Abstract
Objective.
Positive surgical margins in oral cavity squamous cell carcinoma are associated with cost escalation, treatment intensification, and greater risk of recurrence and mortality. The positive margin rate has been decreasing for cT1-T2 oral cavity cancer over the past 2 decades. We aim to evaluate positive margin rates in cT3-T4 oral cavity cancer over time, and determine factors associated with positive margins.
Study Design.
Retrospective analysis of a national database.
Setting.
National Cancer Database 2004 to 2018.
Methods.
All adult patients diagnosed between 2004 and 2018 who underwent primary curative intent surgery for previously untreated cT3-T4 oral cavity cancer with known margin status were included. Logistic univariable and multivariable regression analyses were performed to identify factors associated with positive margins.
Results.
Among 16,326 patients with cT3 or cT4 oral cavity cancer, positive margins were documented in 2932 patients (18.1%). Later year of treatment was not significantly associated with positive margins (odds ratio [OR] 0.98, 95% confidence interval [CI] 0.96–1.00). The proportion of patients treated at academic centers increased over time (OR 1.02, 95% CI 1.01–1.03). On multivariable analysis, positive margins were significantly associated with hard palate primary, cT4 tumors, advancing N stage, lymphovascular invasion, poorly differentiated histology, and treatment at nonacademic or low-volume centers.
Conclusion.
Despite increased treatment at academic centers for locally advanced oral cavity cancer, there has been no significant decrease in positive margin rates which remains high at 18.1%. Novel techniques for margin planning and assessment may be required to decrease positive margin rates in locally advanced oral cavity cancer.
Keywords: Head and Neck Oncology, National Cancer Database, oral cavity, positive surgical margin, squamous cell carcinoma
Upfront surgical resection remains the preferred treatment for both early and locally advanced oral cavity squamous cell carcinoma (SCC).1 Positive surgical margins identified during primary curative intent surgery of oral cavity cancers are associated with greater risk of recurrence, treatment intensification, mortality, and cost to the health care system.2–4 Despite the importance of negative surgical margins in oral cavity cancer, positive surgical margin rates in oral cavity SCC patients are the highest among all solid malignancies that affect both men and women.2
Two recent studies reported decrease in annual positive margin rates in cT1-T2 oral cavity SCC.5,6 This decrease has been attributed to an increasing number of patients treated at high-volume and academic centers. However, many patients present with locally advanced disease,7 and whether the same margin trend exists among patients with cT3-T4 disease remains unknown.
In the present study, we evaluated trends in positive margin rates in locally advanced oral cavity cancer and examined factors associated with positive margins using the National Cancer Database (NCDB). We hypothesized that, despite improved positive margin rates for cT1-T2 oral cavity SCC, the rates for cT3-T4 disease have remained unchanged.
Methods
This study was approved by the University of Missouri- Columbia Institutional Review Board (IRB #2090785). The NCDB, a nationwide clinical oncology database with data from over 1500 hospitals, was utilized to conduct a retrospective analysis. The 2019 participant user file (PUF) was used. All adult patients diagnosed from 2004 to 2018 who underwent primary curative intent surgery for previously untreated cT3-T4 oral cavity SCC with known margin status were included. Patients in NCDB are staged according to American Joint Commission on Cancer (AJCC) staging guidelines in place at the time of their presentation. Primary site international classification of disease (ICD-10) codes included were: C00.0, C00.1, C00.2, C00.3, C00.4, C00.5, C00.6, C00.8, C00.9, C02.0, C02.1, C02.2 C02.3, C02.5, C02.6, C02.7, C02.8, C02.9, C04.1, C04.8, C04.9, C05.0, C06.0, C06.1, C06.2, C06.8, and C06.9 (Supplemental Table S1, available online).
Histology diagnosis codes of SCC were included: 8051 to 8084 and 8120 to 8131.8 Only patients with invasive carcinoma were included. Only patients undergoing oncologic, specimen-producing surgery as defined by NCDB were included. Exclusion criteria included: patients with preoperative radiotherapy or chemotherapy, unreported margin status, unknown clinical T stage, unknown clinical N stage, and those with cT1 or cT2 disease.
The following patient demographic information was collected: age, sex, race, year of diagnosis, zip code distance from treating facility, zip code median income 2012, zip code percentage of population with no high- school degree 2012, and population setting. The following facility information was collected: geographic region, facility type, and surgical volume as defined by prior studies.6 The following patient clinical information was collected: Charlson comorbidity index, oral cavity cancer subsite, clinical stage, pathologically noted lymphovascular invasion, histologic grade, and margin status. Positive margins are defined by excised tissue with tumor identified in the circumferential ink by the reporting institution.
Descriptive statistics were utilized to describe the patient population and clinical information. Univariable followed by multivariable logistic regression analysis using positive margin as a binary outcome was used to investigate associations between patient and facility characteristics with positive margins. Characteristics with a p < .15 on univariable analysis were included on multivariable analysis. Effects are reported as odds ratios (OR) for categorical variables and OR per year for 2 continuous variables: age and year of diagnosis. Statistical significance was defined as p < .05. All statistical analyses were performed using R statistical software (R project).
