Abstract
Introduction:
Radiofrequency ablation is a procedure used to alleviate pain by destroying nerves with by radiofrequency-generated heat. Traditionally, radiofrequency ablation is preceded by diagnostic medial branch block injections, both guided by fluoroscopy. Fluoroscopic visualization of the superolateral aspect of the thoracic transverse process, where thoracic medial branch nerves occur, can be challenging due to anatomical complexities, especially in obese patients. We present a novel technique in which ultrasound was utilized in conjunction with fluoroscopy to perform medial branch block and radiofrequency ablation of the thoracic medial branch nerves.
Case Report:
First, two diagnostic thoracic medial branch nerve blocks were performed under ultrasound guidance. For the subsequent radiofrequency ablation, spinal needles were first advanced under ultrasound guidance to the target thoracic medial branch nerves. The position of those spinal needles was then used to guide the placement of cooled radiofrequency ablation probes using fluoroscopy. The patient reported 100% pain relief following the procedures.
Discussion:
We found that the addition of ultrasound allowed us to overcome the challenge of visualizing the superolateral aspect of thoracic transverse process under fluoroscopy alone. Direct ultrasound visualization allowed us to accurately and safely perform a thoracic medial branch block and radiofrequency ablation in a patient with poor fluoroscopic anatomy, as demonstrated by the patient’s complete pain relief after both medial branch block and radiofrequency ablation. We also theorize that our novel technique allows the provider to directly visualize the pleura, which could reduce the risk of severe pneumothorax associated with thoracic medial branch block and cooled radiofrequency ablation.
Keywords: Chronic pain, interventional pain management, obesity, pneumothorax
Introduction
Radiofrequency ablation (RFA) is a procedure in which heat generated by radiofrequency is conducted via electrode probes to destroy nerves responsible for pain. RFA probes are directed to the appropriate anatomical location based on spinal landmarks visualized via fluoroscopy. RFA is often preceded by diagnostic median branch block (MBB) injections, which predict whether the patient will experience pain relief from RFA therapy. MBB followed by RFA is widely used to treat facetogenic pain innervated by the medial branches of the lumbar, thoracic, and cervical spine. 1 RFA is also used for chronic shoulder, knee, hip, and lower back pain refractory to conservative management. 2
Pain from the thoracic spine is less common than cervical or lumbar spine pain. Thoracic pain prevalence in the general population is estimated at 15%, while lumbar and cervical spine pain prevalence is 56% and 44%, respectively.3,4 Greater mobility in the cervical and lumbar spine likely contributes to these proportions. Thoracic MBB and RFA are less frequent and less studied compared with those in the cervical and lumbar spines.
There is greater patient-to-patient anatomical variation in the location of thoracic medial branches, making it difficult to pinpoint and ablate them successfully. Cooled radiofrequency ablation (C-RFA) creates a larger ablation area, accommodating the varied locations of medial branch nerves. 5 This increases the chance of successful ablation.
The thoracic spine presents challenges for MBB injections and nerve ablation. Identifying the superolateral aspect of the thoracic transverse process (TP) is difficult due to overlapping ribs and transverse processes on fluoroscopic imaging, especially in obese patients. 1 Correct placement is crucial as thoracic medial branch nerves occur there. MBB and C-RFA in the thoracic spine also carry the risk of pneumothorax.
In our case study, ultrasound (US) was used to overcome challenges in thoracic spine MBB and C-RFA under fluoroscopy. Our patient was a 68-year-old male who suffered from long-standing left upper thoracic back pain. We performed a technique involving two US-guided thoracic MBBs that provided greater than 80% relief. That was followed by an US-guided C-RFA technique in which spinal needles were first advanced under US guidance to the target medial branch nerves. The position of those spinal needles was then used to guide the placement of C-RFA probes under fluoroscopy.
Case report
The patient was a 68-year-old obese male with 17 years of constant left upper thoracic back pain extending both inferiorly to his left posterior ribs and anteriorly from there to his left lower lateral and left lower anterior ribs. He previously underwent C3-C6 laminectomy and posterior cervical spinal fusion, which failed to alleviate his pain. Thoracic spine magnetic resonance imaging demonstrated prominent osteophytes at the costovertebral junctions at multiple levels, most prominent at the T8 level where osteophytic spurring focally compressed the adjacent pleura, and multilevel degenerative changes in the thoracic spine and mild spinal canal stenosis at T1-T2 and T2-T3.
