Forced mouth opening TMD model induces significant hypersensitivity and CGRP antagonist attenuates TG sensitization, confirmed by in vivo GCaMP3 Ca2+ imaging of intact TG.
Keywords: In vivo GCaMP Ca2+ imaging, Intact trigeminal ganglion neurons, TMJ injury pain model, FMO, CFA, CGRP
Abstract
Patients with temporomandibular disorders (TMDs) typically experience facial pain and discomfort or tenderness in the temporomandibular joint (TMJ), causing disability in daily life. Unfortunately, existing treatments for TMD are not always effective, creating a need for more advanced, mechanism-based therapies. In this study, we used in vivo GCaMP3 Ca2+ imaging of intact trigeminal ganglia (TG) to characterize functional activity of the TG neurons in vivo, specifically in mouse models of TMJ injury and inflammation. This system allows us to observe neuronal activity in intact anatomical, physiological, and clinical conditions and to assess neuronal function and response to various stimuli. We observed a significant increase in spontaneously and transiently activated neurons responding to mechanical, thermal, and chemical stimuli in the TG of mice with TMJ injection of complete Freund adjuvant or with forced mouth opening (FMO). An inhibitor of the calcitonin gene–related peptide receptor significantly attenuated FMO-induced facial hypersensitivity. In addition, we confirmed the attenuating effect of calcitonin gene–related peptide antagonist on FMO-induced sensitization by in vivo GCaMP3 Ca2+ imaging of intact TG. Our results contribute to unraveling the role and activity of TG neurons in the TMJ pain, bringing us closer to understanding the pathophysiological processes underlying TMJ pain after TMJ injury. Our study also illustrates the utility of in vivo GCaMP3 Ca2+ imaging of intact TG for studies aimed at developing more targeted and effective treatments for TMJ pain.
1. Introduction
Joints endow us with flexible movements like moving away from danger, securing food, intricate communication, and manipulating food or objects. Among those joints, the temporomandibular joint (TMJ) performs essential behaviors such as mastication, speech, and other jaw movement activities. The paramount role of joints is evident as 80% of joint afferent nerve fibers exhibit nociceptive properties.27 The dominance of nociceptors underscores their critical function—specialized nerve endings highly sensitive to potential damage & injury stimuli. Their prevalence indicates an evolutionary response toward immediate detection and mitigation of injury, serving a protective role for joints.45
The protective mechanism provided by joint afferents can become a double-edged sword when injured and inflamed. For instance, excessive mechanical stress can induce disc displacement in TMJ, leading to inflammation.36 Nociceptors could also cause a prolonged increase in excitability after inflammation and become more sensitive to normally innocuous stimuli.7 This peripheral sensitization in TMJ afferents, innervated exclusively by trigeminal ganglion (TG) neurons, results in temporomandibular disorder (TMD)-related pain and discomfort.7,37 As a result, the pain and discomfort occurring as a protective response to prevent further injury can become persistent, even in the absence of an immediate threat to the joint's integrity.10
TMD is the second most common musculoskeletal disorder, affecting 5% to 12% of the US population (National Institutes of Health). It consists of myalgia, arthralgia, and myofascial pain, resulting in headaches, limited jaw motion, and pain-related disability.11 Existing treatments for TMD and/or TMJ pain are not always effective in all patients, underscoring the need for more advanced, mechanism-based therapies. Therapeutic limitations stem from our poor understanding of TMJ pain mechanisms. Because TMJ pain exclusively relies on TG neurons encoding pain signals and relaying them from the periphery to the central nervous system,10 monitoring the activities of TG neurons could provide direct mechanistic information enabling identification of the pathological mechanisms of TMJ pain. We have here confirmed peripheral sensitization in TMJ injury and inflammation using in vivo GCaMP3 Ca2+ imaging of intact TG and have demonstrated the utility of GCaMP3 Ca2+ imaging for unraveling the function and activity pattern of TG neurons in the TMJ pain. Our study shows that in vivo GCaMP3 Ca2+ imaging of intact TG offers unprecedented insights into the functional alterations of TG neurons in TMJ injury and inflammation. Thus, our approach promises to bridge existing knowledge gaps and foster the development of targeted therapeutic interventions.
2. Methods
2.1. Animals
Pirt-GCaMP3 mice, prepared according to methods described previously,22,23 were used with C57BL/6 mice. Mice were housed 3 to 5 mice per cage and exposed to a 12-hour light/dark cycle. They were allowed access to water and mouse chow ad libitum. Male and female mice, aged between 6 and 12 weeks, were used for experiments. All animal procedures were approved by the University of Texas Health at San Antonio Institutional Animal Care and Use Committee. All experiments were performed following the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2. Peptides and drugs
α-Calcitonin gene–related peptide (CGRP) was acquired from Bachem (Bubendorf, Switzerland). Complete Freund adjuvant (CFA) was sourced from Sigma (Burlingame, CA). BIBN-4096 was obtained from Tocris Bioscience/Bio-Techne Corporation (Minneapolis, MN). BIBN-4096, at a concentration of 10 mg/kg, was formulated in a solution containing 5% DMSO and 5% Tween-80 dissolved in 0.9% NaCl. The prepared solution was administered intraperitoneally to the experimental subjects.
2.3. Complete Freund's Adjuvant injection
CFA (1 mg/mL) was emulsified 1:1 with phosphate-buffered saline and was injected once unilaterally into the TMJ intra-articular space (20 μL).16 As a control measure, phosphate-buffered saline was injected on the contralateral side.
2.4. Forced mouth opening
Forced mouth opening (FMO) procedure used in here was based on previous studies with modifications.15,19,39,42,44 Mice were anesthetized with Ketamine/Xylazine (80/10 mg/kg) (Zoetis, KET-00002R2; VetOne, 33197). To apply a constant force, we used a colibri retractor (Fine science tools, 17000-03) for opening mouth. FMO was conducted for 3 hours each day for 5 consecutive days. Control mice underwent the same anesthetic regimen without a sustained mouth-opening procedure.
