Abstract
Background:
Colibacillosis caused by Escherichia coli causes significant economic losses in the livestock sector worldwide and is one of the calves’ leading causes of diarrhea.
Aim:
This study aimed to identify the most frequent E. coli molecularly pathotypes in calves with diarrhea in six provinces of the Cajamarca region in the northern highlands of Peru.
Methods:
Twenty-eight herds of dairy cattle under a semi-intensive rearing system were evaluated; 95 samples were isolated from calves with diarrhea up to the first month of life, 62 males and 33 females, during the rainy season.
Results:
The presence of virulence genes of E. coli strains was more prevalent in males; the astA (89.47%), st (83.15%), and f5 (57.89%) genes were more expressed, and the lt (17.89%) and stx2 (1.05%) genes were less expressed. The eae gene (21.05%) was more present in females.
Conclusion:
When E. coli strains express virulence genes astA, st, and f5 and their atypical double, triple, and quadruple combination between different observed pathotypes, they give rise to the formation of several pathotypes by the horizontal transfer of virulence genes, which can cause colibacillosis processes in more virulent calves, which is one of the most important causes of diarrhea in calves in the region of Cajamarca, compromising the sanitary viability in the herds.
Keywords: Calves, Colibacillosis, E. coli, Pathotypes
Introduction
The most critical and severe disease that attacks new born animals is colibacillosis, caused by several infectious agents (Lee et al., 2019), the primary etiological agent is Escherichia coli (E. coli) which causes enteritis in calves and causes significant health problems in calves (Tutija et al., 2022) and causes significant economic losses in the livestock industry worldwide E. coli bacillosis represents one of the most important causes of morbidity and mortality in calves. Also, it causes a decrease in the growth of the animal and an increase in treatment and control costs (Algammal et al., 2020).
There are several strains, among them enterotoxigenic E. coli (ETEC) which is considered to be an essential cause of diarrhoeal disease in humans, calves, pigs, and poultry (Bernal-Reynaga et al., 2013). Enterohaemorrhagic E. coli (EHEC) is the most frequent strain in apparently healthy calves with diarrhea, with a high implication in the aetiopathogenesis of calf diarrhea, being a reservoir of E. coli in calves and potentially pathogenic to humans (Aref et al., 2018). Meanwhile, enteroaggregative E. coli (EAEC) is associated with childhood malnutrition, regardless of the presence of diarrhea (Havt et al., 2017). However, diarrhoeagenic E. coli is the leading cause of gastroenteritis in children in developing countries and is associated with high levels of antimicrobial resistance, each strain possesses different virulence genes, including eae, tir, and bfpA for enteropathogenic pathotype (EPEC) and ap AA for EAEC. Two different toxins have been described in EHEC, including Stx1/Stx2 (Shiga-like toxin) and ehxA (enterohaemolysin), while an invasion plasmid (vir regulon) has been described in EIEC. There are two enterotoxins, thermostable (ST) and labile-stable (LT), by ETEC (Cabal et al., 2016). Antibiotic resistance is currently one of the greatest threats to health, food security, and development worldwide, accelerated by the misuse and abuse of different drugs in both humans and animals, which prolongs hospital stays and increases medical costs and mortality. Resistance to ampicillin (92.5%), tetracycline (76.6%) and trimethoprim/sulfamethoxazole (70.1%), 51.92% to sulfamethoprim, 26.92% to neomycin, and 9.61% to enrofloxacin has been reported (Cabrera-Gonzáles et al., 2023).
According to the 2012 agricultural census, Peru’s livestock population is 5,156,000 head of cattle. Of this population, 78% is located in the highlands, 11% in the coast, and 10% in the jungle. Livestock activity is complementary to agriculture, forming integral production systems mainly oriented to self-consumption and artisanal production of dairy products, producing 15% of the country’s milk production. Cajamarca is one of the leading milk-producing regions, producing 1 million l of milk per day (more than 360 million l per year), 40% of which goes to processing plants and a significant percentage to large milk companies such as Gloria and Nestlé, one of the most critical activities in the Cajamarca region being the production of milk and its derivatives. The objective was to molecularly identify the most frequent E. coli pathotypes in calves with diarrhea.
