Abstract
Introduction:
Hemorrhagic shock demands swift intervention. Management involves the rapid infusion of blood products to restore circulation and uphold tissue perfusion. The aim of this study was to evaluate the effectiveness of prehospital plasma administration in trauma patients, comparing outcomes with normal saline. This was a meta-analysis of randomized controlled trials.
Methods:
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline, searches were conducted in PubMed, MEDLINE, and the Cochrane Central Register of Controlled Trials from August 1, 2018, to April 4, 2023. The PubMed search string included terms related to blood plasma, prehospital care, emergency medical services, and hemorrhagic shock: (Blood Plasma [MeSH Terms] OR fresh frozen plasma [MeSH Terms] OR plasma OR fresh frozen plasma OR FFP) AND (Prehospital OR emergency care, prehospital [MeSH Terms] OR prehospital emergency care [MeSH Terms] OR prehospital OR prehospital OR EMS OR emergency medical service [MeSH Terms]) AND (hemorrhagic shock [MeSH Terms] OR hemorrhage OR hemorrhage OR hemorrhagic shock OR hemorrhagic shock). Results from the trials were pooled using a random effects model, presented as risk ratios with 95% confidence intervals.
Results:
In the analysis of 760 patients from three studies, outcomes included mortality at 24 h and 28 days, multi-organ failure (MOF), acute lung injury, and vasopressor use within 24 h. Patients were divided into plasma (363) and normal saline (397) groups.
Conclusion:
There is no distinction between prehospital plasma administration and normal saline concerning mortality at 24 and 28 days or the need for vasopressors within 24 h. Moreover, plasma administration did not appear to influence rates of acute lung injury or MOF.
Keywords: Hemorrhagic shock, plasma, resuscitation, shock
INTRODUCTION
Hemorrhagic shock, marked by life-threatening blood volume loss and impaired tissue perfusion, remains a foremost cause of mortality in trauma patients globally.[1] Standard management involves rapid resuscitation, ideally with blood products, to restore circulation and counteract shock-induced coagulopathy (SIC).[1,2] Recent studies highlight the potential benefits of prehospital plasma administration in improving outcomes for hemorrhagic shock patients.[2,3,4,5]
The rationale behind prehospital plasma lies in addressing the pathophysiology of SIC, a consequence of hemorrhagic shock that disrupts the coagulation system, increasing bleeding risk.[6] Plasma, rich in clotting factors, corrects coagulopathy and prevents further bleeding.[6] Early prehospital plasma administration has demonstrated the ability to provide timely resuscitation, improving survival rates, and reducing multi-organ failure (MOF) incidence in trauma patients with hemorrhagic shock.[2]
Notably, a retrospective study by the French Military Health Service associated pre-hospital plasma administration with enhanced survival in combat casualties experiencing hemorrhagic shock.[3] Despite acknowledged concerns about implementation, such as storage and adverse reactions, innovations such as freeze-dried plasma offer extended shelf life and simplified storage.[5,7]
This meta-analysis aims to systematically review the existing evidence on prehospital plasma administration’s efficacy in managing hemorrhagic shock, examining its potential as an early resuscitation strategy and adjunct to traditional approaches.[2,3,4,5,6,7]
This involved searching from August 1, 2018, to April 4, 2023, PubMed, MEDLINE, and the Cochrane Central Register of Controlled Trials were searched without language restrictions. The PubMed search string included terms related to blood plasma, prehospital care, emergency medical services, and hemorrhagic shock.
METHODS
This updated systematic review and meta-analysis was carried out in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[8] The study was also registered in the “International Prospective Register of Systematic Reviews” (PROSPERO) in 2023 (CRD2023414214). A version is available upon requesting the corresponding author.