Results
Between 2004 and 2018, 16,326 patients with cT3 or cT4 oral cavity SCC met inclusion and exclusion criteria for this study; of those, positive margins were documented in 2932 patients (18.1%). The majority of patients (70.5%) had cT4 oral cavity SCC. The most common anatomic subsite was oral tongue (27.5%) with the next most common gum-alveolar ridge (23.9%). The majority of patients were cN0 (47.2%), had no lymphovascular invasion (LVI) (71.4%), and had moderately differentiated histologic grade (61.4%). Most patients were treated at an academic center (68.8%) within 50 miles of their zip code of residence (71.6%). The clinical and facility characteristics are summarized in Table 1.
Table 1. Clinical Characteristics and Relation to Positive Surgical Margins.
| Characteristic | Total Sample (n= 16,326) | Negative Margins (n = 13,394) | Positive Margins (n = 2,932) | Effect Index (95% CI) | p-value |
|---|---|---|---|---|---|
| Age, yr, mean (SD) | 62.3 (12.7) | 62.6 (12.7) | 63.1 (12.5) | 1.00 (1.00–1.01) | 0.039 |
| Male | 64.6 | 64.4 | 65.7 | 1.06 (0.97–1.15) | 0.189 |
| Race | |||||
| Non-Hispanic White | 77.4 | 77.8 | 75.8 | [Reference] | NA |
| Black | 9.1 | 8.7 | 10.5 | 1.24 (1.08–1.41) | 0.002 |
| Hispanic | 7.1 | 6.9 | 7.9 | 1.17 (1.01–1.36) | 0.038 |
| Asian-Pacific Islander | 4.3 | 4.4 | 3.7 | 0.86 (0.69–1.05) | 0.149 |
| Other-unknown | 2.1 | 2.1 | 2.0 | 0.99 (0.74–1.30) | 0.927 |
| Insurance | |||||
| Private | 33.8 | 34.6 | 30.1 | [Reference] | NA |
| Uninsured | 5.6 | 5.5 | 6.1 | 1.29 (1.07–1.54) | 0.005 |
| Medicare-Medicaid or other government | 59.2 | 58.5 | 62.4 | 1.23 (1.12–1.34) | <0.001 |
| Unknown | 1.4 | 1.4 | 1.3 | 1.10 (0.76–1.55) | 0.591 |
| Median income quartiles | |||||
| <$30,000 | 16.2 | 16.1 | 16.6 | [Reference] | NA |
| $30,000-$34,999 | 20.5 | 20.7 | 19.7 | 0.92 (0.80–1.06) | 0.255 |
| $35,000-$45,999 | 28.8 | 28.6 | 30.0 | 1.01 (0.89–1.16) | 0.839 |
| $46,000 | 34.5 | 34.7 | 33.7 | 0.94 (0.83–1.07) | 0.321 |
| Education level of zip code | |||||
| 29% no HSD | 19.8 | 19.8 | 19.8 | [Reference] | NA |
| 20%−28.9% no HSD | 26.0 | 26.0 | 25.7 | 0.99 (0.87–1.12) | 0.873 |
| 14%−19.9% no HSD | 24.8 | 24.8 | 24.8 | 1.00 (0.88–1.14) | 0.982 |
| <14% no HSD | 29.4 | 29.3 | 29.7 | 1.01 (0.90–1.15) | 0.833 |
| Charlson Comorbidity Index | |||||
| 0 | 72.1 | 72.3 | 71.1 | [Reference] | NA |
| 1 | 19.8 | 19.7 | 20.3 | 1.05 (0.95–1.16) | 0.366 |
| 2 | 5.1 | 5.0 | 5.4 | 1.09 (0.91–1.30) | 0.36 |
| 3+ | 3.0 | 3.0 | 3.3 | 1.13 (0.90–1.41) | 0.291 |
| Setting | |||||
| Metropolitan | 77.8 | 77.7 | 78.4 | [Reference] | NA |
| Urban | 19.7 | 19.8 | 18.9 | 0.94 (0.85–1.05) | 0.282 |
| Rural | 2.5 | 2.4 | 2.7 | 1.11 (0.86–1.42) | 0.42 |
| Year of diagnosis | 0.99 (0.98–1.00) | 0.113 | |||
| 2004 | 3.0 | 3.1 | 2.8 | ||
| 2005 | 3.0 | 2.9 | 3.1 | ||
| 2006 | 3.5 | 3.4 | 4.1 | ||
| 2007 | 4.1 | 4.1 | 4.3 | ||
| 2008 | 5.3 | 5.3 | 5.4 | ||
| 2009 | 6.0 | 6.0 | 6.1 | ||
| 2010 | 6.4 | 6.4 | 6.3 | ||
| 2011 | 6.6 | 6.7 | 6.3 | ||
| 2012 | 7.1 | 7.0 | 7.8 | ||
| 2013 | 7.8 | 7.5 | 8.8 | ||