Materials and Methods
Sample collection
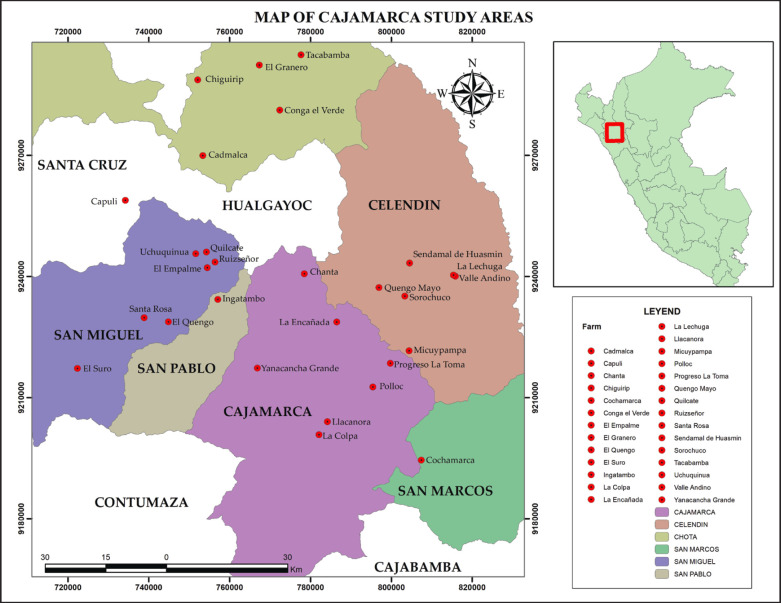
Fecal samples were collected from 28 Holstein and Brown Swiss cattle dairy herds with grazing systems in the Cajamarca region’s six provinces. Ninety-five samples were isolated from calves with diarrhea up to one month of age, 62 males and 33 females. The selection of sampled animals was based on the presence of diarrhea and the total number of calvings, considering that the herds where samples were collected do not use sexed semen. Fecal samples were collected during the rainy season, which is divided into two seasons: rainy and dry, with 15 February to 15 March being the season with the highest frequency of heavy and sudden rains; dry corresponds to autumn-winter between May and September (September 2021 - March 2022) (Fig. 1).
Fig. 1. Geographical distribution of farms where samples were taken.
Each herd consisted of 20 animals, of which 5 were calves. The number of samples taken was the total number of calves born in the herds of both sexes that presented diarrhea. The calves were handled painlessly by trained personnel (veterinarians) with the owner’s express permission. Fecal samples were obtained directly from the rectum using sterile polyethylene bags of first use, collecting approximately 3 g of feces, which were identified and transferred in a technoport box containing cooling ice gels (ThermoSafe—USA) to the laboratory of Biotechnology in Animal Health of the Agricultural Experimental Station Baños del Inca for the isolation and bacteriological identification of E. coli.
Isolation of E. coli
Using a bacteriological loop, 300 ml (μl) aliquot of feces was seeded on MacConkey agar (Becton, Dickinson, and Company/Loveton Circle Sparks, MD 21152, USA). The aliquot was then incubated (Memmert/ICO-50/ Schwabach/Germany) in aerobiosis at 37°C for 24 hours; colonies with morphological growth development typical of E. coli were identified.
DNA extraction from isolated E. coli colonies
Two selected colonies of typically colored (pinkish) E. coli colonies obtained from each fecal sample were seeded on MacConkey agar and deposited on powdered, liquid microbial growth medium (2xYT) (Sigma, REF Y2377) at 37°C for 18 hours for development and multiplication in an incubator (CO incubator2 ICO50-Memmert/Germany); without CO activation, operating as a standard incubator, subsequently, samples were evaluated in a spectrophotometer (PCR MAX Lambda 64272, Bibby Scientific Ltd, UK), using optical density and concentration pre-set in the spectrophotometer, the colony forming units were analyzed at 600 nm. To obtain E. coli genomic DNA, the Wizard® Genomic DNA purification kit (Promega, REF. A1120), following the manufacturer’s instructions, the purified DNA was stored in 1,500 ml (μl) polypropylene microcentrifuge tubes (Eppendorf™); finally, the samples were identified and stored under refrigeration (Samsung refrigerator, RT35K5930S8/PE, Samsung /Mexico) at four°C, for subsequent use in the polymerase chain reaction (PCR) analysis processes.