2.5. Facial von Frey test
To measure facial pain behavior in response to mechanical stimuli, we used the facial von Frey test with paper cups.5 Mice underwent a habituation phase in which they were familiarized with the experimenter's scent and hand touch for 2 days. The mice were then acclimated to a transparent plexiglass chamber equipped with 4-oz paper cups for 2 hours daily over 3 consecutive days. After acclimation, baseline tests were performed to assess the cutaneous sensitivity of the facial area, particularly the temporomandibular region, using von Frey filaments, for 5 to 7 days. The facial von Frey test baseline was determined to be achieved when mice exhibited a threshold between 0.5 and 0.7 g. The exact thresholds were ascertained using the Dixon “up-and-down” methodology.18
2.6. Trigeminal ganglion exposure surgery for in vivo Pirt-GCaMP3 Ca2+ imaging
Mice were anesthetized by intraperitoneal injection of Ketamine (Zoetis, Parsippany-Troy Hills, NJ)/Xylazine (approximately 80/10 mg/kg) (VetOne, Boise, ID) 24 hours after the completion of CFA injection and FMO, and then ophthalmic ointment (Lacri-lube; Allergen Pharmaceuticals, Dublin, Ireland) was applied to the eyes. Hair was shaved from the cheek area to the temporomandibular region, and surgery was performed as previously described.22,40 The right-side dorsolateral skull was exposed by removing skin and muscle. A patch of dorsolateral skull (parietal bone between the right eye and ear) was removed using a dental drill (Buffalo Dental Manufacturing, Syosset, NY) to make a cranial window (∼10 × 10 mm). The TG was then exposed by aspirating overlying cortical tissue. During the surgery, the mouse's body temperature was maintained on a heating pad at 37°C ± 0.5°C and monitored by a rectal probe.
2.7. In vivo Pirt-GCaMP3 Ca2+ imaging of intact trigeminal ganglion
In vivo Pirt-GCaMP3 Ca2+ imaging of intact TG in live mice was performed for 2 to 5 hours immediately after exposure surgery. After the exposure surgery, mice were placed abdomen-down on a custom-designed platform under the microscope. For in vivo Pirt-GCaMP3 Ca2+ imaging of intact TG, the animal's head was fixed by a head holder to minimize movements from breathing and heartbeats. During the imaging session, body temperature was maintained at 37°C ± 0.5°C on a heating pad and was monitored by a rectal probe. Anesthesia was maintained with 1% to 2% isoflurane using a gas vaporizer with pure oxygen. Live images were acquired at 10 frames per cycle in frame-scan mode at ∼4.5 to 8.79 seconds per frame, ranging from 0 to 900 μm, using a 5 × 0.25 numerical aperture dry objective at 512 × 512 pixels or higher resolution with solid diode lasers tuned at 488 nm and emission at 500 to 550 nm.
To evaluate the sensitization of TG in response to different stimuli, we applied mechanical (von Frey filaments), thermal (noxious water and acetone), and chemical (capsaicin) stimuli. von Frey filament (0.4 g) was applied to the different areas of the animal's face divided by TG branches for 1 cycle. Noxious water (4°C and 50°C, 2 mL) and acetone (20 μL) were gently applied with a pipette to the fur skin of the mouse's face for 2 cycles. Capsaicin (500 μM, 10 μL) was injected intracutaneously into the different TG branches.
For the analysis of imaging data, raw image stacks were collected, deconvoluted, and imported into ImageJ (National Institutes of Health). We realigned and corrected optical planes from sequential time points using the stackreg plugin, which is based on rigid-body cross-correlation image alignment. We expressed Ca2+ signal amplitudes as a ratio of Ft (the fluorescence intensity in each frame) to F0 (the average fluorescence intensity observed during the initial 1-4 frames). Each responding cell was confirmed through a meticulous visual examination of the raw imaging data. Cells that exhibited stead-state high Ca2+ or oscillations in amplitude driven by calcium signals in the absence of external stimuli were identified as spontaneous neurons, whereas those that exhibited amplitude changes in response to specific stimuli were termed activated neurons. The cell body diameters of these neurons were quantitatively assessed using the ImageJ software, specifically using the Feret diameter measurement tool. After this evaluation, neurons were categorized based on their diameter: cells with a diameter of <20 μm were considered small, those in the 20 to 25 μm range were considered medium, and neurons with diameters >25 μm were classified as large.
2.8. Statistics
Statistical evaluations were conducted using Prism (GraphPad). Error bars represent mean ± SEM. A P < 0.05 was considered significant. The specific statistical tests used are detailed in the figure legends.
3. Results
3.1. In vivo GCaMP Ca2+ imaging enables monitoring of functional changes and activity patterns of trigeminal ganglion neurons innervating temporomandibular region (V3)
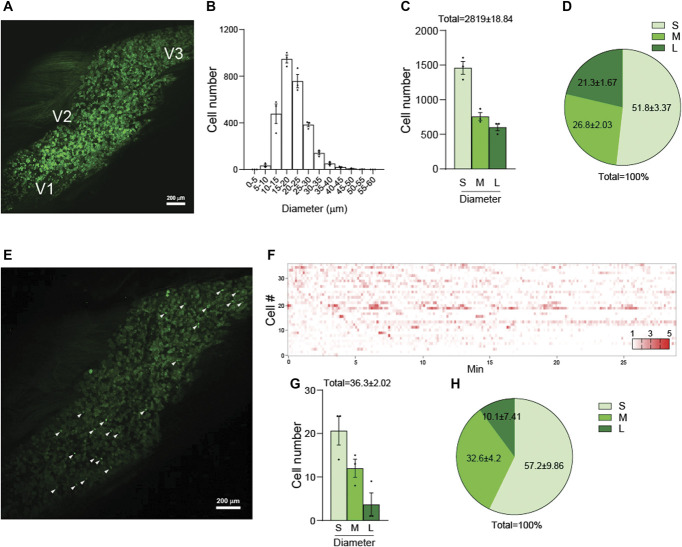
Using Pirt-GCaMP3 mice, in which the genetically encoded Ca2+ indicator GCaMP3 is specifically expressed in >95% of all peripheral sensory neurons under the control of the Pirt Promoter,22,23 we imaged intact TG in vivo (Fig. 1A). We imaged 2819 ± 18.84 neuronal cell bodies in each TG examined. Diameters of most neurons ranged from 10 to 35 μm (Fig. 1B), which we classified into 3 groups, including small-diameter (<20 μm), medium-diameter (20-25 μm), and large-diameter (>25 μm) neurons. Small-diameter TG neurons were the most numerous (Figs. 1C and D). To observe the basal condition of spontaneously activated neurons, before stimulus or drug application, we monitored TG neurons for approximately 25 minutes using in vivo Ca2+ imaging of intact TG. An average of 36.3 neurons exhibited spontaneous activity (Figs. 1E and F). Small-diameter TG neurons exhibited the highest average activation, followed by medium-diameter neurons (Figs. 1G and H).