After failing conservative management, we explored interventional approaches to treat the patient’s pain. We decided to proceed with bilateral T2-T5 MBB to assess the possibility of referred thoracic facetogenic pain as the cause of the patient’s long-standing discomfort. We had initially planned to perform the first MBB injection at the T2 medial branch nerve. However, despite multiple repositioning attempts, fluoroscopic angle changes, and attempts to collimate and magnify the fluoroscopic image, the left-sided T2 transverse process was very difficult to visualize, likely due to the increased amount of soft tissue between the X-ray beam and the image intensifier resulting from patient’s obese body habitus. It was then decided to attempt to visualize using US. We used an i8CX1 transducer. A depth of 4.0 cm, gain setting of 81, dynamic range of 65, acoustic power of 2, and persistence setting of 2 were the ultrasonography parameters used. No US harmonics or compounding imaging were employed. A curvilinear probe was placed in the sagittal orientation, and the T2 superior lateral aspect of the transverse process was identified. By first scanning from lateral to medial, we were able to visualize the transition from rib to TP, which clarified the lateral border of the transverse process. We visualized that transition by appreciating the deeper oval shape of the rib transition to the more superficial characteristic ‘tombstone’ appearance of the transverse process on US. Then, by scanning from cephalad and caudad, we were able to visualize superior and inferior aspects of the TP. Following local anesthesia with lidocaine, an echogenic 22-gauge 80-mm needle was advanced to the superolateral aspect of the T2 transverse process, through which 1 mL of 0.5% bupivacaine was injected. The same steps were repeated for the transverse processes of T3 through T5. The procedure was well tolerated, and there were no apparent complications immediately after the procedure. The patient reported reduction in his pain from 7/10 to 0/10 following bilateral MBB.
Given the patient’s significant pain relief with MBB, it was then decided to proceed with left T2-T5 medial branch C-RFA. Given prior difficulty with visualization, we planned to place 25-gauge spinal needles under US guidance as fluoroscopic markers to indicate the superolateral aspects of the T2, T3, T4, and T5 transverse processes. A curvilinear probe was first placed in the sagittal orientation and the T2 superolateral aspect of the transverse process was identified, as detailed previously. Following local lidocaine, a 25-gauge 3.5" spinal needle was then advanced to the superior lateral aspect of the T2 transverse process under US guidance in a similar fashion as that utilized in MBB. That procedure was repeated for levels T3 through T5. Fluoroscopy was used to confirm the thoracic levels (Figures 1 and 2). The needle entry points were infiltrated with 1% lidocaine at the appropriate points. The 17-gauge 75 mm and 5.5 mm radiofrequency probes were advanced to the superolateral aspects of the transverse process directly adjacent to the previously placed spinal needles. This ensured that the larger caliber RFA needles were advanced directly over bone, reducing risk of pneumothorax associated with the larger caliber C-RFA needles. Correct probe position was verified using anteroposterior and lateral fluoroscopic images (Figures 3 and 4) to confirm the precision of our procedure. After successful stimulation testing, radiofrequency neural ablation with active cooling of the needle tip was subsequently performed. On follow-up 3 days later, the patient reported 100% pain relief. No complications were reported.
Figure 1.
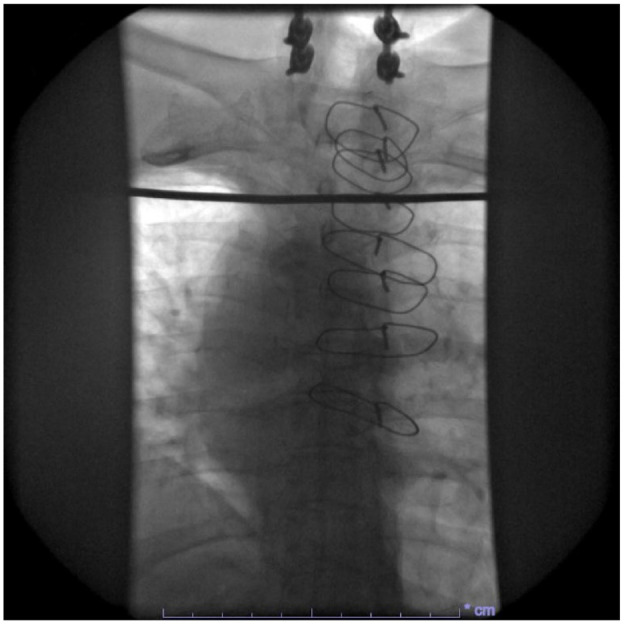
Anteroposterior (AP) fluoroscopy of the patient’s thorax prior to RFA.
