Abstract
Pregnancy-associated pulmonary embolism (PA-PE) is a life-threatening presentation however literature surrounding its optimal management is limited. This case describes a case of PA-PE treated with catheter-directed thrombolysis after clinical deterioration despite standard anticoagulation therapy. Careful multidisciplinary planning is required to successfully manage the deteriorating patient with PA-PE with catheter-directed thrombolysis being considered as potential first-line therapy in these patients.
Keywords: Pregnancy, pulmonary embolism, catheter-directed, multidisciplinary
Background
Massive pulmonary embolism (PE) is one of the leading causes of maternal death,1,2 yet despite this the evidence surrounding optimal management is sparse. Current guidelines suggest systemic anticoagulation with low molecular weight heparin (LMWH).3–5 However, if haemodynamic instability occurs, the decision-making surrounding resuscitative treatment is difficult due to the need to balance risk in both mother and fetus. This case report demonstrates the successful use of catheter-directed thrombolysis/thrombectomy as a first-line option for haemodynamically unstable pregnancy-associated pulmonary embolism (PA-PE).
Case report
A previously well 33-year-old female at 30 weeks’ gestation of her second pregnancy presented with acute shortness of breath to a peripheral hospital. The patient had no past medical history and no regular medication. The baseline venous thromboembolism (VTE) risk was low with the patient's body mass index being 26.8 kg/m2 (weight: 61 kg, height: 161 cm), non-smoker and no alcohol intake in addition to no known family history of thromboembolic disorders. Her first pregnancy was an unplanned and uncomplicated caesarean due to failure to progress in labour. The patient's main VTE risk factor was the physiological changes of pregnancy. Initial investigation with a ventilation-perfusion scan confirmed a large central PE and the decision was to commence therapeutic anticoagulation (enoxaparin 1 mg/kg twice daily) and transfer to a tertiary hospital for early multidisciplinary team (MDT) input.
On initial review to our hospital the patient was mildly tachypneic (Respiratory rate [RR] 27–32 breaths/min) and tachycardic (Heart rate [HR] 110–120 beats/min), with stable oxygen saturation (95% on room air) and blood pressure (105/70 mmHg). Transthoracic echocardiogram (TTE) revealed a severely dilated right ventricle (RV), moderate reduction of RV ejection fraction and septal flattening. The patient was admitted to the intensive care unit (ICU) with the diagnosis of submassive PE for monitoring. The precarious nature of the situation required a multidisciplinary discussion at the beginning of the patient's ICU admission to develop a provisional plan in the event of deterioration. This included planning for potential haemodynamic collapse, consideration of thrombolysis and thrombectomy, and requirement for emergent delivery as well as potential extracorporeal supports. Specialties consulted included obstetric medicine, maternal–fetal medicine (MFM), neonatology, haematology, anaesthesia, radiology, interventional radiology and the extracorporeal membrane oxygenation (ECMO) service. The aim was ideally to prolong the pregnancy as long as possible whilst ensuring the safety of the mother as priority. LMWH was continued after careful consideration based on the gestational age, the imminent risk profile and risk/benefit analysis of all possible therapy options available.