| 2014 | 8.2 | 8.3 | 8.2 | ||
| 2015 | 8.9 | 9.0 | 8.1 | ||
| 2016 | 9.2 | 9.3 | 8.7 | ||
| 2017 | 9.5 | 9.5 | 9.2 | ||
| 2018 | 11.4 | 11.5 | 11.0 | ||
| Subsite | |||||
| Oral tongue | 27.5 | 28.4 | 23.5 | [Reference] | NA |
| Lip | 2.1 | 2.3 | 1.4 | 0.75 (0.53–1.03) | 0.091 |
| Gum-alveolar ridge | 23.6 | 23.9 | 22.4 | 1.13 (1.01–1.27) | 0.038 |
| Floor of mouth | 20.2 | 19.8 | 22.1 | 1.34 (1.19–1.51) | <0.001 |
| Hard palate | 4.3 | 3.8 | 6.2 | 1.96 (1.62–2.36) | <0.001 |
| Other * | 22.3 | 21.8 | 24.3 | 1.34 (1.20–1.51) | <0.001 |
| Clinical T category | |||||
| cT3 | 29.5 | 30.8 | 23.5 | [Reference] | NA |
| cT4 | 70.5 | 69.2 | 76.5 | 1.46 (1.33–1.60) | <0.001 |
| Clinical N category | |||||
| cN0 | 47.2 | 48.5 | 41.2 | [Reference] | NA |
| cN1 | 16.6 | 16.5 | 17.2 | 1.23 (1.09–1.37) | <0.001 |
| cN2 | 35.0 | 33.9 | 39.9 | 1.39 (1.27–1.52) | <0.001 |
| cN3 | 1.2 | 1.1 | 1.7 | 1.80 (1.29–2.48) | <0.001 |
| Lymphovascular invasion | |||||
| Negative | 71.4 | 73.7 | 60.8 | [Reference] | NA |
| Positive | 28.6 | 26.3 | 39.2 | 1.81 (1.63–1.99) | <0.001 |
| Histologic grade | |||||
| Well differentiated | 18.2 | 19.4 | 13.0 | [Reference] | NA |
| Moderately differentiated | 61.4 | 61.3 | 61.8 | 1.50 (1.32–1.71) | <0.001 |
| Poorly differentiated-Undifferentiated | 20.4 | 19.3 | 25.2 | 1.94 (1.68–2.25) | <0.001 |
| Distance to treatment center | |||||
| <=50 miles | 71.6 | 71.0 | 74.4 | [Reference] | NA |
| >50 miles | 28.4 | 29.0 | 25.6 | 0.84 (0.77–0.93) | <0.001 |
| Region | |||||
| South Atlantic | 22.5 | 22.7 | 21.5 | [Reference] | NA |
| New England | 4.3 | 4.2 | 5.0 | 1.27 (1.04–1.56) | 0.02 |
| Middle Atlantic | 14.0 | 14.3 | 13.0 | 0.96 (0.84–1.11) | 0.604 |
| East North Central | 19.0 | 19.3 | 17.9 | 0.98 (0.86–1.11) | 0.755 |
| East South Central | 8.4 | 8.3 | 8.5 | 1.08 (0.91–1.27) | 0.363 |
| West North Central | 9.2 | 9.2 | 9.1 | 1.06 (0.89–1.23) | 0.543 |
| West South Central | 8.0 | 7.9 | 8.5 | 1.14 (0.97–1.34) | 0.117 |
| Mountain | 3.8 | 3.7 | 4.2 | 1.21 (0.97–1.50) | 0.084 |
| Pacific | 10.9 | 10.5 | 12.3 | 1.23 (1.07–1.43) | 0.005 |
| Academic center | |||||
| No | 31.2 | 30.2 | 35.6 | [Reference] | NA |
| Yes | 68.8 | 69.8 | 64.4 | 0.78 (0.72–0.85) | <0.001 |
| Treatment center volume | |||||
| Low (<=20 cases/yr) | 59.3 | 58.0 | 65.3 | [Reference] | NA |
| High (>20 cases/yr) | 40.7 | 42.0 | 34.7 | 0.73 (0.67–0.79) | <0.001 |
The “other” category for “subsite” includes hard palate, retromolar trigone, and mouth (overlapping or no otherwise specified)
Unadjusted rates of positive margins per study year are shown in Figure 1. Positive margin rates from 2004 to 2018 had an undulating pattern; starting at 16.5% in 2004, rising to 20.7% and 20.3% in 2006 and 2013 respectively, and falling to 17.3% in 2018 (OR 0.98 91% CI 0.95–1.00, p = .11). The odds-adjusted rates of treatment at an academic center over the study period are shown in Figure 2. Patients were more likely to be treated at academic centers over the course of the study period (OR 1.02, 95% CI 1.01–1.03, p < .001).
Figure 1.