Data sources and search strategy
The electronic databases PubMed, MEDLINE, and the Cochrane Central Register of Controlled Trials were searched without language restrictions from August 1, 2018, to April 4, 2023. The following is the PubMed search string: (Blood Plasma [MeSH Terms] OR fresh frozen plasma [MeSH Terms] OR plasma OR fresh frozen plasma OR FFP) AND (Prehospital OR emergency care, prehospital [MeSH Terms] OR prehospital emergency care [MeSH Terms] OR prehospital OR prehospital OR EMS OR emergency medical service [MeSH Terms]) AND (hemorrhagic shock [MeSH Terms] OR hemorrhage OR hemorrhage OR hemorrhagic shock OR hemorrhagic shock). To retrieve eligible studies, a thorough manual search of reference lists of retrieved studies, relevant articles, and previous meta-analyses was conducted.
Study selection
The following eligibility criteria were applied: (1) Randomized controlled trials comparing plasma administration to normal saline in the prehospital setting and (2) Trials treating patients with or at risk for hemorrhagic shock. Excluded from the analysis were trials involving interventions other than plasma or conducted in a hospital setting.
Data extraction and assessment of study quality
The search results were imported into the EndNote Reference Library application. The software automatically detected and removed duplicates. Two independent reviewers (FG and NJ) then evaluated the titles and abstracts of the studies and selected those that met the inclusion criteria. The full texts of the selected studies were obtained and thoroughly reviewed to confirm their eligibility. A third reviewer resolved any discrepancies (FA). Mortality at 24 h; mortality at 1 month; acute lung injury; MOF; and vasopressors required within 24 h were extracted from the included trials. The Cochrane risk of bias tool was used to evaluate the quality of included studies.
Statistical analysis
The statistical analysis was conducted using Review Manager (RevMan, version 5.4.1; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The results of the trials were pooled using a random effects model and presented as risk ratios (RRs) with 95% confidence intervals (CIs). The Higgins I2 was used to evaluate heterogeneity across studies, and a value >50% was deemed unacceptable. All results with a P < 0.05 were considered significant.
RESULTS
Results of the literature search
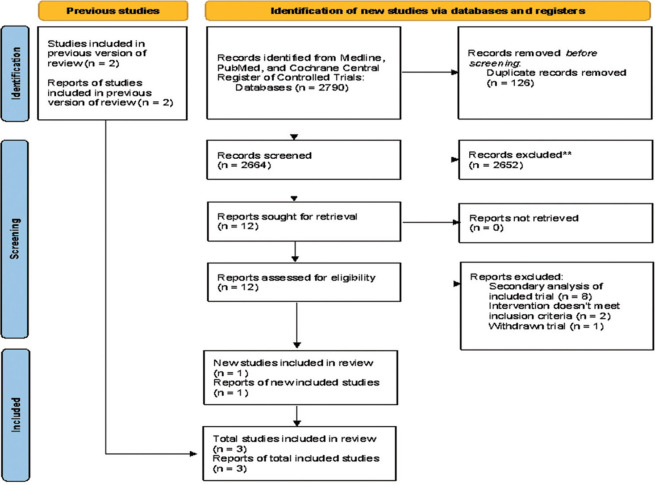
After exclusions, the initial search of two databases and one register yielded 2790 studies, of which one trial was included. From the previous meta-analysis, two studies were included. The PRISMA flowchart contains a summary of the literature search [Figure 1].
Figure 1.
