Using a Heme-based nanozyme as bifunctional redox mediator for Li−O2 batteries. Batteries Supercaps. 2020;3:336–40. [Google Scholar]
- 214.Ling P, Cheng S, Chen N, Qian C, Gao F. Nanozyme-modified metal-organic frameworks with multienzymes activity as biomimetic catalysts and electrocatalytic interfaces. ACS Appl Mater Interfaces. 2020;12:17185–92. [DOI] [PubMed] [Google Scholar]
- 215.Wu JJX, Wei H. Efficient design strategies for nanozymes. Progress Chem. 2021;33:42–51. [Google Scholar]
- 216.Gao L, Chen L, Zhang R, Yan X. Nanozymes: next-generation artificial enzymes. SCIENTIA SINICA Chimica. 2022;52:1649–63. [Google Scholar]
- 217.Chen Z, Yu Y, Gao Y, Zhu Z. Rational design strategies for nanozymes. ACS Nano. 2023;17:13062–80. [DOI] [PubMed] [Google Scholar]
- 218.Zhang Z, Zhang X, Liu B, Liu J. Molecular imprinting on inorganic nanozymes for hundred-fold enzyme specificity. J Am Chem Soc. 2017;139:5412–9. [DOI] [PubMed] [Google Scholar]
- 219.He S, Ma L, Zheng Q, Wang Z, Chen W, Yu Z, Yan X, Fan K. Peptide nanozymes: An emerging direction for functional enzyme mimics. Bioact Mater. 2024;42:284–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.