Abstract
Synthetic cathinone derivatives are commonly considered quasi-legal alternatives for stimulant drugs, such as cocaine and methamphetamine, but some derivatives are increasingly being detected in club drug formulations of Ecstasy or “Molly” as substitutes for methylenedioxymethamphetamine (±-MDMA). Although several studies have evaluated the psychostimulant-like effects of synthetic cathinones, few cathinone compounds have been assessed for MDMA-like activity. In order to determine their likelihood of interchangeability with entactogenic club drugs, the discriminative stimulus effects of methcathinone, 4-fluoromethcathinone, 4-methylmethcathinone, 4-methylethcathinone, 3-fluoromethcathinone, pentedrone, and ethylone were assessed in Sprague-Dawley rats trained to discriminate 1.5 mg/kg racemic methylenedioxymethamphetamine (±-MDMA) from vehicle. Methamphetamine and the cathinones 4-fluoromethcathinone, 4-methylmethcathinone, 4-methylethcathinone, 3-fluoromethcathinone, pentedrone, and ethylone fully substituted for the discriminative stimulus effects of ±-MDMA. In contrast, methcathinone produced a maximum of only 43% ±-MDMA-appropriate responding and higher doses suppressed responding. Most, but not all of the cathinone compounds tested have discriminative stimulus effects similar to those of MDMA as well as psychostimulant-like effects; however, the potency of MDMA versus psychostimulant substitution varies substantially among the compounds, suggesting that a subset of synthetic cathinones are more MDMA-like than psychostimulant-like. These findings further highlight the highly-variable pharmacology of this class of compounds and suggest that those cathinones with MDMA-like effects may also have increased use as club drugs.
Keywords: cathinones, MDMA, entactogens, drug discrimination
Introduction
Synthetic cathinones comprise a broad class of novel psychoactive substances that began appearing in the global drug market around 2010 and continue to be widely used today (UNODC, 2017). Users of synthetic cathinones commonly report feelings of euphoria, stimulation, and decreased appetite when using cathinones (Johnson & Johnson, 2014; Ashrafioun et al., 2016), and cite these effects, as well as the desire to substitute for an illegal drug, as their primary motivations for using them (Ashrafioun et al., 2016). Although some users report using synthetic cathinones as alternatives to traditional, illicit stimulants, a subset of synthetic cathinone users prefers them to cocaine or methamphetamine (Smith & Stoops, 2019). Beyond the intentional users of synthetic cathinones, many users are inadvertently exposed to these drugs through adulterated “Ecstasy”, “Molly”, or other club-drug formulations, believing they are consuming pure methylenedioxymethamphetamine (MDMA) (Palamar et al., 2016; 2017) and potentially leading to underreporting of the actual prevalence of synthetic cathinone use (Oliver et al., 2018). The inadvertent use of synthetic cathinones is especially concerning, given the broad adverse effects associated with their use and the highly-variable potencies of synthetic cathinones (Musselman & Hampton, 2014).
Many of the behavioral pharmacological studies of cathinones have examined their psychostimulant-like effects and similarity to established psychostimulants, such as cocaine and methamphetamine (e.g. Gatch et al., 2013; 2015; Gannon et al., 2017; Nguyen et al., 2017). These studies indicate that cathinones consistently produce discriminative stimulus effects comparable to the psychostimulants methamphetamine and cocaine (Gatch et al., 2013; 2015; 2017; Gannon & Fantegrossi, 2016; Naylor et al., 2015; Smith et al., 2017). These results are not unexpected, as synthetic cathinones are pharmacologically similar to traditional psychostimulants in that they predominately exert their effects by increasing concentrations of synaptic monoamines through direct transmitter release or inhibition of transmitter uptake at presynaptic monoamine transporters (DeFelice et al., 2014). Currently, there has been little research on the MDMA-like effects of cathinones. Because cathinones are found in “Ecstasy” formulations, it is of interest to determine whether they actually produce MDMA-like subjective effects. Given that many synthetic cathinones, like MDMA, are relatively nonselective for the monoamine transporters and promote robust increases in extracellular serotonin through direct or indirect transmitter release (Eshleman et al., 2013; 2017; 2018), it seems likely that a subset of these compounds would demonstrate subjective effects that are more similar to MDMA than predominately dopaminergic psychostimulants. Indeed, some of the non-cathinone street drugs such as MDAI and the benzofurans (“Benzofury”) are actually more potent at producing MDMA-like discriminative stimulus effects than methamphetamine-like discriminative stimulus effects (Dolan et al., 2017; Gatch et al., 2016).
