Abstract
Background
Fatty acid-binding protein 4 (FABP4) is an adipokine that plays significant roles in the development of insulin resistance and atherosclerosis. High levels of soluble tumor necrosis factor receptors (TNFRs) including TNFR1 and TNFR2 are associated with renal dysfunction and increased mortality in patients with diabetes mellitus (DM). However, the association between circulating levels of FABP4 and TNFRs remains unclear.
Methods
We investigated the associations of FABP4 with TNFRs and metabolic markers in Japanese patients with type 1 DM (T1DM, n = 76, men/women: 31/45) and type 2 DM (T2DM, n = 575, men/women: 312/263).
Results
FABP4 concentration was positively correlated with levels of TNFR1 and TNFR2 in both patients with T1DM and those with T2DM. Multivariable regression analyses showed that there were independent associations of FABP4 concentration with body mass index (BMI) and estimated glomerular filtration rate (eGFR) after adjustment for age and sex in both patients with T1DM and those with T2DM. FABP4 concentration was independently associated with circulating levels of TNFR1 and TNFR2 after adjustment for the confounders in patients with T2DM but not in those with T1DM. Similarly, levels of TNFR1 and TNFR2 were independently associated with FABP4 concentration after adjustment for age, sex, systolic blood pressure, duration of DM and levels of eGFR, high-density lipoprotein cholesterol, and C-reactive protein in patients with T2DM but not in those with T1DM.
Conclusion
FABP4 concentration is independently associated with levels of TNFRs in patients with DM, but the association is more evident in patients with T2DM than in those with T1DM.
Keywords: adiposcience, diabetes mellitus, fatty acid-binding protein 4, tumor necrosis factor receptor
Introduction
Diabetes mellitus (DM) is a critical issue in modern public health, and the prevalence of DM is continuously increasing worldwide (1). It has been reported that major causes of death, including cardiovascular disease, stroke, renal dysfunction, and cancers, are triggered by the presence of DM (2, 3). In a recent research field called ‘adiposcience’, new scientific concepts including ‘adipokines’ as various biologically active substances derived from adipose tissue, and metabolically driven chronic and low-grade inflammation referred to as ‘metaflammation’, have emerged to define the unique combination of overlapping mediators and/or states that contribute to immunometabolism and metabolic homeostasis (4, 5).
Type 1 DM (T1DM) is characterized by pancreatic β-cell destruction and absolute insulin deficiency, while type 2 DM (T2DM) is mainly characterized by the presence of insulin resistance and relative insulin insufficiency, which are induced by genetic factors and metabolic dysfunction including obesity (3). Several adipokines and metaflammation in adipose tissue have been reported to reflect inflammatory processes in T2DM (6). However, there has been little investigation of the metaflammation in T1DM (7).
Fatty acid-binding protein 4 (FABP4) is expressed in adipocytes, macrophages, and capillary and injured arterial endothelial cells, and excessive expression of FABP4 is associated with the development of insulin resistance and atherosclerosis, leading to cardiovascular diseases (8, 9, 10). FABP4 has been shown to be secreted from adipocytes in association with lipolysis via a non-classical pathway and acts as an adipokine, though there are no typical secretory signal peptides in the sequence of FABP4 (11). Recently, the endothelium has also been shown to be a major source of circulating FABP4 (12). It has been reported that circulating levels of FABP4 are associated with the development of cardiovascular disease (13, 14, 15), renal dysfunction (16, 17), and cancer (18) and that an elevated circulating level of FABP4 can be a prognostic predictor for cardiovascular death in the general population (19). Furthermore, there are distinct independent associations of FABP4 with renal dysfunction, adiposity, and hypertriglyceridemia in patients with T2DM (20). However, the effects of FABP4 on DM-associated complications have not been elucidated in patients with T1DM.
Tumor necrosis factor α (TNFα) has been shown to be an important cytokine derived from adipose tissue (5). In addition to the ligand itself, it has also been reported that tumor necrosis factor receptors (TNFRs), including TNFR1 and TNFR2, which are cell membrane-bound receptors involved in inflammation and immune response, are released into the extracellular space by enzymatic cleavage as solubilized forms (5). Recent studies have shown that elevated circulating levels of TNFR1 and TNFR2 are associated with renal dysfunction (21, 22, 23, 24, 25, 26) and can be prognostic factors for mortality in patients with T2DM (22, 27). However, the relationships between FABP4 and TNFRs, including TNFR1 and TNFR2, have not been fully addressed despite the fact that their upstream and downstream factors overlap (28, 29). In the present study, we aimed to elucidate the associations of FABP4 with TNFRs and metabolic markers including renal dysfunction in a real-world clinical setting in Japanese patients with T1DM and those with T2DM, two types of DM that have different pathophysiological phenotypes in whole body metabolism.
Materials and methods
The present study was a cross-sectional, single-center study conducted to assess the relationship between pathological conditions and several biomarkers in patients with DM who received standard and homogeneous treatment by diabetologists in Japan. The study conformed to the principles outlined in the Declaration of Helsinki and was approved by the ethics committees of Kure Medical Center and Chugoku Cancer Center (26-06) and Sapporo Medical University (3-1-77). Written informed consent was obtained from all of the subjects.
Study subjects
Japanese patients with DM were recruited at Kure Medical Center and Chugoku Cancer Center during the period from July 1, 2014, to March 31, 2016 (n = 738). Management of diabetes therapy including the use of medication for anti-diabetic drugs was performed by each attending physician. A flow chart of the study patients is shown in Supplementary Figure 1 (see section on supplementary materials given at the end of this article). Prespecified exclusion criteria were patients with DM due to specific causes, gestational DM, and those who were treated with pioglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) agonist, since the FABP4 gene is a target of PPARγ. Patients with T1DM were defined as those diagnosed with T1DM by their attending physicians and those receiving at least insulin therapy. Patients with T2DM were defined as those having DM except for T1DM and DM caused by other factors. After exclusion, a total of 651 patients with T1DM (n = 76) and T2DM (n = 575) were enrolled in the present study.
Measurements
Blood and urine samples were obtained and stored at −80°C until biochemical analyses. Concentrations of FABP4, TNFR1, and TNFR2 were measured using enzyme-linked immunosorbent assay kits for FABP4 (BioVendor, Modrice, Czech Republic), TNFR1 (DRT100; R&D Systems), and TNFR2 (DRT200; R&D Systems), respectively. Two internal serum controls were included in each assay to estimate the inter-assay coefficients of variation. The inter-assay coefficients of variation for FABP4, TNFR1, and TNFR2 were 3.7, 8.0, and 9.4%, respectively (25, 30, 31). Variables of liver function, renal function, and glucose and lipid metabolism were measured as previously described (27). The estimated glomerular filtration rate (eGFR) for Japanese individuals was calculated using an equation (32). A self-administered questionnaire survey was performed to obtain information on current smoking habit, alcohol drinking habit, and family history of DM.
