Abstract
Study Design
The study was a retrospective cross-sectional database analysis.
Objective
Deoxycholic acid (DOC) injections are a novel, in-office procedural alternative to submental liposuction or submentoplasty to address excess submental fat. Post-market safety data regarding this treatment is currently limited. The objective of this study is to analyze adverse events reported in the Manufacturer and User Facility Device Experience (MAUDE) database.
Methods
The MAUDE database was queried for all reports related to adverse events involving deoxycholic acid using the search terms “KYBELLA” and “deoxycholic acid.” Reports were individually reviewed by 2 reviewers and categorized with special attention to adverse events.
Results
A total of 34 medical device reports were identified from the database query. Thirteen of these reports (21 total events) were included in the analysis after excluding duplicates, unrelated adverse events, or events associated with the off-label use of DOC. Reported adverse events include excessive swelling (n = 5, 24%), marginal mandibular nerve weakness (n = 4, 19%), unsatisfactory aesthetic outcome (n = 4, 19%), numbness (n = 3, 14%), dysphagia (n = 1, 5%), infection (n = 1, 5%), and skin necrosis (n = 3, 14%). Two patients required hospitalization for skin necrosis management; both had underlying systemic diseases.
Conclusions
Adverse events following DOC injections included excessive swelling, dysphagia, numbness, infection, unsatisfactory aesthetic outcome, facial nerve weakness, and skin necrosis requiring hospitalization and/or surgery. Patient counseling regarding these adverse events should be discussed when offering DOC injections for submental convexity.
Keywords: deoxycholic acid, submental convexity, MAUDE database, adverse event
Introduction
The accumulation of submental fat (SMF) is a defining feature of the lower third of the aging face. Excess SMF, even in small amounts, has a direct relationship with negative self-perception in health-based surveys. 1 In 2021, the American Society for Dermatologic Surgery conducted a blind survey and 70% of the respondents surveyed that they sought cosmetic procedures due to excess fat under the chin/neck. 2 Although submentoplasty and submental liposuction have been well described and classically thought of as the workhorse for submental fat reduction, these surgeries are not without risks and significant downtime. 3 Nonsurgical treatments such as the subcutaneous infiltration of deoxycholic acid (DOC) have risen substantially in popularity. 4 DOC is a bile acid that contributes to the emulsification and digestion of dietary fat. When injected subcutaneously, the substance works by promoting lysis of the adipocyte cell membrane resulting in apoptosis.5,6 DOC as the product KYBELLA (AbbVie Inc., Chicago, Illinois, U.S.A.) was approved by the US Food and Drug Administration (FDA) in 2015. Phase 3 trials and a 3 year follow-up review captured all adverse events associated with submental DOC injections. Most of the adverse events were inflammatory in nature and injection site-related reactions such as pain, swelling, bruising, bleeding, numbness, erythema, and induration. The more serious adverse events included temporary marginal mandibular nerve (MMN) injury (up to 4.3%), dysphagia thought due to amount infiltrated (1.9%), skin ulceration thought due to poor surgical technique (.2%), and injection site alopecia (.4%).7,8 The 3 year follow-up review did not have any further treatment-associated adverse events. 9 However, with the increase in popularity of DOC injections, it is important to continue to monitor adverse events. This study sought to query the US FDA’s MAUDE database (Manufacturer and User Facility Device Experience) for adverse events associated with DOC for submental convexity. The MAUDE database provides a report of suspected device-associated deaths, serious injuries, and malfunctions associated with FDA-approved medical devices. Further, the FDA mandates certain parties (manufacturers, importers, and device user facilities) and encourages others (health care providers and patients) to report such events. The objective of this study is to further elucidate adverse events associated with DOC injections to the submentum after being on the market for the past 8 years.