The discriminative stimulus effects of MDMA are complex, having both a dopaminergic component and a serotonergic component, such that MDMA has some psychostimulant-like effects and some hallucinogen-like effects (Goodwin and Baker, 2000; Goodwin et al., 2003; Harper et al., 2014; Webster et al., 2017). The effects of psychostimulants in MDMA-drug discrimination are somewhat equivocal. Cocaine substitutes for the discriminative stimulus effects of MDMA, but MDMA does not substitute for cocaine (Khorana et al., 2004). Amphetamine fully substituted for the discriminative stimulus effects of MDMA in one study (Oberlender and Nichols, 1988), but only partially substituted in another (Glennon and Misenheimer, 1989). Likewise, amphetamine-trained rats failed to show amphetamine-appropriate responding following MDMA in one study (Oberlender and Nichols, 1988), but fully generalized to MDMA in others (Evans and Johanson, 1986; Kamien et al., 1986). In our lab, there was full cross-substitution between MDMA and methamphetamine (Gatch et al., 2009). Similarly, serotonergic hallucinogens produce mixed effects in rats trained to discriminate MDMA. LSD and DOM did not substitute for ±-MDMA in one study (Oberlender and Nichols, 1988), whereas in other studies, LSD and DiPT fully substituted for ±-MDMA (Gatch et al., 2009; 2011). MDMA fully substituted for LSD, DOM and DMT (Gatch et al., 2009), for DiPT (Carbonaro, 2012) and for DPT (Fantegrossi et al., 2008).
In terms of cathinones, studies in humans have determined that the subjective effects of mephedrone are similar to those of MDMA (Kapitány-Fövény et al., 2013; Papaseit et al., 2016). It is not surprising then, that MDMA fully substituted for the discriminative stimulus effects of mephedrone (Berquist et al., 2017), although at doses that suppressed response rate. However, some cathinones may have more MDMA-like effects than others. This hypothesis is supported by a recent study in which methylone and butylone fully substituted for the discriminative stimulus effects of MDMA in rats, whereas pentylone did not (Dolan et al., 2018). In another study, dimethylone, dibutylone and clephedrone fully substituted for MDMA, whereas N-ethylpentylone did not (Gatch et al., 2019), and in yet another, MDPV produced only partial substitution in MDMA-trained rats (Harvey and Baker, 2016).
The purpose of the present study was to determine whether a group of commonly used cathinones have MDMA-like subjective effects. Drug discrimination is a useful animal model of the subjective effects of drugs. So, in the present study, the test compounds methcathinone, 4-fluoromethcathinone (flephedrone), 4-methylmethcathinone (mephedrone), 4-methylethcathinone, 3-fluoromethcathinone, pentedrone, and ethylone were tested for substitution in rats trained to discriminate ±-MDMA from saline. Although it is known that stimulants substitute more for S(+)-MDMA and hallucinogens for R(−)-MDMA (Murnane et al., 2009), racemic MDMA was used instead of the + and/or – isomers, since that is what is most likely found on the street.
Methods
Subjects
Male Sprague-Dawley rats were obtained from Envigo. All rats were housed individually and were maintained on a 12:12 light/dark cycle (lights on at 7:00 AM). Body weights were maintained at 320–350 g by limiting food to 15 g/day which included the food received during operant sessions. Water was readily available. All housing and procedures were in accordance with Guidelines for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Discrimination Procedures
Standard behavior-testing chambers (Coulbourn Instruments, Allentown, PA, Model E10–10) were connected to IBM-PC compatible computers via LVB interfaces (Med Associates, East Fairfield, VT). Response levers were positioned to the left and right of the food hopper. A house light was centered over the hopper close to the ceiling and was illuminated only when the levers were active. The computers were programmed in Med-PC for Windows, version IV (Med Associates) for the operation of the chambers and collection of data.
Using a two-lever choice methodology, 55 rats were trained to discriminate ±-MDMA (1.5 mg/kg) from saline. Rats received an injection of either saline or drug and were subsequently placed in the behavior-testing chambers, where food (45 mg food pellets; Bio-Serve, Frenchtown, NJ) was available as a reinforcer for every ten responses on a designated injection-appropriate lever. The pretreatment time was 15 min. Each training session lasted a maximum of 10 min, and the rats could earn up to a maximum of 20 food pellets. The rats received approximately 60 of these sessions before they were used in tests for substitution of the experimental compounds. Rats were used in testing once they had achieved 9 of 10 sessions at 85% injection-appropriate responding for both the first reinforcer and total session. The training sessions occurred on separate days in a double alternating fashion (drug-drug-saline-saline-drug; etc.) until the training phase was complete, after which substitution tests were introduced into the training schedule such that at least one saline and one drug session occurred between each test (drug-saline-test-saline-drug-test-drug; etc.). The substitution tests occurred only if the rats had achieved 85% injection-appropriate responding on the two prior training sessions.