Figure 1.
Visualization and analysis of activated neuronal populations in TG of Pirt-GCaMP3 Mice. (A) Representative image of whole TG captured using in vivo Pirt-GCaMP3 Ca2+ imaging of intact TG. The locations of neuronal cell bodies for V1 (ophthalmic), V2 (maxillary), and V3 (mandibular) are indicated. (B) Histogram depicting the cell number distribution according to cell diameter (μm) (n = 3 mice). (C) Graph showing the cell numbers categorized into 3 groups based on cell diameter: small (<20 μm), medium (20-25 μm), and large (>25 μm) (n = 3 mice). (D) Pie chart representing the percentage composition of each cell diameter size group (n = 3 mice). (E) Representative image of spontaneous activities captured through in vivo Pirt-GCaMP3 Ca 2+ imaging of intact TG. White arrowheads point to spontaneously activated neurons. (F) Representative heatmap displaying the spontaneously activated individual neurons in normal mice. (G) Bar graph showing the number of spontaneously activated neurons, categorized by cell diameter size group (n = 3 mice). (H) Pie chart showing the percentage composition of spontaneously activated neurons according to cell diameter size (n = 3 mice). S: small-diameter TG neurons (<20 μm); M: medium (20-25 μm); L: large (>25 μm). Error bars indicate SEM. TG, trigeminal ganglion.
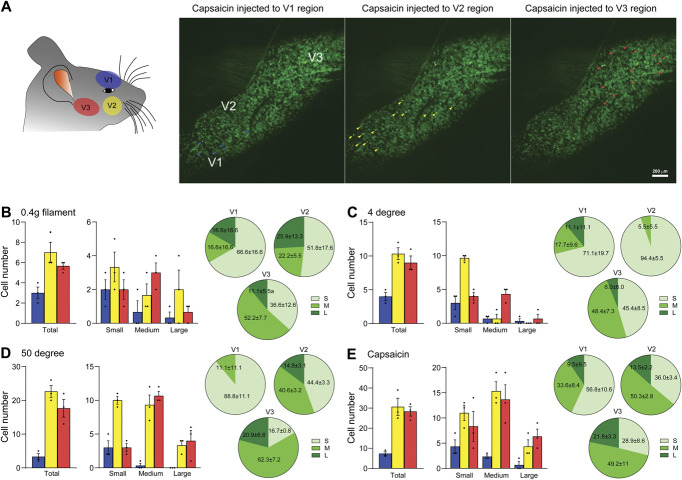
Next, to observe TG neuronal activity in response to various stimuli, we applied von Frey filament (mechanical), hot/cold water (thermal), or capsaicin (chemical) to each orofacial region innervated by ophthalmic (V1), maxillary (V2), and temporomandibular (V3) TG neurons12 during in vivo Pirt-GCaMP3 Ca2+ imaging of intact TG (Fig. 2A). In response to stimuli applied to V1, V2, and V3 regions of the face, TG neurons exhibited a region-specific activation pattern, highlighting a distinct, localized response in the TG neurons associated with each peripheral tissue (Fig. 2A, right panels). V2 and V3 neurons were more responsive to stimuli than V1 neurons (Figs. 2B–E). Neurons activated in response to stimulation in region V3 were primarily medium-diameter neurons (Figs. 2B–E). These results, using Pirt-GCaMP3 mice, indicated that in vivo GCaMP Ca2+ imaging of intact TG in living mice provides a robust tool for capturing neural dynamic responses with various stimuli under various conditions, especially within the V3 region. These techniques and tools will likely provide the capacity for precise and detailed examination of neural signaling and circuit responses.
Figure 2.
Region-specific activation patterns of TG neurons in response to various stimuli. (A) (Left) Schematic illustrating the specific regions stimulated during in vivo GCaMP3 Ca2+ imaging. (Right) Representative images depicting neurons activated after capsaicin injection in the V1, V2, or V3 regions. (B-E) (Left) Graphs showing the number of individual TG neurons activated by various stimuli: (B) 0.4-g von Frey filament, (C) 4°C water, (D) 50°C water, and (E) capsaicin (500 μM, 10 μL) in normal mice (n = 3 mice). Each graph categorizes activated neurons by cell diameter size: small (<20 μm), medium (20-25 μm), and large (>25 μm). (Right) Pie charts representing the percentage composition of each cell diameter size group (n = 3 mice). Error bars indicate SEM. TG, trigeminal ganglion.
3.2. Sensitization of trigeminal ganglion neurons in temporomandibular joint pain animal models
Animal models mimicking the high levels of inflammation observed in the TMJ of TMD patients have been developed using direct injection of chemical agents, including CFA, zymosan, carrageenan, mustard oil, monoiodoacetate, albumin, and formalin, into the TMJ to cause inflammation. It has been confirmed that intra-TMJ injections of CFA cause mechanical hyperalgesia within the TMJ in rodents.11 Consistent with this, we confirmed hypersensitivity lasting up to 7 days in the V3 region of CFA-injected mice by the von Frey test (Fig. 3A). Although the current model is essential for deciphering the inflammatory processes leading to TMJ pain and degeneration, there is a pressing need for a direct translational model that accurately replicates the pathological progression of TMJ pain. This necessity stems from the fact that the use of CFA injections only captures some aspects of the complex disease pathogenesis of TMJ pain. The FMO model involves overloading the TMJ and mechanically mimics the pathogenesis of TMJ pain.15,19,39,42,44 Here, we found that FMO for 3 hours per day for 5 days caused mechanical hypersensitivity in the V3 region, which lasted longer than the CFA-injection model (Fig. 3B). FMO–induced hyperalgesia lasts up to 32 days.44 These results suggest that, as a translational model for understanding the pathophysiology of TMJ pain, the FMO model is more representative of human TMJ pain.
Figure 3.
