Abstract
Background:
Morphological and histological examination of the testes can provide a suitable insight into the health of the reproductive system.
Aim:
The objective of the current study was to investigate the morphological and histological features of the testes of local pigeons (Columba livia domestica) at mature and immature stages of age.
Methods:
Two groups of collected specimens underwent macroscopic and microscopic investigation to evaluate and compare the main general properties of their testes.
Results:
The findings indicated that the testis has an oval shape in both pre-puberty and post-puberty stages, situated on the inner side of the kidney towards the caudal extreme of the lungs. However, the left testis was bigger than those on the right side. In the pre-puberty stage group, the testicular parenchyma was small, and almost collapsed seminiferous tubules containing a single layer of Spermatogonia and Sertoli cells. In contrast, in the post-puberty stage, the parenchyma space between seminiferous tubules was small, and tubules adhered closely to each other. Also, mature cells including sertoli, spermatogonia, and spermatocytes were noticed to spread within the tubules.
Conclusion:
The change in the histological structure of testes before and after maturity may help to evaluate the complexity of the male reproductive system of pigeons and draw attention to the organization of sex hormones and the function of several types of cells within the testes.
Keywords: Testes, Histology, Reproductive cells, Morphology
Introduction
In birds, the reproductive system is generally situated internally, encouraging the notable interest of researchers to investigate the histological and morphological characteristics of bird testes. Many studies have delved into these features throughout numerous bird species (Bull et al., 2007; Blesbois, 2018; Tamilselvan and Singh, 2020). It has been proven there is a clear relationship between the age of the body and the size of the testis across different avian breeds (Tingari and Lake, 1972; Aire, 1982). A recent study (Abd Alkhazraji et al., 2022) observed that the left testicle was longer than the right testicle in domestic fowl, with both testes having an oval shape. Testicles are present in most vertebrates enclosed within a protective tissue capsule that facilitates the entry and exit of blood arteries and nerves to and from the testicles (Razi et al., 2010; Gartner and Hiatt., 2014). Two primary factors that can influence alterations in bird testicular tissue are age and season, thus, the testes undergo significant changes in size annually in many bird species, leading scientists to consider them as one of the most flexible organs in terms of physiology and anatomy among adult vertebrates (Leska et al., 2012; Islam et al., 2013; Gerzilov et al., 2016). However, the morphological and histological characteristics of the pigeon testis at different ages are still poorly understood. Their unique differentiations of the testis can make them a good model for conducting comparative studies on the evolution of vertebrate reproduction. The architecture of the testes seems to be the most important feature in understanding the development of the male reproductive system. Thus, the success of reproductive efficiency in males is closely associated with several factors such as sperm morphology, sperm number, and the time it takes for sperm to mature.
Testes of birds consist of two primary components, the stroma and parenchyma. The stroma contains the capsule that provides support to the testes. It is worth noting that bird testes do not have septula testes that present in mammals which are barriers within the testes that separate them into lobules. Instead, it contains seminiferous tubules or testicular tubes that are responsible for sperm production in birds. The parenchyma of bird testes is the site for creating the interstitial cells and seminiferous tubules. Within the seminiferous tubules, two types of cells can be observed including germ cells and sertoli cells. Sertoli cells play a main role in supporting and nourishing the developing germ cells during the process of spermatogenesis. On the other hand, germ cells, undergo various divisions and differentiations to form mature spermatozoa (Ventela et al., 2003; Trefil et al., 2006; Mustafa et al., 2021).
To the best of our knowledge, there are no previous studies describing the shape and structure of Iraqi pigeon testes, thus, this study aimed to investigate the morphological and histological of the testes in two different ages including pre-puberty and post-puberty of Iraqi pigeons (Columba livia).
Material and Methods
Sixteen pigeons were procured from a reputable pigeon breeder in Fallujah City, carefully selected based on their ages to showcase the structural differences in testes at various stages of birds development. The pigeons were divided into two groups:
Group 1: Eight adult and healthy Iraqi local birds, weighing an average of 337 ± 3.47 g and aged between 24 and 26 weeks.