Statistical analysis
Variables are presented as means ± s.d. for normal distributions or medians (interquartile ranges) for skewed variables. The normality of each variable was tested by the Shapiro–Wilk test. Comparisons between two groups for parametric and nonparametric parameters were performed by the Student’s t-test and the Mann–Whitney U test, respectively. The chi-square test was performed for intergroup differences in the percentages of parameters. Pearson's correlation analysis was performed for the correlation between two variables. Non-normally distributed variables were logarithmically transformed for regression analyses. Multivariable regression analyses were performed to identify independent associations of FABP4, TNFR1, and TNFR2 after adjustment for age, sex, and variables with significant correlations determined by Pearson’s coefficients after consideration of multicollinearity, showing the standardized regression coefficient (β), the percentage of variance for the selected independent predictors explained (R2), and Akaike’s information criterion. A P-value of less than 0.05 was considered statistically significant. The statistical power (1 – β error) as a post hoc analysis was calculated by G*Power 3.1 (33, 34) using sample size, the number of predictors, and the effect size of f2. The f2 was calculated by the adjusted coefficient of determination R2: f2 = R2/(1 − R2). All data were analyzed using EZR (35), R version 3.6.1., and JMP15.2.1 for Windows (SAS Institute, Cary, NC, USA).
Results
Characteristics of the studied patients with DM
Basal characteristics of the enrolled (n = 651) and excluded (n = 87) patients with DM are shown in Supplementary Table 1. The enrolled patients had a significantly higher rate of use of insulin and a lower rate of use of sulfonylureas and α-glucosidase inhibitors than did the excluded subjects. The FABP4 level was significantly lower in the enrolled patients than in the excluded patients. There were no significant differences in the levels of TNFR1 and TNFR2 between the excluded and enrolled patients.
Basal characteristics of the enrolled patients with T1DM and T2DM are shown in Table 1, and those divided by sex are shown in Supplementary Table 2. The patients with T2DM were significantly older than those with T1DM and included a significantly higher percentage of men than did patients with T1DM. Body mass index (BMI) and levels of triglycerides, alanine transaminase (ALT), and γ-glutamyl transpeptidase (γGTP) were significantly higher in patients with T2DM than in patients with T1DM, and fasting glucose level was significantly lower in patients with T2DM than in those with T1DM. There were no significant differences in levels of hemoglobin A1c and aspartate transaminase (AST) between patients with T1DM and those with T2DM.
Table 1.
Characteristics of the enrolled patients with DM. Variables are expressed as number (%), means ± s.d., or medians (interquartile ranges).
| T1DM (n = 76) | T2DM (n = 575) | P | |
|---|---|---|---|
| Age, years | 60 ± 14 | 65 ± 13 | 0.001 |
| Sex, men | 31 (40.8) | 312 (54.3) | 0.028 |
| BMI | 23 ± 4 | 25 ± 4 | <0.001 |
| Systolic BP, mmHg | 135 ± 16 | 139 ± 18 | 0.044 |
| Diastolic BP, mmHg | 77 ± 11 | 78 ± 12 | 0.381 |
| Heart rate, per min | 81 ± 13 | 81 ± 14 | 0.956 |
| Current smoking habit | 10 (13.5) | 111 (17.5) | 0.510 |
| Alcohol drinking habit | 17 (23.0) | 160 (25.6) | 0.672 |
| Duration of DM, years | 12 (5–26) | 14 (6–22) | 0.939 |
| Family history | |||
| DM | 36 (47.4) | 339 (59.0) | 0.064 |
| Comorbidity | |||
| Hypertension | 47 (61.8) | 422 (73.4) | 0.041 |
| Cardiovascular disease | 15 (19.7) | 209 (36.4) | 0.014 |
| Medication | |||
| Anti-diabetic drugs | |||
| Insulin | 76 (100) | 212 (36.9) | <0.001 |
| GLP-1 analogs | 1 (1.3) | 31 (5.4) | 0.160 |
| Sulfonylureas | 0 (0) | 166 (28.9) | <0.001 |
| Biguanides | 11 (14.5) | 309 (53.7) | <0.001 |
| α-glucosidase inhibitors | 5 (6.6) | 95 (16.5) | 0.026 |
| DPP-4 inhibitors | 18 (23.7) | 414 (72.0) | <0.001 |
| Glinides | 0 (0) | 49 (8.5) | 0.004 |
| SGLT2 inhibitors | 0 (0) | 10 (1.7) | 0.616 |
| Anti-dyslipidemic drugs | |||
| Statin | 29 (38.2) | 302 (52.5) | 0.020 |
| Biochemical data | |||
| Total protein, g/dL | 7.2 ± 0.4 | 7.3 ± 0.5 | 0.018 |
| AST, IU/L | 21 (17–25) | 21 (17–28) | 0.378 |
| ALT, IU/L | 18 (13–23) | 19 (14–28) | 0.017 |
| γGTP, IU/L | 21 (15–39) | 25 (18–46) | 0.007 |
| BUN, mg/dL | 17 ± 7 | 17 ± 8 | 0.977 |
