Abstract
As the range of Ixodes scapularis Say expands, host abundance and land use can play important roles in regions where ticks and their associated pathogens are emerging. Small mammal hosts serve as reservoirs of tick-borne pathogens, with Peromyscus leucopus Rafinesque often considered a primary reservoir. A sympatric species Peromyscus maniculatus Wagner is also a competent reservoir and is notoriously difficult to differentiate from P. leucopus. Anthropogenic land use can alter host and habitat availability, potentially changing tick exposure risk. We tested the hypotheses that tick infestation and pathogen prevalence differ between the two Peromyscus spp. and that host-seeking I. scapularis density and pathogen prevalence differ across land use and ecotone gradients. We live trapped small mammals and collected ticks across 3 land-use classifications and ecotones in Maine, an emergent area for tick-borne disease. We tested each small mammal and tick sample for Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti. While both Peromyscus spp. serve as hosts for immature ticks, P. leucopus exhibited a higher tick infestation frequency and intensity. We did not detect any significant difference in pathogen infection prevalence between the two species. The density of I. scapularis nymphs and the density of infected nymphs did not differ significantly between land-use types, though did differ across ecotones. We also noted a significant north/south gradient, with higher tick densities and pathogen prevalence at the southern end of the study area. Our study highlights the potential variability in tick density and pathogen prevalence across fine spatial scales within an emerging region for tick-borne disease.
Keywords: tick, host, wildlife, habitat, ecotone
Introduction
In the United States and Canada, Ixodes scapularis Say (Acari: Ixodidae) is the primary vector of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), the causative agent of Lyme disease, as well as multiple other tick-borne pathogens including Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae) and Babesia microti (Aconoidasida: Piroplasmida) (Piesman and Eisen 2007). In recent decades, sustained range expansion of I. scapularis from endemic areas to novel regions, particularly in the Northeastern and Midwestern United States, has increased the public health burden posed by this vector, and patterns of human disease have reflected this shift in tick distribution (Leger et al. 2013, Sonenshine 2018, Bisanzio et al. 2020, Gardner et al. 2020). At the landscape scale, increases in the spatial distribution and abundance of I. scapularis and its associated pathogens are frequently attributed to changes in climatic conditions and long-term trends of reforestation and fragmentation (Ogden et al. 2004, Simon et al. 2014, Sonenshine 2018). The establishment and persistence of ticks within their introduced range is dependent upon the availability of wildlife hosts among several key factors. In addition to facilitating tick movement across the landscape, wildlife hosts and human activity may also facilitate the ability of I. scapularis to persist in previously uninhabited areas.
The invasion of novel regions by I. scapularis and the establishment of its associated pathogens may occur at varying rates and patterns depending on local factors related to host availability, landscape features, and climate (Hamer et al. 2010, Leighton et al. 2012, Gardner et al. 2020). The white-footed mouse, Peromyscus leucopus Rafinesque (Rodentia: Cricetidae), is a primary reservoir of B. burgdorferi, A. phagocytophilum, B. microti, and several other tick-borne pathogens and is commonly parasitized by immature I. scapularis (Mather et al. 1989, Keesing et al. 2012). In addition to directly influencing pathogen dynamics, P. leucopus can potentially impact the density of ticks with increases in mouse populations leading to subsequent increases in tick numbers (LoGiudice et al. 2003). In recent decades, P. leucopus populations have expanded northward at varying rates and are expected to continue to shift northward over the next several decades (Roy-Dufresne et al. 2013). This shift has resulted in greater sympatry and potential displacement of a closely related species, the woodland deer mouse, Peromyscus maniculatus Wagner (Rodentia: Cricetidae), which currently occurs at higher latitudes than P. leucopus (Long, 1996, Myers et al. 2009). While they occupy similar ecological niches and are notoriously difficult to differentiate morphologically, differences in foraging behavior and habitat selection have been noted (Garman et al. 1994, Lindquist et al. 2003, Cramer 2014, Leo and Millien 2017). Like P. leucopus, P. maniculatus is a competent reservoir of several tick-borne pathogens, including B. burgdorferi and A. phagocytophilum (Rand et al. 1993, Larson et al. 2018). Even with its known ability to serve as a reservoir of tick-borne disease, the relative importance of P. maniculatus plays in sustaining these pathogens and their tick vectors, particularly in emerging regions, has not been widely characterized.
Emergence and persistence of I. scapularis are also dependent on the availability of suitable habitat for both ticks and their wildlife hosts. At the local level, increased abundance of ticks and pathogen prevalence are positively associated with edge ecotones between forests and open-field plant communities (Maupin et al. 1991, Frank et al. 1998). Differences in vegetation assemblages within habitats, particularly those with shrub layers, deciduous leaf litter, and in some instances, invasive plant species, are also associated with elevated I. scapularis abundance (Ostfeld et al. 1995, Lubelczyk et al. 2004). Anthropogenic land-use changes can alter these fine-scale factors and ultimately impact the expansion and persistence of ticks and tick-borne disease (Diuk-Wasser et al. 2021). Previous studies have examined the effects of land cover metrics, including forest cover and fragmentation, on the distribution and abundance of ticks over large spatial scales (Jackson et al. 2006, Li et al. 2012, Zolnik et al. 2015, Piedmonte et al. 2018). Comparisons of tick abundance based upon differing human land uses, such as agricultural vs. reforested, have also been conducted at the broader landscape scale but have been less frequently attempted at finer, property level scales (Diuk-Wasser et al. 2021). Early Lyme disease research identified peridomestic settings as areas associated with particularly high human risk and recreational areas as potential settings for high-risk tick encounters (Falco and Fish 1988, Maupin et al. 1991, Hahn et al. 2018). While the processes that drive tick densities have been examined in many of the areas in which I. scapularis is established, factors including seasonality, habitat suitability, climate, host availability, and human land use may differ in areas where tick populations are emergent (Bouchard et al. 2011, Burtis et al. 2016). Examining some of the local drivers in emerging areas may provide new insight into the establishment and relative risk of tick-borne disease in human-dominated landscapes.
This study addressed two sets of hypotheses related to the local scale drivers of ticks and tick-borne pathogens in an emergent area for tick-borne disease. The first examines the potential differences between the roles of P. leucopus and P. maniculatus in the maintenance of tick populations and in the tick-borne pathogen transmission cycle. The second assesses the effects of specific human land-use types and local-scale ecotones (i.e., forest, edge, open field) on I. scapularis in the lower Penobscot River Valley of Maine. Maine has experienced a prolonged and persistent increase in I. scapularis distribution and abundance, resulting in one of the highest incidences of Lyme disease in the country (Rand et al. 2007, Elias et al. 2020, 2021; CDC 2022). As Maine is on the prevailing edge of I. scapularis’ northward expansion, there exists a north-south gradient of tick abundance within the state, thus making it an ideal location to study the interactions between ticks, wildlife hosts, and their shared ecotones within an emergent area for tick-borne disease. To examine these interactions, we compared tick infestation rates and tick-borne pathogen prevalence between P. leucopus and P. maniculatus to test whether these ubiquitous small mammal species play differing roles in the tick-borne pathogen transmission cycle. We also specifically tested the hypothesis that tick burdens and pathogen prevalence in Peromyscus spp., as well as the host-seeking density of infected I. scapularis nymphs differ across land-use types associated with specific human activities and across local ecotones (i.e., forest, edge, open field) within these land-uses. As P. leucopus and P. maniculatus are nearly indistinguishable morphologically, we also developed an assay to delineate between the two Peromyscus spp., while simultaneously testing for tick-borne pathogens.
