Abstract
Background:
The appropriateness of aortic valve surgery for patients with moderate aortic valve regurgitation undergoing coronary artery bypass graft (CABG), mitral valve replacement (MVR), or both is uncertain. This study aimed to investigate the outcomes of moderate aortic valve regurgitation following these procedures.
Methods:
This retrospective cohort study included 113 eligible participants with moderate aortic valve regurgitation who underwent CABG, MVR, or both procedures between January 2014 and January 2015 at Tehran Heart Center. Echocardiographic index data were extracted from the Tehran Heart Center data center after a 2-year follow-up to examine changes in the patients’ conditions.
Results:
A total of 113 patients (mean [SD] age, 64.7 [9.9] years; 78 [69.0%] female patients) were included in the study and followed up for a mean (SD) of 24 (6) months. Among those patients, 38 (33.6%) experienced improvement, with their aortic valve regurgitation downgraded to mild, while the remaining 75 (66.4%) patients maintained a moderate aortic valve regurgitation level. Notably, combined CABG and MVR procedures were associated with statistically significant improvement, with all cases downgraded to mild aortic valve regurgitation. Baseline characteristics, including diabetes, hypertension, dyslipidemia, smoking, family history of aortic valve regurgitation, and a history of drug use, did not differ statistically significantly between patients with improved aortic valve regurgitation and patients with no changes. Echocardiographic indices related to the aorta, such as aortic valve pressure gradient, showed improvement (P < .001), and ejection fractions before and after surgery remained comparable. Changes in aortic valve regurgitation severity were found to differ statistically significantly between the various procedures (P = .001).
Conclusion:
These findings suggest that it is not likely that moderate aortic valve regurgitation will progress after CABG or MVR. Hence, no support was found for concurrent aortic valve replacement during these procedures.
Keywords: Aortic regurgitation, coronary artery bypass grafting, mitral valve
Key Points:
Recently, the issue of performing concurrent AVR in patients with moderate aortic valve regurgitation undergoing other cardiac surgeries has been the subject of debate. Limited data are available on midterm and long-term follow-up of patients with moderate aortic valve regurgitation who underwent CABG, MVR, or both procedures.
Although AVR is beneficial for recovering cardiac valve function, adding AVR to the primary cardiac operation elongates surgical time and exacerbates surgical risks. Hence, the risks and benefits of adding another surgery should be considered.
In the 2-year follow-up of this study population, approximately two-thirds of the patients showed no change in aortic valve regurgitation severity according to echocardiographic indices, and one-third of patients demonstrated improved aortic valve regurgitation.
These findings suggest that it is not likely that untreated moderate aortic valve regurgitation will progress following CABG, MVR, or both procedures combined. No support was found for concurrent AVR during these procedures.
Introduction
The term aortic valve regurgitation refers to the incomplete closing of the aortic valve in diastole, leading to backward blood flow through the aortic valve.1 Aortic valve regurgitation is a statistically significant contributor to cardiovascular mortality and morbidity and was reported in 13% of men and 8.5% of women in the Framingham Heart Study.2,3 Aortic valve surgery for moderate aortic valve regurgitation in conjunction with another cardiac surgery, particularly coronary artery bypass graft (CABG) or mitral valve replacement (MVR), continues to be controversial.4 Aortic valve replacement (AVR) may also carry a higher risk of mortality when performed alongside other cardiac procedures, such as CABG, because of the risks and possible complications associated with a major combined procedure.5
In accordance with the 2020 American College of Cardiology/American Heart Association guideline for the treatment of patients with valvular heart disease, patients with moderate aortic valve regurgitation who are undergoing other cardiac operations may benefit from concomitant aortic valve surgery (class IIa, level of evidence C).6 The 2021 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines for managing valvular heart disease suggest that the health care team should determine whether to intervene in these patients based on etiology and other clinical characteristics.7
Given the controversy surrounding aortic valve surgery for moderate aortic valve regurgitation at the time of CABG or MVR, the goal of this study was to track changes in moderate aortic valve regurgitation in patients undergoing these procedures.