| Creatinine, mg/dL | 0.72 (0.60–0.90) | 0.80 (0.64–1.00) | 0.114 |
| eGFR, mL/min/1.73 m2 | 70 ± 25 | 67 ± 23 | 0.274 |
| Uric acid, mg/dL | 4.8 ± 1.5 | 5.4 ± 1.3 | <0.001 |
| TC, mg/dL | 190 ± 30 | 182 ± 36 | 0.050 |
| LDL-C, mg/dL | 112 ± 23 | 105 ± 29 | 0.073 |
| HDL-C, mg/dL | 63 ± 17 | 51 ± 14 | < 0.001 |
| Non-HDL-C, mg/dL | 127 ± 26 | 130 ± 33 | 0.430 |
| Triglycerides, mg/dL | 75 (52–100) | 109 (77–169) | <0.001 |
| CRP, mg/dL | 0.07 (0.05–0.13) | 0.11 (0.06–0.20) | 0.009 |
| Glucose, mg/dL | 173 (115–218) | 140 (118–171) | 0.047 |
| Hemoglobin A1c, % | 7.4 (6.7–8.1) | 7.1 (6.5–7.9) | 0.097 |
| C-peptide, ng/mL | 0.15 (0.15–0.71) | 1.69 (1.12–2.54) | <0.001 |
| TNFR1, ng/mL | 1.33 (108–1.79) | 1.61 (1.28–2.18) | <0.001 |
| TNFR2, ng/mL | 2.95 (2.38–4.04) | 3.43 (2.74–4.56) | 0.005 |
| FABP4, ng/mL | 14.1 (9.0–17.3) | 17.6 (11.6–24.4) | 0.001 |
ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CRP, C-reactive protein; DM, diabetes mellitus; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; FABP4, fatty acid-binding protein 4; GLP-1, glucagon-like peptide-1; γGTP, γ-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SGLT2, sodium-glucose co-transporter-2; TC, total cholesterol; TNFR, tumor necrosis factor receptor; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Levels of FABP4 and TNFRs in patients with T1DM and T2DM
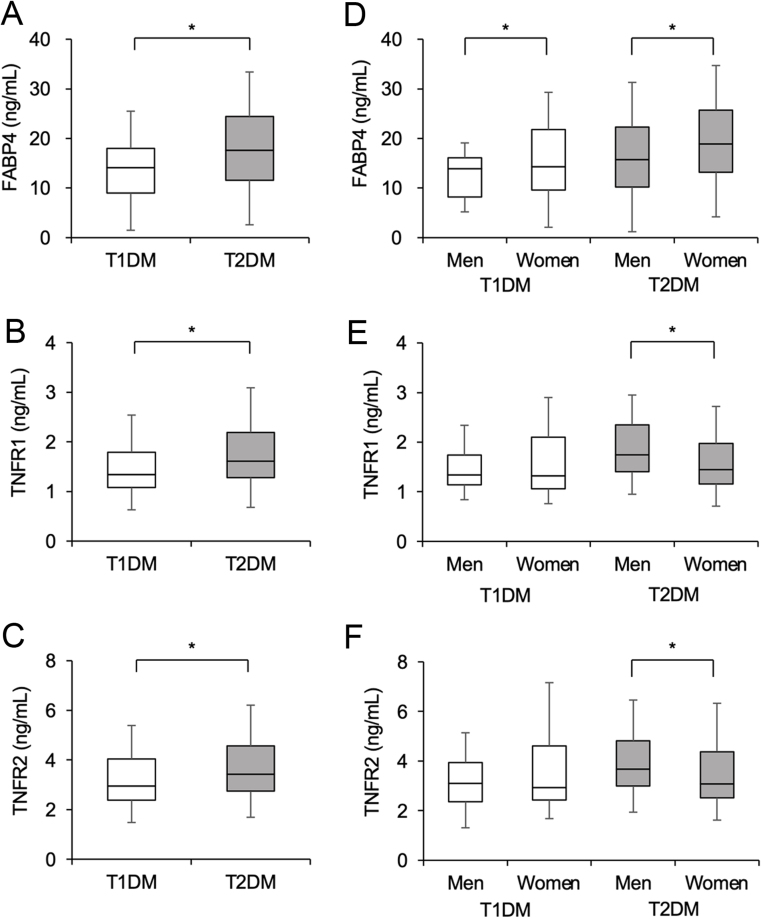
Levels of FABP4 (Fig. 1A), TNFR1 (Fig. 1B), and TNFR2 (Fig. 1C) were significantly higher in patients with T2DM than in those with T1DM. When sex was separately analyzed in the T1DM and T2DM groups, FABP4 concentration was significantly lower in men than in women (Fig. 1D). There were no significant differences in levels of TNFR1 (Fig. 1E) and TNFR2 (Fig. 1F) between men and women in patients with T1DM. In patients with T2DM, levels of TNFR1 and TNFR2 were significantly higher in men than in women.
Figure 1.
Concentrations of FABP4, TNFR1, and TNFR2. (A–C) Concentrations of fatty acid-binding protein 4 (FABP4) (A), tumor necrosis factor receptor (TNFR) 1 (B), and TNFR2 (C) in patients with type 1 diabetes mellitus (T1DM, n = 76) and those with type 2 diabetes mellitus (T2DM, n = 575). (D–F) Concentrations of FABP4 (D), TNFR1 (E), and TNFR2 (F) in patients with T1DM (men/women: 31/45) and those with T2DM (men/women: 312/263) divided by sex. Box-and-whisker plots show the median, the first quartile (Q1), the third quartile (Q3), lower outlier (Q1 – 1.5 × interquartile range (IQR)), and higher outlier (Q3 + 1.5 × IQR). *P < 0.05.
Correlation analyses for FABP4 in patients with T1DM and T2DM
In both patients with T1DM and those with T2DM, FABP4 concentration was positively correlated with BMI and triglyceride levels and was negatively correlated with eGFR (Table 2). The FABP4 concentration was positively correlated with levels of AST, ALT, and γGTP in patients with T1DM but not in those with T2DM. There were significant positive correlations of FABP4 concentration with levels of TNFR1 and TNFR2 in both patients with T1DM and those with T2DM (Table 2).
Table 2.
Correlation analyses for FABP4 in patients with DM.
| Log FABP4 | ||||
|---|---|---|---|---|
| T1DM (n = 76) | T2DM (n = 575) | |||
| r | P | r | P | |
| Age | 0.029 | 0.802 | −0.005 | 0.913 |
| BMI | 0.392 | <0.001 | 0.316 | <0.001 |
| Systolic BP | 0.159 | 0.170 | 0.058 | 0.160 |
| Diastolic BP | 0.013 | 0.914 | 0.021 | 0.611 |
| Heart rate | −0.077 | 0.510 | 0.076 | 0.068 |
| Log (duration of DM) | 0.218 | 0.061 | 0.062 | 0.144 |
| Total protein | 0.004 | 0.716 | −0.043 | 0.305 |
| Log AST | 0.273 | 0.017 | 0.036 | 0.386 |
| Log ALT | 0.248 | 0.031 | 0.062 | 0.124 |
| Log γGTP | 0.270 | 0.019 | 0.054 | 0.191 |
| BUN | 0.459 | <0.001 | 0.231 | <0.001 |
| Log creatinine | 0.506 | <0.001 | 0.272 | <0.001 |
| eGFR | −0.510 | <0.001 | −0.297 | <0.001 |
| Uric acid | 0.187 | 0.106 | 0.203 | <0.001 |
| TC | 0.001 | 0.935 | 0.001 | 0.996 |
| LDL-C | 0.015 | 0.898 | 0.006 | 0.887 |
| HDL-C | −0.178 | 0.250 | −0.124 | 0.003 |
| Non-HDL-C | 0.126 | 0.277 | 0.051 | 0.222 |
| Log triglycerides | 0.344 | 0.002 | 0.128 | 0.002 |
| Log CRP | 0.202 | 0.081 | 0.106 | 0.012 |
| Log glucose | 0.078 | 0.503 | −0.023 | 0.576 |
| Log (hemoglobin A1c) | 0.221 | 0.054 | 0.072 | 0.086 |
| Log C-peptide | 0.184 | 0.112 | 0.202 | <0.001 |
| Log TNFR1 | 0.546 | <0.001 | 0.366 | <0.001 |
| Log TNFR2 | 0.519 | <0.001 | 0.334 | <0.001 |
ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CRP, C-reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FABP4, fatty acid-binding protein 4; γGTP, γ-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TNFR, tumor necrosis factor receptor; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
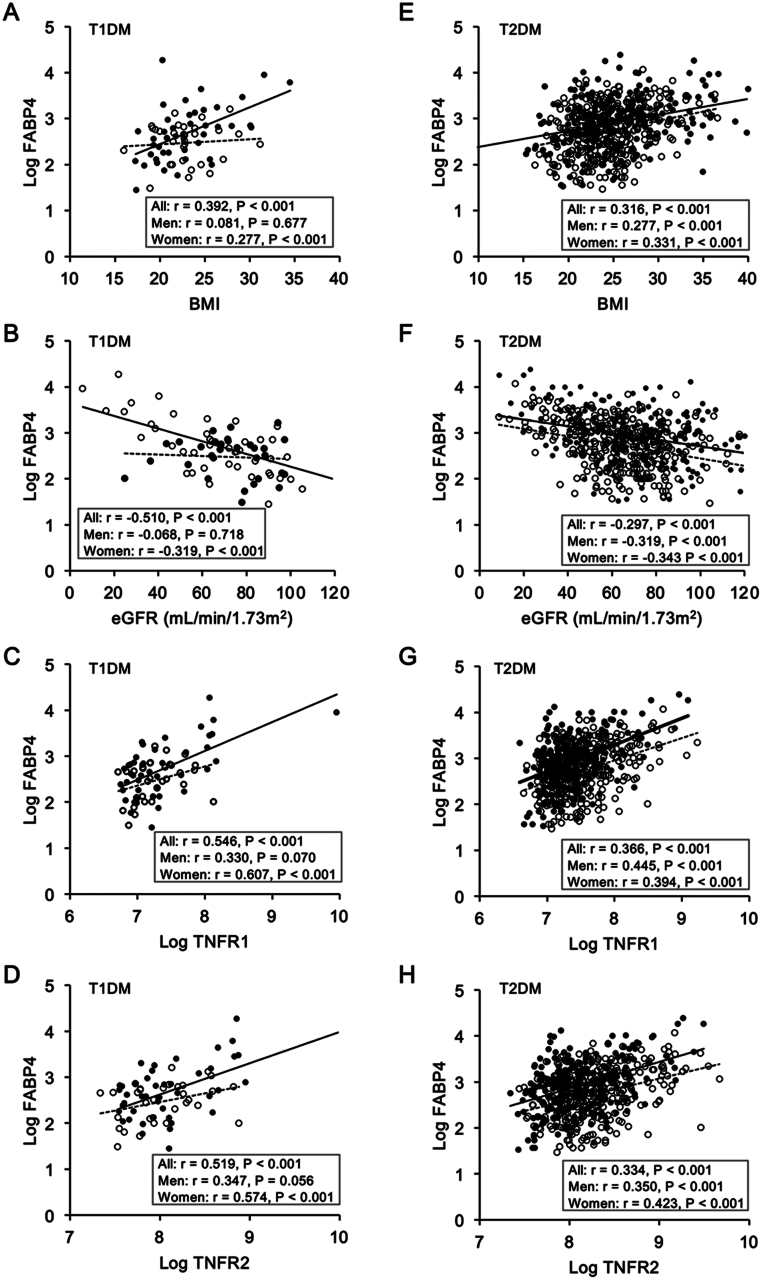
When sex was separately analyzed in patients with T1DM (n = 76, men/women: 31/45), FABP4 concentration was significantly correlated with BMI (Fig. 2A) and with levels of eGFR (Fig. 2B), uric acid, triglycerides, TNFR1 (Fig. 2C) and TNFR2 (Fig. 2D) in women but not in men (Supplementary Table 3). On the other hand, FABP4 concentration was significantly correlated with systolic and diastolic blood pressures and levels of AST, ALT, and γGTP in men but not in women (Supplementary Table 3).
Figure 2.
Correlations of FABP4 levels with metabolic parameters. (A–D) Levels of logarithmically transformed (Log) fatty acid-binding protein 4 (FABP4) were plotted against levels of body mass index (BMI) (A), estimated glomerular filtration rate (eGFR) (B), Log tumor necrosis factor receptor (TNFR)1 (C) and TNFR2 (D) in patients with type 1 diabetes mellitus (T1DM, n = 76).(E–H) Levels of Log FABP4 were plotted against levels of BMI (E), eGFR (F), Log TNFR1 (G), and Log TNFR2 (H) in patients with type 2 diabetes mellitus (T2DM, n = 575). Open circles and broken regression lines: men. Closed circles and solid regression lines: women.
In patients with T2DM (n = 575, men/women: 312/263), FABP4 concentration was significantly correlated with BMI (Fig. 2E) and levels of eGFR (Fig. 2F), uric acid, triglycerides, TNFR1 (Fig. 2G), and TNFR2 (Fig. 2H) in both men and women (Supplementary Table 3).
Multivariable regression analyses for FABP4 in patients with T1DM and T2DM
Multivariable regression analyses using age, sex, and variables with significant correlations after consideration of multicollinearity, including BMI as a marker of obesity, eGFR as an indicator of renal function, and triglycerides as a lipid marker, showed that FABP4 level was independently associated with eGFR but with the level of TNFR1 (Model 1) or TNFR2 (Model 2) in patients with T1DM (Table 3). There was no significant interaction of age, sex, or duration of DM for the association of FABP4 level with the level of TNFR1 (P values: 0.470/0.612/0.563, respectively) or TNFR2 (P values: 0.215/0.620/0.276, respectively) in patients with T1DM. The statistical powers of Models 1 and 2 for FABP4 were both 0.999 in patients with T1DM. When additionally testing at a significance level of 0.01 or 0.001, the statistical powers of multivariable regression analyses for FABP4 in Models 1 and 2 were 0.995/0.967 and 0.995/0.968, respectively, in patients with T1DM.
Table 3.
Multivariable regression analyses for Log FABP4 in patients with DM.
| T1DM (n = 76) | T2DM (n = 575) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| β | P | β | P | β | P | β | P | |
| Age | −0.126 | 0.288 | −0.143 | 0.216 | −0.107 | 0.013 | −0.125 | 0.004 |
| Sex (men) | −0.125 | 0.198 | −0.119 | 0.221 | −0.232 | <0.001 | −0.228 | <0.001 |
| BMI | 0.207 | 0.079 | 0.206 | 0.079 | 0.280 | <0.001 | 0.286 | <0.001 |
| eGFR | −0.335 | 0.048 | −0.366 | 0.016 | −0.238 | <0.001 | −0.273 | <0.001 |
| Log triglycerides | 0.128 | 0.260 | 0.136 | 0.231 | 0.003 | 0.930 | 0.007 | 0.851 |
| Log TNFR1 | 0.106 | 0.180 | – | – | 0.281 | <0.001 | – | – |
| Log TNFR2 | – | – | 0.189 | 0.169 | – | – | 0.247 | <0.001 |
| AIC | 99 | 99 | 736 | 742 | ||||
| R2 | 0.403 | 0.419 | 0.303 | 0.295 | ||||
| Adjusted R2 | 0.367 | 0.368 | 0.296 | 0.288 | ||||
| Statistical power (1 − β error) | 0.999 | 0.999 | 1.000 | 1.000 | ||||
AIC, Akaike’s information criterion; β, standardized regression coefficient; BMI, body mass index; eGFR, estimated glomerular filtration rate; FABP4, fatty acid-binding protein 4; TNFR, tumor necrosis factor receptor; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
In patients with T2DM, the FABP4 level was independently associated with the level of TNFR1 (β = 0.281, P < 0.001) (Model 1) or TNFR2 (β = 0.247, P < 0.001) (Model 2) after adjustment for confounders including age, sex, BMI, and levels of eGFR and triglycerides (Table 3). There was no significant interaction of age, sex, or duration of DM for the association of FABP4 level with the level of TNFR1 (P values: 0.351/0.462/0.141, respectively) or TNFR2 (P values: 0.436/0.441/0.226, respectively) in patients with T2DM. The statistical powers of Models 1 and 2 for FABP4 were all the same (1.000) in patients with T2DM.