Group 2: Eight healthy Iraqi local birds, weighing an average of 243 ± 5.07 g and aged between 3–5 weeks.
Each group underwent a thorough examination both grossly and microscopically to study the testis structure.
The pigeons were administered a combination of high-dose xylazine and ketamine as anesthetic agents in order to euthanize them. Following euthanasia, laparotomy was performed to gain careful access to the testes. The testes were thoroughly inspected in their natural position before being dissected for anatomical measurements.
After removal from the abdomen, the testes were examined under a microscope. Additionally, small samples from each organ were then fixed in 10% formalin for 2 days. The fixed samples were then processed using the paraffin technique, which involved obtaining thin slices (5 μm). To determine the histological composition of the testes, all sections were stained with hematoxyLeska et al., 2012 eosin and subsequently analyzed under a microscope, following the guidelines outlined by Suvarna et al. (2013). The histomorphometric analysis was performed using digital microscopy (Optica).
The collected data were exhibited as mean ± standard error (SE) for statistical analysis. The analysis of difference (AEcho) test has been utilized, with the assistance of SPSS software (version 15), to identify any significant differences.
Ethical approval
This study has received approval from the Research Ethics Committee at the College of Veterinary Medicine, University of Falluja.
Results
Based on the conducted research, it was observed that the left and right testes of local Iraqi pigeons (Columba livia) at two different ages exhibited an oval shape. These organs were located adjacent to the medial side of the kidneys towards the caudal ends of the lungs, spanning both lateral sides of the midline of the body (Fig. 1).
Fig. 1. Macroscopic photo of Iraqi local pigeons shows: the left testis (Blue arrow), right testis (Yellow arrow), right kidney (Blue star), and left kidney (Yellow star).
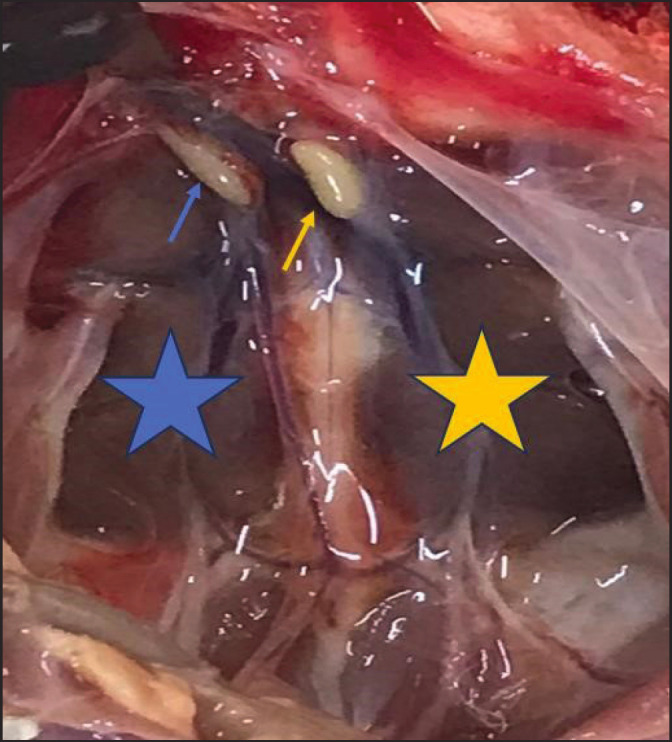
Generally, both the left and right testes in both age groups were located on opposite sides of the median plane. However, they displayed noticeable differences in size. Specifically, the left testis appeared consistently larger than the right testis across both age groups (Figs. 1 and 2).
Macroscopic measurements of the testes at both ages further supported these findings. The measurements revealed that the testis on the left side was consistently larger than the testis that lie on the right side (Tables 1 and 2). Statistical analysis indicated a significant increase at a level of p ≤ 0.05 in the width, length, and weight of the testis on the left side compared to those on the right side.
