Abstract
We describe the case of a 40-year-old patient who sustained an aortic transection in 2016 following a road traffic accident and had two subsequent full-term pregnancies. This case adds to the literature on pregnancy after endovascular aortic repair.
Keywords: Maternal medicine, high-risk pregnancy, traumatic aortic transection, transluminal endovascular aortic repair, pregnancy complications, cardiovascular
Introduction
Acute traumatic aortic transection (ATAT) is life-threatening. Transluminal endovascular aortic repair (TEVAR) is the surgical repair of choice. Management of women with TEVAR in pregnancy presents a challenge due to a lack of available evidence.
History
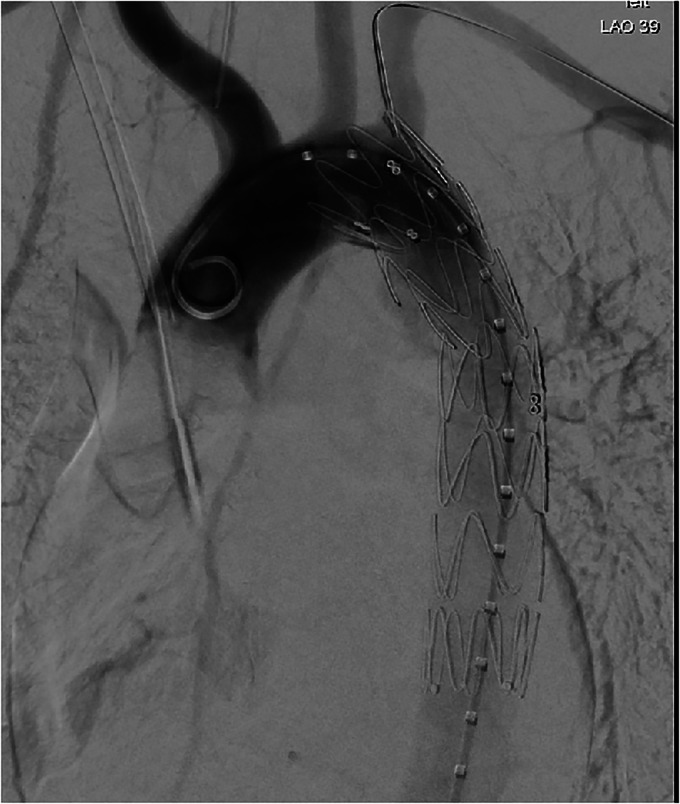
In 2016, the patient sustained an ATAT at the level of T5 during a road traffic accident. She underwent emergency endovascular aortic stent insertion (Figure 1). Postoperative computerised tomography (CT) was satisfactory. She was discharged on ramipril for optimal blood pressure (BP) control. The team recommended imaging every 2–3 years to check for stent leaks. The patient's CT in 2019 was normal. She attended her cardiothoracic appointment in 2020, and a follow-up scan in 1 year was recommended.
Figure 1.
Emergency endovascular aortic stent insertion.
First pregnancy
The patient was referred to our hospital, a stand-alone tertiary-level maternity unit with approximately 8000 deliveries per year, in October 2020 at 6 weeks’ gestation. Ramipril was switched to labetalol 200 mg twice daily. She attended the maternal medicine clinic at 8 weeks. BP was 128/70mmHg, and her cardiothoracic surgeon was informed. He advised there would be a very low risk of spontaneous graft leak during pregnancy, and recommended maintaining good BP control. The patient attended the maternal medicine clinic where she saw the consultant cardiologist at regular intervals.
Investigations and delivery planning
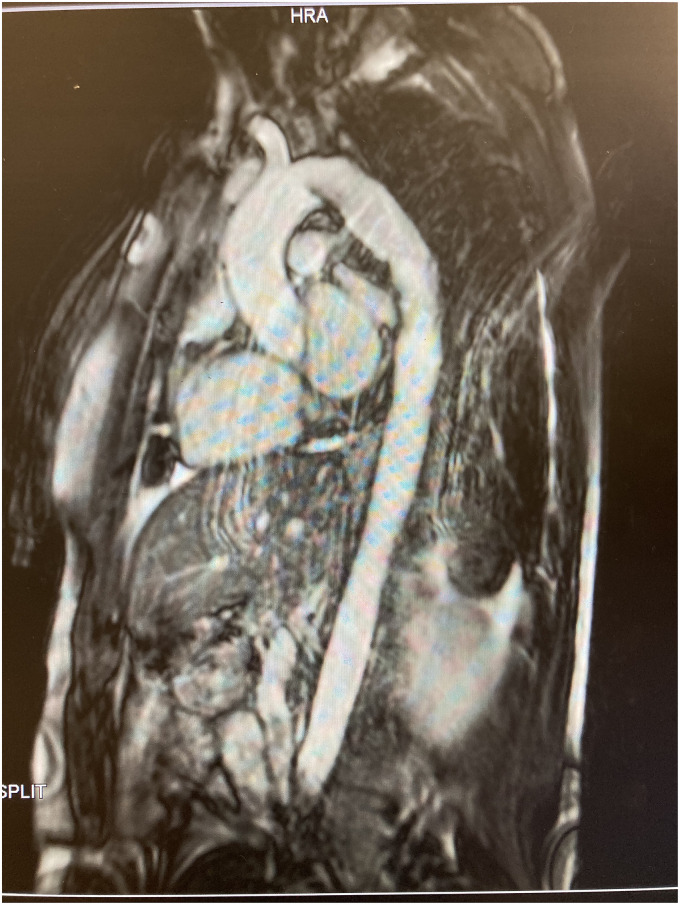
BP remained well controlled at each visit. A Magnetic Resonance Imaging (MRI) thoracic aorta at 23 weeks (Figure 2) showed the stent in the distal aortic arch extending to the proximal descending thoracic aorta, with no evidence of dissection and normal thoracic aorta calibre. Echocardiogram was normal.