After additional adjustment of smoking habits and medications for DM, the FABP4 level was not independently associated with the level of TNFR1 (Model 3) or TNFR2 (Model 4) in patients with T1DM (Supplementary Table 4). On the other hand, the FABP4 level was independently associated with the level of TNFR1 (Model 3) or TNFR2 (Model 4) in patients with T2DM.
Moreover, multivariable regression analyses after adjustment for glucose, C-reactive protein (CRP), cardiovascular disease, and use of statin, in addition to age, sex, BMI, eGFR, and triglycerides, were analyzed to investigate potential confounders. The results (Models 5 and 6) (Supplementary Table 5) were similar to the results of Models 1 and 2 (Table 3).
Correlation and multivariable regression analyses for TNFRs in patients with T1DM and T2DM
There was a strong correlation between concentrations of TNFR1 and TNFR2 in both patients with T1DM (r = 0.956, P < 0.001) and those with T2DM (r = 0.940, P < 0.001) (Table 4). Levels of TNFR1 and TNFR2 were positively correlated with systolic blood pressure, duration of DM, and level of CRP and were negatively correlated with levels of eGFR and high-density lipoprotein cholesterol (HDL-C) in both patients with T1DM and those with T2DM (Table 4).
Table 4.
Correlation analyses for TNFRs in patients with DM.
| Log TNFR1 | Log TNFR2 | |||||||
|---|---|---|---|---|---|---|---|---|
| T1DM (n = 76) | T2DM (n = 575) | T1DM (n = 76) | T2DM (n = 575) | |||||
| r | P | r | P | r | P | r | P | |
| Age | 0.172 | 0.138 | 0.280 | <0.001 | 0.196 | 0.090 | 0.326 | <0.001 |
| BMI | 0.289 | 0.012 | 0.025 | 0.550 | 0.260 | 0.024 | −0.002 | 0.968 |
| Systolic BP | 0.395 | <0.001 | 0.134 | 0.001 | 0.315 | 0.006 | 0.126 | 0.025 |
| Diastolic BP | 0.037 | 0.748 | −0.128 | 0.002 | 0.018 | 0.876 | −0.125 | 0.003 |
| Heart rate | −0.030 | 0.796 | −0.001 | 0.866 | 0.040 | 0.732 | −0.043 | 0.308 |
| Log (duration of DM) | 0.302 | 0.008 | 0.238 | <0.001 | 0.264 | 0.023 | 0.244 | <0.001 |
| Total protein | −0.178 | <0.001 | 0.103 | 0.374 | −0.165 | <0.001 | 0.102 | 0.381 |
| Log AST | 0.339 | 0.003 | 0.900 | 0.005 | 0.340 | 0.003 | 0.079 | 0.058 |
| Log ALT | 0.268 | 0.020 | −0.105 | 0.012 | 0.235 | 0.041 | −0.062 | 0.134 |
| Log γGTP | 0.449 | <0.001 | 0.040 | 0.344 | 0.421 | <0.001 | 0.071 | 0.088 |
| BUN | 0.716 | <0.001 | 0.661 | <0.001 | 0.691 | <0.001 | 0.613 | <0.001 |
| Log creatinine | 0.852 | <0.001 | 0.747 | <0.001 | 0.776 | <0.001 | 0.709 | <0.001 |
| eGFR | −0.762 | <0.001 | −0.655 | <0.001 | −0.713 | <0.001 | −0.641 | <0.001 |
| Uric acid | 0.321 | 0.004 | 0.328 | <0.001 | 0.320 | 0.005 | 0.328 | <0.001 |
| TC | −0.167 | 0.149 | −0.250 | <0.001 | −0.246 | 0.036 | −0.287 | 0.089 |
| LDL-C | −0.120 | 0.301 | −0.245 | <0.001 | −0.158 | 0.174 | −0.279 | <0.001 |
| HDL-C | −0.235 | 0.041 | −0.268 | <0.001 | −0.312 | 0.006 | −0.268 | <0.001 |
| Non-HDL-C | −0.042 | 0.716 | −0.157 | <0.001 | −0.084 | 0.470 | −0.196 | <0.001 |
| Log triglycerides | 0.228 | 0.048 | 0.102 | 0.015 | 0.191 | 0.098 | 0.078 | 0.062 |
| Log CRP | 0.486 | <0.001 | 0.254 | <0.001 | 0.471 | <0.001 | 0.253 | <0.001 |
| Log glucose | 0.014 | 0.908 | 0.012 | 0.769 | 0.028 | 0.809 | 0.001 | 0.992 |
| Log (hemoglobin A1c) | −0.029 | 0.807 | −0.034 | 0.420 | −0.001 | 0.996 | −0.056 | 0.180 |
| Log C-peptide | 0.385 | <0.001 | 0.096 | 0.022 | 0.283 | 0.013 | 0.070 | 0.093 |
| Log TNFR1 | – | – | – | – | 0.956 | <0.001 | 0.940 | <0.001 |
| Log TNFR2 | 0.956 | <0.001 | 0.940 | <0.001 | – | – | – | – |
| Log FABP4 | 0.546 | <0.001 | 0.366 | <0.001 | 0.519 | <0.001 | 0.334 | <0.001 |
ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CRP, C-reactive protein; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FABP4, fatty acid-binding protein 4; γGTP, γ-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; TNFR, tumor necrosis factor receptor.
Multivariable regression analyses showed that FABP4 concentration was not an independent predictor for TNFR1 or TNFR2 after adjustment for age, sex, and variables with significant correlations after consideration of multicollinearity, including systolic blood pressure, duration of DM, and levels of eGFR, HDL-C, and CRP in patients with T1DM (Table 5). The statistical powers for TNFR1 or TNFR2 were 0.999 in patients with T1DM. In patients with T2DM, FABP4 concentration was independently associated with levels of TNFR1 and TNFR2 after adjustment of the confounders. The statistical powers for TNFR1 or TNFR2 were 1.000 in patients with T2DM.
Table 5.