±-MDMA (n=13), methamphetamine (n=6), methcathinone (n=7), 4-fluoromethcathinone (n=6), 4-methylmethcathinone (n=7), 4-methylethcathinone (n=6), 3-fluoromethcathinone (n=8), pentedrone (n=9), and ethylone (n=6) were tested for substitution in ±-MDMA-trained rats. Test sessions lasted for a maximum of 20 min. In contrast with training sessions, both levers were active, such that 10 consecutive responses on either lever led to reinforcement. Data were collected until all 20 reinforcers were obtained, or for a maximum of 20 min. A repeated-measures design was used, such that each rat was tested at all doses of a given drug, including vehicle and training-drug controls. The dose effect of each compound was tested from no effect to full effect or rate suppression (<20% of vehicle control) or adverse effects. The training dose of ±-MDMA was based on earlier reports (Glennon and Misenheimer, 1989; Gatch et al., 2013). Starting doses and pretreatment times were inferred from previously published locomotor activity and drug discrimination testing (Gatch et al., 2013; 2015; 2017). For dose-effect experiments, intraperitoneal (i.p.) injections (1 ml/kg) of vehicle or test compound were administered with a pretreatment of 15 min for each compound, except flephedrone, which had a pretreatment time of 60 min due to its delayed stimulant effects (Gatch et al., 2013). Rats that failed to complete the first fixed ratio were excluded from the analysis of drug-appropriate responding, but were used for analysis of response rate.
Drugs
(±)-3,4-Methylenedioxymethamphetamine hydrochloride, methcathinone HCl, 4-fluoromethcathinone HCl, 4-methylmethcathinone HCl, 4-methylethcathinone HCl, 3-fluoromethcathinone HCl, pentedrone HCl, and ethylone HCl were all supplied by the National Institute on Drug Abuse Drug Supply Program. Optically active cathinones were provided as racemates. All compounds were dissolved in 0.9% NaCl saline solution and administered i.p. in a volume of 1 ml/kg.
Data Analysis
Drug discrimination data are expressed as the mean percentage of drug-appropriate responses occurring in each test period. Graphs for percent drug-appropriate responding and response rate were plotted as a function of dose of test compound (log scale). Percent drug-appropriate responding was shown only if at least 3 rats completed the first fixed ratio. Full substitution was defined as ≥80% drug-appropriate responding and not statistically different from the training drug. Rates of responding were expressed as a function of the number of responses made divided by the total session time. Response rate data were analyzed by one-way repeated measures analysis of variance. Effects of individual doses were compared to the vehicle control value using a priori contrasts. The potencies (ED50 and 95%-confidence intervals) of the test compounds in both assays were calculated by fitting straight lines to the linear portion of the dose-response data for each compound by means of OriginGraph (OriginLab Corporation, Northampton, MA). A one-way ANOVA was conducted on the ED50 values.