Figure 2.
MRI thoracic aorta.
The consultant cardiologist and consultant obstetrician reviewed the patient at 29 weeks’ gestation. As cardiac imaging was reassuring, vaginal delivery was recommended, with caesarean section (CS) for obstetric indications only.
A delivery plan was placed in the chart in consultation with anaesthesiology. Early epidural avoiding adrenaline was recommended. Standard fluid management was advised, with judicious use of vasopressors if required. The second stage was to proceed as normal, and the standard oxytocin regimen was to be given in the third stage, avoiding ergometrine. If CS was required, standard dose spinal or epidural top-up could be given, avoiding adrenaline. If general anaesthetic (GA) was required, measures to maintain normal cardiovascular parameters were advised. Intravenous (IV) labetalol was to be available to keep systolic BP at or less than 120 mmHg. Postpartum care was to proceed as normal, and she was to be discharged on labetalol with cardiology follow-up.
Delivery
The patient was admitted with pains at 38 weeks and 3 days of gestation. She had uneventful epidural placement at L3-4 interspace. BP was checked every 30 min, and was well controlled. The first stage lasted 3 h and 40 min. After 10 min of active pushing, she delivered a baby boy weighing 3.41 kg. The third stage was actively managed with 10 IU of intramuscular oxytocin, and 40 IU of IV oxytocin in one litre of compound sodium lactate. The patient recovered well and was soon discharged home.
Second pregnancy
In December 2021, the patient was referred to our service at 7 weeks’ gestation. She had been taking ramipril 2.5 mg once daily with good BP control, which was switched to labetalol 100 mg twice daily. She attended the maternal medicine clinic at 12 weeks. BP was 110/60mmHg.
The consultant cardiologist noted her last MRI thoracic aorta in March 2021 was normal. Echocardiograms at 20 and 34 weeks were unremarkable. As with her first pregnancy, a delivery plan was placed in the chart. The anaesthesiology consultant recommended an early epidural in labour.
Labour was induced at 39 weeks and 1 day due to maternal history. The patient had prostin gel, and artificial rupture of membranes the following morning. The epidural was sited at 09:50, and the patient delivered a male infant at 10:05 weighing 3.27 kg. The third stage was actively managed. BP was normal throughout. She was discharged home on labetalol with General Practitioner follow-up. A postnatal cardiology review was arranged, and echocardiogram at 12 weeks' postnatal showed stable aortic dimensions.
Discussion
Pregnancy is associated with significant cardiovascular changes, including increased circulating blood volume, stroke volume and cardiac output. 1 Labour is associated with further haemodynamic changes secondary to factors including pain and uterine contractions. 2 Postnatally, fluid shifts occur which risk precipitating heart failure. 2
Hormonal changes in pregnancy affect the integrity of the aorta. 3 Although rare, the risk of aortic dissection is strongly associated with pregnancy – reported incidence is approximately 4- to 25-fold higher than in the nonpregnant populations. 4 The vast majority of dissections occur in the third trimester or postpartum period, but can happen at any stage and therefore there is a need for robust surveillance throughout pregnancy and postpartum. 5 Surveillance should include repeat cross-sectional imaging postnatally. 2
The European Society of Cardiology (ESC) outlines principles for safe management of cardiovascular disease in pregnancy. 2 For aortic pathology, management should be as per aortic diameter on echocardiogram. 2 As hypertension may cause aortic dissection good BP control is recommended. 2 Our patient continued taking labetalol postnatally to maintain BP control. However, other antihypertensives such as enalapril, an angiotensin-converting enzyme, can be prescribed for postnatal women who are breastfeeding in accordance with national guidelines. 6
Vaginal delivery is appropriate for most patients. 2 Of note, our patient had TEVAR 5 years prior to delivery. If this time period was shorter, it is likely the team would have been more cautious.