Table 1. Macro morphometric data for the testis of local Iraqi pigeons during pre-puberty.
| Parameters | Left side (Mean ± SE) | Right side (Mean ± SE) |
|---|---|---|
| Testis length (mm) | 8.24 ± 0.32* | 7.27 ± 0.53 |
| Testis wide (mm) | 5.76 ± 0.51* | 4.39 ± 0.29 |
| Testis height (mm) | 4.34 ± 0.18 | 3.85 ± 0.15 |
| Testis weight (g) | 4.64 ± 0.19* | 2.93 ± 0.18 |
(٭) denote significant differences at level p ≤ 0.05 between the right and left.
The histological study finding of this study on the testes of local Iraqi pigeons (C. livia) at pre-puberty and post-puberty stages showed that these testes consist of the stroma and the parenchyma.
The stroma of pigeon testis in the current study was found composed of the interstitial connective tissue and capsule. However, the septula testes were absent in the testes of pigeons at the two ages examined. Also, the parenchyma within the testes was formed of the seminiferous tubules in addition to interstitial cells.
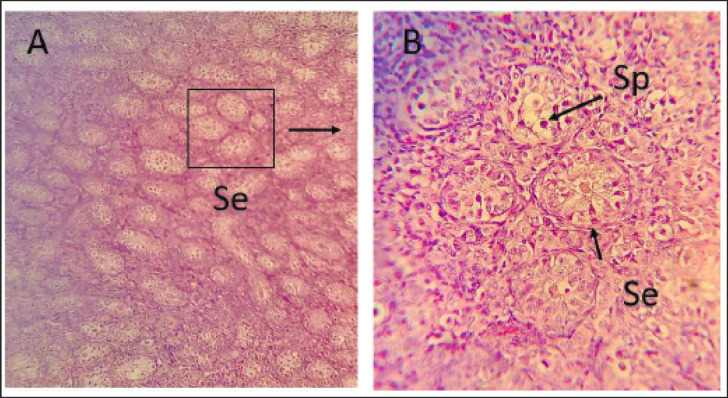
In the pre-puberty age group, distinct signs of regression were observed in the testicular parenchyma. The seminiferous tubules appeared small and many of them collapsed. These tubules were predominantly rounded in shape and comprised one layer of spermatogonia (types A and B) and sertoli cells. The space between the seminiferous tubules, known as the parenchyma space, was relatively large (Fig. 3).
Fig. 3. Photomicrographs of testis of local Iraqi pigeon during pre-puberty showing seminiferous tubule with few germs cells generation (Se), Spermatogonia (Sp), Interstitial cell (Red arrow). (H and E stain 10X (A) 40X (B).
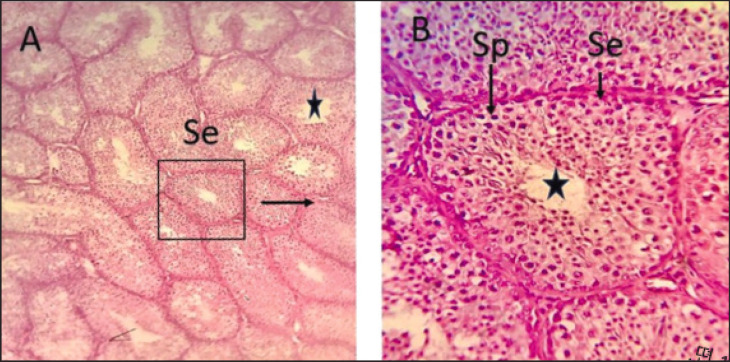
The tunica albuginea was a thin connective tissue layer that was primarily composed of collagen fibers surrounding the testes. It also contains elastic fibers that are distributed in different directions. Furthermore, numerous smooth muscle fibers were observed, particularly in the inner region of the tunica albuginea (Figs. 4 and 5).
Fig. 4. Photomicrographs of testis of local Iraqi pigeon during post-puberty showing seminiferous tubule with germ cells generation (Se), Spermatogonia (Sp), lumen (black star). (H & E stain 10X (A) 40X (B).