Methods
Study Locations
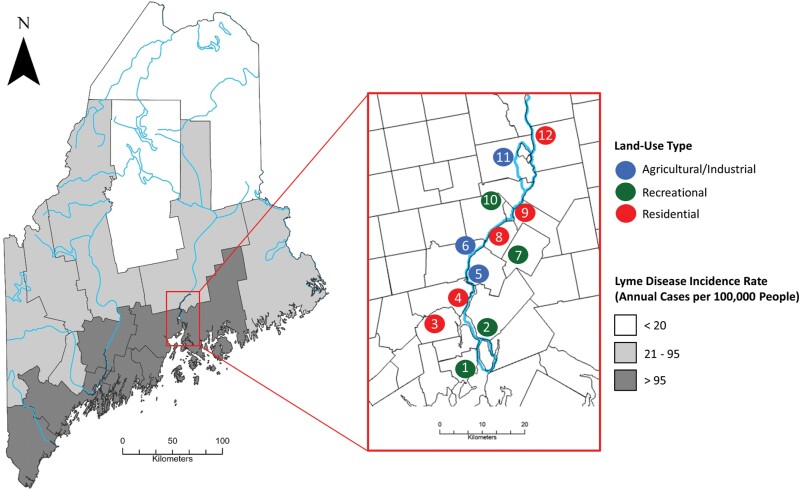
We conducted tick dragging and small mammal trapping at 12 study sites along the Penobscot River in Maine, encompassing 3 different land-use classifications: agricultural/industrial, residential, and recreational (Fig. 1). As I. scapularis densities in Maine change along a latitudinal gradient, with higher densities in the southern and coastal parts of the state, study sites were interspersed along the Penobscot River within a distance of approximately 50 km from the Maine coast where the mouth of the river empties into the Atlantic Ocean, with an average distance of approximately 6 km from one another. Agricultural/industrial sites (n = 3) included a research farm, a commercial sand and gravel mining site, and a mixed-use site composed of a commercial marina and municipal gravel depot. While the land-use purpose differed between agricultural/industrial sites, they supported similar levels of human activity, presence and use of large equipment, as well as property characteristics (area and availability of different ecotones). For these reasons, we grouped them into a single land-use category. Recreational sites (n = 4) included conservation lands that permitted and encouraged recreation through the construction of walking/hiking trails. We also sampled residential sites (n = 5), composed of single-family dwellings on low-density lots.
Fig. 1.
Small mammal trapping / tick collection sites and major rivers of Maine, USA. Maine counties shaded based upon incidence rates of Lyme disease (annual cases per 100,000 people, averaged across years 2007–2021; Maine CDC 2023).
We chose study sites based on the availability of ecotones (i.e., forest, edge, open field) within each site, lot size (a minimum of 5 acres), as well as a relatively uniform forest structure and tree species composition. At each site, we sampled ticks and small mammals within an approximately 5-acre parcel with transects distributed among 3 local ecotones (forest, edge, and open field). Forest structure and composition were relatively consistent between sample sites, with forests primarily consisting of a mixture of tree age classes, including mature trees, saplings, and seedlings. Forest canopy composition included a diverse mixture of coniferous and deciduous species, with red oak (Quercus rubra), balsam fir (Abies balsamea), red maple (Acer rubrum), sugar maple (Acer saccharum), and to a lesser extent birch (Betula spp.), aspen (Populus spp.), and spruce (Picea spp.) dominating. The understories included a subset of immature saplings of the dominant canopy species, as well as winterberry (Ilex verticillata), sumac (Rhus spp.), and a number of unidentified fern species. Three sites contained small infestations of Japanese barberry (Berberis thunbergii), an invasive plant associated with increased tick abundance (Lubelczyk et al. 2004, Elias et al. 2006, Williams and Ward 2010, Williams et al. 2017). Though present, barberry was not abundant and did not encompass a significant portion of the understory at any of these 3 sites. Open field ecotone at each site contained a mixture of grasses managed at varying levels, with some areas mowed frequently and grasses kept below 10 cm and others mowed infrequently with grasses reaching heights as high as 50 cm. We conducted sampling in open fields in which grasses were minimally managed, with grass heights ranging from approximately 20 to 50 cm within sites. The edge ecotone encompassed an approximately 10 m wide interface between open fields and forest ecotones centered on the forest edge.
Small Mammal Trapping and On-Host Tick Collection
To test the hypothesis that the infestation frequency (the number of tick-infested mice) and mean intensity (mean number of ticks on infested mice) of I. scapularis infestation on Peromyscus spp. differed between species and based upon land use and ecotone, we live trapped small mammals at each of the 12 study sites from June through October 2017 and 2018. We handled all animals in accordance with approved University of Maine Institutional Animal Care and Use Committee (IACUC) protocols (IACUC protocol #A2016-04-01). We conducted small mammal trapping sessions at each site 3 times per year in an attempt to overlap with I. scapularis activity. We conducted the first trapping session in late June when nymphal I. scapularis are becoming active, the second in early August when both nymphal and larval I. scapularis are active, and a third session in early October when adult I. scapularis are most active. Within each of the study sites, we established 9 transects (3 transects per ecotone type) and deployed 10 Sherman LFA Folding Live Capture traps (H.B. Sherman Traps, Tallahassee, FL, USA) per transect. In each transect, we placed 10 traps at 10 m intervals, forming a 100 m transect. In forested and open ecotones, we placed transects parallel to one another with approximately 10 m between them. Due to the limited width of edge ecotone, we placed transects end-to-end with approximately 20 m between them. Although there was some variability between sites, we attempted to maintain a distance of at least 75 m between ecotone types. We baited traps with peanut butter and deployed them approximately 2 h before sunset (1830 h in June, 1800 h in August, 1630 h in October), checking for captures each morning within 2 h of sunrise (0650 h in June, 0730 h in August, 0830 h in October). During each of the trapping sessions, we deployed traps for 2 consecutive nights resulting in 2,160 trap nights per trapping session and a total of 6,480 trap nights. We did not deploy traps on rainy nights or if there was a forecast for rain. In instances of rain or forecasted rain, we delayed the deployment of traps until the next clear night.
Upon first capture, we anesthetized each individual small mammal by isoflurane inhalation. We transferred animals from the trap to a prepared gallon zipper-locking bag containing a small metal tea strainer with a cotton ball saturated in 1 mL of isoflurane (Piramal, Bethlehem, PA, USA, NDC 66794-013-25) and exposed animals until they could no longer right themselves; approximately 30–45 s (Mathews et al. 2002, West et al. 2014). Once anesthetized, we recorded demographics (species, sex, and age class) and standard body measurements (mass, body length, tail length, ear length, and hind-foot length) of each individual (Lindquist et al. 2003, Reid 2006, Stephens et al. 2014,et al.). We determined age class by body mass (juveniles were 12 g or less, subadults were 13–18 g, and adults were greater than 18 g). We then examined each individual for the presence of ticks of all life stages, systematically inspecting the head and neck, and collected all tick samples into vials of 70% ethanol (Brunner and Ostfeld 2008). Following tick sampling, we collected a tissue sample for pathogen testing and species identification by snipping a 3-mm portion from the outer margin of the left ear and placing it into a vial of 70% ethanol. Ear snips were also used to identify recaptures, for which we did not recollect measurements, tissue samples, or attached ticks upon initial recapture or during subsequent trapping sessions. We did not tag or mark individual small mammal captures in a way that would allow for individual identification, as estimation of population density was not a goal of this study. Upon completion of sampling, we released each small mammal at the site of capture.
Host-Seeking Tick Collection and Species Identification
To test the hypothesis that land-use and ecotone influence I. scapularis nymphal density and the density of infected nymphs (DIN), we collected host-seeking ticks from forest interiors, edge ecotones, and open fields within each study site. We conducted sampling once at each site during July or early August in 2017, 2018, and 2019. We sampled each site between 0900 h and 1200 h and only under dry, clear weather conditions. We did not conduct field collections if it was raining or if the ground was wet from previous weather events. Within each ecotone type, we dragged a 1 m2 drag cloth at ground level along 5 separate 100 m transects, stopping every 10 m to examine the drag cloth for ticks. Within forested and open ecotones, transects were established parallel to one another and spaced approximately 20 m apart. Due to the limited width of edge ecotone, we arranged transects end-to-end with approximately 20 m between them. We attempted to avoid overlapping tick sampling transects with small mammal trapping transects, as we did not want to remove ticks that may naturally encounter and attach to the small mammals that we were sampling. We removed all ticks attached to the drag cloths with forceps and stored them in vials of 70% ethanol. We transported all ticks back to the University of Maine where they were visually sorted by life stage. We identified both the on-host and host-seeking ticks to species and life stages using published keys (Clifford et al. 1961, Keirans and Litwak 1989, Durden and Keirans 1996).