Molecular identification of E. coli
PCR technique was used, using primers (F5’-TCAGCGCGAAGTCTTTCTTTCTTTATACC-3’, R5’-CGTCGGTAATCACCATTCCC-3’), to amplify the uidA gene (248 bp). The thermal profile was: denaturation 94°C/2’, 25 cycles of denaturation 94°C/30”, hybridisation 55°C/30”, extension 72°C/45”; final extension 72°C/2’. DNA fragments amplified to identify E. coli strains were separated by molecular weight by electrophoresis (1.5% agarose). Fragment analysis was performed by gel staining with Sybr Green (Thermo Fisher) and observed on a UV transilluminator at 302 nm Labnet U1001 Taiwan. Amplification of the uidA gene was used as it is specific for E. coli detection.
Determination of the virulence gene profile
For the amplification of virulence genes (f5, st, lt, eae, stx1, stx2, hylA, and astA ), 2 PCR-multiplex (eae, hlyA, stx1, and astA), (lt, st, and f5) and a regular PCR (stx 2), with different primer pairs for each gene and with a different thermal profile (Tables 1 and 2); the procedure was performed with a thermal cycler (PCRmax Alpha-UK), the amplified DNA fragments were separated by molecular weight (bp) by 1.5% electrophoresis, the analysis was performed by agarose gel staining with Syber Green (Thermo Fisher) and observed in a 302 nm UV transilluminator—Labnet U1001 Taiwan. For the identification of virulence genes, the virulence genes were analyzed with the GelAnalyzer 23.1.
Table 1. Primer sequence for the identification of the virulence genetic profile of E. coli (Awad et al., 2020).
| Pathotype | Generation | Primer sequence | PCR amplification (bp) |
|---|---|---|---|
| EPEC | MAR (eae) | F: TCAATGCAGTTCCGTTATCAGTT | 482 bp |
| R: GTAAAGTCCGTTACCCCCCCCCCCCCCCCAACCTG | |||
| STEC | Stx1 (stx1), | F: CGATGTTACGGTTTGTGTGTGTTACTGTGTGTGACAGC | 244 bp |
| R: AATGCCACGCTTCCCAGAATTG | |||
| Stx2 (stx2) | F: CCATGACAACGGACGGACAGCAGTT | 779 bp | |
| R: CCTGTCAACTGAGCAGCAGCAGCAGCACTTTTTG | |||
| HlyA (hlyA) | F: AGCTGCAAGTGCGGGGGGTCTG | 569 bp | |
| R: TACGGTTATGCCTGCAAGTTCAC | |||
| ETEC | LT (lt) | F: GGGCACACACACAGATTATATATATACCGTGC | 450 bp |
| R: CGGTTCTCTCTATATTATTATTATTCCCTGTT | |||
| STREET | F: ATTTTTTTTMTTTCTTGTATTRTCTT | 190 bp | |
| R: CACCCCGGTACARGCAGGATT | |||
| Fimbria f5 (k99) | F: TATTATATATCTTAGGTGGTGGTGGTGGTATGG | 314 bp | |
| R: GGTATATCCTTTAGCAGCAGCAGCAGTATTTATTC | |||
| EAEC | ESTE1 (astA) | F: TGCCATCATCATCAACACACACACAGTATATATATCCG | 102 bp |
| R: ACGGCTTTTGTAGTCCTTCCTTCCAT |
Table 2. Components and thermal amplification profile of virulence genes of E. coli (Awad et al., 2020).
| Virulence genes | Mix components: PCR/volume (μl) | Thermal profiling—PCR |
|---|---|---|
| eae, hlyA, stx1 and astA | 5 μl of Master Mix 5 μl of template and 0,5 μl for each F and R primer (4 μl total) 11 μl of ultrapure water |
Initial denaturation 94°C, 5 minutes/1 cycle 35 cycles Denaturation 94°C, 30 seconds Alignment 62°C, 30 seconds Extension 72°C, 1 minute Final extension 72°C, 5 minutes / 1 cycle |
| stx2 | 5 μl of Master Mix 5 μl of template and 1 μl for each F and R primer (2 μl in total) 13 μl of ultrapure water |
Initial denaturation 95°C, 3 minute / 1 cycle 35 cycles Denaturation 95°C, 20 second Alignment 58°C, 40 second Extension 72°C, 90 second Final extension 72°C, 5 minute / 1 cycle |
| lt, st and f5 | 5 μl of Master Mix 5 μl of template and 0,5 μl for each F and R primer (3 μl in total) 12 μl of ultrapure water |
Initial denaturation 95°C, 5 minutes / 1 cycle 40 cycles Denaturation 95°C, 45 seconds Alignment 50°C, 1 minute Extension 72°C, 90 seconds Final extension 72°C, 5 minutes / 1 cycle |
Statistical analysis
Results were analyzed using Graph Pad Prism 9.3.1 software (Prism Software, Irvine, CA, USA). The normality of the data was determined by Kolmogorov-Smirnoff analysis of variance (ANOVA) (p ≤ 0.05), followed by Tukey’s post-hoc test for evaluations between virulence genes related to the identification of E. coli pathotypes. The information obtained was considered statistically significant at p ≤ 0.05.