Significantly, this study revealed a notable increase at a level of p ≤ 0.05 in the left testicle compared to the right testicle during both age groups. This difference is presented in Tables 3 and 4.
Table 3. Micro morphometric data for the testes of local Iraqi pigeons during pre-puberty.
| Parameters | Left side (Mean ± SE) | Right side (Mean ± SE) |
|---|---|---|
| Seminiferous tubule | 220.58 ± 1.54* | 211.83 ± 1.74 |
| Spermatogonia | 3.85 ± 0.24* | 3.64 ± 0.16 |
| Spermatogonia nuclei | 3.56 ± 0.23 | 3.48 ± 0.13 |
| Sertoli cells | 6.02 ± 0.12 | 6.23 ± 0.12 |
| Interstitial cells | 3.57 ± 0.63 | 3.26 ± 0.28 |
| Interstitial nuclei | 2.36 ± 0.36 | 2.11 ± 0.11 |
| Capsule thickness | 10.47 ± 1.12* | 8.85± 0.74 |
(٭) denote significant differences at level p ≤ 0.05 between the right and left.
In contrast, the post-puberty age group showed a different pattern. The majority of seminiferous tubules in the testicular parenchyma were densely packed and exhibited a convoluted arrangement. There was very little interstitial space between these tubules, which contained interstitial cells. Additionally, both sertoli and spermatogenic cells were observed within the seminiferous tubules (Fig. 4). In the seminiferous tubules, a variety of spermatogenic cell types were observed, including primary and secondary spermatocytes, spermatogonia, spermatids, and mature sperm cells, specifically, the spermatogonia were located within the tubules at their basement membrane.
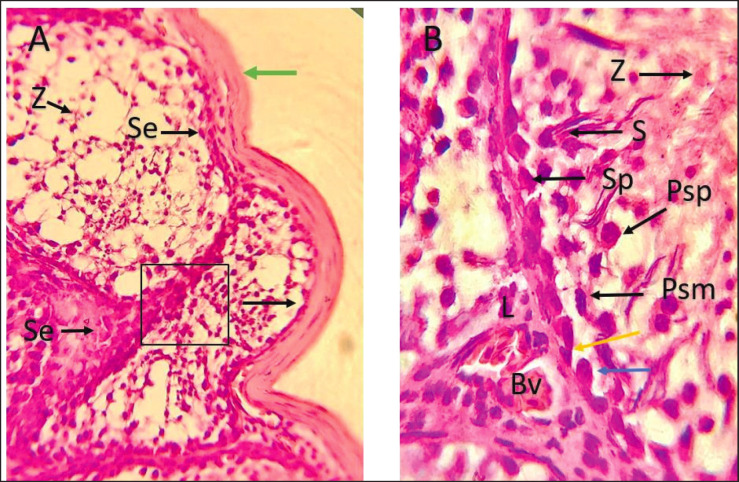
Depending on the morphological features of the cytoplasm and nucleus, two distinct types of spermatogonia were identified including type A (SpA) and type B (SpB). Moreover, spermatocytes were noticed diffused in the middle region of seminiferous tubules in various meiotic phases such as prophase and metaphase. Primary spermatocytes were the largest among the spermatogenic cells, while the secondary spermatocytes moved closer to the inner surface of the seminiferous tubules occupying a more advanced site and being smaller in size compared to primary spermatocytes. This information is illustrated in Figure 5.
Fig. 2. Macroscopic photo of Iraqi local pigeons shows: left testis (black arrow), right testis (blue arrow), and ileum (I).