Figure 2.
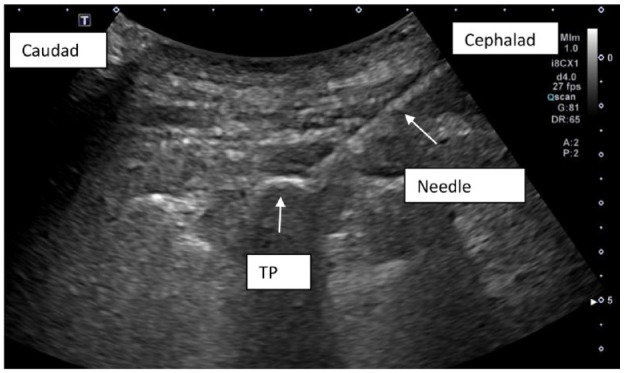
Ultrasound image demonstrating echogenic 22-gauge 80-mm needle advanced to the superior lateral aspect of the T2 transverse process (TP).
Figure 3.
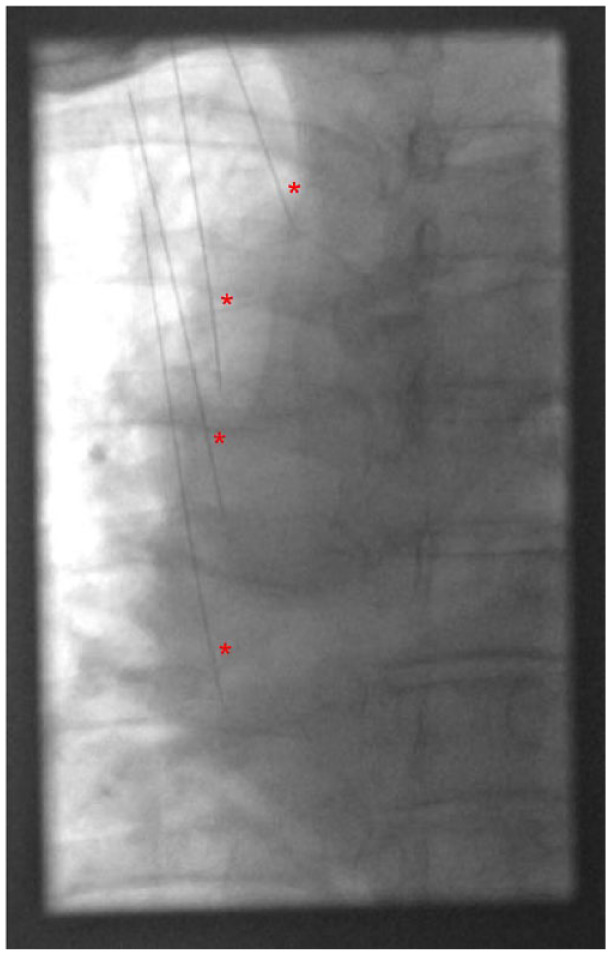
Anteroposterior (AP) fluoroscopy of the patient’s thorax after ultrasound-guided placement of spinal needles at the superolateral aspects of the T2, T3, T4, and T5 transverse processes prior to placement of C-RFA probes. Spinal needles are indicated by the red * symbols.
Figure 4.