Molecular Pathogen Detection and Peromyscus Species Delineation
To test the hypotheses that pathogen prevalence was affected by land use and ecotone and differed between Peromyscus spp., we tested all Peromyscus ear tissue samples and all host-seeking I. scapularis nymphs for the presence of B. burgdorferi sensu lato. We also used a molecular test that we developed to delineate between P. leucopus and P. maniculatus, while simultaneously testing for B. burgdorferi sensu lato, A. phagocytophilum, and B. microti. We extracted DNA from tick samples and performed a multiplex quantitative polymerase chain reaction (qPCR) assay for pathogen detection using the procedures described in Rounsville et al. (2021). For pathogen testing and Peromyscus species delineation of small mammal tissue samples, we first extracted DNA using the protocol of the Qiagen (Germantown, MD, USA) DNeasy Blood and Tissue Kit (Cat no. 69506) extraction kit. We then performed a multiplex qPCR assay to detect B. burgdorferi sensu lato, A. phagocytophilum, and B. microti and simultaneously delineate between P. leucopus and P. maniculatus.
For the initial reaction components, we used 5 µl Quantabio (Beverly, MA, USA) PerfeCTa qPCR ToughMix, 3 µl of primer and probe mixture (Table 1), and 2 µl of tissue sample DNA extract. We completed each qPCR in at least duplicate on a BioRad CFX 1,000 thermal cycler (Hercules, CA, USA) with the following conditions: an initial 95°C burn-in for 3 min followed by 45 cycles of 95°C denaturation (15 s) and 60°C annealing-extension (1 min). We also used negative DNA extraction and PCR controls, as well as synthetic positive controls from the target pathogens obtained from Integrated DNA Technologies (Coralville, IA, USA). To reduce the possibility of inaccurate Peromyscus spp. assignments, we used tissue samples from two voucher specimens each of P. leucopus and P. maniculatus as positive controls. These voucher specimens were sourced from the University of New Hampshire mammal collection. We designed a single forward and reverse primer pair that will amplify a portion of the cytochrome oxidase subunit I gene from both Peromyscus spp. and two individual fluorescently labeled probes that detect and report either P. leucopus (Cy5) or P. maniculatus (Cy5.5), respectively. These probes typically produced amplicons with critical thresholds (CTs) of 15–17 and we recorded the species of origin of a sample if it produced an amplicon with one of these probes with a CT less than 25. If the Peromyscus amplicon produced a CT of greater than 25 or none at all, we considered that particular sample to either have failed in DNA extraction or that the tissue sample was not from a Peromyscus spp., and removed it from the study. We recorded a tissue sample as positive for a pathogen if all of the following were true: pathogen DNA amplified in both the sample and duplicate, the amplicon produced a CT of less than 37.5, the difference between the two runs is less than 3 CT’s, and both amplifications appear to have logarithmic growth. We applied differing thresholds for mice and pathogens to account for the higher concentration of mouse DNA in the tissue samples. We reran samples producing inconsistent results in at least duplicate and recorded samples as positive if they produced a positive pathogen result in half or more of the total runs for that sample.
Table 1.
Quantitative PCR primers and probes used to detect selected pathogens and delineate between Peromyscus leucopus and Peromyscus maniculatus
| Target | Type | Sequence (5ʹ-3ʹ) | Conc. (nM) | Ref. |
|---|---|---|---|---|
| Borrelia burgdorferi sensu lato | FWD | CGAGTCTTAAAAGGGCGATTTAGT | 350 | Xu et al. (2016) |
| REV | GCTTCAGCCTGGCCATAAATAG | 350 | ||
| Probe | FAM—AGATGTGGTAGACCCGAAGCCGAGTG | 175 | ||
| Anaplasma phagocytophilum | FWD | ATGGAAGGTAGTGTTGGTTATGGTATT | 650 | Hojgaard et al. (2014) |
| REV | TTGGTCTTGAAGCGCTCGTA | 650 | ||
| Probe | HEX—TGGTGCCAGGGTTGAGCTTGAGATTG | 350 | ||
| Babesia microti | FWD | CGCGTGGCGTTTATTAGACTT | 400 | Wang et al. (2015) |
| REV | CAAAGCCATGCGATTCGC | 400 | ||
| Probe | ROX—CCAACCCTTCGGGTAATCGGTGATTC | 225 | ||
| Peromyscus | FWD | TTCTACTAACCACAAAGACATCGGGAC | 350 | This manuscript |
| REV | TGATCGTCACCTAATAAGGCACC | 350 | ||
| Peromyscus leucopus | Probe | Cy5—GTATTTATTATTCGGAGCCTGAGCAGGA | 275 | This manuscript |
| Peromyscus maniculatus | Probe | Cy5.5—CCTATTGTTTGGGGCATGAGCTGGG | 275 | This manuscript |
Statistical Analyses
We conducted all statistical analyses using R version #4.0.5 (R Core Team 2021). As we captured P. maniculatus at only 4 of our 12 study sites with the majority coming from 1 site, we confined the species comparison to this 1 site. Due to zero inflation in the tick counts collected from Peromyscus spp., we used a 2-stage hurdle model in the package “glmmTMB” (Brooks et al. 2017) to assess differences in on-host tick infestation between the 2 species. Using an initial binary model to distinguish between zero and nonzero values, the hurdle model then uses a zero-truncated negative binomial model to fit the nonzero values. Our model first assessed the difference in infestation frequency (number of tick-infested mice) of Peromyscus spp. with I. scapularis. The second stage of the model tested that, given the presence of ticks, there was a significant difference in the intensity of the infestation (mean number of ticks on infested mice). Tick burden was the response variable, Peromyscus species, sex, and age class were the main fixed effects, and month and year were random effects. We used a generalized linear mixed effects model in the package “lme4” (Bates et al. 2015) to analyze the difference in B. burgdorferi sensu lato prevalence between the Peromyscus spp.
Due to the low number of P. maniculatus observed across study sites, we confined our analysis of the effects of land-use, ecotone, and distance from the mouth of the river on I. scapularis infestation to P. leucopus only, again using a 2-stage hurdle model. Due to the low number of P. leucopus captures in the “open” ecotone (n = 5), we compared “edge” and “forest” ecotones only. In this model, tick burden was included as the response variable, fixed effects included land use, ecotone, and distance up river, and month and year were included as random effects. We used a generalized linear mixed effects model in the package “lme4” (Bates et al. 2015) to analyze the impact of land use, ecotone, and distance up river on B. burgdorferi prevalence in P. leucopus. While we report the infection prevalence for A. phagocytophilum and B. microti among the Peromyscus spp., we did not include these pathogens in the analysis, as blood samples were not available for testing. We used linear mixed effects models in the package “nlme” (Pinheiro et al. 2022) to analyze the impact of land-use, ecotone, and distance up river on the density of host-seeking nymphs (DON) and DIN, with year included as a random effect. Due to the low number of host-seeking I. scapularis in the “open” ecotone, we made comparisons of DON, and DIN between “edge” and “forest” ecotones. We performed a square root transformation on DIN prior to running the mixed effects models to satisfy the model assumption of normally distributed residuals.
Results
Tick Infestation and Pathogen Prevalence Between Peromyscus spp.