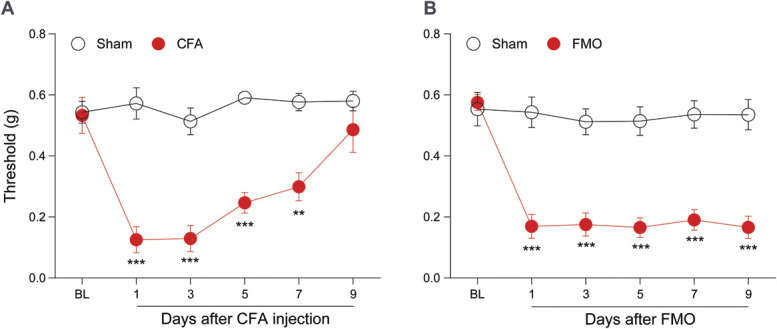
Mechanical hypersensitivity in the orofacial V3 region of CFA-injected and FMO models. (A) Graph depicting the TMJ and orofacial withdrawal thresholds, as measured by von Frey filament, after CFA injection (n = 6 mice per group). (B) Graph depicting the TMJ and orofacial withdrawal thresholds determined by von Frey filament after FMO (n = 7 mice per group). Error bars indicate SEM. **P < 0.01; ***P < 0.001; 2-way analysis of variance with Bonferroni multiple comparison post hoc test. CFA, complete Freund adjuvant; BL, baseline; FMO, forced mouth opening; TMJ, temporomandibular joint.
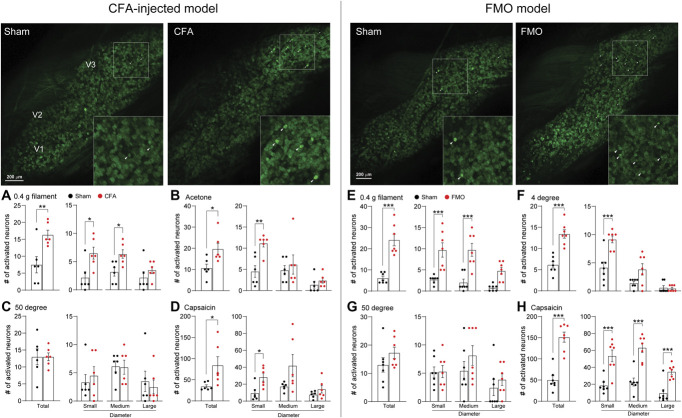
Peripheral sensitization of nociceptors usually takes 2 forms. One is a sustained spontaneous activity, and the other is a response to innocuous stimuli.10 To assess whether CFA injection or FMO alters the spontaneous activity of TG neurons, we first observed the activity of TG neurons using in vivo Pirt-GCaMP3 Ca2+ imaging of intact TG. The total number of spontaneously activated neurons increased in both the CFA and the FMO groups compared with the sham controls, and the increases were because of increases in 2 subgroups, small-diameter (<20 μm) and medium-diameter (20-25 μm) neurons (Figs. 4A and B). We next asked whether TG neurons were sensitized in response to noxious or nonnoxious stimuli. For this, we applied von Frey filament (mechanical), acetone (thermal), hot/cold water (thermal), or capsaicin (chemical) to the V3 region. After application of 0.4 g filament to the V3 region, activation of small-diameter neurons but not of medium-diameter neurons was increased in CFA-injected mice, whereas activation of both small- and medium-diameter neurons was increased in FMO mice (Figs. 5A and B). When we applied acetone to the V3 region of CFA-injected mice, the number of activated neurons was increased because of an increase in activation of small-diameter neurons (Fig. 5C). Cold (4°C) water induced an increase in activated neurons of FMO mice (Fig. 5D), but hot (50°C) water did not induce any changes in activated neurons of V3 regions of either CFA-injected or FMO mice (Figs. 5E and F). Capsaicin injection into the V3 region increased the number of activated TG neurons in both CFA-injected and FMO mice (Figs. 5G and H). In CFA-injected mice, small- and medium-diameter neurons contributed to the increase of activated neurons, whereas in FMO mice, all 3 subgroups contributed to the capsaicin-induced increase (Figs. 5G and H). These data indicate that CFA injection and FMO differentially sensitize TG neurons to various stimuli and suggest that FMO causes behavioral and cellular changes related to TMJ pain symptoms.
Figure 4.
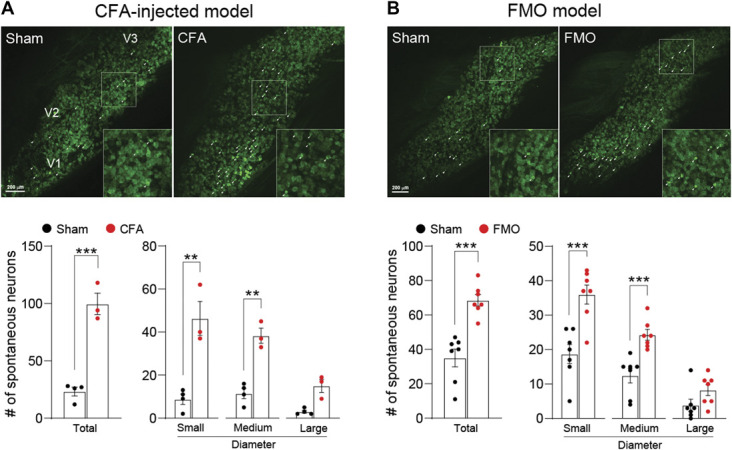
Spontaneous activity increase of TG neurons after CFA-TMJ injection or FMO. (A) (Upper) Representative images of spontaneous activities in in vivo Pirt-GCaMP3 Ca2+ imaging of intact TG in CFA-TMJ–injected mice. V1 (ophthalmic), V2 (maxillary), and V3 (mandibular) indicate the location of neuronal cell bodies in images of intact TG. White arrowheads indicate spontaneously activated neurons. (Bottom) Number of total, small, medium, and large spontaneously activated neurons from each group in CFA-TMJ–injected mice (n = 6 mice per group). (B) (Upper) Representative images of spontaneous activities in FMO mice. (Bottom) Number of total, small, medium, and large spontaneously activated neurons from each group in FMO mice (n = 7 mice per group). Error bars indicate SEM. **P < 0.01; ***P < 0.001; 2-tailed Student t test. CFA, complete Freund adjuvant; FMO, forced mouth opening; TG, trigeminal ganglion; TMJ, temporomandibular joint.