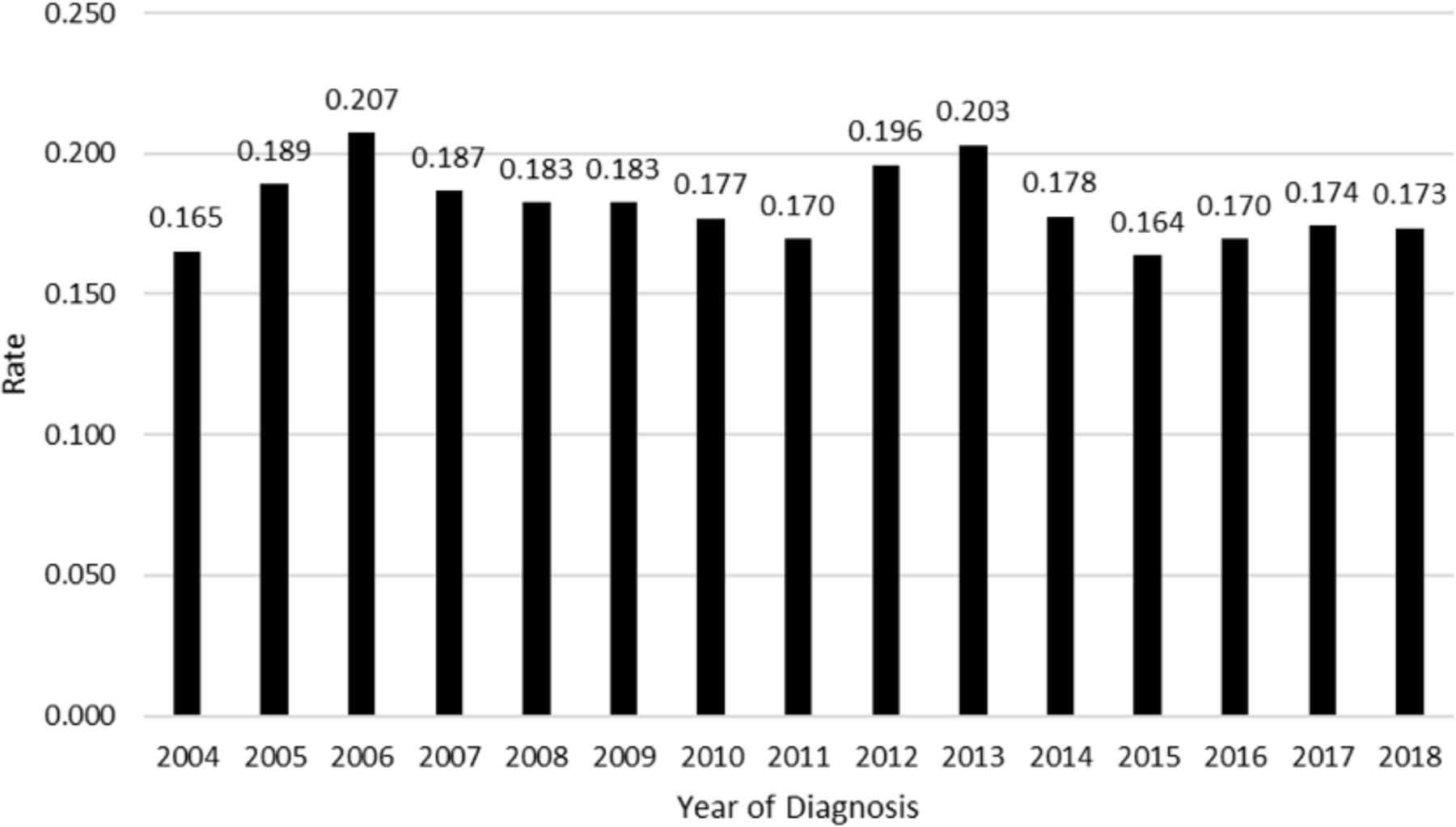
Unadjusted rate of positive margins for cT3-T4 oral cavity squamous cell carcinoma (SCC). Risk of positive surgical margins in oral cavity SCC 2004 to 2018 patients as a percentage.
Figure 2.