Methods
Design, Population, and Data Collection
This retrospective cohort investigation was conducted at the Tehran Heart Center (THC) in Tehran, Iran, with 113 eligible participants. The study aimed to collect and analyze data encompassing demographic, pharmacologic, and echocardiographic characteristics of patients diagnosed with moderate aortic valve regurgitation who underwent CABG, MVR, or both procedures between January 2014 and January 2015. The enrolled patients were selected consecutively. The data were retrieved from the THC data center. To ensure the validity of the study, certain exclusion criteria were applied. Patients with a history of AVR, mild or severe aortic valve regurgitation, or moderate to severe aortic valve stenosis were excluded from the analysis. To determine the status of aortic valve regurgitation before cardiac surgery, patients first underwent a transthoracic echocardiogram. In rare cases, when the echocardiographic view was not suitable or judgment was difficult, a transesophageal echocardiogram was performed to ensure accuracy. After cardiac surgery, follow-up echocardiography was performed using the transthoracic echocardiogram approach in all patients. Moderate aortic valve regurgitation was defined based on specific echocardiographic indices, including a central jet width ranging from 25% to 64% of the left ventricular outflow tract and a vena contracta measuring 3 to 6 mm.7,8 Consequently, data from a total of 125 patients were extracted and evaluated for the availability of key echocardiographic indices related to aortic valve regurgitation. The assessment was performed at a mean (SD) follow-up period of 24 (6) months after surgery. In instances where echocardiographic data were not available, the individuals in question were contacted and invited to THC for a comprehensive echocardiographic examination. Twelve patients did not respond to the invitation and were therefore excluded from the study.
Ethical Considerations
All procedures that involved human participants were conducted in accordance with the ethical standards of the Medical Ethical Committee of the THC, and the study was performed in accordance with the 2013 revised Declaration of Helsinki. Informed consent was obtained from participants who were invited to THC because of the unavailability of their echocardiographic data.
Statistical Analysis
Means with SDs are reported for continuous variables with normal distribution. For categorical variables, absolute frequencies and percentages are reported. Pearson χ2 tests and Fisher exact tests were used to compare categorical variables between the group with improved aortic valve regurgitation and the group with no change. Independent sample t tests and Mann-Whitney U tests were performed to analyze continuous variables. Paired-sample t tests were performed to compare echocardiographic characteristics before and after surgery. The normality of the data was assured using the abovementioned descriptive central tendency, dispersion measures, and histograms. A 2-sided P < .05 was regarded as statistically significant. The statistical analyses were performed using SPSS Statistics for Windows, version 23.0, software (IBM Corp).
Results
Between January 2014 and January 2015, a total of 125 participants with moderate aortic valve regurgitation underwent CABG, MVR, or both procedures. Among these participants, 113 patients (mean [SD] age, 64.7 [9.9] years; 78 [69.0%] female patients) met the inclusion criteria and were included in the study. After undergoing these procedures, 38 (33.6%) patients exhibited an improvement, with their aortic valve regurgitation being downgraded to mild. Conversely, in the remaining 75 (66.4%) patients, aortic valve regurgitation status remained at moderate. With the exception of sex and dyslipidemia, baseline characteristics (Table I) were not found to differ statistically between the 2 groups. Although the P values for age, hypertension, cigarette smoking status, family history of aortic valve regurgitation, and history of drug use did not reach statistical significance, they demonstrated clinical significance worth noting.
TABLE I.