Results
In ±MDMA–trained rats, ±MDMA dose-dependently increased drug-appropriate responding to 96% at the training dose of 1.5 mg/kg (ED50 = 0.67±0.05 mg/kg) as shown in the left panels of Figure 1. Potencies are shown in Table 1. ±-MDMA (n=13) increased response rate compared to vehicle control following 0.32, 0.75 and 1.5 mg/kg [F(4,48)=3.013, p=0.027]. Methamphetamine (ED50 = 0.86±0.07 mg/kg) also fully substituted for the discriminative stimulus effects of ±-MDMA (Figure 1, right panels). Methamphetamine (n=6) decreased response rate following 2.5 mg/kg such that 2 of 6 rats did not complete the first fixed ratio [F(5,25)=12.347, p=0.001].
Figure 1. MDMA-like discriminative stimulus effects of cathinones.
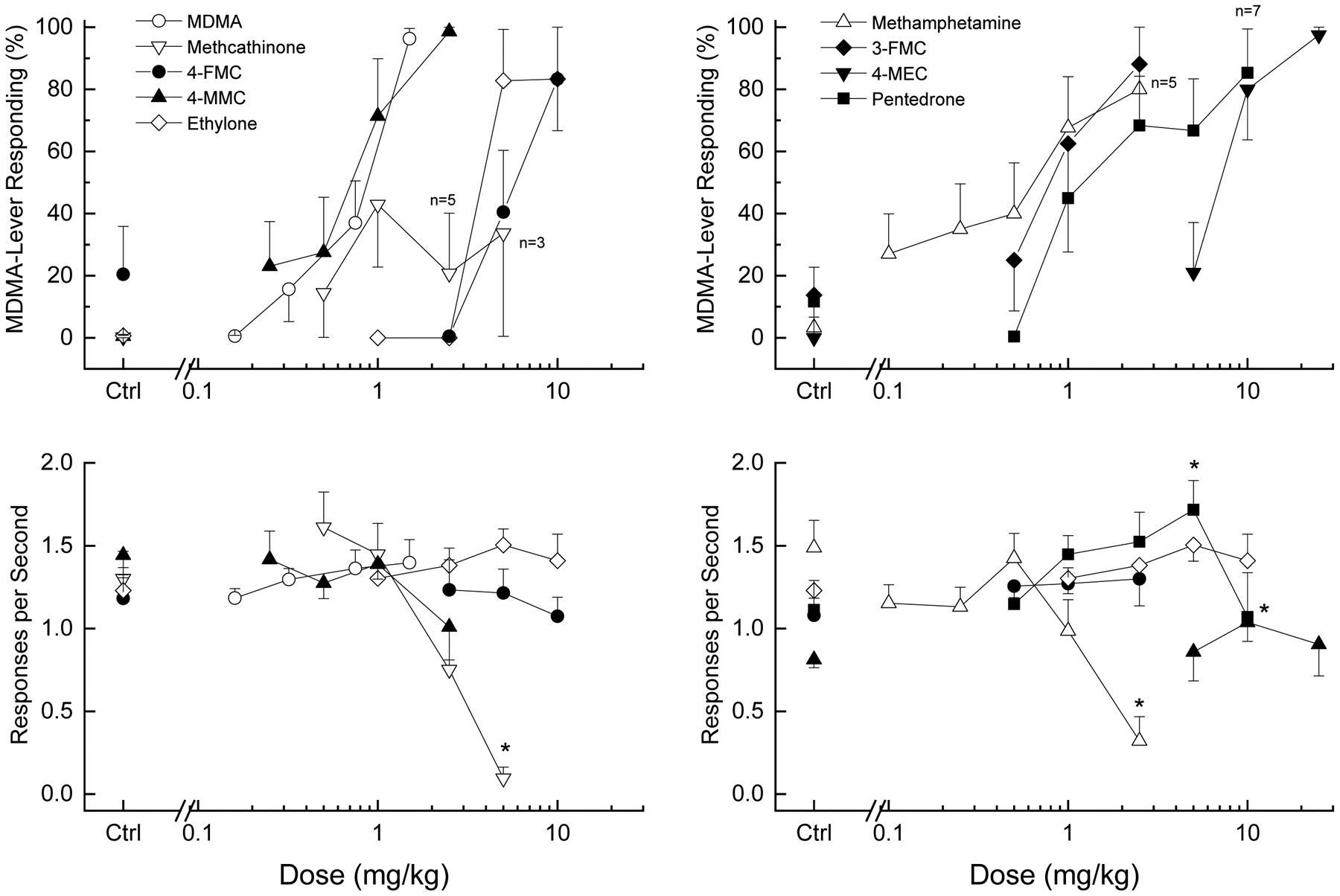
Top panels show percentage of total responses made on the drug-appropriate lever. Bottom panels show rate of responding in responses per second (r/s). Sample size of MDMA n=13, 4-methylethcathinone (4-MEC), flephedrone, ethylone n=6, methcathinone, 4-methylmethcathinone (4-FMC, mephedrone) n=7, 3-fluoromethcathinone (3-FMC) n=8, pentedrone n=9, unless otherwise shown. Ctrl indicates vehicle and training drug control values. * indicates response rate different from vehicle control (p < 0.05).
Table 1.
Potencies at producing MDMA- and methamphetamine-like (Meth) discriminative stimulus effects. ED50 values were calculated from total session data. DAT/SERT selectivity refers to relative potency for inhibition of transmitter uptake by DAT over SERT.
| Training Drug | MDMA (mg/kg) |
Meth (mg/kg) |
MDMA/Meth Potency Ratio |
DAT/SERT |
|---|---|---|---|---|
| Test Compound | ||||
| MDMA | 0.67±0.05 | 2.09±0.06 | 3.12 | 0.88 |
| Methamphetamine | 0.86±0.07 | 0.33±0.13 | 0.37 | 111.00 |
| Methcathinone | -- | 0.34±0.082 | NA | 136.00 |
| Flephedrone | 5.27±0.08 | 2.85±0.081 | 0.54 | >36 |
| Mephedrone | 0.59±0.17 | 1.42±0.061 | 2.41 | 5.2 |
| Pentedrone | 2.00±0.11 | 2.67±0.182 | 1.34 | 51 |
| Ethylone | 4.57±0.06 | 5.01±0.083 | 1.10 | .27 |
| 4-MEC | 6.04±0.10 | 8.69±0.132 | 1.44 | .23 |
| 3-FMC | 0.86±0.10 | 0.74±0.072 | 0.86 | 60.3 |
The cathinones 4-fluoromethcathinone (ED50 = 5.27±0.08 mg/kg), 4-methylmethcathinone (0.59±0.17 mg/kg), 4-methylethcathinone (6.04±0.10), 3-fluoromethcathinone (0.86±0.10), pentedrone (2.00±0.11 mg/kg), and ethylone (4.57±0.06 mg/kg) fully substituted for the discriminative stimulus effects of ±-MDMA. 4-Fluoromethcathinone increased response rate at 1 mg/kg [F(4,20)=4.89, p=0.006], and pentedrone increased response rate at 5 mg/kg [F(5,40)=4.008, p=0.005]. The other test compounds did not alter response rate.
In contrast, methcathinone produced a maximum of 43% ±-MDMA-appropriate responding at 1 mg/kg. Higher doses produced less ±-MDMA-appropriate responding (21–37%) and decreased response rate [F(4,24)=12.708, p<0.001], such that 2 of 7 rats failed to complete the first fixed ratio following 2.5 mg/kg and 4 of 7 following 5 mg/kg.