Materials and Methods
This study was a retrospective cross-sectional database analysis that was determined by the University of Arkansas for Medical Sciences Institutional Review Board to be exempt from review. The MAUDE database was queried for all reports of adverse events using the search terms “KYBELLA” and “deoxycholic acid” between 2012 and 2023 (web address: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm; Date of access: April 29, 2023). Data gathered included event report date, manufacturer, complication type, and description of the adverse event. Two reviewers (A.C.G. and A.R.K.) independently reviewed the data. The events were categorized for statistical analysis. Reports were individually reviewed and categorized with special attention to adverse events. Given the nature of the MAUDE database, this study is retrospective and descriptive in nature.
Results
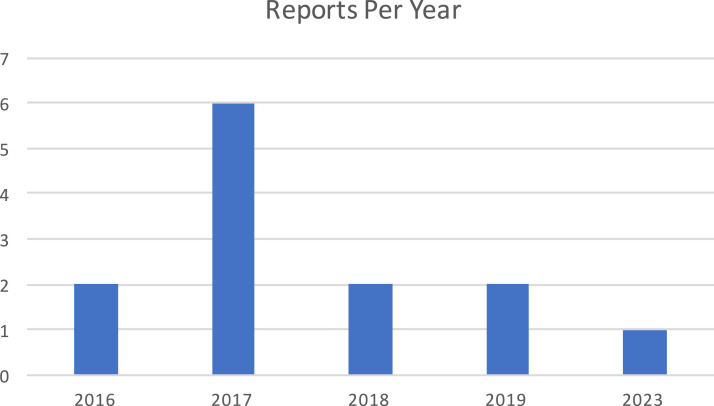
Thirty-four medical device reports were identified from the MAUDE database. Twenty-one of these cases were excluded as they were either duplicates, unrelated adverse events, or events associated with the off-label use of DOC. Of the 13 included cases, there were a total of 21 adverse events reported (see Figure 1 for a breakdown of the cases per year).
The most morbid of the complications were 3 reports (14%) of skin necrosis, all requiring surgical interventions and/or hospitalization. Of the 3 incidents, the 2 requiring hospitalization did have suspected concomitant underlying systemic diseases, systemic lupus erythematous, and acute myeloid leukemia. The latter required washout and debridement. The third patient with skin necrosis required excision and subsequent laser treatments. There were 4 events (19%) resulting in unsatisfactory aesthetic outcome; the injections resulted in excess sagging of the skin, asymmetry, suboptimal results, and paradoxical hyperplasia, respectively. There were 4 events (19%) of marginal mandibular nerve (MMN) weakness, all of which are unknown if they were temporary or permanent. Three reports (14%) noted numbness; 2 of the 3 did resolve after several months. The most common adverse event noted was excessive swelling (n = 5, 24%); most of these were not aesthetically pleasing to the patient, 1 patient had resulting dysphagia from the edema (n = 1, 5%). One patient experienced infection at the injection site (n = 1, 5%). See Table 1 for a summary of adverse events.
Figure 1.
Adverse events reports per year.
Table 1.
Adverse Events of Deoxycholic Acid Injection.
| Adverse Event | No. (n = 21) | % | Required Surgery (n = 2) | Required Hospitalization (n = 2) |
|---|---|---|---|---|
| Excessive swelling | 5 | 24 | ||
| MMN weakness | 4 | 19 | ||
| Unsatisfactory aesthetic outcome | 4 | 19 | ||
| Skin necrosis | 3 | 14 | 2 | 2 |
| Numbness | 3 | 14 | ||
| Dysphagia | 1 | 5 | ||
| Infection | 1 | 5 |
MMN = Marginal mandibular nerve.