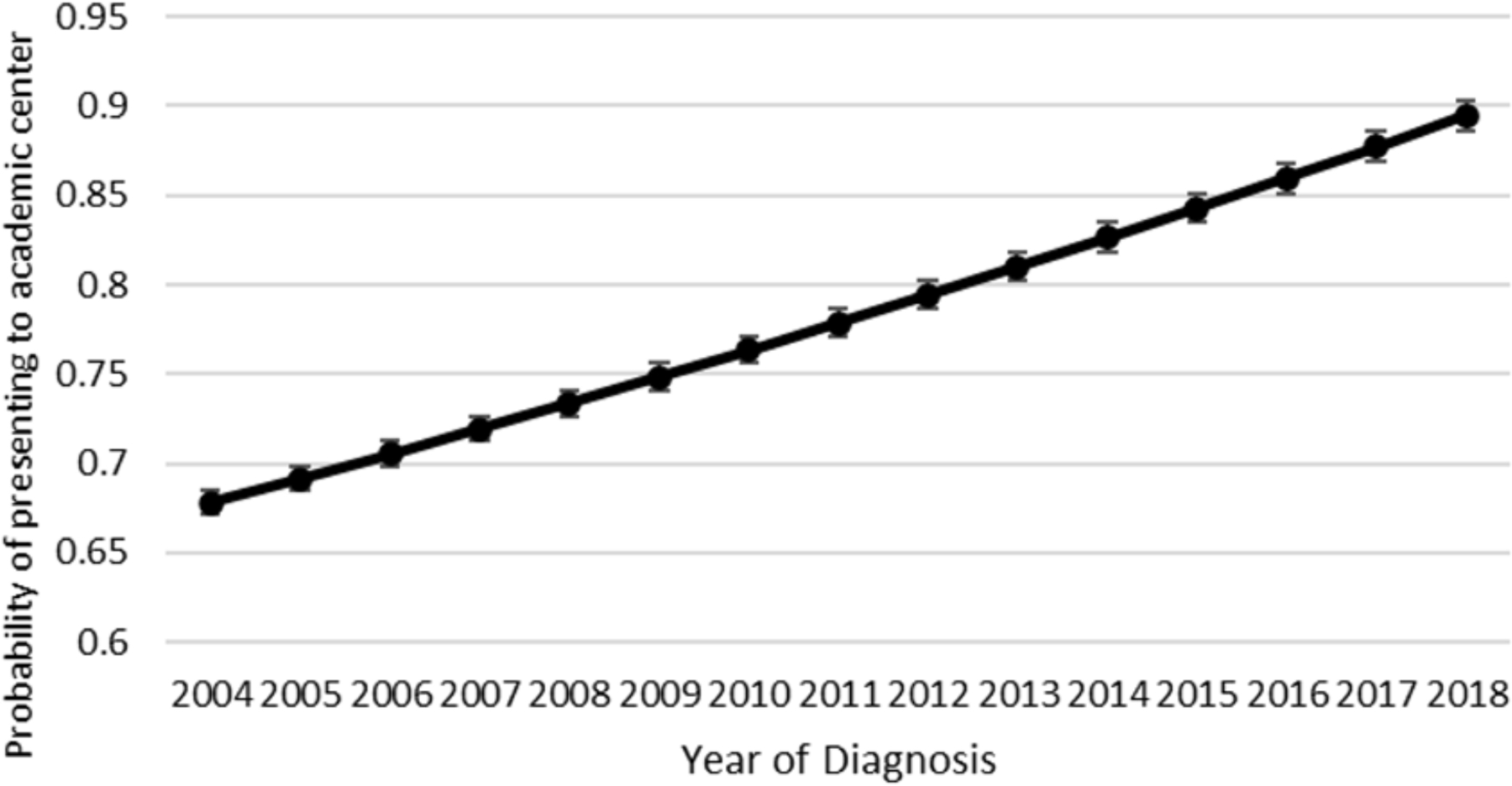
Univariable adjusted probability of treatment at an academic center. Probability of patients with oral squamous cell carcinoma (cT3, cT4) who present to academic centers for treatment versus nonacademic centers over time.
Multivariable logistic regression analysis to explore the associations between clinical and facility characteristics with positive surgical margins is shown in Table 2. There was no statistically significant decrease in positive margin status associated with year of diagnosis (OR 0.98, 95% CI 0.95–1.00, p = .11). However, positive margins were significantly associated with cT4 tumors (OR 1.40, 95% CI 1.22–1.61, p < .001), hard palate primary tumors (OR 1.96, 95% CI 1.51–2.54, p < .001), advancing clinical N stage (for cN2, OR 1.29, 95% CI 1.14–1.46, p < .001), LVI (OR 1.62, 95% CI 1.44–1.81, p < .001), and moderate- poorly differentiated histology (OR 1.99, 95% CI 1.62–2.47, p < .001). Positive margins were also associated with “other” oral cavity subsite (OR 1.27, 95% CI 1.08–1.50, p = .003) which includes cheek, vestibule, retromolar trigone, and overlapping or not otherwise specified areas of the mouth. Additionally, treatment at an academic center (OR 0.86, 95% CI 0.76–0.98, p = .022), as well as high volume centers (OR 0.79, 95% CI 0.70–0.90, p < .001), were associated with a decreased likelihood of positive margins. Odds ratios for all factors examined on multivariable analysis are presented in Figure 3.