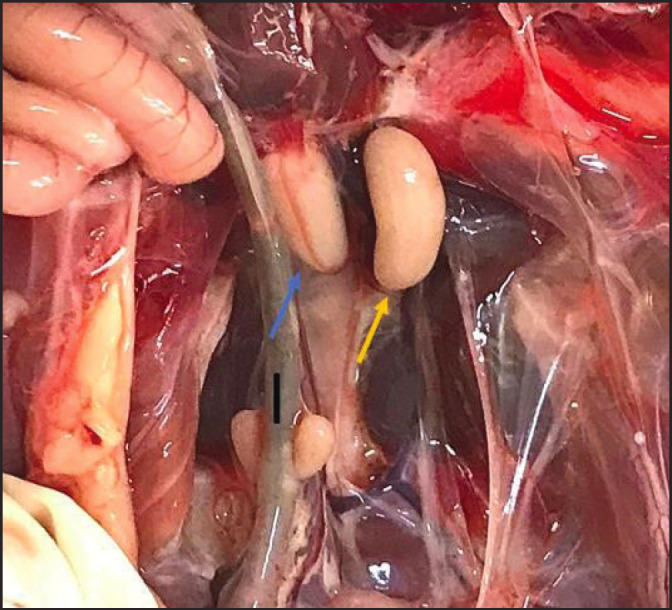
Table 2. Macro morphometric data for the testis of local Iraqi pigeons during post-puberty.
| Parameters | Left side (Mean ± SE) | Right side (Mean ± SE) |
|---|---|---|
| Testis length (mm) | 17.53 ± 0.74* | 14.32 ± 0.63 |
| Testis wide (mm) | 10.75 ± 0.42* | 9.57 ± 0.29 |
| Testis height (mm) | 8.97 ± 0.63 | 7.18 ± 0.15 |
| Testis weight (g) | 8.89 ± 0.18 | 6.45 ± 0.13 |
(٭) denote significant differences at level p ≤ 0.05 between the right and left.
The findings indicated that in the pre-pubertal stage of C. livia, there was an expansion of the interstitial space. Numerous interstitial cells were observed, and within the interstitial regions comprising collagenous connective tissue, Leydig cells were dispersed. The leydig cells showed a polygonal shape and contained a great spherical nucleus (Fig. 3A and Fig. 6). On the contrary, Leydig cells in the post-puberty stage were (Figs. 7 and 8).
Fig. 6. Photomicrographs of testis of local Iraqi pigeon during pre-puberty showing seminiferous tubule with few germs cells generation (Se), Spermatogonia (Sp), Spermatogonia type A (SpA), Spermatogonia type B (SpB), Interstitial cell (Red arrow), Myoid cell (green arrow), Sertoli cell (S). (H & E stain 100X).
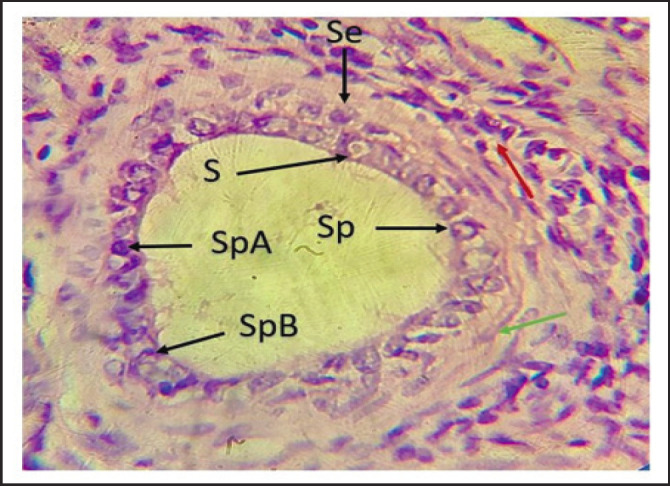
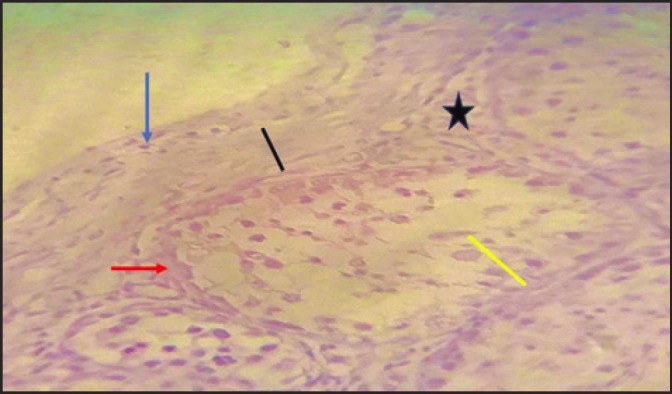
Fig. 7. Photomicrographs during post-puberty showing seminiferous tubule (Red arrow), Capsule (tunica albuginea) (Black arrow), Nucleus of the smooth muscle of capsule (Blue arrow), spermatogenesis (Yellow arrow), Interstadial space (star). (H & E stain 40X).