We trapped 833 P. leucopus and 34 P. maniculatus (867 total) over the course of 6,480 trap nights during 2017 and 2018 across our 12 study sites (Table 2). While P. leucopus captures were represented at each of the sample sites, P. maniculatus was captured from only 4 of the sites, with the majority coming from one site. From this site, we trapped 61 P. leucopus and 25 P. maniculatus and collected a total of 160 immature I. scapularis specimens (114 larvae and 46 nymphs) from the Peromyscus spp. during the 2-year sampling period. We collected I. scapularis from 77% (47/61 mice) of sampled P. leucopus and from 28% of P. maniculatus (7/25 mice). We detected a mean I. scapularis infestation intensity of 2.41 ticks per mouse (± 0.35 SE) on P. leucopus and 0.52 ticks per mouse (± 0.21 SE) on P. maniculatus. Hurdle model analysis demonstrated that both the frequency (Z = 2.513, P = 0.012) and intensity (Z = -2.141, P = 0.032) of I. scapularis infestation was significantly higher for P. leucopus than P. maniculatus (Table 3). Across Peromyscus age-classes, infestation frequency was not significantly different, though we did detect a significantly higher mean infestation intensity in Peromyscus adults (2.08 ticks per mouse ± 0.37 SE) than juveniles (1.00 tick per mouse ± 0.33 SE; Z = −2.522, P = 0.012). We compared 61 P. leucopus and 25 P. maniculatus for the presence of B. burgdorferi. Among P. leucopus samples, 35 (57%) were positive for B. burgdorferi, whereas 14 (56%) P. maniculatus samples were positive. We did not detect any significant difference in the pathogen prevalence between Peromyscus spp. for B. burgdorferi (Z = 0.198, P = 0.843).
Table 2.
Mean Ixodes scapularis infestation intensity and pathogen infection among Peromyscus leucopus and Peromyscus maniculatus
| Peromyscus leucopus | Peromyscus maniculatus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site | N (Mice) | Mean tick infestation (SE) | B. burgdorferi infected (prevalence) | A. phagocytophilum infected (prevalence) | B. microti infected (prevalence) | N (Mice) | Mean tick infestation (SE) | B. burgdorferi infected (prevalence) | A. phagocytophilum infected (prevalence) | B. microti infected (prevalence) |
| 1 | 93 | 1.28 (0.22) | 44 (0.47) | 39 (0.42) | 9 (0.10) | 0 | 0 | 0 | 0 | 0 |
| 2 | 64 | 2.56 (0.44) | 40 (0.63) | 10 (0.16) | 4 (0.06) | 5 | 2.2 (1.07) | 4 (0.80) | 1 (0.20) | 0 (0) |
| 3 | 61 | 2.41 (0.35) | 35 (0.57) | 1 (0.02) | 2 (0.03) | 25 | 0.52 (0.21) | 14 (0.56) | 2 (0.08) | 0 (0) |
| 4 | 53 | 1.17 (0.32) | 19 (0.48) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | 0 |
| 5 | 83 | 2.21 (0.43) | 54 (0.65) | 12 (0.14) | 3 (0.04) | 0 | 0 | 0 | 0 | 0 |
| 6 | 101 | 1.09 (0.25) | 29 (0.29) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 | 0 |
| 7 | 52 | 1.04 (0.25) | 24 (0.46) | 4 (0.08) | 1 (0.02) | 0 | 0 | 0 | 0 | 0 |
| 8 | 84 | 1.29 (0.26) | 43 (0.51) | 18 (0.21) | 0 (0) | 0 | 0 | 0 | 0 | 0 |
| 9 | 56 | 1.66 (0.31) | 29 (0.52) | 10 (0.18) | 1 (0.02) | 3 | 2 (2.00) | 2 (0.67) | 2 (0.67) | 0 (0) |
| 10 | 40 | 0.75 (0.29) | 13 (0.33) | 1 (0.03) | 0 (0) | 0 | 0 | 0 | 0 | 0 |
| 11 | 101 | 0.88 0.27) | 29 (0.29) | 2 (0.02) | 0 (0) | 1 | 0 | 1 (1.00) | 0 (0) | 0 (0) |
| 12 | 45 | 1.93 (0.63) | 14 (0.31) | 3 (0.07) | 0 (0) | 0 | 0 | 0 | 0 | 0 |
Table 3.
Hurdle model analysis of Ixodes scapularis infestation rates on Peromyscus leucopus and Peromyscus maniculatus with sex, and age-class as additional fixed effects and year and month included as random effects
| Zero-inflation model (infestation frequency) | ||||
|---|---|---|---|---|
| Estimate | Std. Error | Z | P | |
| Species—P. maniculatus | 1.955 | 0.778 | 2.513 | 0.012* |
| Sex—Male | −0.671 | 0.707 | −0.950 | 0.342 |
| Age-Class—Juvenile | −3.281 | 10.659 | −0.308 | 0.758 |
| Age-Class—Subadult | −0.088 | 0.682 | −0.128 | 0.898 |
| Conditional model (infestation intensity) | ||||
|---|---|---|---|---|
| Estimate | Std. Error | Z | P | |
| Species—P. maniculatus | −0.884 | 0.413 | −2.141 | 0.032* |
| Sex—Male | 0.069 | 0.188 | 0.366 | 0.715 |
| Age-Class—Juvenile | −1.036 | 0.411 | −2.522 | 0.012* |
| Age-Class—Subadult | −0.157 | 0.196 | −0.799 | 0.424 |
* P < 0.05.
Tick Infestation and Pathogen Prevalence of Peromyscus leucopus Across Land-Use and Ecotone
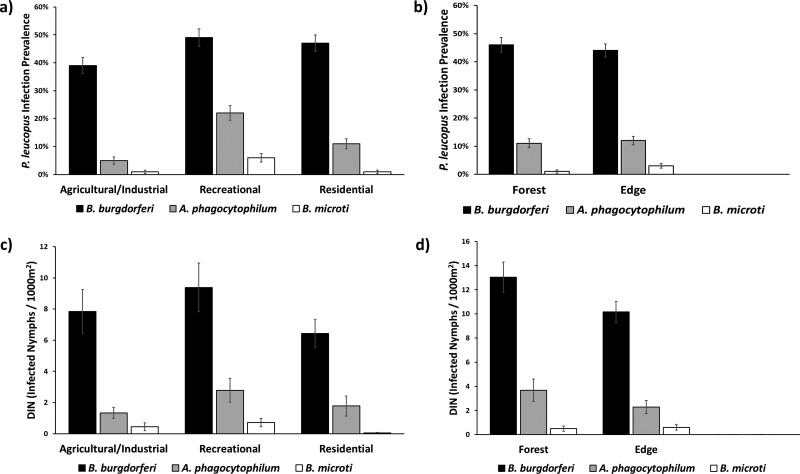
From the 833 P. leucopus trapped across the 12 study sites, we collected I. scapularis from 53% (159/299 mice) of P. leucopus in residential sites, 47% (116/249 mice) in recreational sites, and 38% (109/285 mice) in agricultural/industrial sites. Our hurdle model analysis indicated a significant difference in I. scapularis infestation frequency, with higher rates of infestation in residential settings than in recreational (Z = 3.6, P < 0.001) or agricultural/industrial sites (Z = 3.643, P < 0.001). Though the mean infestation intensity was higher in residential sites (1.66 ticks per mouse ± 0.16 SE) than in recreational (1.47 ticks per mouse ± 0.16 SE) or agricultural/industrial (1.34 ticks per mouse ± 0.18 SE) sites, the infestation intensity was not statistically different across land-use type (Table 4). Within edge and forest ecotones, we collected ticks from 48% (219/457 mice) and 44% (165/376 mice) of P. leucopus, respectively. We detected a significantly higher mean I. scapularis infestation intensity in edge ecotones (1.68 ticks per mouse ± 0.14 SE) than in forest ecotones (1.27 ticks per mouse ± 0.14 SE; Z = −3.527, P < 0.001). The infestation frequency was not significantly different across ecotones (Table 4). The distance north from the mouth of the Penobscot River was significant in determining tick infestation frequency, with more P. leucopus parasitized by ticks at sites closer to the mouth of the river (Z = 4.978, P < 0.001; Table 4). Pathogen infection prevalence was relatively evenly distributed across land-use types (Fig. 2a). Mixed effects modeling showed that pathogen prevalence was not significantly different across land-use types for B. burgdorferi (Table 5). Among the different ecotones, pathogen prevalence was also relatively evenly distributed (Fig. 2b). The prevalence of B. burgdorferi was not significantly different between edge and forest ecotones (Table 5).
Table 4.