Discussion
All of the cathinone compounds tested in the present study have previously been shown to fully substitute for the discriminative stimulus effects of psychostimulants such as cocaine and methamphetamine in studies reviewed in more detail below. A natural question to raise, given the adulteration of Ecstasy with synthetic cathinones, is whether these compounds produce discriminative stimulus effects comparable to both the entactogenic club drug MDMA and the psychostimulant methamphetamine, or whether some of the compounds are more MDMA-like or more methamphetamine-like. The data from the current study, alongside previous reports of the MDMA- and methamphetamine-like discriminative stimulus effects of synthetic cathinones, support the notion that many synthetic cathinones may produce greater MDMA-like subjective effects relative to methamphetamine.
MDMA and methamphetamine fully cross-substituted for each other as previously reported (Gatch et al., 2009), suggesting some overlap in subjective effects. However, it should be noted that relatively large doses were required that also produced substantial rate-decreasing effects. The training dose of MDMA was 1.5 mg/kg, but a dose of 3 mg/kg was needed to produce full substitution in methamphetamine-trained rats; conversely the training dose of methamphetamine was 1 mg/kg, but a dose of 2.5 mg/kg was necessary to produce full substitution in MDMA-trained rats. These findings suggest whereas there are some similarities in the discriminative-stimulus effects of methamphetamine and MDMA, they are not identical.
Previous studies evaluating the dopamine-mediated discriminative stimulus effects of MDMA have demonstrated that MDMA’s discriminative stimulus is more serotonergic at lower doses, but becomes more dopaminergic at higher doses (Webster et al., 2017; Harper et al., 2014). These dose-dependent aspects of MDMA’s discriminative stimulus may explain why higher doses of MDMA are needed to substitute for methamphetamine, which is a predominately dopaminergic compound (Munzar and Goldberg, 2000). Similar to the potency differences in MDMA and methamphetamine cross-substitution, cathinone compounds do not all produce equivalent psychostimulant-like and MDMA-like effects. Even though all have fully substituted for the discriminative stimulus effects of methamphetamine, there has been a wide range in the degree and potency of MDMA-like effects (illustrated in Table 1). Some compounds substitute with similar potency in methamphetamine- and MDMA-trained rats, whereas others have more potent and/or efficacious methamphetamine-like effects, and yet others have more potent MDMA-like effects.
Starting with the latter group, several cathinones produce more potent MDMA-like effects, although none of the cathinones have been more efficacious by failing to substitute for methamphetamine. Other, non-cathinone, synthetic designer street drugs such as MDAI and the benzofurans 6-APDB and 5-APB are both more potent and/or efficacious at producing MDMA-like effects than methamphetamine-like effects (Dolan et al., 2017; Gatch et al., 2016). The synthetic cathinones clephedrone and methylone were 10-fold and 2-fold, respectively, more potent in MDMA-trained rats than in methamphetamine-trained rats (Gatch et al., 2019; Dolan et al., 2018), and mephedrone was 2.4-fold more potent in MDMA-trained rats in the present study than in methamphetamine-trained rats in our previous study (Gatch et al., 2013). In rodents, mephedrone was previously reported to substitute for 1.5 mg/kg MDMA (Harvey and Baker 2016), amphetamine (Harvey et al., 2017), and cocaine (Gannon and Fantegrossi, 2016); however, these psychostimulant discriminative stimulus effects were not replicated in in monkeys (Smith et al., 2017). When mephedrone was trained as a discriminative stimulus (3.2 mg/kg), cocaine, MDMA, and methamphetamine only produced partial substitution (Erwin et al., 2018; Varner et al., 2013), but in other studies, cocaine fully substituted for mephedrone (DeLarge et al., 2017) as did MDMA (Berquist et al., 2017), although at doses that suppressed response rate.
Conversely, other cathinones have been more potent and/or efficacious in producing methamphetamine-like effects. Flephedrone was 2-fold more potent in methamphetamine-trained rats than in MDMA-trained rats (Gatch et al., 2013). Compounds that were less efficacious (failed to substitute) in MDMA include methcathinone (43% DAR, present study), N-ethylpentylone (50% DAR, Gatch, 2019), methylenedioxypyrovalerone (50–60%, Harvey & Baker, 2016), and pentylone (75%, Dolan et al., 2108). Each of these compounds fully substitute for methamphetamine and cocaine (Smith et al., 2017; Gatch et al., 2015), often at lower doses than those that failed to produce substitution in MDMA in the present study.