Discussion
This study aimed to investigate the histological and morphological aspects of the testis in Iraqi local pigeons (C. livia domestica). The macroscopic findings indicated that there are differences in the size between the left and right testis in both stages including the pre-puberty and post-puberty of the selected specimens. These variations were noticed in terms of the shape and size of the testis, as well as, the structure of the seminiferous tubules.
The observations associated with the form and size of testis in two ages of the current study aligned with the finding made recently by Abd Alkhazraji et al. (2022). They described the testes of Iraqi local Roosters and they have oval shapes and found the left testis bigger than those in the right one. However, the consequences of this study differ from previous findings of Razi et al. (2010), who reported that the testes of Iranian roosted were equal in length.
The local Iraqi pigeon (C. livia) contains testes similar to those of other birds composed of the stroma, which primarily creates the interstitial connective tissue and the capsule, as well as having segmented lobes instead of septula testes (Mustafa, et al., 2021).
The histological investigation of this study showed some differentiations of the testicular parenchyma of the pre-puberty and post-puberty ages in some histological features. The structural changes in the testes occur depending on the reproductive cycle and age as described in a previous study on the testes of Muscovy drakes (Gerzilov et al., 2016).
Fig. 5. Photomicrographs during post-puberty showing seminiferous tubule with germ cells generation, Spermatogonia (Sp), Spermatogonia type A (Yellow arrow), Spermatogonia type B (Blue arrow), Primary spermatocyte in prophase (Psp), Primary spermatocyte in metaphase (Psm), Secondary spermatocyte (Red arrow), spermatid (Z), Interstitial cells (L), Capsule (tunica albuginea) (green arrow), Sertoli cells (S), and blood vessel (Bv). (H & E stain 40X (A) 100X (B).
Table 4. Micro morphometric data for the testes of local Iraqi pigeons during post-puberty.
| Parameters | Left side (Mean ± SE) | Right side (Mean ± SE) |
|---|---|---|
| Seminiferous tubule | 223.64 ±1. 62* | 206.33 ± 1.74 |
| Spermatogonia | 4.93 ± 0.75* | 4.61 ± 0.24 |
| Spermatogonia nuclei | 3.72 ± 0.2 | 3.22 ± 0.13 |
| Primary spermatocyte | 5.59 ± 0.3* | 4.84 ± 0.27 |
| Primary spermatocyte nuclei | 4.78 ± 0.22* | 4.18 ± 0.24 |
| Spermatids | 3.98 ± 0.37* | 3.35 ± 0.16 |
| Spermatids nuclei | 3.75 ± 0.24* | 3.12 ± 0.24 |
| Sertoli cells | 6.94 ± 0.2 | 6.21 ± 0.15 |
| Interstitial cells | 4.82 ± 0.33 | 4.72 ± 0.13 |
| Interstitial nuclei | 3.74 ± 0.12 | 3.34 ± 0.22 |
| Capsule thickness | 51.05 ± 0.62* | 49.33 ± 0.27 |
(٭) denote significant differences at level p ≤ 0.05 between the right and left.
In the current findings, two types of spermatogonia were found including spermatogonia type A (SpA) and type B (SpB) that were diffused close to the seminiferous tube on its basement membrane in the pre-puberty age group. The majority of germ cells in birds are comprised of spermatogonia. According to studies by Geraert (1991), and Mfoundou et al. (2022), the maturation and normal sexual activity of gonads in most bird species are influenced by favorable conditions, including an appropriate photoperiod.