Hurdle model analysis of Ixodes scapularis infestation rates on Peromyscus leucopus with land-use, ecotone, and distance up river included as fixed effects and year and month included as random effects
| Zero-inflation model (infestation frequency) | ||||
|---|---|---|---|---|
| Estimate | Std. Error | Z | P | |
| Land-use—Recreational | 0.775 | 0.215 | 3.600 | <0.001* |
| Land-use—Ag/Industrial | 0.662 | 0.182 | 3.643 | <0.001* |
| Ecotone—Forest | 0.155 | 0.154 | 1.010 | 0.312 |
| Distance Up River | 0.245 | 0.049 | 4.978 | <0.001* |
| Conditional model (infestation intensity) | ||||
|---|---|---|---|---|
| Estimate | Std. Error | Z | P | |
| Land-use—Recreational | 0.002 | 0.086 | 0.024 | 0.981 |
| Land-use—Ag/Industrial | 0.119 | 0.104 | 1.599 | 0.110 |
| Ecotone—Forest | −0.229 | 0.065 | −3.527 | <0.001* |
| Distance Up River | −0.008 | 0.020 | −0.405 | 0.686 |
“Residential” is the reference level for the land-use category and “Edge” is the reference level for the ecotone category.
* P < 0.05.
Fig. 2.
a) Pathogen infection prevalence among Peromyscus leucopus across different land-use types. b) Pathogen infection prevalence among P. leucopus in forest and edge ecotones. c) Density of infected nymphs (DIN) across different land use types. d) Density of infected nymphs (DIN) across forest and edge ecotones.
Table 5.
Mixed effects model assessing differences in pathogen infection prevalence in Peromyscus leucopus for Borrelia burgdorferi across land use categories and ecotone type
| Borrelia burgdorferi | ||||
|---|---|---|---|---|
| Estimate | Std. Error | Z | P | |
| Land use—Recreational | −0.281 | 0.200 | −1.408 | 0.159 |
| Land use—Ag/Industrial | −0.312 | 0.175 | −1.784 | 0.075 |
| Ecotone—Forest | 0.084 | 0.146 | 0.571 | 0.568 |
| Distance Up River | −0.175 | 0.046 | −3.755 | <0.001* |
“Residential” is the reference level for the land-use category and “Edge” is the reference level for the ecotone category.
* P < 0.05.
Host-Seeking Tick Collection and Pathogen Prevalence
We collected and tested a total of 1,190 I. scapularis nymphs during tick dragging sessions in 2017, 2018, and 2019. The density of nymphs (DON) per 1,000 m2 was slightly higher in recreational sites (24.5 nymphs/1,000 m2) than agricultural/industrial (22.5 nymphs/1,000 m2) or residential (19.7 nymphs/1,000 m2), however, our mixed effects modeling showed these densities were not statistically different (Table 6). We did not detect significant variation across ecotone (Table 6), with forested ecotones maintaining a DON of 34.1 nymphs/1,000 m2 and edges with a DON of 30.2 nymphs/1,000 m2. Distance up river did significantly affect DON, with higher questing nymphal densities at sites closer to the mouth of the river (Table 6). Across the entire study area and over 3 years (2017–2019), we detected B. burgdorferi in 420 nymphs (35.3%), A. phagocytophilum in 90 nymphs (7.6%), and B. microti in 20 nymphs (1.6%). We also noted annual differences in infection prevalence, with B. burgdorferi detected in 34% of I. scapularis nymphs in 2017, 31% of nymphs in 2018, and 41% in 2019. The DIN was relatively even across land-use types with 9.39 infected nymphs/1,000m2 (SE = 1.57) in recreational sites, 7.85 infected nymphs/1,000m2 (SE = 1.40) in agricultural/industrial sites, and 6.43 infected nymphs/1,000m2 (SE = 0.89) in residential sites (Fig. 2c). We detected no significant difference in DIN across land-use type (Table 7). We detected a significant difference in DIN across ecotones for B. burgdorferi, with forested ecotones (13.03 infected nymphs/1,000m2 ± 1.25 SE) maintaining a higher DIN than edge ecotones (10.17 infected nymphs/1,000m2 ± 0.86 SE; Table 7; Fig. 2d). We did not detect a significant difference in DIN across ecotones for A. phagocytophilum or B. microti. The distance from the mouth of the Penobscot River significantly influenced DIN for all 3 pathogens, with higher DIN at sites closer to the mouth of the river (Table 7).
Table 6.
Summary of mixed effects model assessing the impacts of land-use, ecotone, and distance up river on the density of Ixodes scapularis nymphs (DON) with year as a random effect
| Mixed effects model | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | DF | t-value | P | |
| Land-use—Recreational | 1.519 | 2.597 | 65 | 0.585 | 0.560 |
| Land-use—Ag/Industrial | 3.269 | 2.659 | 65 | 1.229 | 0.223 |
| Ecotone—Forest | 3.889 | 2.101 | 65 | 1.851 | 0.069 |
| Distance Up River | −3.071 | 0.627 | 65 | −4.902 | <0.001* |
“Residential” is the reference level for the land-use category and “Edge” is the reference level for the ecotone category.
* P < 0.05.
Table 7.
Mixed effects model assessing differences in density of infected nymphs for Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti across land-use categories and ecotone type with year as a random effect
| Borrelia burgdorferi | ||||
|---|---|---|---|---|
| Estimate | Std. Error | t-value | P | |
| Land-use—Recreational | 0.029 | 0.188 | 0.153 | 0.879 |
| Land-use—Ag/Industrial | 0.341 | 0.192 | 1.773 | 0.081 |
| Ecotone—Forest | 0.373 | 0.152 | 2.451 | 0.017* |
| Distance Up River | −0.354 | 0.045 | −7.807 | <0.001* |
| Anaplasma phagocytophilum | ||||
| Estimate | Std. Error | t-value | P | |
| Land-use—Recreational | −0.052 | 0.289 | −0.179 | 0.859 |
| Land-use—Ag/Industrial | 0.035 | 0.295 | 0.119 | 0.906 |
| Ecotone—Forest | 0.313 | 0.234 | 1.339 | 0.185 |
| Distance Up River | −0.397 | 0.069 | −5.705 | <0.001* |
| Babesia microti | ||||
| Estimate | Std. Error | t-value | P | |
| Land-use—Recreational | 0.271 | 0.176 | 1.543 | 0.128 |
| Land-use—Ag/Industrial | 0.336 | 0.180 | 1.869 | 0.066 |
| Ecotone—Forest | −0.032 | 0.142 | −0.226 | 0.822 |
| Distance Up River | −0.166 | 0.042 | −3.912 | <0.001* |
“Residential” is the reference level for the land-use category and “Edge” is the reference level for the ecotone category. DF = 65 for all comparisons.
* P < 0.05.
Discussion
The complex relationships between ticks, wildlife hosts, and their shared ecotones are important to the ability of both vector and pathogen to persist in previously uninhabited areas. Here, we demonstrate that, while similar morphologically and in their ecological roles, P. leucopus and P. maniculatus differ in tick infestation frequency and intensity but not in pathogen prevalence within our study area in an emergent region for tick-borne disease. This supports previous studies that found higher rates of I. scapularis parasitization on P. leucopus in other emerging regions for tick-borne disease (Bouchard et al. 2011, Larson et al. 2018). The tick encounter rate and the subsequent number of ticks that feed upon the two Peromyscus spp. is regulated in part by mouse densities and the occurrence and abundance of other potential host species. Although Peromyscus spp. were not marked and recaptured and thus we were unable to calculate population size estimates, P. leucopus was far more abundant than P. maniculatus or any other small mammal species within the confines of the study. Though both species are relatively ubiquitous within their ranges, P. leucopus tends to favor deciduous and mixed forests, as well as fragmented landscapes (Adler et al. 1987, Anderson et al. 2006) while P. maniculatus is more of a habitat generalist (Garman et al. 1994). As our study sites primarily contained deciduous and mixed forests and were relatively fragmented, they may have favored the presence of P. leucopus. While P. leucopus was infested more frequently and intensively with immature I. scapularis, we found no significant difference in pathogen infection prevalence between the Peromyscus spp. Although this may be a result of the small number of infected P. maniculatus in our study, it contradicts findings in the Midwest that noted a significantly higher B. burgdorferi infection prevalence in P. maniculatus (Larson et al. 2021).