Finally, some compounds have been approximately equipotent at producing methamphetamine- and MDMA-like discriminative stimuli. In the current study, pentedrone, ethylone, 4-MEC, and 3-FMC fully substituted for MDMA-like discriminative stimuli, and have previously demonstrated methamphetamine-like discriminative stimulus effects (Gatch et al., 2015; 2017). Previously-evaluated compounds producing equipotent cross-substitution include 4-fluoramphetamine (Dolan et al., 2017), butylone (Dolan et al., 2018), dimethylone, and dibutylone (Gatch, et al., 2019). It should be noted that 4-MEC failed to substitute for methamphetamine in another study which used a different pretreatment time and narrower range of doses (Naylor et al., 2015). Taken together, these studies suggest that the discriminative stimulus effects of cathinones are broad, and their overlap with psychostimulants and MDMA are not completely equivalent. It should also be pointed out that these compounds have been tested in relatively few rats and largely in one laboratory, and there may be differences in sensitivity between individual subjects. Further testing will be necessary to confirm the robustness of these results.
Despite their structural variability, the synthetic cathinones typically fall into one of two synaptic mechanistic categories: amphetamine-like monoamine transporter substrates or cocaine-like monoamine uptake inhibitors (reviewed in De Felice, Glennon, & Negus, 2014), and vary substantially in their affinity and selectivity for the dopamine (DAT) or serotonin (SERT) transporters. Many synthetic cathinones, including methcathinone, flephedrone, 3-FMC, and pentedrone, bind preferentially to DAT relative to SERT and promote increases in synaptic dopamine at nanomolar to low-micromolar concentrations (Blough et al., 2018; Eshleman et al, 2013; 2017; Simmler et al., 2013; 2014). Although there are no SERT-selective synthetic cathinones (i.e. >10-fold selectivity), many, including mephedrone, ethylone, and 4-MEC, are nonselective and bind to and exert their effects at DAT and SERT at comparable concentrations (Eshleman et al., 2013; 2017). Given that MDMA is nonselective for DAT versus SERT (Eshleman et al., 2017) and its discriminative stimulus is mediated by both dopamine and serotonin (Goodwin & Baker, 2000; Harper et al., 2014), it seems reasonable that nonselective cathinones would produce more-potent or equipotent substitution for MDMA relative to methamphetamine. Although this was the case for mephedrone, ethylone, and 4-MEC, pentedrone and 3-FMC are 50–60-fold more-selective for DAT over SERT, yet were equipotent in their MDMA- and methamphetamine-like discriminative stimulus effects (Table 1), and mephedrone was 5-fold more selective for DAT, yet produced the second highest MDMA discrimination ratio. This discrepancy may potentially result from differences in transporter pharmacodynamics and their resulting influence on discriminative stimulus effects, as pentedrone is a monoamine transporter blocker whereas mephedrone is a substrate, and ethylone and 4-MEC exhibit DAT blockade and highly-efficacious serotonin release (Eshleman et al., 2017; 2018). Although 3-FMC is a transporter substrate, it is not a particularly efficacious dopamine and 5HT releasing agent (Eshleman et al., 2017), much like mephedrone (Eshleman et al., 2018), suggesting that the capacity to directly release greater quantities of monoamines in vitro may be more important in mediating the relative stimulant vs entactogenic discrimination potency of novel drugs than simple relative transporter potency. As such, monoamine transporter selectivity may generally serve as a useful initial guide for predicting how a novel cathinone compound’s in vitro pharmacology may translate into subjective effects; however, the limited SERT selectivity (< 10-fold over DAT) of most synthetic cathinones combined with the complex interplay between selectivity, mechanism of action, and release efficacy may limited the overarching applicability of this selectivity approach. Although relative DAT/SERT potency is strongly related to synthetic cathinone reinforcement (Gannon et al., 2018), this ratio in isolation may have less applicability in determining relative discriminative stimulus effects. Testing of additional cathinones with a range of relative DAT/SERT potency will be helpful in resolving this empirical question, as will evaluating how the discriminative stimulus effects of mechanistically-varying synthetic cathinones generalize to MDMA in MDMA substitution tests.
In summary, many of the cathinone compounds tested to date have discriminative stimulus effects similar to both those of methamphetamine and MDMA. This is not entirely related to chemical structures, since ring substitutions do not consistently appear to make cathinones less methamphetamine-like and more MDMA-like; however, relative DAT versus SERT selectivity may underlie the differential discriminative stimulus effects in some, but not all, cathinones. Because many cathinones have both psychostimulant- and MDMA-like effects, they may be substituted on the street for either traditional psychostimulants like methamphetamine and cocaine or club drugs like MDMA. Some of the compounds that are more methamphetamine-like may also have abuse liability similar to that of methamphetamine and may be more likely to drive compulsive use and addiction than compounds that are more like MDMA. So far, there is no evidence that providers of illicit recreational compounds deliberately target different cathinones for different target population; however, given the highly variable abuse potential and toxicity among synthetic cathinones and their growing prevalence as adulterants, research on the distribution of these compounds will be important.