Table 2. Multivariable Analysis of Positive Surgical Margin Association.
| Characteristic | Effect Index (95% CI) | p-value |
|---|---|---|
| Age, yr, mean (SD) | 1.00 (0.99–1.00) | 0.581 |
| Race | ||
| Non-Hispanic White | [Reference] | NA |
| Black | 1.18 (0.98–1.41) | 0.076 |
| Hispanic | 1.08 (0.86–1.37) | 0.488 |
| Asian-Pacific Islander | 0.93 (0.70–1.21) | 0.588 |
| Other-unknown | 0.97 (0.67–1.37) | 0.848 |
| Insurance | ||
| Private | [Reference] | NA |
| Uninsured | 1.08 (0.84–1.38) | 0.521 |
| Medicare-Medicaid or other government | 1.23 (1.08–1.41) | 0.002 |
| Unknown | 1.17 (0.71–1.85) | 0.521 |
| Year of diagnosis | 0.98 (0.95–1.00) | 0.11 |
| Subsite | ||
| Oral tongue | [Reference] | NA |
| Lip | 1.02 (0.63–1.57) | 0.939 |
| Gum-alveolar ridge | 1.14 (0.96–1.36) | 0.145 |
| Floor of mouth | 1.10 (0.93–1.30) | 0.287 |
| Hard palate | 1.98 (1.52–2.56) | <0.001 |
| Other * | 1.28 (1.09–1.51) | 0.003 |
| Clinical T category | ||
| cT3 | [Reference] | NA |
| cT4 | 1.39 (1.21–1.60) | <0.001 |
| Clinical N category | ||
| cN0 | [Reference] | NA |
| cN1 | 1.20 (1.03–1.40) | 0.021 |
| cN2 | 1.28 (1.13–1.45) | <0.001 |
| cN3 | 1.58 (0.98–2.48) | 0.05 |
| Lymphovascular invasion | ||
| Negative | [Reference] | NA |
| Positive | 1.60 (1.42–1.80) | <0.001 |
| Histologic grade | ||
| Well differentiated | [Reference] | NA |
| Moderately differentiated | 1.69 (1.41–2.04) | <0.001 |
| Poorly differentiated-Undifferentiated | 2.00 (1.63–2.48) | <0.001 |
| Distance to treatment center | ||
| <=50 miles | [Reference] | NA |
| >50 miles | 0.94 (0.82–1.06) | 0.319 |
| Academic center | ||
| No | [Reference] | NA |
| Yes | 0.86 (0.76–0.98) | 0.022 |
| Treatment center volume | ||
| Low (<=20 cases/yr) | [Reference] | NA |
| High (>=20 cases/yr) | 0.73 (0.67–0.79) | <0.001 |
The “other” category for “subsite” includes hard palate, retromolar trigone, and mouth (overlapping or no otherwise specified)
Figure 3.
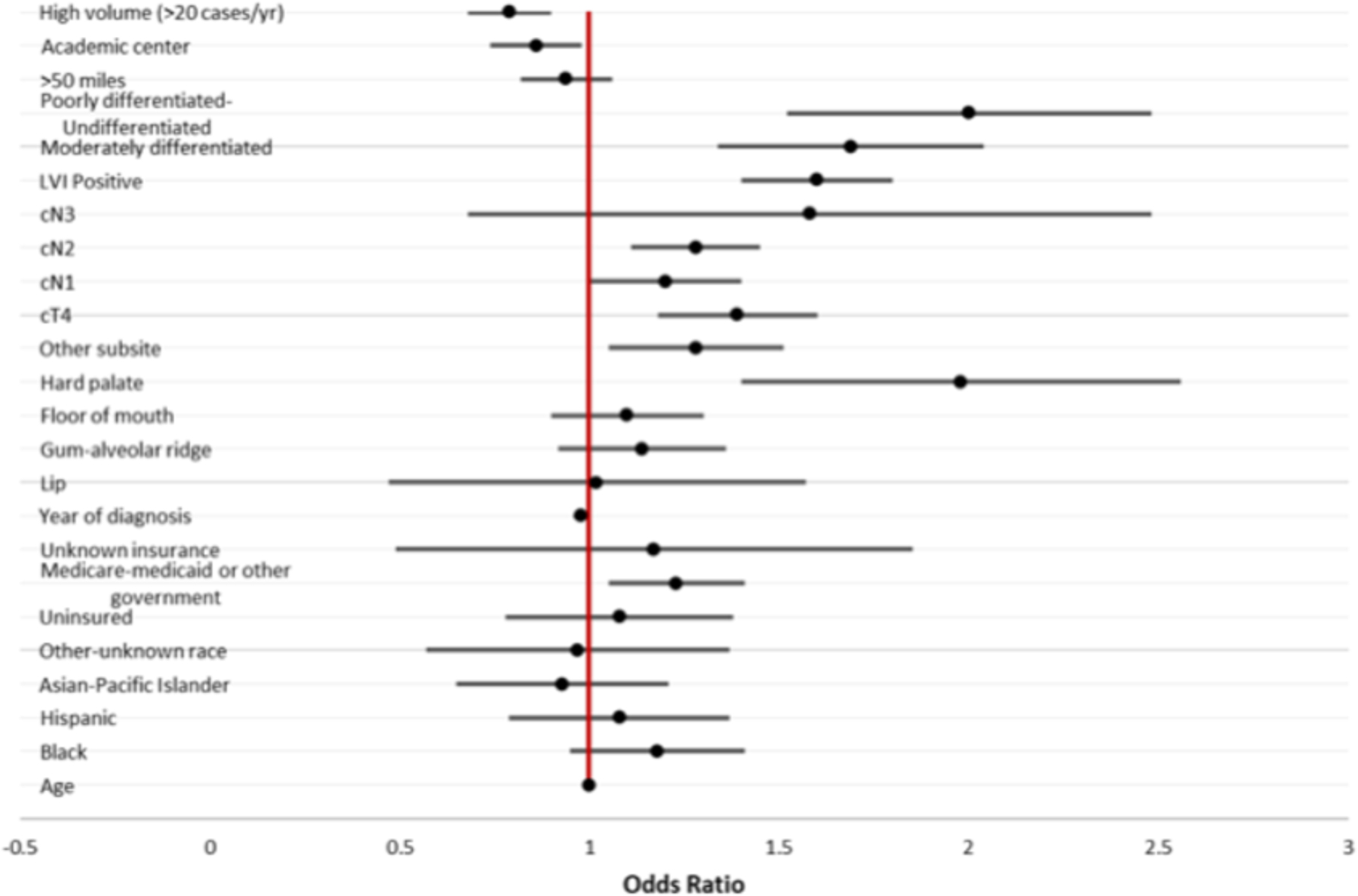
Multivariable logistic regression of factors associated with positive surgical margins in oral cavity squamous cell carcinoma 2004 to 2018. Variables were retained if p < .15 on univariable logistic regression.