On the other hand, active spermatogenesis was designated through the present large numbers of spermatocytes, spermatogonia, and spermatids. Worthy of note, there were some spermatids still connected to the sertoi cell, while several spermatozoa were gathering inside the lumen of the seminiferous tubules. This accumulation is a good indicator of an increase the sexual activity (Lin et al., 1993; Czubaszek et al., 2019).
The seminiferous epithelium in the pre-puberty stage of age showed a prominent existence of many spermatogonia that diffused within the seminiferous tubules, suggesting that the activity of the testes still had not commenced. In contrast, it was noticed that the lumen of the seminiferous tubules in the post-puberty group was filled with numerous spermatozoa, which means completing spermatogenesis. While the empty lumen of the seminiferous tubules in the pre-puberty group confirms the nonappearance of spermatogenesis yet (Khatun et al., 2021).
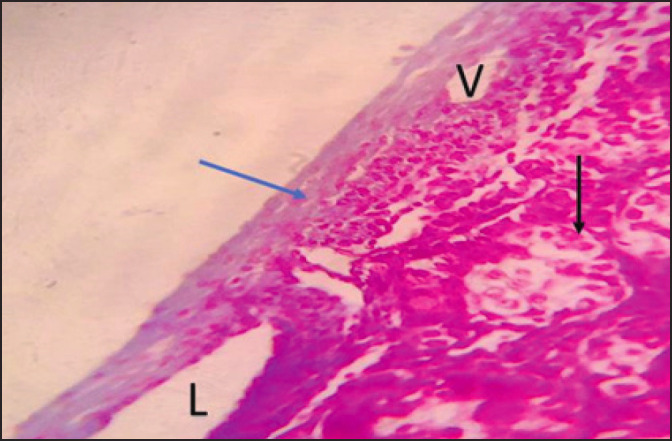
Fig. 8. Photomicrographs during post-puberty showing seminiferous tubule (Black arrow), the tunica albuginea (Collagen fibers) (Blue arrow), venule (V), and lymph vessels over connective tissue. (Masson Tricom stain 40X).
Conclusion
The current study investigated differences in the shape and form of the testis at two stages of bird age. The findings provide knowledge of the histological and morphological aspects of the bird testes in the local Iraqi pigeon.
Acknowledgments
The authors would express their appreciation to their dedicated team for their invaluable contributions to this study. In addition, special acknowledgment to the College of Veterinary Medicine, University of Falluja, Iraq, College of Veterinary Medicine, University of Tikrit, Iraq, and University of Baghdad, College of Education for Pure Science Ibn Al Haitham, Iraq.
Conflict of interest
The authors confirm that they have no conflicts of interest.
Authors’ contributions
All authors have contributed equally.
Funding
No external sources have funded this research, so the author personally supports this work in a self-sustaining manner.
Data availability
Data are available upon request. Please contact the author (Oday Al-Juhaishi) at oday_jassim@tu.edu.iq for access to the data.