Given the similarity in B. burgdorferi infection prevalence, P. maniculatus may serve as a “rescue host” in emerging regions like Maine, facilitating the expansion and maintenance of tick-borne pathogens in the absence of the primary reservoir, P. leucopus. Previous studies have noted the ability of other small mammal species, including shrews and eastern chipmunks, to act as rescue hosts for B. burgdorferi when white-footed mouse populations are low (LoGiudice et al. 2003, Brunner et al. 2008). Though P. leucopus is now abundant in much of southern and coastal Maine, P. maniculatus is more common in the central and northern parts of the state. As I. scapularis continues its northward expansion, suitable hosts like P. maniculatus may act as rescue hosts in the absence of P. leucopus. Although B. burgdorferi can be maintained in enzootic cycles among I. scapularis and rescue hosts prior to the arrival of P. leucopus, establishment of P. leucopus may increase the efficiency of the transmission cycle within small mammal communities by increasing the opportunities for ticks to feed on a highly competent reservoir, resulting in higher infection prevalence within tick and small mammal host populations (Hamer et al. 2010, Simon et al. 2014). The arrival of and subsequent colonization by P. leucopus may thus increase the reservoir importance of P. maniculatus during the period of sympatry. Our study adds to the growing body of literature identifying P. maniculatus as a potential host for I. scapularis and reservoir of tick-borne pathogens along the northern edge of tick and tick-borne pathogen range expansion.
Though land-use change and land cover features are important factors in the emergence and maintenance of tick-borne disease (Gardner et al. 2020, Diuk-Wasser et al. 2021), we found mixed results for the impacts of specific land-use activities and local, site-scale ecotone types on I. scapularis abundance and infection prevalence. The frequency of I. scapularis infestation on P. leucopus was highest among residential settings, however, the intensity of infestation did not significantly differ between land-use types. The B. burgdorferi infection prevalence among P. leucopus also did not significantly differ across land-use types. Previous studies have identified peridomestic settings as particularly high-risk areas for human exposure to tick-borne pathogens, partially due to a combination of available tick habitat and high levels of human activity (Connally et al. 2006, 2009). While we did find moderate evidence for elevated tick infestation and pathogen prevalence among small mammals in residential settings, neither the density of host-seeking nymphs (DON) nor the DIN was significantly different across land-use types. The moderate differences in tick infestation and pathogen prevalence between land-use types may be a function of site selection, as each site was similar in size, vegetation community structure, and all fell within a relatively fragmented landscape mosaic. It seems, that provided ample suitable ecotone within a site, the type of human activity had little effect on tick abundance or pathogen infection prevalence.
Within site-scale ecotone types, edge ecotones have frequently been identified as areas of elevated risk for human exposure to ticks and tick-borne pathogens (Frank et al. 1998, Jackson et al. 2006). While we found that tick infestation intensity on P. leucopus was highest in edge ecotones, DIN for B. burgdorferi was significantly higher in forested ecotones. The difference we detected in tick infestation on P. leucopus may be a function of the arbitrary distance-based definition we used to designate the edge ecotone. Klein and Cameron (2012) noted the importance of characterizing the gradient of changes in vegetation in defining the edge ecotone, rather than relying on an arbitrary distance-based definition. Additionally, while we attempted to place traps at a sufficient distance to minimize individual P. leucopus overlap between ecotones, it is possible that individual mice may have utilized multiple ecotones. Our host-seeking results support previous studies that have contradicted the paradigm that edge ecotones harbor higher I. scapularis abundance than forest interiors (Horobik et al. 2006, Mason et al. 2022). Though these edge ecotones may not support greater tick densities, they may still pose a significant tick encounter risk as areas of high human activity.
As an emergent region along the expansion front for I. scapularis and its associated pathogens, Maine exhibits a north/south gradient of tick abundance and pathogen infection prevalence (Rounsville et al. 2021). We found that the location of each study site in relation to the distance from the mouth of the river also had significant impacts on both on-host and host-seeking tick densities, as well as pathogen infection prevalence. We detected higher tick densities and infection rates at sites closer to the mouth of the river indicating a north/south gradient of tick abundance and infection prevalence across a relatively small spatial scale. Our selection of sites along the Penobscot River may have influenced tick and small mammal host dynamics, as landscape features, including rivers, have been positively correlated with the invasion of I. scapularis and dispersal by white-tailed deer and migratory birds (Cortinas and Kitron 2006, Talbot et al. 2019, Gardner et al. 2020). Our study highlights the potential for variability in tick density and pathogen prevalence across fine spatial scales within an emerging region for tick-borne disease.
Given the expanding geographical distribution of ticks and tick-borne pathogens, understanding the ecological relationships between ticks, wildlife hosts, and their shared ecotones is critical. We observed significant differences in regard to tick infestation frequency and intensity between closely related and sympatric Peromyscus spp. As these two Peromyscus spp. are notoriously difficult to differentiate, we developed a molecular method for delineating between P. leucopus and P. maniculatus, while simultaneously testing for tick-borne pathogens. Though much of the focus of tick-borne pathogen research has focused on P. leucopus, we highlight the importance of considering the role of P. maniculatus, particularly along the northern front of tick-borne disease range expansion. Future research should also examine the expanding distribution of P. leucopus in Maine and other areas of overlap with P. maniculatus to further elucidate the ways P. leucopus affects the efficiency of the tick-borne pathogen transmission cycle. We examined I. scapularis abundance and infection prevalence across both an ecotone and land-use gradient, finding varying impacts of human land-use and fine-scale local ecotones. As human behavior plays a significant role in tick encounter risk, future studies should attempt to incorporate the nuanced relationships between host availability and changing landscape dynamics in order to better understand the underlying mechanisms and facilitate targeted approaches to tick management.
Acknowledgments
We thank the individuals and organizations that allowed our access to their property to conduct this work. This research was supported by the State of Maine and funded by University of Maine Cooperative Extension.
Contributor Information
Griffin M Dill, University of Maine Cooperative Extension Diagnostic and Research Laboratory, 17 Godfrey Drive, Orono, ME 04473, USA.
Thomas F Rounsville, University of Maine Cooperative Extension Diagnostic and Research Laboratory, 17 Godfrey Drive, Orono, ME 04473, USA.
Ann M Bryant, University of Maine Cooperative Extension Diagnostic and Research Laboratory, 17 Godfrey Drive, Orono, ME 04473, USA.
Eleanor Groden, School of Biology and Ecology, University of Maine, 5722 Deering Hall, Orono, ME 04469, USA.
Allison M Gardner, School of Biology and Ecology, University of Maine, 5722 Deering Hall, Orono, ME 04469, USA.