Ethical approval
Rectal fecal samples were obtained from animals without anesthesia or tranquilizers by certified and well trained Veterinarians at the Faculty of Veterinary Sciences of the National University of Cajamarca, Peru (Clinica Veterinaria Ministerio de Agricultura) in propylene bags following established protocols. Fecal samples were referred to the Biotechnology in Animal Health laboratory for coproparasitological analysis after obtaining the farmers’ authorization.
Results
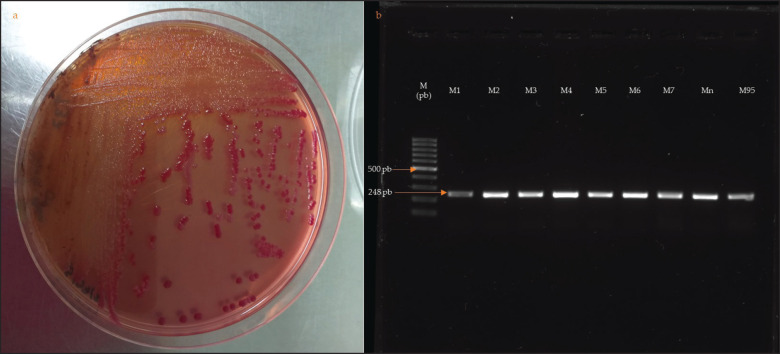
Ninety-five fecal samples from calves with diarrhea were selected by characteristic growth on MacConkey agar and molecularly by PCR targeting amplification of the uid A gene (Fig. 2).
Fig. 2. (a): Growth of colonies on MacConkey II Sorbitol. (b): Amplification of the uidA gene (lane 2, lane 3, lane 95, [248 bp]) in 1.5% agarose, with 100 bp molecular marker.
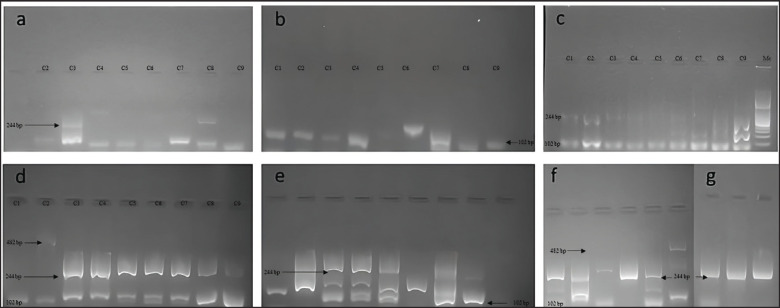
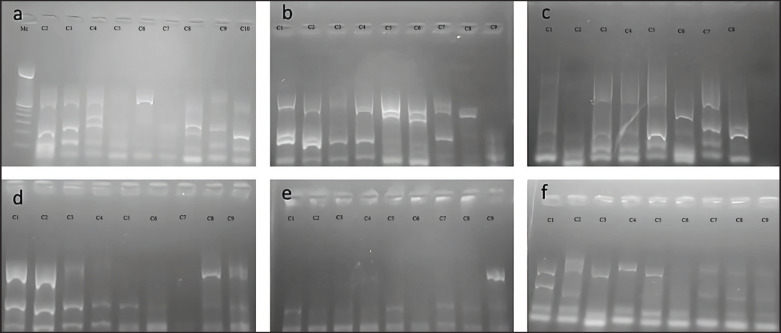
Single and multiplex PCR techniques targeting the amplification of the virulence genes eae, hlyA, stx1, astA (Fig. 3), stx 2 (Fig. 4), lt, st, f5 (Fig. 5) were optimized to identify pathogenic pathotypes of E. coli present in calf diarrhea samples.