Demographic and Pharmacologic Characteristics of the Study Population, by Aortic Valve Regurgitation Status
| No. % | ||||
|---|---|---|---|---|
| Characteristic | Total (N = 113) | Improved aortic valve regurgitation (n = 38) | No change (n = 75) | P valuea |
| Age, mean (SD), y | 64.7 (9.9) | 63.6 (11.6) | 65.3 (8.9) | .39 |
| Sex | .04 | |||
| Male | 35 (31.0) | 7 (18.4) | 28 (37.3) | |
| Female | 78 (69.0) | 31 (81.6) | 47 (62.7) | |
| Diabetes | 35 (31.0) | 11 (28.9) | 24 (32.0) | .74 |
| Hypertension | 78 (69.0) | 25 (65.8) | 53 (70.7) | .59 |
| Dyslipidemia | 56 (49.6) | 14 (36.8) | 42 (56.0) | .05 |
| Cigarette smoking status | 31 (27.4) | 12 (31.6) | 19 (25.3) | .48 |
| Family history of aortic valve regurgitation | 28 (24.8) | 12 (31.6) | 16 (21.3) | .23 |
| Diuretic use | 41 (36.3) | 15 (39.5) | 26 (34.7) | .61 |
| Angiotensin-converting enzyme inhibitor/ angiotensin receptor blocker use | 77 (68.1) | 28 (73.7) | 49 (65.3) | .36 |
| Nitrate use | 82 (72.6) | 24 (63.2) | 58 (77.3) | .11 |
| Statin use | 98 (86.7) | 35 (92.1) | 63 (84.0) | .23 |
A 2-sided P < .05 was regarded as statistically significant.
Among the total patient cohort, 96 (85.0%) individuals underwent CABG, 11 (9.7%) underwent MVR, and 6 (5.3%) underwent both procedures concurrently. Within the CABG group, 30 (31.3%) patients experienced a statistically significant improvement in their aortic valve regurgitation status, with their condition downgraded to mild. In the remaining 66 (68.7%) patients from the same group, however, the severity of aortic valve regurgitation remained moderate, which was consistent with the preoperative measurements. Similarly, within the MVR group, 9 (81.8%) patients did not exhibit a change in their aortic valve regurgitation status after surgery, and only 2 (18.2%) patients experienced regression to mild aortic valve regurgitation. Notably, in the small subgroup of patients who underwent both CABG and MVR, all individuals demonstrated a notable improvement, with their aortic valve regurgitation status being downgraded to mild (Table II). The changes in aortic valve regurgitation severity were statistically significantly different between the various procedures (P = .001).
TABLE II.
Aortic Valve Regurtitation Status After Surgery, by Type of Surgery
| No. % | ||||
|---|---|---|---|---|
| Type of surgery | Total (N = 113) | Improved aortic valve regurgitation (n = 38) | No change (n = 75) | P valuea |
| CABG | 96 (85.0) | 30 (78.9) | 66 (88.0) | .001 |
| MVR | 11 (9.7) | 2 (5.3) | 9 (12.0) | .001 |
| CABG-MVR | 6 (5.3) | 6 (15.8) | 0 | .001 |
CABG, coronary artery bypass graft; MVR, mitral valve replacement.
A 2-sided P < .05 was regarded as statistically significant.
The mean (SD) ejection fraction measured before and after the procedures was 44.7% (9.8%) and 45.0% (8.9%), respectively. These values, along with the mean (SD) diameter of the ascending aorta before (34.7 [4.3] mm) and after (34.6 [3.3] mm) surgery, were found to be comparable. The mean (SD) aortic valve pressure gradient (AVPG), however, was statistically significantly reduced, from 8.9 (4.8) mm Hg to 7.1 (3.3) mm Hg (Cohen d = 0.44; P < .001). The mean (SD) aortic root diameter was statistically significantly reduced, from 31.9 (3.8) mm to 31.7 (4.1) mm, during these 2 years of follow-up after cardiac surgery (Cohen d = 0.20; P = .02) (Table III). Furthermore, there was a small but statistically significant reduction in the mean (SD) left ventricular end-diastolic dimension (from 52.1 [6.7] mm to 50.9 [6.1] mm; Cohen d = 0.19; P = .02) and intraventricular septum (from 10.1 [1.3] mm to 9.7 [1.6] mm; Cohen d = 0.25; P = .01) after the surgery. The remaining echocardiographic indices, as presented in Table III, did not exhibit statistically significant differences following the procedures.
TABLE III.