Figure 5.
Differential activation patterns of TG neurons in CFA-TMJ–injected or FMO mice in response to various mechanical, thermal, and chemical stimuli. (Upper left and right) Representative images of in vivo GCaMP Ca2+ imaging of intact TG neurons activated by 0.4-g von Frey filament onto V3 region. Number of activated TG neurons in response to various stimuli in CFA-TMJ–injected (A-D) (n = 6 mice per group) and FMO (E-H) (n = 7 mice per group) mice. The graph represents the number of individual neurons activated by 0.4-g von Frey filament (A and E), acetone (B), 4°C water (F), 50°C water (C and G), and capsaicin (D and H). Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001; 2-tailed Student t test. CFA, complete Freund adjuvant; FMO, forced mouth opening; TG, trigeminal ganglion; TMJ, temporomandibular joint.
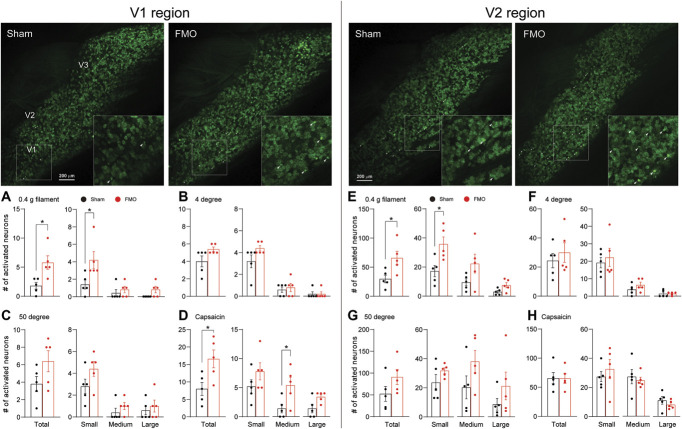
Persistent pain resulting from trigeminal nerve injury and orofacial inflammation can spread to adjacent orofacial regions.38 Notably, patients with TMDs show comorbidity with headache disorders.30 We, therefore, investigated whether FMO leads to the sensitization of adjacent orofacial regions, including the V1 and V2 regions, similar to the observations in the V3 region. We replicated the stimuli used in the V3 region to the V1 and V2 regions of FMO mice during in vivo GCaMP3 imaging of intact TG. Our results showed an increase in the activation of TG neurons in response to a 0.4-g filament stimulus and capsaicin injection applied to V1 region but not to cold (4°C) and hot (50°C) water (Figs. 6A–D). In the V2 region, a 0.4-g filament stimulus increased activated neurons only (Fig. 6E), whereas there were no differences in response to noxious temperature stimuli and capsaicin injection (Figs. 6F–H). These results may indicate that TMJ pain spreads to adjacent orofacial regions at the level of TG.
Figure 6.
Activation patterns of TG neurons in FMO mice in response to different stimuli in V1 and V2 regions. (Upper left and right) Representative images of in vivo GCaMP Ca2+ imaging of intact TG neurons activated by 0.4-g von Frey filament onto V1 (left) and V2 (right) region. Number of activated TG neurons in response to various stimuli to V1 region (A-D) (n = 5 mice per group) and V2 region (E-H) (n = 5 mice per group). The graph represents the number of individual neurons activated by 0.4-g von Frey filament (A and E), 4°C water (B and F), 50°C water (C and G), and capsaicin (D and H). Error bars indicate SEM. *P < 0.05; 2-tailed Student t test. FMO, forced mouth opening; TG, trigeminal ganglion.
3.3. Calcitonin gene–related peptide antagonist alleviates forced mouth opening–induced sensitization of trigeminal ganglion neurons
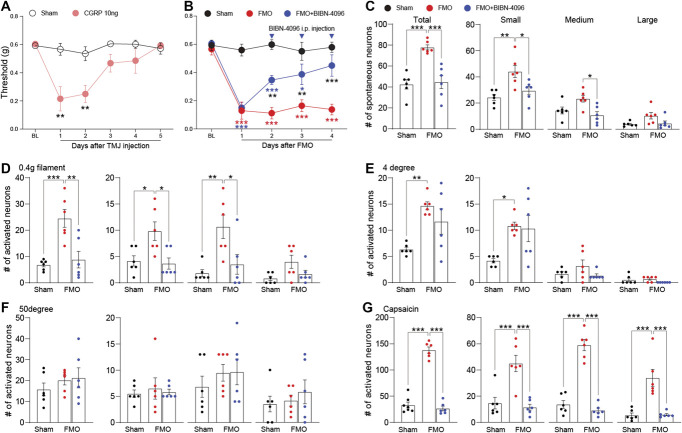
CGRP levels are elevated in the synovial tissue of patients with TMD and are associated with TMD symptoms.1,2,20,25,35 Thus, CGRP is thought to contribute to the pathogenesis of TMD, and indeed, CGRP antagonists have been shown to alleviate TMD symptoms in preclinical studies.6,21,31,41 To determine if artificially increasing CGRP levels induced pain behavior, we administered CGRP directly into the TMJ and tested for pain behavior. CGRP injection induced hypersensitivity (Fig. 7A), which lasted up to 2 days after CGRP injection. Conversely, to determine whether a CGRP receptor antagonist (BIBN-4096) inhibited pain behavior in the FMO model, we induced mechanical hypersensitivity with FMO and then intraperitoneally injected a CGRP receptor antagonist. Hypersensitivity was gradually attenuated by successive injections (Fig. 7B). We used GCaMP3 Ca2+ imaging to determine whether the CGRP receptor antagonist attenuated FMO-induced sensitization of TG neurons. The antagonist was administered on day 4 after FMO under the same conditions as in Figure 7B, and GCaMP Ca2+ imaging was performed 2 hours later. We found that the increases in activated neurons seen previously (Figs. 4B and 5E–H) were attenuated by the CGRP antagonist (Figs. 7C–G). However, the increased number of activated neurons resulting from application of cold (4°C) water was not reduced by the CGRP antagonist (Fig. 7E). These results confirm the sensitization of TG neurons in the FMO-induced TMJ pain and indicate that GCaMP Ca2+ imaging enables mechanistic studies with various drugs and stimuli.