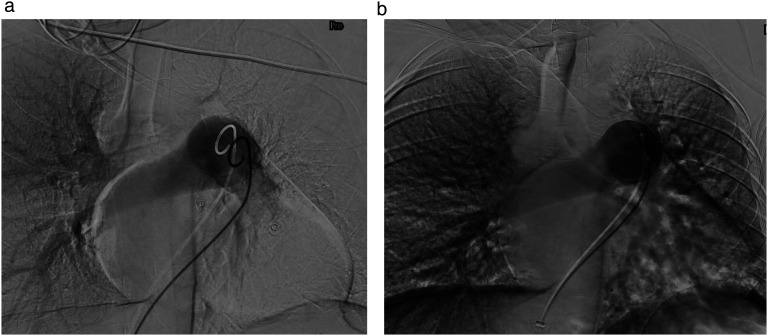
Initially, the patient remained stable but less than 24 h into her ICU admission the patient developed presumptive obstructive shock (Blood pressure [BP] 79/59 mmHg, HR 125 beats/min, RR 36 breaths/min, mildly raised lactate peak 2.1 mmol/L) requiring vasopressor support. Computer tomography (CT) pulmonary angiogram confirmed a near occlusive large central PE within the left main pulmonary trunk in addition to significant embolus in the right main pulmonary artery. A second MDT swiftly progressed the patient towards interventional radiology, with a plan for left pulmonary artery thrombectomy and catheter-directed thrombolysis. In the event of haemodynamic collapse, the agreed back-up plan was for initiation of advanced life support, immediate delivery within 5 min and initiation of ECMO in the interventional radiology suite. Steroids for fetal lung maturity were given in case delivery of the fetus was required. The 2-h procedure was performed under local anaesthetic only. Right common femoral vein access was obtained, and the left femoral artery was also wired (but not dilated) in case emergent ECMO was required. The pulmonary outflow trunk was cannulated with a 5F pigtail catheter. Angiography demonstrated occlusive thrombus in the left main pulmonary artery and extensive thrombus in the right main pulmonary artery (Figure 1(a)). Pulsed catheter thrombolysis was performed with intra-arterial alteplase (5 mg bolus) delivered directly to the left main pulmonary artery thrombus. After a dwell time of 5 min, catheter thrombectomy was performed using the Indigo Lightning system (Penumbra Inc., Alameda, CA, USA). Post-thrombectomy, small volume residual thrombus remained. Therefore, a 5F Unifuse infusion catheter was left across the left main and lower lobar pulmonary artery enabling continuous alteplase infusion (1 mg/h) until repeat pulmonary angiogram. The patient returned to the ICU without the need for ongoing haemodynamic support.
Figure 1.
(a) Angiography demonstrating occlusive thrombus in the left main pulmonary artery (pre-thrombolysis/thrombolectomy). (b) Angiography demonstrating complete resolution of thrombus in the left main pulmonary artery (post-thrombolysis/thrombolectomy).
Repeat scanning 24 h later demonstrated complete resolution of the main and lobar PE (Figure 1(b)) and a repeat TTE showed improvement in RV dilation. The patient was discharged from the ICU 2 days later and discharged home at day 7 on LMWH. Two months later at 39 weeks and 1 day of gestation, a healthy baby boy was delivered via planned caesarean section with an anticoagulation pause to allow safe administration of a neuraxial block (birthweight 2990 g, 5-min Apgar score 9).
Discussion
Evidence for the management of high- and intermediate-risk PA-PE is limited. Despite PA-PE accounting for up to 10% of maternal deaths in Australia, 3 treatment guidelines are based on case reports, case series or extrapolated from non-pregnant individuals which is problematic.2,5 Currently, the preferred first-line recommendation for unstable PA-PE is systemic thrombolysis3–6 with other therapies such as catheter-directed thrombolysis/thrombectomy, thoracotomy/surgical embolectomy or ECMO typically utilised as salvage procedures.5–7
Systemic thrombolysis in pregnancy is associated with risks including maternal bleeding, fetal toxicity, fetal loss, and premature delivery.7,8 However, catheter-directed thrombolysis/thrombectomy, although seldom used, can reduce bleeding risk and lower in-hospital mortality when compared with systemic thrombolysis.9–11 Rather than being a treatment option only when systemic thrombolysis is contraindicated or failed, it should be considered in institutions where the appropriate expertise is present. Many review articles propose risk stratification, stage of presentation (antepartum, peripartum or post-partum) and presence of contraindications be considerations in determining a particular patient's first-line options, rather than utilising systemic thrombolysis in all cases.3,4,12
In our case report, the centre in which we operate has the availability of interventional radiological procedures. This was with the knowledge that in the event of cardiac arrest, the patient would have undergone resuscitation, hysterotomy and ECMO. If implementation of these supports was not practical or possible, the alternative of systemic thrombolysis may have been utilised. Surgical embolectomy had been considered, however, its increased risk of bleeding, time to organise theatre and the coordination of skilled persons made this option less feasible in the time critical emergency. 12
Once stabilised, the next critical period is delivery of the fetus. The highest risk of maternal bleeding occurs post-partum (within 72 h of delivery). 7 Therefore, the method and timing of delivery is important in risk minimisation. Although elective caesarean delivery may be recommended, no significant benefit has been proven.3,12,13 Caesarean delivery offers a theoretical risk reduction in the ability to control appropriate cessation of anticoagulation, therefore enabling safe administration of neuraxial blocks if required. 3
Overall, all PA-PE admitted to hospital should have a clearly documented plan in the event of deterioration. The physiological changes which occur during pregnancy warrant the need for more tailored therapy for those presenting with PA-PE. This case report adds to the literature to support catheter-directed thrombolysis/thrombectomy as first-line treatment for massive PA-PE in institutions that have the expertise to do so in a timely manner.