Echocardiographic Characteristics of Participants Before and After Surgery
| Mean (SD) | ||||
|---|---|---|---|---|
| Echocardiographic index | Before surgery | After surgery | Cohen d | P valuea |
| AVPG, mm Hg | 8.9 (4.8) | 7.1 (3.3) | 0.44 | <.001 |
| Aortic root diameter, mm | 31.9 (3.9) | 31.1 (4.1) | 0.20 | .02 |
| Ascending aorta diameter, mm | 34.7 (4.3) | 34.6 (3.3) | 0.02 | .68 |
| Left ventricular end-diastolic dimension, mm | 52.1 (6.7) | 50.9 (6.1) | 0.19 | .02 |
| Left ventricular end-systolic dimension, mm | 35.9 (6.8) | 35.5 (7.1) | 0.06 | .27 |
| Intraventricular septum, mm | 10.1 (1.6) | 9.7 (1.6) | 0.25 | .01 |
| Posterior wall, mm | 9.7 (1.4) | 9.5 (1.2) | 0.15 | .12 |
| Ejection fraction, % | 44.7 (9.8) | 45 (8.9) | 0.03 | .56 |
| Left atrium size, mm | 39.1 (5.9) | 38.6 (5.8) | 0.09 | .20 |
AVPG, aortic valve pressure gradient.
A 2-sided P < .05 was regarded as statistically significant.
Based on the aortic valve regurgitation status, Table IV presents the echocardiographic indices of the participants before and after surgery. Among participants with no change in aortic valve regurgitation status, mean (SD) AVPG was 8.7 (4.6) mm Hg before surgery and 7.5 (3.3) mm Hg after surgery. Among patients with improved aortic valve regurgitation, the mean (SD) AVPG decreased from 8.5 (4.8) mm Hg to 6.2 (3.1) mm Hg. The rest of the indices are also listed in Table IV.
TABLE IV.
Echocardiographic Characteristics of Participants Before and After Surgery, Stratified by Aortic Valve Regurgitation Status
| No change, mean (SD) (n = 75) | Improved aortic valve regurgitation, mean (SD) (n = 38) | |||||
|---|---|---|---|---|---|---|
| Echocardiographic index | Before surgery | After surgery | P valuea | Before surgery | After surgery | P valuea |
| AVPG, mm Hg | 8.7 (4.6) | 7.5 (3.3) | <.001 | 8.5 (4.8) | 6.2 (3.1) | <.001 |
| Aortic root diameter, mm | 31.9 (3.8) | 31.7 (4.1) | .44 | 31.7 (4.1) | 30.1 (3.7) | .02 |
| Ascending aorta diameter, mm | 34.9 (4.1) | 34.8 (3.0) | .76 | 34.2 (4.6) | 34.1 (3.6) | .78 |
| Left ventricular end-diastolic dimension, mm | 51.5 (6.6) | 51.6 (5.7) | .79 | 53.2 (6.7) | 49.6 (6.5) | .001 |
| Left ventricular end-systolic dimension, mm | 35.5 (6.8) | 35.9 (6.6) | .34 | 36.9 (7.6) | 34.8 (7.9) | .003 |
| Intraventricular septum, mm | 10 (1.5) | 9.8 (1.6) | .31 | 10.4 (1.8) | 9.5 (1.7) | .003 |
| Posterior wall, mm | 9.6 (1.3) | 9.6 (1.2) | .93 | 9.9 (1.5) | 9.4 (1.1) | .01 |
| Ejection fraction, % | 45.2 (9.8) | 44.9 (8.9) | .57 | 43.6 (9.9) | 45.3 (9.1) | .22 |
| Left atrium size, mm | 39.4 (5.8) | 38.6 (5.6) | .22 | 38.9 (6.2) | 38.5 (6.3) | .58 |
AVPG, aortic valve pressure gradient.
A 2-sided P < .05 was regarded as statistically significant.