Fig. 3. Single and multiplex PCR techniques targeting the amplification of virulence genes. (a): lanes 3 and 8 positive for the stx1 gene (244 bp), lanes 2 to 9 positive for the astA gene (102 bp). (b): lanes 1 to 9 positive for the astA gene except lane 5 (102 bp). (c): lanes 1,2 and 9 positive for the stx1 gene (244 bp), lanes 1 to 9 positive for the astA gene (102 bp). (d): lanes 1 to 9 positive for the astA gene (102 bp); lanes 3, 4, 5, 6, 7, and 8 positive for the stx1 gene (244 bp); lanes 2 positive for the eae gene (482 bp). (e): lanes 3, 4, 5, and 8 positive for the stx1 gene (244 bp), lanes 1 to 8 positive to the astA gene (102 bp). (f): lanes 2,5 and 6 positive to the astA gene (102 bp); lanes 1, 2, 4, and 5 positive for the stx1 gene (244 bp); lane 6 positive for the eae gene (482 bp); g. lanes 1, 2, and 3 positive to the eae gene (244 bp).
Fig. 4. Single and multiplex PCR techniques targeting the amplification of virulence genes. (a): Lane 3 stx 2 gene positive (799 bp). (b, c, d, e, f, g): all lanes negative.
Fig. 5. Single and multiplex PCR techniques targeting the amplification of virulence genes. (a): Lanes 1, 2, 3, 5, and 8 positive to Lt gene (450 bp), lanes 1 to 9 positive to st gene (190 bp), lanes 3, 7, and 8 positive to f5 gene (314 bp). (b): lanes 1, 2, 4, 5, 6, 7, and 8 positive to Lt gene (450 bp), lanes 1 to 9 minus 8 positive to st gene (190 bp), lanes 1 to 7 positive to f5 gene (314 bp). (c): lanes 1, 3, 4, 5, 7, and 8 positive to Lt gene (450 bp), lanes 1 to 9 minus 8 positive to st gene (190 bp), lanes 1 to 7 positive to f5 gene (314 bp); lane 6 positive for Lt gene (450 bp), the rest negative, lanes 1 to 8 positive for st gene (190 bp), lanes 1, 3, 4, 5, 7, and 8 positive for f 5 gene (314 bp) (d): lanes 1, 2, 8, and 9 positive to the Lt gene (450 bp), lanes 1 to 9 minus 7 positive to the st gene (190 bp), lanes 1 to 9 minus 1 and 7 positive to the f5 gene (314 bp). (e): lane 9 positive to Lt gene (450 bp), lanes 1 to 9 positive to st gene (190 bp), lanes 1 to 9 minus 6 positive to f 5 gene (314 bp). (f): lanes 1 to 9 positive to st gene (190 bp), lanes 1 to 5 positive to Lt gene (450 bp), lane 1 positive to f 5 (314 bp). Prevalence of amplified virulence genes of E. coli strains according to sex.
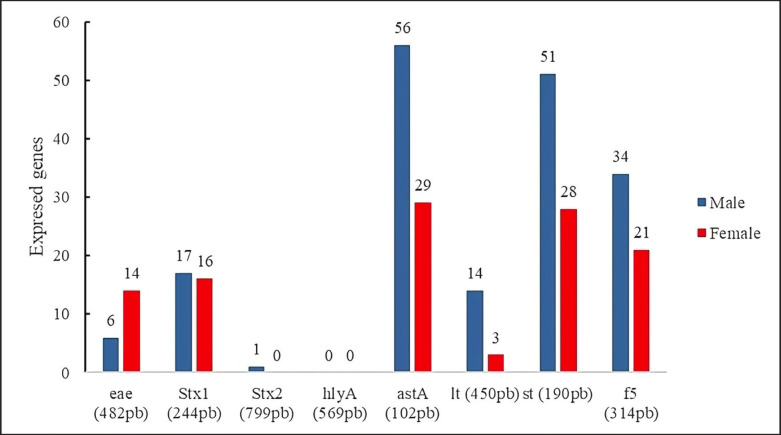
The results showed that the presence of virulence genes of E. coli strains isolated from diarrhea did not show significant statistical differences between sexes; the most prevalent genes were astA (89.47%) (males, n = 56/95; females, n = 29/95).
Next, the st gene was expressed in lower proportion (83.15%) (males, n = 51/95; females, 28/95), f 5 (57.89%) (males, n = 34/95; females, n = 21/95).