Flowchart of the literature search
Study characteristics and quality assessment
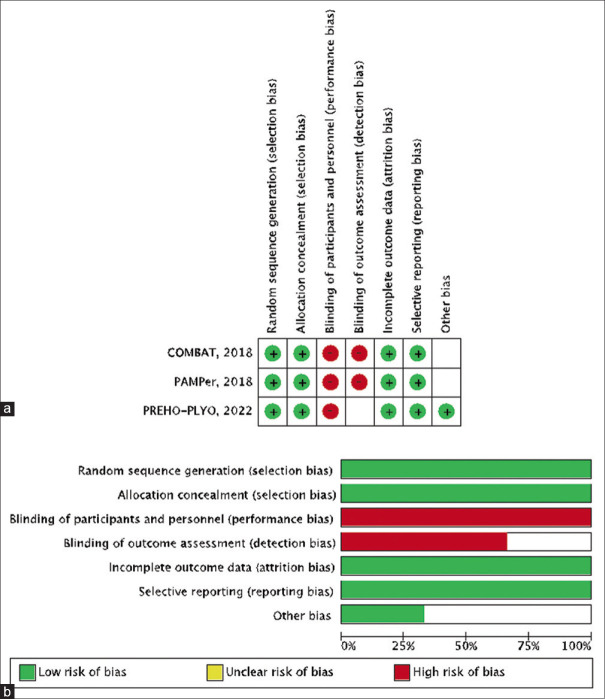
Seven hundred sixty patients with hemorrhagic shock or at risk of hemorrhagic shock participated in the trials, with 363 in the plasma group and 397 in the normal saline group. All patients were monitored for 1 month after admission. Table 1 provides a summary of the baseline characteristics of the included studies. Figure 2 depicts a quality assessment of all included studies, which reveals that all trials are of high methodological quality.
Table 1.
Summary of study characteristics
| Study name, year | |||
|---|---|---|---|
|
| |||
| COMBAT, 2018 | PAMPer, 2018 | PREHO-PLYO, 2022 | |
| Study design | RCT | RCT | RCT |
| Population (P) | 65 | 230 | 68 |
| Population (C) | 60 | 271 | 66 |
| Median age (P) | 33.0 (25.0–51.0) | 44.0 (21.0–59.0) | 36.6 (26.8–49.5) |
| Median age (C) | 32.5 (25.5–42.0) | 46.0 (29–60.0) | 33.6 (25.2–47.6) |
| ISS (P) | 27.0 (10.0–41.0) | 22.0 (14.0–33.0) | 29 (12.0–48.0) |
| ISS (C) | 27.0 (11.5–36.0) | 21.0 (12.0–29.0) | 25.0 (9.0–41.0) |
All data are presented in number or median and IQR. P: Plasma group, C: Control group, IQR: Interquartile range, RCT: Randomized controlled trial, ISS: Injury severity score
Figure 2.
(a) Detailed quality assessment of the included trials. (b) Summary of quality assessment showing either high risk or unclear risk of bias in blinding of participants, personnel, and outcome assessment in all three trials. All other domains were of low risk or unclear risk
Results of the meta-analysis
The results of our meta-analysis are depicted in Figures 3a, b, and 4a-c, which include Forest plots that illustrate the effect size of each result.
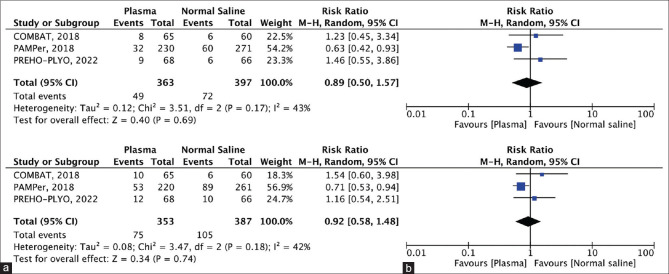
Figure 3.
(a) Forest plot showing mortality at 24 h, (b) Forest plot showing mortality at 1 month
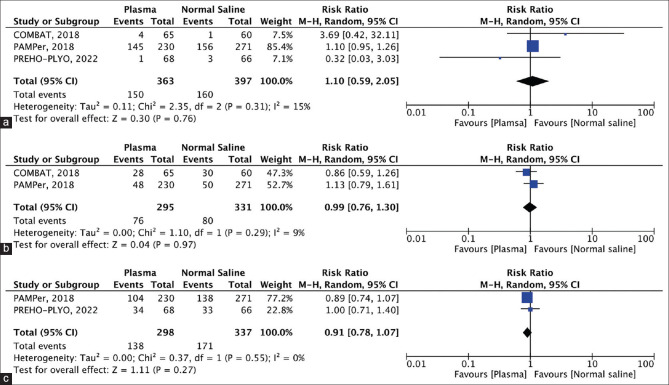
Figure 4.