Multivariable regression analyses for Log TNFRs in patients with DM.
| T1DM (n = 76) | T2DM (n = 575) | |||||||
|---|---|---|---|---|---|---|---|---|
| Log TNFR1 | Log TNFR2 | Log TNFR1 | Log TNFR2 | |||||
| β | P | β | P | β | P | β | P | |
| Age | −0.258 | <0.001 | −0.179 | 0.040 | −0.029 | 0.417 | 0.051 | 0.177 |
| Sex (men) | 0.087 | 0.166 | 0.068 | 0.376 | 0.110 | <0.001 | 0.111 | <0.001 |
| Systolic BP | 0.224 | <0.001 | 0.141 | 0.073 | 0.071 | 0.015 | 0.054 | 0.071 |
| Log (duration of DM) | 0.107 | 0.085 | 0.096 | 0.116 | 0.045 | 0.152 | 0.034 | 0.295 |
| eGFR | −0.685 | <0.001 | −0.600 | <0.001 | −0.561 | <0.001 | −0.516 | <0.001 |
| HDL-C | −0.067 | 0.288 | −0.167 | 0.034 | −0.124 | <0.001 | −0.134 | <0.001 |
| Log CRP | 0.242 | <0.001 | 0.229 | 0.009 | 0.193 | <0.001 | 0.200 | <0.001 |
| Log FABP4 | 0.085 | 0.247 | 0.104 | 0.249 | 0.187 | <0.001 | 0.173 | <0.001 |
| AIC | 38 | 40 | 212 | 156 | ||||
| R2 | 0.781 | 0.669 | 0.551 | 0.540 | ||||
| Adjusted R2 | 0.754 | 0.629 | 0.545 | 0.519 | ||||
| Statistical power (1 − β error) | 0.999 | 0.999 | 1.000 | 1.000 | ||||
AIC, Akaike’s information criterion; β, standardized regression coefficient; BP, blood pressure; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; FABP4, fatty acid-binding protein 4; HDL-C, high-density lipoprotein cholesterol; TNFR, tumor necrosis factor receptor; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Discussion
The present study showed that circulating FABP4 concentration was independently associated with levels of TNFR1 and TNFR2 after adjustment for age, sex, BMI, and levels of eGFR and triglycerides in patients with T2DM. Similarly, both levels of TNFR1 and TNFR2 were independently associated with FABP4 levels after adjustment for age, sex, systolic blood pressure, duration of DM, and levels of eGFR, HDL-C, and CRP in patients with T2DM. We confirmed our hypothesis that FABP4 and TNFRs are associated with each other independent of metabolic markers, including renal dysfunction. To the best of our knowledge, there were two previous studies that focused on the association between levels of FABP4 and TNFR1. In a study using 81 Spanish women, including 43 morbidly obese patients (BMI > 40), circulating FABP4 levels were significantly higher in obese subjects than in non-obese subjects and were associated with TNFR1 levels after adjustment for age and BMI (28). The other study, using 282 human immunodeficiency virus 1-infected patients, showed that circulating FABP4 levels were positively correlated with TNFR1 levels (29). In the present study, there was a strong correlation between levels of TNFR1 and TNFR2. Furthermore, FABP4 concentration was independently associated with levels of not only TNFR1 but also TNFR2 in patients with T2DM. Taken together, these findings support the notion that circulating FABP4 is significantly associated with soluble TNFRs in patients with metabolic disorders, suggesting a link between FABP4 and TNF bioactivity, including insulin resistance, lipolysis, and metaflammation (5).
Distinct independent associations of FABP4 with adiposity and renal dysfunction were confirmed in both patients with T1DM and those with T2DM (Table 3). However, the association between FABP4 and TNFRs was not found in patients with T1DM after adjustment for the confounders. Although the precise reasons for the discrepant results in the T1DM and T2DM groups were not elucidated in the present study, there are at least two possibilities. First, since the number of patients with T1DM (n = 76) and the number of patients with T2DM (n = 575) who were enrolled in this study were different, the difference of statistical power may be involved in the discrepant results. Indeed, the correlation coefficients of FABP4 with TNFR1 (T1DM vs T2DM: r = 0.546 vs r = 0.366) and TNFR2 (T1DM vs T2DM: r = 0.519 vs r = 0.334) were even larger in patients with T1DM than in those with T2DM (Table 2). The small number of patients with T1DM may also have influenced the discrepant results between men (n = 31) and women (n = 45) in simple correlation analyses for FABP4 (Fig. 2, Supplementary Table 3). Second, although T1DM and T2DM share the same aspects of impaired insulin action, their upstream pathophysiological mechanisms are substantially different, with insulin deficiency underlying T1DM and insulin resistance being central to the pathogenesis in most patients with T2DM. Considering that FABP4 is particularly associated with insulin resistance (8, 9, 10), it is plausible that differences in these correlations are due to differences in glucose metabolism and insulin resistance. Nevertheless, further studies are needed to clarify the association between FABP4 and TNFRs using a sufficient number of patients with T1DM.
Regarding insights into possible biological mechanisms linking FABP4 and TNF-related molecules, it has been reported that the inhibition of FABP4 decreased the gene expression of TNFα in the adipose tissue of obese mice (36) and that the knockdown of FABP4 suppressed the inflammatory response by downregulating the elevated levels of cellular inflammatory factors including TNFα in cigarette smoke extract-mediated 16HBE cells (37). FABP4 has also been shown to promote lipolysis and inflammation in differentiated 3T3-L1 adipocytes with elevated gene expression of TNFα (38). Since chronic inflammation has been reported to be one of the leading features of DM and DM-related complications (39, 40), inflammation and/or metaflammation in patients with DM may be a pivotal common pathway linking circulating FABP4 and TNFRs. Indeed, we previously showed that adenovirus-mediated overexpression of FABP4 in human coronary artery endothelial cells increased inflammatory cytokines, including TNFα (41). Furthermore, treatment of human renal glomerular endothelial cells or mouse podocytes with palmitate-bound recombinant FABP4 significantly increased the gene expression of inflammatory cytokines, including TNFα, and the effects of FABP4 in podocytes were attenuated in the presence of an anti-FABP4 antibody (42). The findings of significant correlations between circulating levels of FABP4 and TNFRs in patients with DM may be implicated in underlying mechanisms other than adipocyte-derived pathophysiological mechanisms. Since the distinct mechanisms of the association between circulating levels of FABP4 with TNFRs have not yet been elucidated, more detailed in vivo and in vitro studies are needed.
FABP4 possibly passes through the glomerulus filtration barrier since the molecular mass of FABP4 is about 15 kDa (10). On the other hand, the molecular weights of TNFR1 and TNFR2 are 55 kDa and 75 kDa, respectively (21), which are similar to that of albumin (69 kDa). Hence, the metabolic kinetics and excretion pathways of FABP4 and TNFRs regarding renal dysfunction are considered to be different. It has recently been reported that ectopic expression of FABP4 in glomerular endothelial cells is associated with proteinuria and renal dysfunction (16) and that urinary FABP4 is a possible biomarker of glomerular damage (17, 42). It has also been reported that exposure of kidney organ culture to TNFR1 or TNFR2 increases apoptosis mainly in tubules (43) and that individual knockout mouse models of TNFR1 or TNFR2 have a delay in the fibrotic response in a mouse model of tubulointerstitial fibrosis (44). Elucidation of the upstream molecular mechanisms between elevated FABP4 and TNFRs with renal dysfunction in patients with DM may contribute to the development of novel approaches against DM-associated renal damage.