Conclusion
The use of catheter-directed thrombectomy/thrombolysis in PA-PE requires an MDT approach including specialties such as obstetric medicine, MFM, neonatology, haematology, interventional radiology, intensive care, ECMO services, anaesthesia and neonatology. Its use in this niche population may be advantageous due to the ability to avoid the risk of significant haemorrhage/fetal loss when compared to systemic thrombolysis.
Acknowledgements
Not applicable.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This study was approved by Monash Health research support services under NHMRC Ethical Considerations in Quality Assurance and Evaluation Activities (2014) guideline.
Informed consent: Written consent was obtained from the patient for their anonymised information to be published in their article.
Guarantor: SL is the guaranteed author for this paper.
Contributorship: SL, WM and TC wrote the first draft. All authors edited this and approved the final draft.
ORCID iD: Suzanne Luong https://orcid.org/0000-0003-0636-1344
References
- 1.Australian Institute of Health and Welfare. Maternal deaths in Australia [Internet]. Canberra: Australian Institute of Health and Welfare. https://www.aihw.gov.au/reports/mothers-babies/maternal-deaths-in-australia (2020, accessed 7 September 2024). [Google Scholar]
- 2.Rodriguez D, Jerjes-Sanchez C, Fonseca S, et al. Thrombolysis in massive and submassive pulmonary embolism during pregnancy and the puerperium: a systematic review. J Thromb Thrombolysis 2020; 50: 929–941. [DOI] [PubMed] [Google Scholar]
- 3.Society of Obstetric Medicine of Australia and New Zealand. Position statement on pulmonary embolism in pregnancy and post-partum. https://www.somanz.org/content/uploads/2021/06/SOMANZ_PE_Guide_2021-Final-20210622.pdf (2021, accessed 7 September 2024).
- 4.Royal College of Obstetricians and Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: Acute management (green-top 37b). London: RCOG. https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/thrombosis-and-embolism-during-pregnancy-and-the-puerperium-acute-management-green-top-guideline-no-37b/ (2015, accessed 7 September 2024). [Google Scholar]
- 5.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Heart J 2019; 41: 543–603. [DOI] [PubMed] [Google Scholar]
- 6.Makowska A, Treumann T, Venturini Set al. et al. Pulmonary embolism in pregnancy: a review for clinical practitioners. J Clin Med 2024; 13: 2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martillotti G, Boehlen F, Robert-Ebadi H, et al. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemostasis 2017; 15: 1942–1950. [DOI] [PubMed] [Google Scholar]
- 8.Te Raa GD, Ribbert LSM, Snijder RJet al. et al. Treatment options in massive pulmonary embolism during pregnancy: a case-report and review of literature. Thromb Res 2009; 124: 1–5. [DOI] [PubMed] [Google Scholar]
- 9.Arora S, Lahewala S, Patel P, et al. Abstract 15240: catheter-directed thrombolysis versus systemic thrombolysis in pulmonary embolism: predictors of in-hospital mortality and Major bleeding. Circulation 2016; 134: A15240-A. [Google Scholar]
- 10.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011; 123: 1788–1830. [DOI] [PubMed] [Google Scholar]
- 11.Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest 2007; 132: 657–663. [DOI] [PubMed] [Google Scholar]
- 12.Blondon M, Martinez de Tejada B, Glauser F, et al. Management of high-risk pulmonary embolism in pregnancy. Thromb Res 2021; 204: 57–65. [DOI] [PubMed] [Google Scholar]
- 13.O'Shaughnessy F, O'Reilly D, Ní Áinle F. Current opinion and emerging trends on the treatment, diagnosis, and prevention of pregnancy-associated venous thromboembolic disease: a review. Transl Res 2020; 225: 20–32. [DOI] [PubMed] [Google Scholar]