(a) Forest plot showing multi-organ failure, (b) Forest plot showing acute lung injury, (c) Forest plot showing vasopressors needed within 24 h
Mortality at 24 h: Each of the three included studies [Figure 3a] evaluated 24-h mortality (plasma group, 363 patients, 49 events; control group, 397 patients, 74 events). Plasma was favored over normal saline in the prehospital setting; however, this difference was not statistically significant (RR =0.98, CI =0.50–1.57; P = 0.69).
Mortality at 1 month: All three trials reported mortality at 1 month (plasma group, 353 patients, 75 events; normal saline group, 387 patients, 105 events) [Figure 3b]. Plasma administration reduced the incidence of mortality at 1 month compared to normal saline administration in the prehospital setting, however, the result was not statistically significant (RR =0.92, CI =0.58–1.48; P = 0.74).
MOF: Data on the incidence of MOF at 28 days were reported in the three studies (plasma group, 363 patients, 150 events; normal saline group, 397 patients, 160 events) [Figure 4a]. MOF at 28 days was higher in the plasma group compared to the control group; however, this was not statistically significant (RR =1.10. CI =0.59–2.05; P = 0.76).
Acute lung injury: Two of the three included studies [Figure 4b] reported on acute lung injury (plasma group, 295 patients, 76 events; normal saline group, 331 patients, 80 events). Plasma administration did not significantly reduce the incidence of acute lung injury compared to normal saline (RR =0.99, CI =0.76–1.30; P = 0.97).
Vasopressors are needed within 24 h: Two of the three studies reported adequate data on the use of vasopressors within 24 h between the two groups (plasma group, 298 patients, 138 events; normal saline group, 337 patients, 171 events) [Figure 4c]. Although not statistically significant, the use of vasopressors was lower in the plasma group compared to the control group (RR =0.91, CI =0.78–1.07; P = 0.27).
DISCUSSION
Fluid resuscitation strategies are constantly changing with the emergence of new studies, with the earlier approach of immediate aggressive fluid resuscitation slowly disappearing and the appearance of more novel fluids appearing, choosing the appropriate fluid is becoming of more importance.[9] With the myriad number of available fluids, each with its own physiological and therapeutic advantages,[10] our study aims to assess the effectiveness of prehospital plasma infusion in patients with hemorrhagic shock. The data consists of 760 patients from three studies. Patients were divided into two groups, those receiving plasma (363) and those receiving normal saline (397). Our analysis revealed that the use of plasma did not significantly reduce mortality at 24 h, mortality at 28 days, MOF, acute lung injury, or vasopressors needed within 24 h with all outcomes being statically insignificant.