Figure 7.
Evaluation of CGRP-induced hypersensitivity and its attenuation by a CGRP receptor antagonist in FMO mice. (A) Graph showing head withdrawal thresholds tested by von Frey filament after CGRP-TMJ injection (n = 5 mice per group). (B) Head withdrawal thresholds observed in FMO mice treated with BIBN-4096 (CGRP receptor antagonist, 10 mg/kg intraperitoneally) (n = 6 mice per group). (C) Number of total, small, medium, and large spontaneously activated neurons in FMO mice after BIBN-4096 treatment (n = 6 mice per group). (D-G) Number of individual TG neurons activated by 0.4-g von Frey filament (D), 4°C water (E), 50°C water (F), and capsaicin (G) after BIBN-4096 treatment in FMO mice. Error bars indicate SEM. *P < 0.05; **P < 0.01; ***P < 0.001; (A) 2-tailed Student t test; (B) 2-way analysis of variance with Tukey multiple comparison post hoc test; (C-G) 1-way analysis of variance with Tukey multiple comparison post hoc test. CGRP, calcitonin gene-related peptide; FMO, forced mouth opening; TMJ, temporomandibular joint; Veh, vehicle.
4. Discussion
TMJ involves daily jaw movements, such as chewing, swallowing, speech, and other automatic movements, including yawning, grinding, or clenching. TMJ dysfunction in patients with TMD can devastate daily-life activities in humans.36 However, available treatment methods and options only provide limited effectiveness. The unmet therapeutic needs of patients with TMD stem from our poor understanding of the pathophysiological mechanisms of TMJ pain. Here, we showed that in vivo GCaMP3 Ca2+ imaging of intact TG allows the simultaneous monitoring of nearly 3000 neurons in living mice and could be used to determine whether the sensitization of TG neurons occurs in clinically relevant translational TMJ pain animal models. Moreover, we confirmed that a CGRP receptor antagonist alleviates FMO-induced hypersensitivity of TG neurons. In addition, our use of in vivo GCaMP3 Ca2+ imaging of intact TG has provided nuanced insights into TG neuronal sensitization in TMJ pain, laying a foundational step towards enhanced therapeutic strategies, as evidenced by the mitigating effects of CGRP antagonist on FMO-induced hypersensitivity.
The articular disk of TMJ has a limited ability to redistribute joint stress, making this joint susceptible to damage from overloading. Overloading causes disk displacement or joint degeneration, which leads to inflammation and the release of inflammatory mediators.7 This inflammation can cause peripheral sensitization of nociceptors in the TMJ,27 ultimately resulting in pain and discomfort related to TMD.7 Because of the role that inflammation plays in the development of TMD-related pain, CFA injection into TMJ in mice provides a reasonable TMJ pain model. However, this model has limitations. CFA injections are limited in simulating the complexity of chronic inflammatory responses. Although CFA can induce acute inflammatory reactions and histological modifications in the TMJ, including extracellular matrix accumulation and soft tissue alterations, it does not cause cartilage and bone degradation. Furthermore, this model is not fully representative of the underlying causes driving the sustained pain and degeneration experienced by human patients because it does not encompass the unidentified foundational elements contributing to the persistence of these conditions.11 Because excessive TMJ activity and prolonged mouth opening are risk factors in development of TMD,7 the FMO model of TMD-related pain is attractive.15,19,39,42,44 FMO causes prolonged hypersensitivity in the V3 region of mice, which lasted for at least 32 days after FMO, which is longer than the CFA-injection model. In addition, FMO causes TMJ injury but also significantly induces masticatory muscle injury.44 These characteristics suggest that FMO is sufficient to induce sensitization of TG neurons innervating TMJ. The changes in thermal pain thresholds have been observed in patients with TMD who showed lower thermal pain thresholds to heat or cold stimuli.8,43 In particular, the hypersensitivity to cold sensation (Fig. 5), but not to heat sensation, is consistent with a lower threshold to cold stimuli, but not to heat stimuli, in the V3 region of TMD patients.8 This symptom similarity suggests that our TMJ pain model using GCaMP3 Ca2+ imaging and FMO is suitable for preclinical testing of the efficacy of therapeutic compounds.
Peripheral sensitization of the afferents originating from the TG significantly contributes to ongoing craniofacial pain.37 Peripheral neuropeptides, receptors, and ion channels activating or sensitizing the nociceptive afferents have been identified.4,17 TRPV1, TRPA1, and TRPV4 have been identified as contributing to the sensitization of TG in animal models of TMJ pain.9,26,41 However, direct studies on the sensitization of TG neurons in TMJ pain animal models still need to be completed. Here, we monitored the spontaneous activity and response to stimulation of TG neurons in live mice in TMJ pain animal models and showed that the neurons were more highly sensitized than in controls. We also confirmed that CGRP inhibitors attenuated FMO-induced TG sensitization. To our knowledge, this is the first time that the sensitization of TG neurons has been measured directly in a TMJ pain model in vivo.
Neuropeptides are known to contribute to nociceptor sensitization.3,24 In peripheral tissue, neuropeptides, including CGRP and substance P, are released from sensory neurons in a process of neurogenic inflammation and can activate their specific receptors to promote inflammation or sensitize nociceptors.29,32 CGRP has been associated with craniofacial pain, including TMD.33,37 In particular, in migraine, an increase in CGRP levels of peripheral tissues activates receptors on nociceptors and then sensitizes nociceptors.14 New medications have been developed based on this mechanism and are now commercially available for patients with migraine.13 The effectiveness of these drugs in migraine continues to be confirmed.34 In TMD, the relationship between CGRP levels and TMD symptoms has also been confirmed,1,2,20,25,35 and preclinical studies have shown antinociceptive effects of CGRP antagonists.6,21,31,41 However, the role of CGRP in TMJ pain remains unclear. In this context, our confirmation of the effect of CGRP antagonists through in vivo GCaMP Ca2+ imaging represents a significant step forward in understanding CGRP-related mechanisms in TMJ pain. As the systemic treatment of CGRP antagonists in this study did not completely exclude the involvement of the central nervous system, future studies are needed to investigate the mechanisms behind the effects of CGRP antagonists that we have identified.