Discussion
The objective of this retrospective cohort study was to assess the midterm outcomes of moderate aortic valve regurgitation in patients who underwent CABG, MVR, or both procedures without concurrent AVR. Overall, 75 (66.4%) patients showed no statistically significant changes in aortic valve regurgitation status, while the remaining 38 patients experienced a down-grade to mild aortic valve regurgitation. Among the surgeries performed, CABG accounted for 85.0%, while MVR and combined CABG-MVR procedures accounted for 9.7% and 5.3%, respectively. A statistically significant difference was observed between the outcomes of these procedures, with all patients in the combined CABG-MVR group experiencing improvement in aortic valve regurgitation status. Conversely, the majority of patients who underwent CABG or MVR alone exhibited no statistically significant change in aortic valve regurgitation severity during the 2-year follow-up period. Furthermore, the ejection fractions before and after the procedures remained comparable, and there was a statistically significant improvement in certain aortic indices, such as AVPG and aortic root diameter. Figure 1 illustrates the study design of and the prominent findings from the current investigation.
Fig. 1.
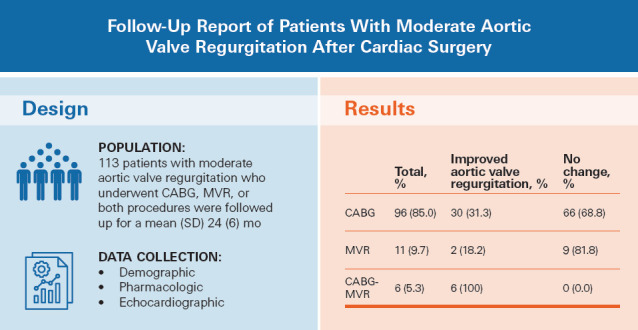
Illustration of the study design of and prominent findings from the current investigation.
CABG, coronary artery bypass graft; MVR, mitral valve replacement.
In the present study, aortic valve regurgitation severity did not progress during a mean follow-up period of 2 years, and it also improved in one-third of the patients. Underlying characteristics, such as dyslipidemia, nitrate consumption, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker consumption, although not statistically significant, were notably higher among individuals who did not show any improvement in aortic valve regurgitation severity. This finding may suggest a role for underlying comorbidities in accelerating aortic valve regurgitation progression.
Physicians must carefully weigh the benefits and risks associated with concurrent AVR when determining the treatment approach for patients with moderate aortic valve regurgitation undergoing other cardiac operations. Untreated aortic valve regurgitation has the potential to progress and lead to statistically significant left ventricular dysfunction, ultimately requiring additional interventions.9 In addition, the inclusion of AVR during the primary cardiac procedure extends the duration of surgery and amplifies surgical risks. It exposes the patient to potential complications associated with prosthetic cardiac valves, whether mechanical or biological, including prosthetic valve endocarditis, long-term anticoagulation requirements, and bioprosthetic valve failure.9,10
Recently, performing concurrent aortic valve surgery on patients with moderate aortic valve regurgitation who are undergoing other cardiac surgeries has been the subject of debate. The 2014 American College of Cardiology/American Heart Association Guidelines for the Management of Patients with Valvular Heart Disease suggested that concurrent aortic valve surgery could be performed in these patients (class IIa), whereas previous versions classified concurrent aortic valve surgery as class IIb in this population.11,12 As mentioned, the current guidelines also suggest that patients with moderate aortic valve regurgitation may benefit from concurrent aortic valve surgery during other cardiac operations (class IIa).6
Previously, proponents of AVR during CABG had claimed that patients with mild aortic valve disease would exhibit deteriorating symptoms and progression of valvular disease.5 Studies aimed at determining the progression course of moderate aortic valve regurgitation, however, have found that the rate of progression is slow and suggested against prophylactic AVR during other cardiac operations.4,13 Furthermore, previous studies have indicated that a history of CABG seems not to be considered a risk factor for further AVR in patients who may require surgical intervention in the future.14,15 In another study, Ward et al9 found that untreated moderate aortic valve regurgitation did not adversely affect the long-term survival of patients undergoing cardiac surgery. In the present study, aortic valve regurgitation severity did not progress during a mean follow-up period of 2 years and improved in one-third of the patients. Although not statistically significant, nitrate consumption and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker consumption were notably higher among patients who did not exhibit any improvement in aortic valve regurgitation severity. This finding may suggest that underlying comorbidities contribute to disease progression. Accordingly, further research to investigate the specific association of factors such as dyslipidemia could uncover the significance of these underlying comorbidities in the course of aortic valve regurgitation after cardiac surgery. The severity of aortic valve regurgitation was more likely to improve in female patients than in male patients undergoing cardiac surgery. In addition, more female patients were included in this retrospective cohort study. It also should be noted that patients were selected consecutively. The possibility of sex-specific responses could be further explored through future investigations with more homogeneous populations.