Author contributions
Griffin Dill (Conceptualization [lead], Data curation [lead], Formal analysis [lead], Funding acquisition [lead], Investigation [lead], Methodology [lead], Project administration [lead], Resources [lead], Supervision [lead], Validation [lead], Visualization [lead], Writing—original draft [lead], Writing—review & editing [lead]), Thomas Rounsville Jr. (Investigation [equal], Methodology [equal], Validation [equal], Writing—review & editing [equal]), Ann Bryant (Investigation [equal], Methodology [equal], Validation [equal], Writing—review & editing [equal]), Eleanor Groden (Conceptualization [equal], Methodology [equal], Project administration [equal], Supervision [equal], Writing—review & editing [equal]), and Allison Gardner (Conceptualization [equal], Methodology [equal], Project administration [equal], Supervision [equal], Writing—review & editing [equal])
References
- Adler GH, Wilson ML.. 1987. Demography of a habitat generalist, the white-footed mouse, in a heterogeneous environment. Ecology 68(6):1785–1796. https://doi.org/ 10.2307/1939870 [DOI] [PubMed] [Google Scholar]
- Anderson CS, Meikle DB, Cady AB, et al. 2006. Annual variation in habitat use by white-footed mice, Peromyscus leucopus: the effects of forest patch size, edge and surrounding vegetation type. Can. Field-Nat. 120(2):192–198. [Google Scholar]
- Bates D, Mächler M, Bolker B, et al. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67(1):1–48. [Google Scholar]
- Bisanzio D, Fernandez MP, Martello E, et al. 2020. Current and future spatiotemporal patterns of Lyme disease reporting in the Northeastern United States. JAMA Netw. Open. 3(3):e200319. https://doi.org/ 10.1001/jamanetworkopen.2020.0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Beauchamp G, Nguon S, et al. 2011. Associations between Ixodes scapularis ticks and small mammal hosts in a newly endemic zone in southeastern Canada: implications for Borrelia burgdorferi transmission. Ticks Tick Borne Dis. 2(4):183–190. https://doi.org/ 10.1016/j.ttbdis.2011.03.005 [DOI] [PubMed] [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, et al. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9(2):378–400. [Google Scholar]
- Brunner JL, Ostfeld RS.. 2008. Multiple causes of variable tick burdens on small‐mammal hosts. Ecology 89(8):2259–2272. https://doi.org/ 10.1890/07-0665.1 [DOI] [PubMed] [Google Scholar]
- Burtis JC, Sullivan P, Levi T, et al. 2016. The impact of temperature and precipitation on blacklegged tick activity and Lyme disease incidence in endemic and emerging regions. Paras. Vect. 9(1):1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2022. Lyme disease data and surveillance [accessed 2023 Mar 9]. cdc.gov/lyme/datasurveillance/index.html
- Clifford CM, Anastos G, Elbl A.. 1961. The larval ixodid ticks of the eastern United States (Acarina-Ixodidae). Entomol. Soc. Am. 2:215. [Google Scholar]
- Connally NP, Durante AJ, Yousey-Hindes KM, et al. 2009. Peridomestic Lyme disease prevention: results of a population-based case–control study. Am. J. Prev. Med. 37(3):201–206. https://doi.org/ 10.1016/j.amepre.2009.04.026 [DOI] [PubMed] [Google Scholar]
- Connally NP, Ginsberg HS, Mather TN.. 2006. Assessing peridomestic entomological factors as predictors for Lyme disease. J. Vector Ecol. 31(2):364–370. https://doi.org/ 10.3376/1081-1710(2006)31[364:apefap]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Cortinas MR, Kitron U.. 2006. County-level surveillance of white-tailed deer infestation by Ixodes scapularis and Dermacentor albipictus (Acari: Ixodidae) along the Illinois River. J. Med. Entomol. 43(5):810–819. https://doi.org/ 10.1603/0022-2585(2006)43[810:csowdi]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Cramer MJ. 2014. Seeds of doubt: feeding preferences of white-footed deer mice (Peromyscus leucopus noveboracensis) and woodland deer mice (Peromyscus maniculatus gracilis) on maple (genus Acer) seeds. Can. J. Zool. 92(9):771–776. https://doi.org/ 10.1139/cjz-2014-0090 [DOI] [Google Scholar]
- Diuk-Wasser M, VanAcker MC, Fernandez MP.. 2021. Impact of land use changes and habitat fragmentation on the eco-epidemiology of tick-borne diseases. J. Med. Entomol. 58(4):1546–1564. [DOI] [PubMed] [Google Scholar]
- Durden LA, Keirans JE.. 1996. Key to the nymphs of the genus Ixodes of the United States. In: Nymphs of the genus Ixodes (Acari: Ixodidae) of the United States: taxonomy, identification key, distribution, hosts, and medical/veterinary importance. Lantham (MD): Entomological Society of America. p. 5. [Google Scholar]
- Elias SP, Gardner AM, Maasch KA, et al. 2021. A generalized additive model correlating blacklegged ticks with white-tailed deer density, temperature, and humidity in Maine, USA, 1990–2013. J. Med. Entomol. 58(1):125–138. [DOI] [PubMed] [Google Scholar]
- Elias SP, Lubelczyk CB, Rand PW, et al. 2006. Deer browse resistant exotic-invasive understory: an indicator of elevated human risk of exposure to Ixodes scapularis (Acari: Ixodidae) in southern coastal Maine woodlands. J. Med. Entomol. 43(6):1142–1152. [DOI] [PubMed] [Google Scholar]
- Elias SP, Maasch KA, Anderson NT, et al. 2020. Decoupling of blacklegged tick abundance and Lyme disease incidence in Southern Maine, USA. J. Med. Entomol. 57(3):755–765. [DOI] [PubMed] [Google Scholar]
- Falco RC, Fish D.. 1988. Ticks parasitizing humans in a Lyme disease endemic area of southern New York State. Am. J. Epidemiol. 128(5):1146–1152. https://doi.org/ 10.1093/oxfordjournals.aje.a115057 [DOI] [PubMed] [Google Scholar]
- Frank DH, Fish D, Moy FH.. 1998. Landscape features associated with Lyme disease risk in a suburban residential environment. Landsc. Ecol. 13(1):27–36. [Google Scholar]
- Gardner AM, Pawlikowski NC, Hamer SA, et al. 2020. Landscape features predict the current and forecast the future geographic spread of Lyme disease. Proc. Biol. Sci. 287(1941):20202278. https://doi.org/ 10.1098/rspb.2020.2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman SL, O’Connell Jr AF, Connery JH.. 1994. Habitat use and distribution of the mice Peromyscus leucopus and P. maniculatus on Mount Desert Island, Maine. Can. Field-Nat. 108(1):67–71. [Google Scholar]
- Hahn MB, Bjork JK, Neitzel DF, et al. 2018. Evaluating acarological risk for exposure to Ixodes scapularis and Ixodes scapularis-borne pathogens in recreational and residential settings in Washington County, Minnesota. Ticks Tick Borne Dis. 9(2):340–348. https://doi.org/ 10.1016/j.ttbdis.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Tsao JI, Walker ED, et al. 2010. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. Ecohealth. 7(1):47–63. https://doi.org/ 10.1007/s10393-010-0287-0 [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J.. 2014. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick Borne Dis. 5(3):349–351. https://doi.org/ 10.1016/j.ttbdis.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Horobik V, Keesing F, Ostfeld RS.. 2006. Abundance and Borrelia burgdorferi-infection prevalence of nymphal Ixodes scapularis ticks along forest–field edges. EcoHealth. 3(4):262–268. https://doi.org/ 10.1007/s10393-006-0065-1 [DOI] [Google Scholar]
- Jackson LE, Hilborn ED, Thomas JC.. 2006. Towards landscape design guidelines for reducing Lyme disease risk. Int. J. Epidemiol. 35(2):315–322. https://doi.org/ 10.1093/ije/dyi284 [DOI] [PubMed] [Google Scholar]
- Keesing F, Hersh MH, Tibbetts M, et al. 2012. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerg. Infect. Dis. 18(12):2013–2016. https://doi.org/ 10.3201/eid1812.120919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans JE, Litwak TR.. 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J. Med. Entomol. 26(5):435–448. https://doi.org/ 10.1093/jmedent/26.5.435 [DOI] [PubMed] [Google Scholar]
- Klein GP, Cameron GN.. 2012. Effect of habitat gradients on space use by white-footed mice (Peromyscus leucopus). J. Mammal. 93(3):706–715. https://doi.org/ 10.1644/11-mamm-a-258.1 [DOI] [Google Scholar]
- Larson SR, Bron GM, Lee X, et al. 2021. Peromyscus maniculatus (Rodentia: Cricetidae): An overlooked reservoir of tick-borne pathogens in the Midwest, USA? Ecosphere 12(11):e03831. [Google Scholar]