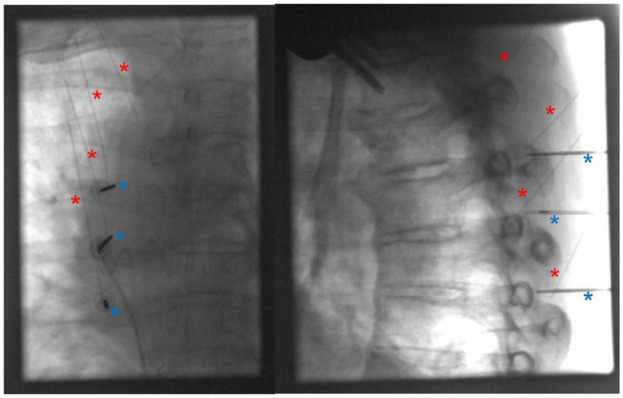
Anteroposterior (AP, left) and lateral view (right) fluoroscopy of the patient’s thorax demonstrating placement of spinal needles and C-RFA probes at the superolateral aspects of the T2, T3, T4, and T5 transverse processes. Spinal needles are indicated by the red * symbols, and C-RFA needles are indicated by blue * symbols. Only three C-RFA needles are depicted because our equipment only allows for three C-RFA probes to be placed at a time. C-RFA of the last medial branch nerve was performed separately.
Discussion
US guidance for cervical and lumbar MBB and RFA have previously been described. 6 However, evidence in the current literature of US guidance for MBB and RFA in the thoracic spine in particular is scarce, and the feasibility and accuracy of US-guided thoracic MBB have not been fully demonstrated. 7 US-guided thoracic facet joint intra-articular injection was first described by Stulc et al. in a cadaveric model. 8 This study describes a technique for thoracic facet joint injections evaluated the intra-articular contrast spread of such injections using an iodinated contrast agent. The study found that 16 of 20 of injections demonstrated intra-articular contrast spread. A technique for US-guided thoracic medial branch blocks was also suggested by Moon et al., though their method was not validated. 9
One reason we took that novel approach was the increased anatomical precision offered by US. Unlike fluoroscopy, which can sometimes obscure the transition from rib to transverse process, US provides clearer visualization of these osseous landmarks. 10 While there is conflicting evidence regarding the ideal exact target for MBB needle and C-RFA probe placement,7,11,12 we believe that our technique allows for more accurate placement of the instrument to the desired target by the provider. That enhanced visibility is particularly advantageous in patients with an obese body habitus, where traditional fluoroscopic imaging may be compromised by excess adipose tissue. By facilitating better localization of the target area, US enables more accurate and safer placement of C-RFA probes at the superior and lateral aspects of the thoracic transverse processes, where the medial branch nerves are typically located.
We posit that US-guided thoracic MBB and our C-RFA probe placement may decrease the possible severity of pneumothorax associated with this procedure. MBB and C-RFA both involve introducing sharp instruments into the thoracic spine, which risks piercing the adjacent pleural cavity. The spinal needles we utilized were 25-gauge 3.5” spinal needles, which are of significantly lower caliber than the 17-gauge 75 mm and 5.5 mm radiofrequency probes used to perform C-RFA. By using the lower caliber spinal needles to first identify the location of our target lesion, we theoretically reduce the risk of more severe pneumothorax that could result from piercing the pleural cavity with the larger caliber radiofrequency probes. Another possible safety improvement afforded by US-guided MBB is the reduction in radiation exposure to both patient and provider.
Despite the benefits, US guidance in MBB and C-RFA also presents some drawbacks. First, widespread proficiency in performing US-guided interventions may require additional training and education for health care providers who are less familiar with this imaging modality. In addition, the technique we described in our study involves the insertion of additional needles for US guidance before placing the C-RFA probes. While these needles are smaller in gauge and inserted under direct ultrasonographic visualization, their placement still carries inherent risks of procedural complications and patient discomfort.
Our technique, which utilizes US alongside fluoroscopy, begs the question of whether US guidance alone can be considered for MBB and C-RFA. While US alone would eliminate the radiation exposure associated with those procedures, US without fluoroscopy presents its own technical challenges. First, acoustic shadowing of bone can render deeper structures, including vertebral disks and the spinal cord, difficult to identify. 7 Furthermore, contrast-enhanced fluoroscopic guidance is the only reliable way to identify intravascular uptake of injectate, necessitating contrast-enhanced fluoroscopic guidance. 13 Our technique mitigates those drawbacks associated with US guidance alone and affords the provider the benefits of both imaging modalities.