The findings of this study may help us move 1 step closer to clarifying the controversy surrounding the decision to perform concurrent aortic valve surgery for patients undergoing other cardiac procedures.
Limitations and Future Directions
This study, which represents 1 of the pioneer investigations into the progression of moderate aortic valve regurgitation following CABG, MVR, or combined CABG-MVR, has certain limitations that should be acknowledged. First, this study may be underpowered and is limited by the relatively short follow-up period. In this context, further studies with longer follow-up would be beneficial. In addition, this is a retrospective analysis conducted at a single center. To validate these findings, larger-scale randomized clinical trials are necessary. Finally, this study did not consider the association of the etiology of aortic valve regurgitation (ie, functional or rheumatic) with aortic valve regurgitation outcomes after cardiac surgery.
Regarding patient selection, patients were enrolled through sequential selection, which could have introduced selection bias. Although propensity score matching was considered, the small sample size limited the feasibility of this approach, a choice that may affect the strength of the conclusions.
Another limitation involves the consideration of sex and dyslipidemia. Although these factors were originally suggested to be coincidental, further regression analyses revealed sex as the only statistically significant factor of the current investigation, with female participants demonstrating greater improvement in aortic valve regurgitation severity than male participants. The small sample size and lack of statistical power, however, do not allow for strong conclusions to be drawn about the influence of sex or dyslipidemia on aortic valve regurgitation outcomes. More robust studies are needed to clarify the role of these factors in aortic valve regurgitation progression. In addition, although no studies directly link these variables to aortic valve regurgitation treatment, their potential influence should be explored in future research.
Future research in this field would be beneficial for physicians and surgeons seeking to make well-informed decisions regarding the treatment of these patients. Furthermore, conducting long-term followup studies that consider the etiology of aortic valve regurgitation could provide valuable insights into patient outcomes.
Conclusion
This study aimed to provide insights into the necessity of concurrent aortic valve surgery in patients with moderate aortic valve regurgitation undergoing other cardiac operations. During the 2-year follow-up period, approximately two-thirds of the patients showed no statistically significant change in aortic valve regurgitation severity based on echocardiographic indices, while one-third demonstrated improvements in the degree of aortic valve regurgitation. Collectively, because it is not likely for untreated moderate aortic valve regurgitation to progress following CABG or MVR surgeries, no support was found for concurrent aortic valve surgery during these procedures.
Abbreviations and Acronyms
- AVPG
aortic valve pressure gradient
- AVR
aortic valve replacement
- CABG
coronary artery bypass graft
- MVR
mitral valve replacement
- THC
Tehran Heart Center
Article Information
Open Access: © 2024 The Authors. Published by The Texas Heart Institute®. This is an Open Access article under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC, https://creativecommons.org/licenses/by-nc/4.0/), which permits use and distribution in any medium, provided the original work is properly cited, and the use is noncommercial.
Author Contributions: All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by Arash Jalali and Manouchehr Ziafat. The first draft of the manuscript was written by Arya Afrooghe and Mohammadreza Babaei, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest/Disclosure: The authors of this work have no competing interests to declare.
Funding/Support: This study was financially supported by the Tehran Heart Center.