TMD and primary headaches have an interrelationship in their onset or severity.30 Consistent with this, we found the mechanical sensitization of neurons from uninjured branches, innervating V1 and V2, at the TG level using in vivo GCaMP imaging. Previous studies have advanced the understanding of the mechanisms mediating the spreading sensitization of nociceptors in sensory ganglia.28,38 The spread of sensitization from injured regions to uninjured regions can be mediated by crosstalk between neurons or between neurons and satellite glial cells at the level of sensory ganglia. Gap junctions, which are formed by connexins, allow direct transmission of secondary messengers within sensory ganglia. Nitric oxide released from sensitized primary afferent neurons could alter the excitability of uninjured trigeminal nerve branches. The spread of sensitization in the sensory ganglia could be indirectly mediated by paracrine neuropeptides such as substance P and CGRP. CGRP released from TG neurons could activate other neurons, satellite glial cells, and schwann cells, increasing nitric oxide and several cytokines, including tumor necrosis factor α and interleukin 1β. However, how the sensitization spreads from the injured branch to the uninjured branch in the TMD remains to be needed further investigation. Our GCaMP3 in vivo imaging with the FMO model may provide an opportunity to reveal the unidentified mechanism of the spreading pain in TMDs.
This study confirmed that FMO, an established TMJ pain model, induces significant hypersensitivity in the V3 region, accompanied by TG sensitization, as confirmed by in vivo GCaMP3 Ca2+ imaging of intact TG. The finding reported here are the first confirmation of TG sensitization in an animal model of TMJ pain and of the antinociceptive effect of a CGRP antagonist that attenuates TG sensitization, suggesting that in vivo GCaMP Ca2+ imaging of intact TG may provide the means to fully elucidate the pathogenesis of TMD and contribute to finding new therapeutic targets in preclinical studies.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgements
Author contributions: Conceptualization: H. Son, M.-K. Chung, D. Perez, F. Amarista, E. Ellis, and Y. S. Kim. Resources and mouse strains: J. Shannonhouse and R. Gomez. Experimentation and acquisition of data: H. Son, Y. Zhang, J. Shannonhouse, and R. Gomez. Data analysis: H. Son. Writing the manuscript: H. Son, M.-K. Chung, D. Perez, F. Amarista, E. Ellis, and Y. S. Kim. Funding acquisition and supervising project: Y. S. Kim.
This work was supported by National Institutes of Health grants NIDCR DE031477 and NS128574 (Y.S.K.) and a Rising STAR Award (Y.S.K.) from the University of Texas System and NIH T32DE014318 COSTAR award (J.S.).
Data availability: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Hyeonwi Son, Email: sonh@uthscsa.edu.
John Shannonhouse, Email: shannonhouse@uthscsa.edu.
Yan Zhang, Email: ZhangY12@uthscsa.edu.
Ruben Gomez, Email: gomezr0@uthscsa.edu.
Felix Amarista, Email: amaristaroja@uthscsa.edu.
Daniel Perez, Email: PerezD5@uthscsa.edu.
Edward Ellis, Email: ellise3@uthscsa.edu.
Man-Kyo Chung, Email: MChung@umaryland.edu.
References
- [1].Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Neuropeptides in the arthritic TMJ and symptoms and signs from the stomatognathic system with special consideration to rheumatoid arthritis. J Orofac Pain 1995;9:215–25. [PubMed] [Google Scholar]
- [2].Appelgren A, Appelgren B, Kopp S, Lundeberg T, Theodorsson E. Relation between intra-articular temperature of the arthritic temporomandibular joint and presence of calcitonin gene-related peptide in the joint fluid: a clinical study. Acta Odontol Scand 1993;51:285–91. [DOI] [PubMed] [Google Scholar]
- [3].Ashina M, Hansen JM, Do TP, Melo-Carrillo A, Burstein R, Moskowitz MA. Migraine and the trigeminovascular system-40 years and counting. Lancet Neurol 2019;18:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burgos-Vega CC, Quigley LD, Trevisan dos Santos G, Yan F, Asiedu M, Jacobs B, Motina M, Safdar N, Yousuf H, Avona A, Price TJ, Dussor G. Non-invasive dural stimulation in mice: a novel preclinical model of migraine. Cephalalgia 2019;39:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cady RJ, Glenn JR, Smith KM, Durham PL. Calcitonin gene-related peptide promotes cellular changes in trigeminal neurons and glia implicated in peripheral and central sensitization. Mol Pain 2011;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cairns BE. Pathophysiology of TMD pain: basic mechanisms and their implications for pharmacotherapy. J Oral Rehabil 2010;37:391–410. [DOI] [PubMed] [Google Scholar]
- [8].Carvalho GF, Chaves TC, Florencio LL, Dach F, Bigal ME, Bevilaqua-Grossi D. Reduced thermal threshold in patients with temporomandibular disorders. J Oral Rehabil 2016;43:401–8. [DOI] [PubMed] [Google Scholar]
- [9].Chen Y, Williams SH, McNulty AL, Hong JH, Lee SH, Rothfusz NE, Parekh PK, Moore C, Gereau RW, Taylor AB, Wang F, Guilak F, Liedtke W. Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. PAIN 2013;154:1295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chichorro JG, Porreca F, Sessle B. Mechanisms of craniofacial pain. Cephalalgia 2017;37:613–26. [DOI] [PubMed] [Google Scholar]
- [11].Chung MK, Wang S, Alshanqiti I, Hu J, Ro JY. The degeneration-pain relationship in the temporomandibular joint: current understandings and rodent models. Front Pain Res 2023;4:1038808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Edvinsson JCA, Viganò A, Alekseeva A, Alieva E, Arruda R, De Luca C, D'Ettore N, Frattale I, Kurnukhina M, Macerola N, Malenkova E, Maiorova M, Novikova A, Řehulka P, Rapaccini V, Roshchina O, Vanderschueren G, Zvaune L, Andreou AP, Haanes KA; European Headache Federation School of Advanced Studies (EHF-SAS). The fifth cranial nerve in headaches. J Headache Pain 2020;21:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies: successful translation from bench to clinic. Nat Rev Neurol 2018;14:338–50. [DOI] [PubMed] [Google Scholar]
- [14].Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A, Ashina M, van den Maagdenberg AMJM, Dodick DW. Migraine. Nat Rev Dis Prim 2022;8:2. [DOI] [PubMed] [Google Scholar]