Discussion
In this NCDB study including patients from 2004 to 2018, the rate of positive surgical margins for cT3-T4 oral cavity SCC was 18.1%. There was no significant improvement in positive margin rates over the study period despite an increase in patients being treated at academic facilities. Surgical subsite, T and N stage, lymphovascular invasion, histologic grade, Medicare-Medicaid or other government insurance, and treatment at a nonacademic or low-volume center were identified as factors associated with positive surgical margins.
Robinson et al, who performed a NCDB analysis of patients with cT1-T2 disease, found the overall positive margin rate to be 7.9%.5 They found the annual positive margin rate for early-stage disease to be decreasing from 2004 to 2016. These investigators attributed this, in part, to the fact that more patients were receiving treatment at academic centers. We appreciated a similar increase in the likelihood of patients with cT3-T4 disease presenting at academic centers, from 67% to 89% across the study period, yet the positive margin rate remained unchanged. However, on multivariable analysis of positive surgical margin association. Treatment at an academic center (OR 0.86; 0.76–0.98) and treatment at high volume center (OR 0.79; 0.70–0.90) was associ in a lower likelihood of positive surgical margins. Our data suggest that treatment of locally advanced tumors at high-volume academic centers may lower the risk for positive surgical margins.
Characteristics of more advanced disease such as later T and N stage, lymphovascular invasion, and poorly differentiated histology, were associated with positive surgical margins (Table 2). Previous studies identified these factors to be similarly associated with positive margins in cT1-T2 disease.5,6 In addition, the odds of a positive surgical margin were nearly 2 times greater for tumors with a hard palate primary compared to those of the oral tongue. A similar increase in odds of positive margin for hard palate tumors was appreciated in cT1-T2 disease and was attributed to inability to perform frozen section analysis on bony portions of resection specimens.5 Worsening histopathologic characteristics, such as poorly differentiated histology and lymphovascular invasion, may increase the likelihood that microscopic disease extends beyond visible or palpable tumor margins. Surgeons resecting hard palate and advanced-stage tumors may need a more aggressive approach to achieve clear surgical margins.
Final margin status may be impacted by margin-sampling technique. In a specimen-based approach, the surgeon will bring the resected tumor to the pathologist and help orient and identify anatomical areas of concern. This may allow for more targeted margin analysis and improve communication between surgeon and pathologist regarding margin sampling sites. One prospective randomized trial found that the specimen-based approach decreased the likelihood of positive margins compared to the tumor-bed, defect-driven approach.9 In addition, Maxwell et al determined that the specimen-driven approach was associated with improved locoregional control.10 Though the NCDB does not include data on approach to margin analysis, future prospective work should continue to investigate the differences in positive margins rates for cT3-T4 diseases based on approach to margin analysis.
Our findings highlight the need for a continued emphasis on advanced surgical techniques, innovation, and technology to improve rates of positive surgical margin in oral cavity cancer. Increasing access to reconstructive surgeons may influence margin rates as patients who underwent flap reconstruction have been found to have decreased positive margins. Intraoperative adjuncts may also help to ensure negative margins. Intraoperative ultrasound can aid in determining the amount of invasion by unmasking differences in tissue density that may not be visible or palpable to the surgeon.11 Molecular markers and fluorescence-guided surgery have the ability to highlight seemingly normal cells that may have malignant potential.12–14 Though data on how these adjuncts impact patient prognosis is lacking, one randomized trial found that fluorescence visualization-guided surgery did not improve local control rates in patients with early stage oral cavity cancers.15 Additionally, neoadjuvant immunotherapy may decrease tumor size resulting in downstaging and a lower likelihood of positive margins, though the data is limited.16 Further studies are necessary to determine impact on intraoperative adjuncts and neoadjuvant therapies on positive margin rates and survival outcomes.