- Larson SR, Lee X, Paskewitz SM.. 2018. Prevalence of tick-borne pathogens in two species of Peromyscus mice common in northern Wisconsin. J. Med. Entomol. 55(4):1002–1010. https://doi.org/ 10.1093/jme/tjy027 [DOI] [PubMed] [Google Scholar]
- Leger E, Vourc’h G, Vial L, et al. 2013. Changing distributions of ticks: causes and consequences. Exp. Appl. Acarol 59(1-2):219–244. https://doi.org/ 10.1007/s10493-012-9615-0 [DOI] [PubMed] [Google Scholar]
- Leighton PA, Koffi JK, Pelcat Y, et al. 2012. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J. Appl. Ecol. 49(2):457–464. https://doi.org/ 10.1111/j.1365-2664.2012.02112.x [DOI] [Google Scholar]
- Leo S, Millien V.. 2017. Microsatellite markers reveal low frequency of natural hybridization between the white-footed mouse (Peromyscus leucopus) and deer mouse (Peromyscus maniculatus) in southern Quebec, Canada. Genome 60(5):454–463. [DOI] [PubMed] [Google Scholar]
- Li S, Hartemink N, Speybroeck N, et al. 2012. Consequences of landscape fragmentation on Lyme disease risk: a cellular automata approach. PLoS One 7(6):e39612. https://doi.org/ 10.1371/journal.pone.0039612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist ES, Aquadro CF, McClearn D, et al. 2003. Field identification of the mice Peromyscus leucopus noveboracensis and P. maniculatus gracilis in central New York. Can. Field-Nat. 117(2):184–189. https://doi.org/ 10.22621/cfn.v117i2.680 [DOI] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt K, et al. 2003. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. PNAS 100(2):567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CA. 1996. Ecological replacement of the deer mouse, Peromyscus maniculatus, by the white-footed mouse, P. leucopus, in the Great Lakes region. The Can. field-Nat. 110(2):271–277. https://doi.org/ 10.5962/p.357451 [DOI] [Google Scholar]
- Lubelczyk CB, Elias SP, Rand PW, et al. 2004. Habitat associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Environ. Entomol. 33(4):900–906. [Google Scholar]
- Maine CDC (Center for Disease Control and Prevention). 2023. Lyme disease—reports and publications. [accessed 2024 Feb 10].maine.gov/dhhs/mecdc/infectious-disease/epi/vector-borne/lyme/index.shtml
- Mason SD, Sherratt SC, Kruguer SM, et al. 2022. Multi-scale analysis of habitat fragmentation on small-mammal abundance and tick-borne pathogen infection prevalence in Essex County, MA. PLoS One 17(6):e0269768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather TN, Wilson ML, Moore SI, et al. 1989. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am. J. Epidemiol. 130(1):143–150. [DOI] [PubMed] [Google Scholar]
- Mathews F, Honess P, Wolfensohn S.. 2002. Use of inhalation anaesthesia for wild mammals in the field. Vet. Rec. 150(25):785–787. https://doi.org/ 10.1136/vr.150.25.785 [DOI] [PubMed] [Google Scholar]
- Maupin GO, Fish D, Zultowsky J, et al. 1991. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am. J. Epidemiol. 133(11):1105–1113. https://doi.org/ 10.1093/oxfordjournals.aje.a115823 [DOI] [PubMed] [Google Scholar]
- Myers P, Lundrigan BL, Hoffman SMG, et al. 2009. Climate-induced changes in the small mammal communities of the Northern Great Lakes Region. Glob Chang Biol 15(6):1434–1454. 10.1111/j.1365-2486.2009.01846.x [DOI] [Google Scholar]
- Ogden NH, Lindsay LR, Beauchamp G, et al. 2004. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the Laboratory and Field. J. Med. Entomol. 41(4):622–633. https://doi.org/ 10.1603/0022-2585-41.4.622 [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Cepeda OM, Hazler KR, et al. 1995. Ecology of Lyme disease: habitat associations of ticks (Ixodes scapularis) in a rural landscape. Ecol. Appl. 5(2):353–361. https://doi.org/ 10.2307/1942027 [DOI] [Google Scholar]
- Piedmonte NP, Shaw SB, Prusinski MA, et al. 2018. Landscape features associated with blacklegged tick (Acari: Ixodidae) density and tick-borne pathogen prevalence at multiple spatial scales in central New York state. J. Med. Entomol. 55(6):1496–1508. https://doi.org/ 10.1093/jme/tjy111 [DOI] [PubMed] [Google Scholar]
- Piesman J, Eisen L. 2007. Prevention of tick-borne diseases. Ann. Rev. Entomol. 53(1):323–343. 10.1146/annurev.ento.53.103106.093429 [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, R Core Team. 2022. nlme: linear and nonlinear mixed effects models. R package version 3.1-160.
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Rand PW, Lacombe EH, Dearborn R, et al. 2007. Passive surveillance in Maine, an area emergent for tick-borne diseases. J. Med. Entomol. 44(6):1118–1129. [DOI] [PubMed] [Google Scholar]
- Rand PW, Lacombe EH, Smith Jr RP, et al. 1993. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetares: Spirochaetaceae) in the wild. J. Med. Entomol. 30(3):614–618. [DOI] [PubMed] [Google Scholar]
- Reid FA. 2006. Field guide to mammals of North America, north of Mexico. vol. 4. Boston (MA): Houghton Mifflin Harcourt. [Google Scholar]
- Rounsville TF, Dill GM, Bryant AM, et al. 2021. Statewide passive surveillance of Ixodes scapularis and associated pathogens in Maine. Vect. Borne Zoon. Dis. 21(6):406–412. https://doi.org/ 10.1089/vbz.2020.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Dufresne E, Logan T, Simon JA, et al. 2013. Poleward expansion of the white-footed mouse (Peromyscus leucopus) under climate change: implications for the spread of Lyme disease. PLoS One 8(11):e80724. https://doi.org/ 10.1371/journal.pone.0080724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Marrotte R, Millien V, et al. 2014. 2014. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol. Appl. 7(7):750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. 2018. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 15(3):478. https://doi.org/ 10.3390/ijerph15030478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RB, Anderson EM, Wendt SR, et al. 2014. Wendt SR. Field identification of sympatric Peromyscus leucopus noveboracensis and P. maniculatus gracilis in Wisconsin from external measurements. Am. Midl. Nat. 171(1):139–146. https://doi.org/ 10.1674/0003-0031-171.1.139 [DOI] [Google Scholar]
- Talbot B, Slatculescu A, Thickstun CR, et al. 2019. Landscape determinants of density of blacklegged ticks, vectors of Lyme disease, at the northern edge of their distribution in Canada. Sci. Rep. 9(1):16652. https://doi.org/ 10.1038/s41598-019-50858-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wormser GP, Zhuge J, et al. 2015. Utilization of a real-time PCR assay for diagnosis of Babesia microti infection in clinical practice. Ticks Tick Borne Dis. 6(3):376–382. https://doi.org/ 10.1016/j.ttbdis.2015.03.001 [DOI] [PubMed] [Google Scholar]
- West G, Heard D, Caulkett N.. 2014. Zoo animal and wildlife immobilization and anesthesia. Hoboken (NJ): John Wiley & Sons. [Google Scholar]
- Williams SC, Linske MA, Ward JS.. 2017. Long-term effects of Berberis thunbergii (Ranunculales: Berberidaceae) management on Ixodes scapularis (Acari: Ixodidae) abundance and Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) prevalence in Connecticut, USA. Environ. Entomol. 46(6):1329–1338. https://doi.org/ 10.1093/ee/nvx146 [DOI] [PubMed] [Google Scholar]
- Williams SC, Ward JS.. 2010. Effects of Japanese barberry (Ranunculales: Berberidaceae) removal and resulting microclimatic changes on Ixodes scapularis (Acari: Ixodidae) abundances in Connecticut, USA. Environ. Entomol. 39(6):1911–1921. https://doi.org/ 10.1603/EN10131 [DOI] [PubMed] [Google Scholar]
- Xu G, Mather TN, Hollingsworth CS, et al. 2016. Passive surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts. Vect. Borne Zoon. Dis. 16(8):520–527. https://doi.org/ 10.1089/vbz.2015.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnik CP, Falco RC, Kolokotronis SO, et al. 2015. No observed effect of landscape fragmentation on pathogen infection prevalence in blacklegged ticks (Ixodes scapularis) in the Northeastern United States. PLoS One 10(10):e0139473. https://doi.org/ 10.1371/journal.pone.0139473 [DOI] [PMC free article] [PubMed] [Google Scholar]