The present study has several limitations. First, since the present study was a cross-sectional study, the association of FABP4 with TNFRs does not prove causality. Second, since patients were recruited from a single hospital, the possibility of sample selection bias cannot be ruled out. Third, since only Japanese people were enrolled, the results obtained in the present study might not be applicable to other races. Fourth, since only patients with DM were enrolled in the present study, comparisons of subjects with and those without DM were not investigated. Finally, other TNF-related proteins including TNFα could not be measured in the present study.
In conclusion, FABP4 concentration is independently associated with levels of TNFRs in patients with DM, but the association is more evident in patients with T2DM than in those with T1DM. Measurement of FABP4 and TNFRs in patients with DM might be useful for predicting prognosis, including renal dysfunction. Furthermore, understanding the mechanisms behind the link between FABP4 and TNFRs may enable the development of new therapeutic strategies for DM-associated complications, including renal dysfunction.
Supplementary Materials
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
MT (22K08313), TG (23K07681), and MF (23K07993) were supported by grants from the Japan Society for the Promotion of Science.
Author contribution statement
MT: conceptualization, data curation, investigation, formal analysis, visualization, roles/writing – original draft. TG: investigation, resources. NK: investigation, resources. MM: investigation, resources. TS: investigation, resources. MK: investigation, resources. EI: investigation, resources. KE: investigation, resources. YS: supervision. MF: conceptualization, data curation; formal analysis, supervision, visualization, roles/writing – original draft, and writing – review and editing. All authors read and approve the final version of the manuscript.
References
- 1.Ogurtsova K da Rocha Fernandes JD Huang Y Linnenkamp U Guariguata L Cho NH Cavan D Shaw JE & Makaroff LE. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice 201712840–50. ( 10.1016/j.diabres.2017.03.024) [DOI] [PubMed] [Google Scholar]
- 2.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB.et al.2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. European Heart Journal 202041255–323. ( 10.1093/eurheartj/ehz486) [DOI] [PubMed] [Google Scholar]
- 3.Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, Osawa H, Taguchi A, Tanizawa Y, Tobe K, et al.Japanese clinical practice guideline for diabetes 2019. Diabetology International 202011165–223. ( 10.1007/s13340-020-00439-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017542177–185. ( 10.1038/nature21363) [DOI] [PubMed] [Google Scholar]
- 5.Sethi JK & Hotamisligil GS. Metabolic Messengers: tumour necrosis factor. Nature Metabolism 202131302–1312. ( 10.1038/s42255-021-00470-z) [DOI] [PubMed] [Google Scholar]
- 6.Freitas Lima LC Braga VA do Socorro de Franca Silva M Cruz JC Sousa Santos SH de Oliveira Monteiro MM & Balarini CM. Adipokines, diabetes and atherosclerosis: an inflammatory association. Frontiers in Physiology 20156304. ( 10.3389/fphys.2015.00304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verrijn Stuart AA Schipper HS Tasdelen I Egan DA Prakken BJ Kalkhoven E & de Jager W. Altered plasma adipokine levels and in vitro adipocyte differentiation in pediatric type 1 diabetes. Journal of Clinical Endocrinology and Metabolism 201297463–472. ( 10.1210/jc.2011-1858) [DOI] [PubMed] [Google Scholar]
- 8.Furuhashi M Fucho R Gorgun CZ Tuncman G Cao H & Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. Journal of Clinical Investigation 20081182640–2650. ( 10.1172/JCI34750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuhashi M & Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nature Reviews. Drug Discovery 20087489–503. ( 10.1038/nrd2589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuhashi M. Fatty acid-binding Protein 4 in cardiovascular and metabolic diseases. Journal of Atherosclerosis and Thrombosis 201926216–232. ( 10.5551/jat.48710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mita T, Furuhashi M, Hiramitsu S, Ishii J, Hoshina K, Ishimura S, Fuseya T, Watanabe Y, Tanaka M, Ohno K, et al.FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring) 201523359–367. ( 10.1002/oby.20954) [DOI] [PubMed] [Google Scholar]
- 12.Inouye KE Prentice KJ Lee A Wang ZB Dominguez-Gonzalez C Chen MX Riveros JK Burak MF Lee GY & Hotamisligil GS. Endothelial-derived FABP4 constitutes the majority of basal circulating hormone and regulates lipolysis-driven insulin secretion. JCI Insight 20238. ( 10.1172/jci.insight.164642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuhashi M, Fuseya T, Murata M, Hoshina K, Ishimura S, Mita T, Watanabe Y, Omori A, Matsumoto M, Sugaya T, et al.Local production of fatty acid-binding Protein 4 in epicardial/perivascular fat and macrophages is linked to coronary atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 201636825–834. ( 10.1161/ATVBAHA.116.307225) [DOI] [PubMed] [Google Scholar]
- 14.Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Matsumoto M, Tanaka M, Moniwa N, Ohnishi H, Saitoh S, Shimamoto K, et al.Circulating fatty acid-binding Protein 4 concentration predicts the progression of carotid atherosclerosis in a general population without medication. Circulation Journal 2018821121–1129. ( 10.1253/circj.CJ-17-1295) [DOI] [PubMed] [Google Scholar]
- 15.Fuseya T, Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Mita T, Ishimura S, Watanabe Y, Hoshina K, Tanaka M, et al.Elevation of circulating fatty acid-binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovascular Diabetology 201413126. ( 10.1186/s12933-014-0126-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M Furuhashi M Okazaki Y Mita T Fuseya T Ohno K Ishimura S Yoshida H & Miura T. Ectopic expression of fatty acid-binding protein 4 in the glomerulus is associated with proteinuria and renal dysfunction. Nephron. Clinical Practice 2014128345–351. ( 10.1159/000368412) [DOI] [PubMed] [Google Scholar]