The administration of other fluids or blood products particularly red blood cells along with plasma has been heavily studied, but rarely plasma alone. Murad et al. found that plasma administration along with blood products in a 1:3 ratio in trauma patients requiring massive transfusion was associated with a significant reduction in mortality, albeit the results were highly heterogeneous.[10] These findings are in accordance with our analysis that found that even though plasma was more effective compared to normal saline in the prehospital settings, the difference was not statistically significant. However, a meta-analysis that included two of the three studies in our analysis found that prehospital plasma infusion does to an extent reduce 24-h mortality.[4] On the other hand, Murad et al. also found that plasma transfusion in cases of trauma not requiring massive transfusion was associated with an adverse effect on mortality, however, their finding was also not statistically significant and associated with high heterogeneity.[10] Our study analyzed both mortality at 24 h and at 28 days and found that there was no difference between the two fluids. However, it is worth noting that the study group in our analysis included a wide spectrum in severity of trauma, with the heaviest study having a median injury severity score (ISS) of 22.[2]
Another outcome that was assessed was the risk of developing MOF after resuscitation. Our results found that there was no difference between the two fluids in causing MOF at 28 days. On the contrary, a prospective cohort study found that early transfusion of fresh frozen plasma (FFP) correlates strongly with an increased risk of developing MOF.[11] The reason behind this increase in mortality may be due to the robust presence of biologically active mediators present in plasma thus leading to uncontrolled immunomodulation and tissue injury.[12,13] The same concept can be applied to transfusion-related acute lung injury (TRALI), in which donor antibodies in the transfused blood products trigger an immune reaction that leads to inflammation in the lungs, causing difficulty breathing and low oxygen levels.[14] In terms of plasma administration, there was also no difference in causing acute lung injury compared to normal saline. Despite our findings, a study has shown that FFP transfusion has been found to have a greater risk of triggering TRALI, almost tripling the risk in mechanically ventilated patients in one study.[15] However, certain steps can be taken to mitigate this risk, particularly the usage of male-only donors, the introduction of a low-risk TRALI donor strategy, and screening for antibodies in donors among others.[14]
Although it remains a controversial topic, the use of vasopressors as an adjunct to fluid resuscitation is used as a last line in trauma patients with hypotension. Vasopressors, such as norepinephrine or vasopressin, can help maintain adequate blood pressure and improve tissue perfusion, but they should be used cautiously and titrated to achieve the desired effect while minimizing adverse effects.[16] Ultimately, however, our study found no difference between the two. Although influenced by multiple confounders, a multicenter prospective study compared patients who did and did not require early vasopressors and found that less early vasopressors were required in patients that received FFP.[17]
The limitations of this meta-analysis include the fact that it was based on only three studies, which may not provide a comprehensive representation of the evidence. This limited number of studies may also limit the power of the analysis to detect significant differences between the two treatments and could be a result of the differences that lie within them. These differences such as transportation time, number of crystalloids received, and Glasgow Coma Scale on arrival could have all affected our results. There is a possibility of important demographic and physiological confounders that contributed to the conclusions drawn. These may also include the nature of the injury, the degree of resuscitation performed, and the time from injury to treatment. For example, there was a 4-point difference in the median ISS score between the plasma and control group in the PREHO-PYLO study. This variation in the degree of severity could contribute to the nature of the response in each respective therapy. There were also important differences in the prehospital transport times between the studies as the mode of transport varied. The PAMPer study, which focused on air-transported patients, had a transport time range of 31–70 min while the COMBAT study, focusing on ground transport, was 16–22 min.[2,3] In addition, the significant heterogeneity of the included studies can affect the generalizability of the results and the ability to draw meaningful conclusions. Moreover, the lack of statistically significant findings may indicate that the study did not have sufficient power or that there is a true lack of effect. All in all, the limitations of the study should be carefully considered when interpreting the results, and further research is needed to provide a more robust evidence base for the comparison of prehospital plasma administration versus normal saline to be made.
CONCLUSION
Statistically speaking, there is no difference between prehospital plasma administration and normal saline when assessing mortality at 24-h and 28 days or the need for adjunct vasopressors within 24 h. Furthermore, plasma administration did not seem to influence the rate of acute lung injury or MOF.
Availability of data and materials
The dataset used/or analyzed is available following any request send to the correspondence.
Authors’ contributions
Faisal AlGhamdi: FGA (Conceptualization, Writing, Title and Abstract Evaluation), Nasser AlJoaib: NA (Conceptualization, Title and Abstract Evaluation, Analysis), Annas Ghafoor: AG (Conceptualization, Writing, Manuscript Review), Fandi Alanazi: FA (Conceptualization, Discrepancy Resolution, Writing), Nisreen Maghraby: NM (Conceptualization, Writing, Manuscript Review).