TEVAR is a relatively new procedure, with previous cases undergoing open repair. 7 It has become the treatment of choice due to lower mortality. 7 Long-term prognosis is unclear, but a recent study found favourable outcomes after 90 months. 8 There are a limited number of case studies on pregnancy after TEVAR. Two patients had an uncomplicated antenatal course. One had a spontaneous vaginal delivery at term, 9 and one had an elective CS. 10 In a third case, the patient presented with chest pain at 17 weeks and required endovascular dilation for a collapsed stent. She had an elective CS at term. 11
Our patient had a structurally normal aorta and no cardiac disease prior to her accident. As the current literature focuses on patients with pre-existing aortic pathology, we must be reflective in our comparisons.8,12 In particular, women who require aortic surveillance in pregnancy are usually those with congenital or familial aortopathies which pre-dispose them to aortic aneurysm or dissection, for example, women with Marfan syndrome. 2 We believe that our patient's cardiac risks were likely to be lower than these patients in pregnancy.
Our case adds to the literature on pregnancy after TEVAR. We recommend that patients with TEVAR should be seen in a maternal medicine clinic, with imaging of the graft during and after pregnancy. Good BP control throughout pregnancy and labour is essential. Signs and symptoms of graft dysfunction should be taken seriously. A delivery plan should be placed in the chart to ensure the team can safely support the patient during labour.
Acknowledgements
We are grateful to Mr. David Healy for his advice and input in the case.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The National Maternity Hospital does not require ethical approval for reporting individual case studies.
Informed consent: Written informed consent was obtained from the patient for their anonymised written information and figures to be published in this article.
Guarantor: Dr. Ruth Roseingrave, lead author, is the guarantor for this case study.
Contributorship: Dr. Ruth Roseingrave researched the literature, conceived the case study and was responsible for drafting the article. Dr. Carla Canniffe provided research and guidance from an expert cardiology perspective, and Dr. Siobhan Corcoran and Professor Fionnuala McAuliffe from a maternal medicine perspective. All authors reviewed and edited the manuscript, and approved the final version.
ORCID iD: Ruth Roseingrave https://orcid.org/0000-0003-4243-5996
References
- 1.Nelson-Percy C. Handbook of obstetric medicine. 5th ed. Florida: CRC Press, 2015. [Google Scholar]
- 2.European Society of Cardiology. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018; 39: 3165–3241. [DOI] [PubMed] [Google Scholar]
- 3.Manalo-Estrella P, Barker AE. Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol 1967; 83: 336–341. [PubMed] [Google Scholar]
- 4.Kamel H, Roman MJ, Pitcher Aet al. et al. Pregnancy and the risk of aortic dissection or rupture: a cohort-crossover analysis. Circulation 2016; 134: 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wanga S, Silversides C, Dore A, et al. Pregnancy and thoracic aortic disease: managing the risks. Can J Cardiol 2016; 32: 78–85. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Clinical Excellence. Hypertension in pregnancy: diagnosis and management, https://www.nice.org.uk/guidance/ng133/resources/hypertension-in-pregnancy-diagnosis-and-management-pdf-66141717671365 (2019). [Google Scholar]
- 7.Xenos ES, Minion DJ, Davenport DL, et al. Endovascular versus open repair for descending thoracic aortic rupture: institutional experience and meta-analysis. Eur J Cardiothorac Surg 2009; 35: 282–286. [DOI] [PubMed] [Google Scholar]
- 8.Skrypnik D, Bischoff MS, Meisenbacher K, et al. A 10-year single-center experience with the GORE TAG conformable thoracic stent graft in the treatment of thoracic aortic disease. J Endovasc Ther 2022; 29: 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlechta B, Wiedemann D, Eppel Wet al. et al. Uncomplicated vaginal delivery 6 years after stent graft repair of an acute traumatic aortic transection. Interact Cardiovasc Thorac Surg 2012; 14: 120–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duiella SF, Gaia G, Bonanomi C, et al. Transluminal endovascular aortic repair and pregnancy: a case report. J Integr Cardiol 2015; 1: 35–36. DOI: 10.15761/JIC.1000112 [DOI] [Google Scholar]
- 11.Khandanpour N, Mehta TA, Adiseshiah Met al. et al. Are aortic stent grafts safe in pregnancy? Case Rep Radiol 2015; 2015: 190878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isselbacher EM, Preventza O, Hamilton J, et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022; 146: e334–e482. https://www.ahajournals.org/doi/10.1161/CIR.0000000000001106#d1e1202 . [DOI] [PMC free article] [PubMed] [Google Scholar]