Discussion
Excess submental fat (SMF) resulting in submental convexity is a common cause of negative self-perception and a common motivator for patients seeking cosmetic procedures. Although submentoplasty and submental liposuction treatments have been well established for many years, novel non-surgical treatments have risen in popularity. Since its US FDA approval in 2015, KYBELLA (AbbVie Inc.) deoxycholic acid (DOC) injections have been used in over 1600 patients. 10 One systematic review by Vertuan et al demonstrated that DOC injections significantly reduced SMF and increased patient satisfaction. 6 With such promising results, DOC injections may continue to become more common. Of equal importance is increasing literature about the potential adverse events of DOC injections. The most common adverse events consist of injection site reactions such as swelling, pain, and bruising. 11 However, more severe side effects have occurred with DOC injections to the submentum. The prescribing information from the KYBELLA website notes potential side effects such as temporary MMN injury with median resolution within 44 days, possible injection into vulnerable anatomic structures such as salivary glands, muscle, and lymph nodes, temporary dysphagia in the setting of injection site edema, injection site hematoma/bruising, alopecia, ulceration, necrosis, and/or infection. 12 The results of this study somewhat mirror what is present in the literature.7,8 The MAUDE database found 13 reports of adverse events between 2012 and 2023 consisting of excessive swelling, dysphagia, numbness, infection, unsatisfactory aesthetic outcome, MMN weakness, and skin necrosis requiring hospitalization and/or surgery. The most morbid side effect in this study was noted to be skin necrosis. Two cases required surgical intervention and 2 required hospitalization. The 2 requiring surgical intervention required excision and follow-up laser treatments and multiple debridements, respectively. The latter, in addition to another case, required hospitalization. Interestingly, both patients requiring hospitalization had suspected underlying systemic disease. Nevertheless, these cases shed light on the potential severity of adverse events associated with DOC injections. The KYBELLA prescribing information provides health care professionals with injection techniques to prevent MMN weakness and skin necrosis. They recommend injection into the pre-platysmal fat compartment of the submentum. The approved technique recommends a 1 centimeter (cm) grid pattern with care to not inject too superficially to prevent ulceration and necrosis. They further advise to stay 1–1.5 cm away from the angle and inferior border of the mandible as to avoid the MMN. 12 Any provider interested in offering DOC injections to their patients should familiarize themselves with these recommendations to attempt to mitigate these adverse events. One side effect of DOC injections the MAUDE database did not report but is worth noting is injection site alopecia. Incidence rates of alopecia are variable but have been reported to be as high as 15% in male patients. 13 Although treatment-induced alopecia has been reported to be primarily temporary with regrowth occurring in 6 weeks to 12 months, the regrowth pattern may be cicatricial which can be aesthetically unappealing to certain patients.14,15
There are potential limitations of this study. The MAUDE database, as previously described, is composed of both mandated and voluntary reports. Due to the nature of the database, there is potential for underreporting of events. It is difficult to postulate any accurate population analyses from the data. Further, the reported events themselves can be biased and incomplete. The events cannot be followed through time so some outcomes are unknown. For example, it is unknown whether the 3 events of MMN weakness were temporary or permanent in nature. Despite its limitations, the MAUDE database does provide important information on the adverse events of FDA-approved medical devices and it is important for both patient and provider to be aware of such events.
Conclusions
With the increase in popularity of deoxycholic acid (DOC) injections for submental fat, it is imperative to understand the possible adverse events to aid in patient counseling. Although the majority are noted to be due to injection site reactions, there are potential side effects of higher morbidity. This study found that DOC treatments can result in excessive swelling, dysphagia, numbness, infection, unsatisfactory aesthetic outcome, MMN weakness, and skin necrosis requiring hospitalization and/or surgery. Patient counseling regarding the risks and benefits of DOC injections should be discussed when offering DOC for submental convexity.