Source of funding:
Funding was provided by the Addiction Treatment Discovery Program of the National Institute on Drug Abuse (NIH N01DA-13-8908)
Footnotes
Conflicts of interest: none declared
References
- Ashrafioun L, Bonadio FA, Baik KD, Bradbury SL, Carhart VL, Cross NA, Davis AK, Feuille M, Harper AR, Lackey JH, Lang B, Lauritsen KJ, Leith J, Osborn LA, Rosenberg H, Stock J, Zaturenskaya M (2016). Patterns of Use, Acute Subjective Experiences, and Motivations for Using Synthetic Cathinones (“Bath Salts”) in Recreational Users. J Psychoactive Drugs 48:336–343. [DOI] [PubMed] [Google Scholar]
- Berquist MD 2nd, Thompson NA, Baker LE(2017). Evaluation of training dose in male Sprague-Dawley rats trained to discriminate 4-methylmethcathinone. Psychopharmacology (Berl). 234:3271–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough BE, Decker AM, Landavazo A, Namjoshi OA, Partilla JS, Baumann MH, Rothman RB (2018). The dopamine, serotonin and norepinephrine releasing activities of a series of methcathinone analogs in male rat brain synaptosomes. Psychopharmacology (Berl). 2018 Oct 20. doi: 10.1007/s00213-018-5063-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Forster MJ, Gatch MB (2013). Discriminative stimulus effects of N,N-diisopropyltryptamine. Psychopharmacology 226:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, & Negus SS (2014). Synthetic cathinones: Chemical phylogeny, physiology, and neuropharmacology. Life Sci 97: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLarge AF, Erwin LL, Winsauer PJ (2017). Atypical binding at dopamine and serotonin transporters contribute to the discriminative stimulus effects of mephedrone. Neuropharmacology 119:62–75. [DOI] [PubMed] [Google Scholar]
- Dolan SB, Chen Z, Huang R, Gatch MB (2018). “Ecstasy” to addiction: Mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA. Neuropharmacology 133:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan SB, Forster MJ, Gatch MB (2017). Discriminative stimulus and locomotor effects of para-substituted and benzofuran analogs of amphetamine. Drug Alcohol Depend 180:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin LL, Nilges MR, Bondy ZB, Winsauer PJ (2018). Effects of cocaine on the discriminative stimulus and reinforcing effects of mephedrone in male rats. Psychopharmacology (Berl). 2018 Nov 17. doi: 10.1007/s00213-018-5110-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Nagarajan S, Wolfrum KM, Reed JF, Swanson TL, Nilsen A, Janowsky A (2018). Structure-activity relationships of bath salt components: substituted cathinones and benzofurans at biogenic amine transporters. Psychopharmacology (Berl). 2018 Nov 5. doi: 10.1007/s00213-018-5059-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A (2013). Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 85: 1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, Janowsky A. (2017). Structure-activity relationships of substituted cathinones, with transporter binding, uptake, and release. J Pharmacol Exp Ther 360: 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Johanson C-E (1986). Discriminative stimulus properties of (+)-3,4- methylenedioxymethamphetamine and (+)-3,4- methylenedioxyamphetamine in pigeons, Drug Alcohol Depend 18, 159–164. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC (2008) Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): Possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav 88: 358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamien JB, Johanson C-E, Schuster CR, Woolverton WL, (1986). The effects of (+/−)-methylenedioxymethamphetamine and (+/−)- methylenedioxyamphetamine in monkeys trained to discriminate (+)- amphetamine from saline, Drug Alcohol Depend 18: 139–147. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Fantegrossi WE (2016). Cocaine-like discriminative stimulus effects of mephedrone and naphyrone in Mice. J Drug Alcohol Res 5: 236009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT (2017). Individual differences in the relative reinforcing effects of 3,4-methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. J Pharm Exp Ther 361:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Baumann MH, Walther D, Jiminez-Morigosa C, Sulima A, Rice KC, & Collins GT (2018). The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 43: 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2015). Comparative behavioral pharmacology of three pyrrolidine-containing synthetic cathinone derivatives. J Pharm Exp Ther 354:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2016). Locomotor, discriminative stimulus, and place conditioning effects of MDAI in rodents. Behav Pharmacol 27:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2017). Locomotor activity and discriminative stimulus effects of a novel series of synthetic cathinone analogs in mice and rats. Psychopharmacology 234: 1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ (2019). Locomotor activity and discriminative stimulus effects of five novel synthetic cathinone analogs in mice and rats. Drug Alcohol Depend 199: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. (2011). Abuse liability profile of three substituted tryptamines. J Pharm Exp Ther 338:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge M, Carbonaro T, Forster MJ (2009). Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology 204:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ (2013). Locomotor stimulant and discriminative stimulus effects of “Bath Salt” cathinones. Behav Pharmacol 24:437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Misenheimer BR (1989). Stimulus effects of N-monoethyl-1-(3,4-methylendioxyphenyl)-2- aminopropane (MDE) and N-hydroxy-1-(3,4- methylenedioxyphenyl)-2- aminopropane (N-OH MDA) in rats trained to discriminate MDMA from saline. Pharmacol Biochem Behav 33, 909–912. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Baker LE (2000). A three-choice discrimination procedure dissociates the discriminative stimulus effects of d-amphetamine and (+/−)-MDMA in rats. Exp Clin Psychopharmacol 8:415–23. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Pynnonen DM, Baker LE (2003). Serotonergic-dopaminergic mediation of MDMA’s discriminative stimulus effects in a three-choice discrimination. Pharmacol Biochem Behav 74:987–95. [DOI] [PubMed] [Google Scholar]