Limitations
The use of a national database is beneficial in terms of large sample size and generalizability, but also has limitations. As individual institutions submit their own data, there is an inherent limitation in its quality and consistency. We are also limited to the variables recorded by the NCDB and could not account for confounders that were not included. We are unable to differentiate between tumor bed margins and specimen-based margins. We are limited by the lack of granularity in margin reporting in the NCDB. The NCDB also does not disclose region data for patients under 40 in order to maintain confidentiality. Additionally, given the high variability of government insurance, we chose to exclude it from our multivariate analysis. Overall, it is unlikely that these limitations would significantly impact our findings.
Conclusions
Over the past 2 decades, despite an increased proportion of oral cavity SCC patients being treated at academic centers, the annual rate of positive surgical margins remains unchanged. Advanced T and N staging, worse histologic grade, hard palate primaries, and treatment at a nonacademic or low-volume center were independent predictors of positive surgical margins. Novel approaches to the care of the locally advanced oral cavity SCC patient such as intraoperative adjuncts may be required to decrease the rate of positive surgical margins in this patient population.
Supplementary Material
Footnotes
This article was presented at the 2023 Triological Society Combine Sections Meeting; January 26–28, 2023; Coronado, California.
Disclosures
Competing interests: None.
Supplemental Material
Additional supporting information is available in the online version of the article.
References
- 1.ACS. Treatment Options for Oral Cavity Cancer by Stage. 2021. Accessed March 23, 2021. https://www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/treating/by-stage.html2022 [Google Scholar]
- 2.Orosco RK, Tapia VJ, Califano JA, et al. Positive surgical margins in the 10 most common solid cancers. Sci Rep 2018;8(1):5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchakjian MR, Ginader T, Tasche KK, Pagedar NA, Smith BJ, Sperry SM. Independent predictors of prognosis based on oral cavity squamous cell carcinoma surgical margins. Otolaryngol Head Neck Surg 2018;159(4):675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loree TR, Strong EW. Significance of positive margins in oral cavity squamous carcinoma. Am J Surg 1990;160(4):410–414. [DOI] [PubMed] [Google Scholar]
- 5.Robinson EM, Lam AS, Solomon I, et al. Trends in positive surgical margins in cT1-T2 oral cavity squamous cell carcinoma. Laryngoscope. 2022;132(10):1962–1970. doi: 10.1002/lary.30033 [DOI] [PubMed] [Google Scholar]
- 6.Luryi AL, Chen MM, Mehra S, Roman SA, Sosa JA, Judson BL. Positive surgical margins in early stage oral cavity cancer: an analysis of 20,602 cases. Otolaryngol Head Neck Surg 2014;151(6):984–990. [DOI] [PubMed] [Google Scholar]
- 7.Gao W, Guo C-B. Factors related to delay in diagnosis of oral squamous cell carcinoma. J Oral Maxillofac Surg 2009; 67(5):1015–1020. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. International Classification of Diseases for Oncology (ICD-O)–3rd edition, 1st revision. WHO; 2013. [Google Scholar]
- 9.Amit M, Na’ara S, Leider-Trejo L, et al. Improving the rate of negative margins after surgery for oral cavity squamous cell carcinoma: a prospective randomized controlled study. Head Neck. 2016;38(S1):E1803–E1809. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell JH, Thompson LD, Brandwein-Gensler MS, et al. Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg 2015;141(12):1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarabichi O, Bulbul MG, Kanumuri VV, et al. Utility of intraoral ultrasound in managing oral tongue squamous cell carcinoma: systematic review. Laryngoscope. 2019;129(3):662–670. [DOI] [PubMed] [Google Scholar]
- 12.Lee YJ, Krishnan G, Nishio N, et al. Intraoperative fluorescence-guided surgery in head and neck squamous cell carcinoma. Laryngoscope. 2021;131(3):529–534. [DOI] [PubMed] [Google Scholar]
- 13.Marsden M, Weyers BW, Bec J, et al. Intraoperative margin assessment in oral and oropharyngeal cancer using label-free fluorescence lifetime imaging and machine learning. IEEE Trans Biomed Eng 2021;68(3):857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weyers BW, Marsden M, Sun T, et al. Fluorescence lifetime imaging for intraoperative cancer delineation in transoral robotic surgery. Transl Biophotonics. 2019;1(1–2):e201900017. doi: 10.1002/tbio.201900017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durham JS, Brasher P, Anderson DW, et al. Effect of fluorescence visualization-guided surgery on local recurrence of oral squamous cell carcinoma: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg 2020; 146(12):1149–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenfeld JD, Hanna GJ, Jo VY, et al. Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol 2020;6(10):1563–1570. doi: 10.1001/jamaoncol.2020.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