Research quality and ethics statement
As our study is a meta-analysis, no approval from the Institutional Review Board/Ethics Committee is required. The authors followed applicable EQUATOR Network (https://www.equator-network.org/) guidelines during the conduct of this research project.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Leighton JL, You D, Schneider P. Limiting blood loss in orthopaedic trauma: Strategies and effects. Injury. 2020;51(Suppl 2):S123–7. doi: 10.1016/j.injury.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–26. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 3.Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: A randomised trial. Lancet. 2018;392:283–91. doi: 10.1016/S0140-6736(18)31553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coccolini F, Pizzilli G, Corbella D, Sartelli M, Agnoletti V, Agostini V, et al. Pre-hospital plasma in haemorrhagic shock management: Current opinion and meta-analysis of randomized trials. World J Emerg Surg. 2019;14:6. doi: 10.1186/s13017-019-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barelli S, Alberio L. The role of plasma transfusion in massive bleeding: Protecting the endothelial glycocalyx? Front Med (Lausanne) 2018;5:91. doi: 10.3389/fmed.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feuerstein SJ, Skovmand K, Møller AM, Wildgaard K. Freeze-dried plasma in major haemorrhage: A systematic review. Vox Sang. 2020;115:263–74. doi: 10.1111/vox.12898. [DOI] [PubMed] [Google Scholar]
- 7.Frith D, Goslings JC, Gaarder C, Maegele M, Cohen MJ, Allard S, et al. Definition and drivers of acute traumatic coagulopathy: Clinical and experimental investigations. J Thromb Haemost. 2010;8:1919–25. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 9.Lira A, Pinsky MR. Choices in fluid type and volume during resuscitation: Impact on patient outcomes. Ann Intensive Care. 2014;4:38. doi: 10.1186/s13613-014-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murad MH, Stubbs JR, Gandhi MJ, Wang AT, Paul A, Erwin PJ, et al. The effect of plasma transfusion on morbidity and mortality: A systematic review and meta-analysis. Transfusion. 2010;50:1370–83. doi: 10.1111/j.1537-2995.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JL, Moore EE, Kashuk JL, Banerjee A, Cothren CC, Biffl WL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145:973–7. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 12.Pati S, Potter DR, Baimukanova G, Farrel DH, Holcomb JB, Schreiber MA. Modulating the endotheliopathy of trauma: Factor concentrate versus fresh frozen plasma. J Trauma Acute Care Surg. 2016;80:576–84. doi: 10.1097/TA.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 13.Middelburg RA, van Stein D, Briët E, van der Bom JG. The role of donor antibodies in the pathogenesis of transfusion-related acute lung injury: A systematic review. Transfusion. 2008;48:2167–76. doi: 10.1111/j.1537-2995.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 14.Müller MC, van Stein D, Binnekade JM, van Rhenen DJ, Vlaar AP. Low-risk transfusion-related acute lung injury donor strategies and the impact on the onset of transfusion-related acute lung injury: A meta-analysis. Transfusion. 2015;55:164–75. doi: 10.1111/trf.12816. [DOI] [PubMed] [Google Scholar]
- 15.Kashuk JL, Moore EE, Johnson JL, Haenel J, Wilson M, Moore JB, et al. Postinjury life threatening coagulopathy: Is 1: 1 fresh frozen plasma: packed red blood cells the answer? J Trauma. 2008;65:261–70. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 16.Gupta B, Garg N, Ramachandran R. Vasopressors: Do they have any role in hemorrhagic shock? J Anaesthesiol Clin Pharmacol. 2017;33:3–8. doi: 10.4103/0970-9185.202185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sperry JL, Minei JP, Frankel HL, West MA, Harbrecht BG, Moore EE, et al. Early use of vasopressors after injury: Caution before constriction. J Trauma. 2008;64:9–14. doi: 10.1097/TA.0b013e31815dd029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used/or analyzed is available following any request send to the correspondence.