- Harvey EL, Baker LE (2016). Differential effects of 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) in rats trained to discriminate MDMA or a d-amphetamine + MDMA mixture. Psychopharmacology (Berl). 233:673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EL, Burroughs RL, Baker LE (2017). Effects of D1 and D2 receptor antagonists on the discriminative stimulus effects of methylendioxypyrovalerone and mephedrone in male Sprague-Dawley rats trained to discriminate D-amphetamine. Behav Pharmacol 28:586–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper DN, Langen AL, Schenk S (2014). A 3-lever discrimination procedure reveals differences in the subjective effects of low and high doses of MDMA. Pharmacol Biochem Behav 116:9–15. [DOI] [PubMed] [Google Scholar]
- Johnson PS, Johnson MW (2014). Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs 46: 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitány-Fövény M, Kertész M, Winstock A, Deluca P, Corazza O, Farkas J, Zacher G, Urbán R, Demetrovics Z (2013). Substitutional potential of mephedrone: an analysis of the subjective effects. Hum Psychopharmacol 28:308–16. [DOI] [PubMed] [Google Scholar]
- Khorana N, Pullogurla MR, Young R, Glennon RA (2004). Comparison of the discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) and cocaine: asymmetric generalization, Drug Alcohol Depend 74: 281–287. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR (2000). Dopaminergic involvement in the discriminative stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 148(2):209–16. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Murai N, Howell LL, Fantegrossi WE (2009). Discriminative stimulus effects of psychostimulants and hallucinogens in S(+)-3,4-methylenedioxymethamphetamine (MDMA) and R(−)-MDMA trained mice. J Pharmacol Exp Ther 331:717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman ME, Hampton JP (2014). “Not for human consumption”: a review of emerging designer drugs. Pharmacotherapy 34: 745–57. [DOI] [PubMed] [Google Scholar]
- Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL (2015). Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend 149:280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011). Guide for the care and use of laboratory animals, 8th edn. The National Academies Press, Washington, D.C. [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Vandewater SA, Taffe MA (2017). Escalation of intravenous self-administration of methylone and mephedrone under extended access conditions. Addict Biol 22:1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE, 1988. Drug discrimination studies with MDMA and amphetamine. Psychopharmacology 95: 71–76. [DOI] [PubMed] [Google Scholar]
- Oliver CF, Palamar JJ, Salomone A, Simmons SJ, Philogene-Khalid HL, Stokes-McCloskey N, Rawls SM (2019). Synthetic cathinone adulteration of illegal drugs. Psychopharmacology (Berl) 236:869–879. doi: 10.1007/s00213-018-5066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Vincenti A, Cleland CM. Detection of “bath salts” and other novel psychoactive substances in hair samples of ecstasy/MDMA/”Molly” users (2016). Drug Alcohol Depend 161:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Gerace E, Di Corcia D, Vincenti M, Cleland CM (2017). Hair testing to assess both known and unknown use of drugs amongst ecstasy users in the electronic dance music scene. Int J Drug Policy 48:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaseit E, Pérez-Mañá C, Mateus JA, Pujadas M, Fonseca F, Torrens M, Olesti E, de la Torre R, Farré M (2016). Human pharmacology of mephedrone in comparison with MDMA. Neuropsychopharmacology. 41:2704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, & Liechti ME (2013). Pharmacological characterization of designer cathinones in vitro. Brit J Pharmacol, 168: 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, & Liechti ME (2014). Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology, 79: 152–160. [DOI] [PubMed] [Google Scholar]
- Smith DA, Negus SS, Poklis JL, Blough BE, Banks ML (2017). Cocaine-like discriminative stimulus effects of alpha-pyrrolidinovalerophenone, methcathinone and their 3,4-methylenedioxy or 4-methyl analogs in rhesus monkeys. Addict Biol. 22:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Stoops WW (2019). Synthetic cathinone use among polysubstance users: indirect indicator of indiscriminate drug taking or preferred drug of abuse? J Drug Issues 49: 369–386. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) (2017). Global Synthetic Drugs Assessment: Amphetamine-type Stimulants and New Psychoactive Substances. United Nations, New York, New York. [Google Scholar]
- Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, Winsauer PJ (2013). Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology (Berl). 225:675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JI, Harper DN, Schenk S (2017). Generalization of serotonin and dopamine ligands to the discriminative stimulus effects of different doses of ±3,4-methylenedioxymethamphetamine. Behav Pharmacol 28:245–54. [DOI] [PubMed] [Google Scholar]