- [15].Fujisawa T, Kuboki T, Kasai T, Sonoyama W, Kojima S, Uehara J, Komori C, Yatani H, Hattori T, Takigawa M. A repetitive, steady mouth opening induced an osteoarthritis-like lesion in the rabbit temporomandibular joint. J Dent Res 2003;82:731–5. [DOI] [PubMed] [Google Scholar]
- [16].Ge JF, Gao WC, Cheng WM, Lu WL, Tang J, Peng L, Li N, Chen FH. Orcinol glucoside produces antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress. Eur Neuropsychopharmacol 2014;24:172–80. [DOI] [PubMed] [Google Scholar]
- [17].Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med 2010;16:1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gonzalez-Cano R, Boivin B, Bullock D, Cornelissen L, Andrews N, Costigan M. Up-Down reader: An open source program for efficiently processing 50% von frey thresholds. Front Pharmacol 2018;9:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hawkins JL, Durham PL. Prolonged jaw opening promotes nociception and enhanced cytokine expression. J Oral Facial Pain Headache 2016;30:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Holmlund A, Ekblom A, Hansson P, Lind J, Lundeberg T, Theodorsson E. Concentrations of neuropeptides substance P, neurokinin A, calcitonin gene-related peptide, neuropeptide Y and vasoactive intestinal polypeptide in synovial fluid of the human temporomandibular joint. A correlation with symptoms, signs and arthroscopic findings. Int J Oral Maxillofac Surg 1991;20:228–31. [DOI] [PubMed] [Google Scholar]
- [21].Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. PAIN 2017;158:543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Saijilafu, Young LA, He S, LaVinka PC, Zhou F, Bergles D, Hanani M, Guan Y, Spray DC, Dong X. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 2016;91:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, Dubner R, Wei F, Dong X. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 2014;81:873–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci 1993;13:2273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu ZM, Peng YJ, Long X, Li J, Ke J, Fang W. Mutual effect between neuropeptides and inflammatory cytokines in neurogenic SMSCs of human temporomandibular joint. J Huazhong Univ Sci Technol Med Sci 2014;34:602–7. [DOI] [PubMed] [Google Scholar]
- [26].Luo Y, Suttle A, Zhang Q, Wang P, Chen Y. Transient receptor potential (TRP) ion channels in orofacial pain. Mol Neurobiol 2021;58:2836–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McDougall JJ. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Res Ther 2006;8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Messlinger K, Balcziak LK, Russo AF. Cross-talk signaling in the trigeminal ganglion: role of neuropeptides and other mediators. J Neural Transm 2020;127:431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 2002;302:839–45. [DOI] [PubMed] [Google Scholar]
- [30].Romero-Reyes M, Klasser G, Akerman S. An update on temporomandibular disorders (TMDs) and headache. Curr Neurol Neurosci Rep 2023;23:561–70. [DOI] [PubMed] [Google Scholar]
- [31].Romero-Reyes M, Pardi V, Akerman S. A potent and selective calcitonin gene-related peptide (CGRP) receptor antagonist, MK-8825, inhibits responses to nociceptive trigeminal activation: role of CGRP in orofacial pain. Exp Neurol 2015;271:95–103. [DOI] [PubMed] [Google Scholar]
- [32].Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 2014;94:1099–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Russo AF, Hay DL. CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev 2023;103:1565–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sacco S, Amin FM, Ashina M, Bendtsen L, Deligianni CI, Gil-Gouveia R, Katsarava Z, MaassenVanDenBrink A, Martelletti P, Mitsikostas D-D, Ornello R, Reuter U, Sanchez-Del-Rio M, Sinclair AJ, Terwindt G, Uluduz D, Versijpt J, Lampl C. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention: 2022 update. J Headache Pain 2022;23:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sato J, Segami N, Kaneyama K, Yoshimura H, Fujimura K, Yoshitake Y. Relationship of calcitonin gene-related peptide in synovial tissues and temporomandibular joint pain in humans. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2004;98:533–40. [DOI] [PubMed] [Google Scholar]
- [36].Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med 2008;359:2693–705. [DOI] [PubMed] [Google Scholar]
- [37].Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol 2011;97:179–206. [DOI] [PubMed] [Google Scholar]
- [38].Shinoda M, Kubo A, Hayashi Y, Iwata K. Peripheral and central mechanisms of persistent orofacial pain. Front Neurosci 2019;13:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sobue T, Yeh WC, Chhibber A, Utreja A, Diaz-Doran V, Adams D, Kalajzic Z, Chen J, Wadhwa S. Murine TMJ loading causes increased proliferation and chondrocyte maturation. J Dent Res 2011;90:512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Son H, Zhang Y, Shannonhouse J, Ishida H, Gomez R, Kim YS. Mast-cell-specific receptor mediates alcohol-withdrawal-associated headache in male mice. Neuron 2024;112:113–23.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Suttle A, Wang P, Dias FC, Zhang Q, Luo Y, Simmons L, Bortsov A, Tchivileva IE, Nackley AG, Chen Y. Sensory neuron-TRPV4 modulates temporomandibular disorder pain via CGRP in mice. J Pain 2023;24:782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tanaka E, Aoyama J, Miyauchi M, Takata T, Hanaoka K, Iwabe T, Tanne K. Vascular endothelial growth factor plays an important autocrine/paracrine role in the progression of osteoarthritis. Histochem Cell Biol 2005;123:275–81. [DOI] [PubMed] [Google Scholar]
- [43].Vierck CJ, Wong F, King CD, Mauderli AP, Schmidt S, Riley JL. Characteristics of sensitization associated with chronic pain conditions. Clin J Pain 2014;30:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang GYF, Shi XQ, Wu W, Gueorguieva M, Yang M, Zhang J. Sustained and repeated mouth opening leads to development of painful temporomandibular disorders involving macrophage/microglia activation in mice. PAIN 2018;159:1277–88. [DOI] [PubMed] [Google Scholar]
- [45].Woolf CJ, Ma Q. Nociceptors-noxious stimulus detectors. Neuron 2007;55:353–64. [DOI] [PubMed] [Google Scholar]