Further investigation is necessary to better discern the benefit provided by the addition of US to thoracic MBB and C-RFA. Comparative studies investigating the differences in pain relief and functional outcomes between US-guided and fluoroscopy-guided procedures would provide insights into the efficacy of US in this context. Similarly, investigations into the incidence and severity of pneumothorax associated with our US-guided technique compared with traditional fluoroscopy-guided approaches would help assess its safety profile. While fluoroscopy was used in addition to US in our procedure to confirm correct instrument placement and thus increase the precision of our procedure, exploration into alternative techniques that utilize US alone for the placement of C-RFA probes may also expand the repertoire of options available to health care providers treating thoracic spinal pain.
Acknowledgments
The authors report no acknowledgments.
Footnotes
Contributors: KJY contributed to conceptualization, project administration writing of the original draft, and review and editing of subsequent drafts. PDM contributed to conceptualization, review and editing of drafts, and supervision. WFS contributed to supervision and review and editing of drafts.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: As a case report and literature review, this manuscript did not require ethical approval from our institution’s institutional review board.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Permission from patient(s) or subject(s) obtained in writing for publishing their case report: Yes.
Permission obtained in writing from patient or any person whose photograph is included for publishing their photographs and images: Yes.
Confirm that you are aware that permission from a previous publisher for reproducing any previously published material will be required should your article be accepted for publication and that you will be responsible for obtaining that permission.: State Yes or No.
Guarantor: KJY.
ORCID iD: Kevin J Yang  https://orcid.org/0000-0002-1030-2729
https://orcid.org/0000-0002-1030-2729
References
- 1. Gungor S, Candan B. The efficacy and safety of cooled-radiofrequency neurotomy in the treatment of chronic thoracic facet (zygapophyseal) joint pain: a retrospective study. Medicine 2020; 99: e19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ye Y, Gabriel RA, Mariano ER. The expanding role of chronic pain interventions in multimodal perioperative pain management: a narrative review. Postgrad Med 2022; 134: 44920210608. [DOI] [PubMed] [Google Scholar]
- 3. Manchikanti L, Singh V, Datta S, et al. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009; 12: E35–E70. [PubMed] [Google Scholar]
- 4. Linton SJ, Hellsing AL, Halldén K. A population-based study of spinal pain among 35-45-year-old individuals. Prevalence, sick leave, and health care use. Spine (Phila Pa 1976) 1998; 23: 1457–1463. [DOI] [PubMed] [Google Scholar]
- 5. Cedeno DL, Vallejo A, Kelley CA, et al. Comparisons of lesion volumes and shapes produced by a radiofrequency system with a cooled, a protruding, or a monopolar probe. Pain Physician 2017; 20: E915–E922. [PubMed] [Google Scholar]
- 6. Wong MJ, Rajarathinam M. Ultrasound-guided axial facet joint interventions for chronic spinal pain: a narrative review. Can J Pain 2023; 7: 219361720230517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreira-Silva N, Ribas R, Hurdle MFB, et al. Ultrasound-guided procedures for the management of chronic thoracic back pain: a technical review. J Ultrasound 2024; 27: 120230830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stulc SM, Hurdle MF, Pingree MJ, et al. Ultrasound-guided thoracic facet injections: description of a technique. J Ultrasound Med 2011; 30: 357–362. [DOI] [PubMed] [Google Scholar]
- 9. Moon SH, Lee S, Lee JI. Ultrasound-guided intervention in thoracic spine. J Korean Orthop Assoc 2015; 50: 93–106. [Google Scholar]
- 10. Zeballos JL, Voscopoulos C, Kapottos M, et al. Ultrasound-guided retrolaminar paravertebral block. Anaesthesia 2013; 68: 649–651. [DOI] [PubMed] [Google Scholar]
- 11. Chua WH, Bogduk N. The surgical anatomy of thoracic facet denervation. Acta Neurochir (Wien) 1995; 136: 140–144. [DOI] [PubMed] [Google Scholar]
- 12. Koutp A, Sadoghi P, Petritsch J, et al. Anatomic-topographic investigation of the branches of the Dorsal Ramus of thoracic spinal nerves. Pain Med 2022; 23: 1869–1874. [DOI] [PubMed] [Google Scholar]
- 13. Sullivan WJ, Willick SE, Chira-Adisai W, et al. Incidence of intravascular uptake in lumbar spinal injection procedures. Spine 2000; 25: 481–486. [DOI] [PubMed] [Google Scholar]