Compliance with Ethics Guidelines: All the procedures in the study that involved human participants were performed in accordance with the ethical standards of the Medical Ethical Committee of the Tehran Heart Center, and the study was performed in accordance with the revised 2013 Declaration Helsinki. Informed consent was obtained from participants invited to Tehran Heart Center because of the unavailability of their echocardiographic data.
Data Availability: The datasets generated or analyzed during the current study are available from the corresponding author upon reasonable request. Due to Tehran Heart Center policies regarding patients’ data and also because our patients did not sign informed consent to make their data publicly available, the authors cannot publicly deposit their datasets. Data requests should be sent to sadeghianhakimeh@yahoo.com.
References
- 1.Dewaswala N, Chait R. StatPearls. StatPearls Publishing; 2023. [Accessed November 4, 2024]. Aortic Regurgitation.https://www.ncbi.nlm.nih.gov/books/NBK555944/ Updated August 8, 2023. [PubMed] [Google Scholar]
- 2.Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83(6):897–902. doi: 10.1016/s0002-9149(98)01064-9. doi: [DOI] [PubMed] [Google Scholar]
- 3.Ravalli F, Kossar AP, Takayama H, Grau JB, Ferrari G. Aortic valve regurgitation: pathophysiology and implications for surgical intervention in the era of TAVR. Struct Heart. 2020;4(2):87–98. doi: 10.1080/24748706.2020.1719446. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisenberg D, Omelchenko A, Shapira Y, et al. Mid-term echocardiographic progression of patients with moderate aortic regurgitation: implications for aortic valve surgery. J Heart Valve Dis. 2013;22(2):192–194. [PubMed] [Google Scholar]
- 5.Ahmed AAM, Graham ANJ, Lovell D, O'Kane HO. Management of mild to moderate aortic valve disease during coronary artery bypass grafting. Eur J Cardiothorac Surg. 2003;24(4):535–539. doi: 10.1016/S1010-7940(03)00469-X. doi: [DOI] [PubMed] [Google Scholar]
- 6.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/ AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–e227. doi: 10.1161/CIR.0000000000000923. doi: [DOI] [PubMed] [Google Scholar]
- 7.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi: 10.1093/eurheartj/ehab395. doi: [DOI] [PubMed] [Google Scholar]
- 8.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/ AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–e227. doi: 10.1161/CIR.0000000000000923. doi: [DOI] [PubMed] [Google Scholar]
- 9.Ward A, Malaisrie SC, Andrei A-C, et al. Fate of moderate aortic regurgitation after cardiac surgery. J Thorac Cardiovasc Surg. 2022;164(6):1784–1792.e1. doi: 10.1016/j.jtcvs.2020.12.114. doi: [DOI] [PubMed] [Google Scholar]
- 10.Goldstone AB, Chiu P, Baiocchi M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. New Eng J Med. 2017;377(19):1847–1857. doi: 10.1056/NEJMoa1613792. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. Bonow RO, Carabello BA, Kanu C, et al. ACC/ AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114(5):e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. doi: [DOI] [PubMed] [Google Scholar]
- 12.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521–e643. doi: 10.1161/CIR.0000000000000031. doi: [DOI] [PubMed] [Google Scholar]
- 13.Patel R, Kamath A, Varadarajan P, Krishnan S, Pai RG. Slow rate of progression of grade 1 and 2+ aortic regurgitation. J Heart Valve Dis. 2012;21(3):328–330. [PubMed] [Google Scholar]
- 14.Sundt TM, Murphy SF, Barzilai B, et al. Previous coronary artery bypass grafting is not a risk factor for aortic valve replacement. Ann Thorac Surg. 1997;64(3):651–657. doi: 10.1016/s0003-4975(97)00622-x. doi: [DOI] [PubMed] [Google Scholar]
- 15.Jegaden O, Lapeze J, Farhart F, de Gevigney G. Aortic valve stenosis after previous coronary bypass: transcatheter valve implantation or aortic valve replacement? J Cardiothorac Surg. 2012;7:47. doi: 10.1186/1749-8090-7-47. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]