Footnotes
Author’s Contributions: A. Celeste Gibson contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript. Carissa Saadi contributed to the conception, design, and implementation of the research, to the analysis of the results, and to the writing of the manuscript. Anvesh Kompelli contributed to the conception, design, and implementation of the research, to the analysis of the results, and to the writing of the manuscript. Vijay Patel contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript. Robert A. Saadi contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript. Tom Shokri contributed to the conception, design, and implementation of the research, to the analysis of the results, and to the writing of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Anna Celeste Gibson https://orcid.org/0000-0002-8283-5823
References
- 1.Baumann L, Shridharani SM, Humphrey S, Gallagher CJ. Personal (self) perceptions of submental fat among adults in the United States. Dermatol Surg. 2019;45(1):124-130. doi: 10.1097/DSS.0000000000001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ASDS Consumer Survey on Cosmetic Dermatologic Procedures. asdsnet. 2021. https://www.asds.net/medical-professionals/practice-resources/consumer-survey-on-cosmetic-dermatologic-procedures Accessed May 3, 2023. [Google Scholar]
- 3.Mangat DS, Harmych BM, Been MJ. Rhytidectomy and facial liposuction. In: Flint P, Haughey B, eds. Cummings Otolaryngology - Head and Neck Surgery. 6th ed. Pennsylvania, PA: Elsevier Saunders; 2015:409-424. [Google Scholar]
- 4.Sturm A, Shokri T, Ducic Y. Nonsurgical rejuvenation of the neck. Facial Plast Surg Clin North Am. 2022;30(3):407-417. doi: 10.1016/j.fsc.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Walker P, Lee D. A phase 1 pharmacokinetic study of ATX-101: Serum lipids and adipokines following synthetic deoxycholic acid injections. J Cosmet Dermatol. 2015;14(1):33-39. doi: 10.1111/jocd.12122. [DOI] [PubMed] [Google Scholar]
- 6.Vertuan M, Honório HM, Queiroz TP, Santos PL. Is deoxycholic acid able to reduce submental fat and increase patient satisfaction when compared to placebo groups? A systematic review. J Plast Reconstr Aesthet Surg. 2022;75(11):4281-4289. doi: 10.1016/j.bjps.2022.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Dayan SH, Schlessinger J, Beer K, et al. Efficacy and safety of ATX-101 by treatment session: pooled analysis of data from the phase 3 REFINE trials. Aesthet Surg J. 2018;38(9):998-1010. doi: 10.1093/asj/sjy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDiarmid J, Ruiz JB, Lee D, Lippert S, Hartisch C, Havlickova B. Results from a pooled analysis of two European, randomized, placebo-controlled, phase 3 studies of ATX-101 for the pharmacologic reduction of excess submental fat. Aesthetic Plast Surg. 2014;38(5):849-860. doi: 10.1007/s00266-014-0364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey S, Cohen JL, Bhatia AC, Green LJ, Green JB, Bowen B. Improvements in submental contour up to 3 Years after ATX-101: Efficacy and safety follow-up of the phase 3 REFINE trials. Aesthet Surg J. 2021;41(11):NP1532-NP1539. doi: 10.1093/asj/sjab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frequently Asked Questions . Have Questions about Kybella? Find Answers Here. MyKybella.com; 2023. https://www.mykybella.com/frequently-asked-questions Accessed 3 May 2023. [Google Scholar]
- 11.Farina GA, Cherubini K, de Figueiredo MAZ, Salum FG. Deoxycholic acid in the submental fat reduction: A review of properties, adverse effects, and complications. J Cosmet Dermatol. 2020;19(10):2497-2504. 10.1111/jocd.13619. [DOI] [PubMed] [Google Scholar]
- 12.Full Prescribing Information . Rxabbvie.com. 2023. https://www.rxabbvie.com/pdf/kybella_pi.pdf Accessed May 3, 2023. [Google Scholar]
- 13.Shridharani SM, Chandawarkar AA. Abstract: Alopecia in male patients treated with ATX-101 (deoxycholic acid) for submental fullness. Plastic and Reconstructive Surgery - Global Open;5(9S):194-195. 10.1097/01.GOX.0000526446.07066.a5. [DOI] [Google Scholar]
- 14.Issa NT, Kaiser M, Martinez-Velasco A, Tosti A. Alopecia after cosmetic injection procedures: A review. Dermatol Surg. 2022;48(8):855-861. 10.1097/DSS.0000000000003498. [DOI] [PubMed] [Google Scholar]
- 15.Shridharani SM, Tisch GM. Commentary on: Improvements in submental contour up to 3 Years after ATX-101: Efficacy and safety follow-up of the phase 3 REFINE trials. Aesthet Surg J. 2021;41(11):NP1540-NP1542. 10.1093/asj/sjab135. [DOI] [PubMed] [Google Scholar]