References
- Abd Alkhazraji K.I., Zghair F.S., abd Alameer Naser R. Microscopic and macroscopic study on Iraqi local rooster genital tract. Rev Electron Vet. 2022;2022:210–8. [Google Scholar]
- Aire T.A. The rete testis of birds. J. Anat. 1982;135:97–100. [PMC free article] [PubMed] [Google Scholar]
- Blesbois E. Skinner M.K. In: Encyclopedia of Reproduction. Vol. 6. Cambridge, MA: Academic Press; 2018. Bird reproduction overview; pp. 579–585. [Google Scholar]
- Bull M.L., Martins M.R.F.B., Cesario M.D., Padovani C.R., Mendes A.A. Anatomical study on domestic fowl (Gallus domesticus) reproductive system. Int. J. Morph. 2007;25(4):709–716. [Google Scholar]
- Czubaszek M., Andraszek K., Banaszewska D. Influence of the age of the individual on the stability of boar sperm genetic material. Theriogenology. 2019;15:176–182. doi: 10.1016/j.theriogenology.2019.11.018. [DOI] [PubMed] [Google Scholar]
- Gartner L.P., Hiatt J.L. 6th. Saunders Elsevier; 2014. Color Atlas and Text of Histology. [Google Scholar]
- Geraert P.A. Métabolisme énergétique du poulet de chair en climat chaud. I.N.R.A. Prouction. Animals. 1991;4(3):257–267. [Google Scholar]
- Gerzilov V., Bochukov A., Penchev G., Petrov P. Testicular development in the Muscovy duck (Cairina moschata) Bulgarian J. Vet. Med. 2016;19(1):8–18. [Google Scholar]
- Islam F., Ishishita S., Uno Y., Mollah B., Skrikulnath K., Matsuda Y. Male hybrid sterility in the mule duck is associated with meiotic arrest in primary spermatocytes. J. Poult. Sci. 2013;50:311–320. [Google Scholar]
- Khatun P., Haque Z., Das S.K. Microscopic features of gonadally inactive testis of khaki campbell duck (Anas platyrhynchos domesticus) in Bangladesh. Turkish J. Agricult. Food Sci. Technol. 2021;9(1):146–149. [Google Scholar]
- Leska A., Kiezun J., Kaminska B., Dusza L. Seasonal changes in the expression of the androgen receptor in the testes of the domestic goose (Anseranser f. domestica). Gen. Comp. Endocrinol. 2012;179(1):63–70. doi: 10.1016/j.ygcen.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Lin M., Jones R.C. Spermiogenesis and spermiation in the Japanese quail (Coturnix coturnix japonica) J. Anat. 1993;183:525. [PMC free article] [PubMed] [Google Scholar]
- Mfoundou J.D., Guo Y., Yan Z., Wang X. Morpho-histology and morphometry of chicken testes and seminiferous tubules among yellow-feathered broilers of different ages. Vet. Sci. 2022;9:485. doi: 10.3390/vetsci9090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa F.E.Z.A., Elhanbaly R. Histological, histochemical, immunohistochemical, and ultrastructural characterization of the testes of the dove. Zygote. 2021;29(1):33–41. doi: 10.1017/S0967199420000477. [DOI] [PubMed] [Google Scholar]
- Razi M., Hassanzadeh S.H., Najafi G.R., Feyzi S.A., Amin M., Moshtagion M., Janabaz H., Amin M. Histological and anatomical study of the White Rooster of testis, epididymis and ductus deferens. Int. J. Vet. Res. 2010;4(4):229–236. [Google Scholar]
- Suvarna S.K., Layton C., Bancroft J.D. Theory and practice of histological techniques. 7th. Oxford, UK: Churchill Livingstone Elsevier; 2013. pp. 72–80. [Google Scholar]
- Tamilselvan S., Singh B. Histological and histometrical studies on ductus deferens of Guinea fowl (Numida meleagris) Indian J. Vet. Anat. 2020;32(2):59–61. [Google Scholar]
- Tingari M.D., Lake P.E. Ultrastructural evidence for resorption of spermatozoa and testicular fluid in the esculent ducts of the testis of the domestic fowl (Gallus domesticus) J. Reprod. Fert. 1972;31:373–381. doi: 10.1530/jrf.0.0310373. [DOI] [PubMed] [Google Scholar]
- Trefil P., Micakova A., Mucksova J., Hejnar J., Poplstein M., Bakst M., Kalina J., Brillard J.P. Restoration of spermatogenesis and male fertility by transplantation of dispersed testicular cells in the chicken. Biol. Reprod. 2006;75:575–581. doi: 10.1095/biolreprod.105.050278. [DOI] [PubMed] [Google Scholar]
- Ventela S., Toppari J., Parvinen M. Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing. Mol. Biol. Cell. 2003;14:2768–2780. doi: 10.1091/mbc.E02-10-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request. Please contact the author (Oday Al-Juhaishi) at oday_jassim@tu.edu.iq for access to the data.