- 17.Okazaki Y, Furuhashi M, Tanaka M, Mita T, Fuseya T, Ishimura S, Watanabe Y, Hoshina K, Akasaka H, Ohnishi H, et al.Urinary excretion of fatty acid-binding protein 4 is associated with albuminuria and renal dysfunction. PLoS One 20149e115429. ( 10.1371/journal.pone.0115429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S Wu D Fan Z Yang J Li Y Meng Y Gao C & Zhan H. FABP4 in obesity-associated carcinogenesis: novel insights into mechanisms and therapeutic implications. Frontiers in Molecular Biosciences 20229973955. ( 10.3389/fmolb.2022.973955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito N, Furuhashi M, Koyama M, Higashiura Y, Akasaka H, Tanaka M, Moniwa N, Ohnishi H, Saitoh S, Ura N, et al.Elevated circulating FABP4 concentration predicts cardiovascular death in a general population: a 12-year prospective study. Scientific Reports 2021114008. ( 10.1038/s41598-021-83494-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuhashi M, Sakuma I, Morimoto T, Higashiura Y, Sakai A, Matsumoto M, Sakuma M, Shimabukuro M, Nomiyama T, Arasaki O, et al.Independent and distinct associations of FABP4 and FABP5 with metabolic parameters in type 2 diabetes mellitus. Frontiers in Endocrinology (Lausanne) 202011575557. ( 10.3389/fendo.2020.575557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, et al.Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. Journal of the American Society of Nephrology 201223516–524. ( 10.1681/ASN.2011060628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, et al.Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. Journal of the American Society of Nephrology 201223507–515. ( 10.1681/ASN.2011060627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohda T, Maruyama S, Kamei N, Yamaguchi S, Shibata T, Murakoshi M, Horikoshi S, Tomino Y, Ohsawa I, Gotoh H, et al.Circulating TNF receptors 1 and 2 predict mortality in patients with end-stage renal disease undergoing dialysis. Scientific Reports 2017743520. ( 10.1038/srep43520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gohda T, Nishizaki Y, Murakoshi M, Nojiri S, Yanagisawa N, Shibata T, Yamashita M, Tanaka K, Yamashita Y, Suzuki Y, et al.Clinical predictive biomarkers for normoalbuminuric diabetic kidney disease. Diabetes Research and Clinical Practice 201814162–68. ( 10.1016/j.diabres.2018.04.026) [DOI] [PubMed] [Google Scholar]
- 25.Kamei N, Yamashita M, Nishizaki Y, Yanagisawa N, Nojiri S, Tanaka K, Yamashita Y, Shibata T, Murakoshi M, Suzuki Y, et al.Association between circulating tumor necrosis factor-related biomarkers and estimated glomerular filtration rate in type 2 diabetes. Scientific Reports 2018815302. ( 10.1038/s41598-018-33590-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gohda T, Kamei N, Kubota M, Tanaka K, Yamashita Y, Sakuma H, Kishida C, Adachi E, Koshida T, Murakoshi M, et al.Fractional excretion of tumor necrosis factor receptor 1 and 2 in patients with type 2 diabetes and normal renal function. Journal of Diabetes Investigation 202112382–389. ( 10.1111/jdi.13351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakoshi M, Kamei N, Suzuki Y, Kubota M, Sanuki M, Tashiro H, Iwasawa T, Kato K, Tanaka M, Furuhashi M, et al.Circulating tumor necrosis factor-related biomarkers predict kidney function decline in Japanese patients with diabetes: an observational cohort study. Diabetes Research and Clinical Practice 2023206111017. ( 10.1016/j.diabres.2023.111017) [DOI] [PubMed] [Google Scholar]
- 28.Terra X, Quintero Y, Auguet T, Porras JA, Hernandez M, Sabench F, Aguilar C, Luna AM, Del Castillo D, Richart C, et al.FABP 4 is associated with inflammatory markers and metabolic syndrome in morbidly obese women. European Journal of Endocrinology 2011164539–547. ( 10.1530/EJE-10-1195) [DOI] [PubMed] [Google Scholar]
- 29.Escote X, Megia A, Lopez-Dupla M, Miranda M, Veloso S, Alba V, Domingo P, Pardo P, Vilades C, Peraire J, et al.A study of fatty acid binding protein 4 in HIV-1 infection and in combination antiretroviral therapy-related metabolic disturbances and lipodystrophy. HIV Medicine 201112428–437. ( 10.1111/j.1468-1293.2010.00903.x) [DOI] [PubMed] [Google Scholar]
- 30.Xu A Wang Y Xu JY Stejskal D Tam S Zhang J Wat NM Wong WK & Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clinical Chemistry 200652405–413. ( 10.1373/clinchem.2005.062463) [DOI] [PubMed] [Google Scholar]
- 31.Ishimura S, Furuhashi M, Watanabe Y, Hoshina K, Fuseya T, Mita T, Okazaki Y, Koyama M, Tanaka M, Akasaka H, et al.Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One 20138e81318. ( 10.1371/journal.pone.0081318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, et al.Revised equations for estimated GFR from serum creatinine in Japan. American Journal of Kidney Diseases 200953982–992. ( 10.1053/j.ajkd.2008.12.034) [DOI] [PubMed] [Google Scholar]
- 33.Faul F Erdfelder E Lang AG & Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 200739175–191. ( 10.3758/bf03193146) [DOI] [PubMed] [Google Scholar]
- 34.Faul F Erdfelder E Buchner A & Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior Research Methods 2009411149–1160. ( 10.3758/BRM.41.4.1149) [DOI] [PubMed] [Google Scholar]
- 35.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplantation 201348452–458. ( 10.1038/bmt.2012.244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furuhashi M, Tuncman G, Gorgun CZ, Makowski L, Atsumi G, Vaillancourt E, Kono K, Babaev VR, Fazio S, Linton MF, et al.Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007447959–965. ( 10.1038/nature05844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W Zhang Y & Zhu Q. Cigarette smoke extract-mediated FABP4 upregulation suppresses viability and induces apoptosis, inflammation and oxidative stress of bronchial epithelial cells by activating p38 MAPK/MK2 signaling pathway. Journal of Inflammation 2022197. ( 10.1186/s12950-022-00304-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dou HX Wang T Su HX Gao DD Xu YC Li YX & Wang HY. Exogenous FABP4 interferes with differentiation, promotes lipolysis and inflammation in adipocytes. Endocrine 202067587–596. ( 10.1007/s12020-019-02157-8) [DOI] [PubMed] [Google Scholar]
- 39.Tsalamandris S Antonopoulos AS Oikonomou E Papamikroulis GA Vogiatzi G Papaioannou S Deftereos S & Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. European Cardiology 20191450–59. ( 10.15420/ecr.2018.33.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez LL Garrie K & Turner MD. Type 2 diabetes - an autoinflammatory disease driven by metabolic stress. Biochimica et Biophysica Acta. Molecular Basis of Disease 201818643805–3823. ( 10.1016/j.bbadis.2018.08.034) [DOI] [PubMed] [Google Scholar]
- 41.Fuseya T Furuhashi M Matsumoto M Watanabe Y Hoshina K Mita T Ishimura S Tanaka M & Miura T. Ectopic fatty acid-binding Protein 4 expression in the vascular endothelium is involved in neointima formation after vascular injury. Journal of the American Heart Association 20176. ( 10.1161/JAHA.117.006377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka M, Moniwa N, Nogi C, Kano T, Matsumoto M, Sakai A, Maeda T, Takizawa H, Ogawa Y, Asanuma K, et al.Glomerular expression and urinary excretion of fatty acid-binding protein 4 in IgA nephropathy. Journal of Nephrology 202336385–395. ( 10.1007/s40620-022-01551-2) [DOI] [PubMed] [Google Scholar]
- 43.Al-Lamki RS Wang J Vandenabeele P Bradley JA Thiru S Luo D Min W Pober JS & Bradley JR. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB Journal 2005191637–1645. ( 10.1096/fj.05-3841com) [DOI] [PubMed] [Google Scholar]
- 44.Guo G Morrissey J McCracken R Tolley T & Klahr S. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. American Journal of Physiology 1999277F766–F772. ( 10.1152/ajprenal.1999.277.5.F766) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a