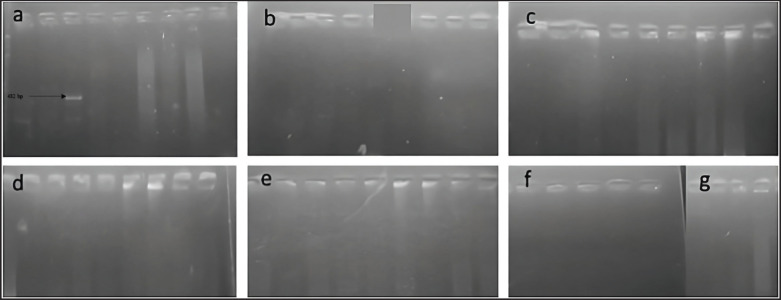
It could also be observed that the genes lt (17.89%) (males, n = 14/64; females, 3/64), stx 2 (1.05%) (males, n = 1/95; females, n = 0/ 95) were expressed in lower proportion. Interestingly, the virulence gene eae (21.05%) was more prevalent in females (n = 14/95) than in males (n = 6/95). The hly A gene did not amplify in the processed samples (Fig. 6).
Fig. 6. Hly A gene did not amplify in the processed samples.
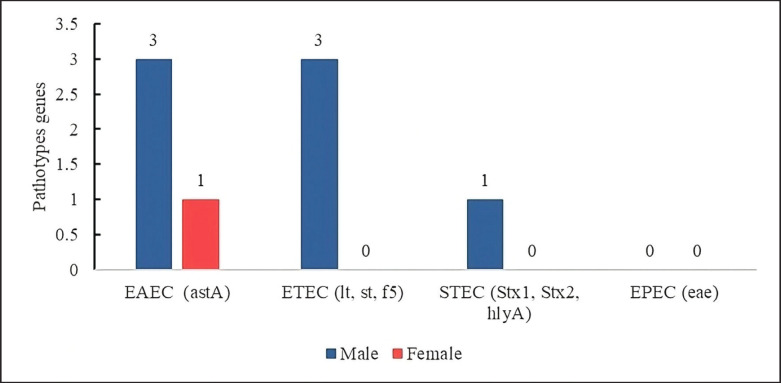
In the virulence gene identification profile of the E. coli isolates, the presence of 3 pathotypes that do not express genes of other pathotypes was observed: the EAEC (4.21%, n = 4/95), which mainly expressed the astA gene more frequently in males (n = 3/4) than in females (n = 1/4).
On the other hand, other pathotypes were only expressed in males as ETEC (3.15%), males 3/95, females 0/95 expressing the lt, st, and f5 genes; and shigatoxigenic (STEC) (1.05%) expressing the stx 1 gene mainly in males (n = 1/95), the EPEC was not expressed in the samples (Fig. 7).
Fig. 7. Strains of pure pathotypes of E. coli present in calf diarrhea.
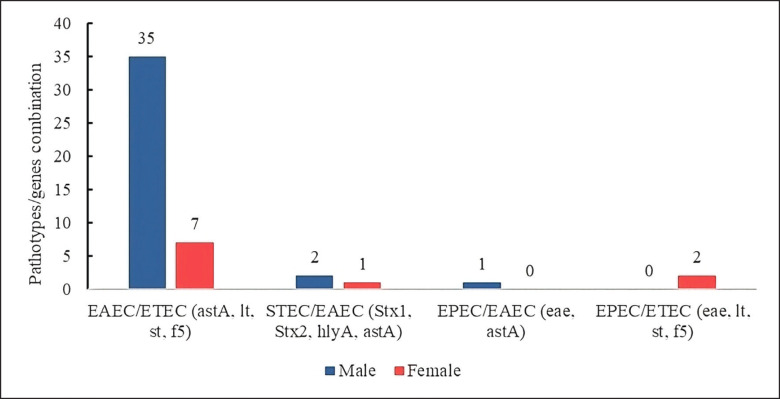
Hybrid pathotypes of E. coli expressing a combination of virulence genes between pathotypes were also present. Double pathotypes formed by EAEC/ETEC strains (44.21%) expressing common genes such as astA, lt, st, and f5 were more prevalent in males (n = 35/95) than in females (n = 7/95).
Another pathotype combination was STEC/EAEC (3.15%), expressing Stx1, Stx2, hlyA, and astA genes, more prevalent in males (n = 2/95) than in females (n = 1/95); the pathotype combination EPEC/EAEC (1.05%) and EPEC/ETEC (2.10%) were expressed in males (n = 1/95) and females (n = 2/95), respectively (Fig. 8).
Fig. 8. E. coli hybrid strains showing virulence genes of two pathotypes.
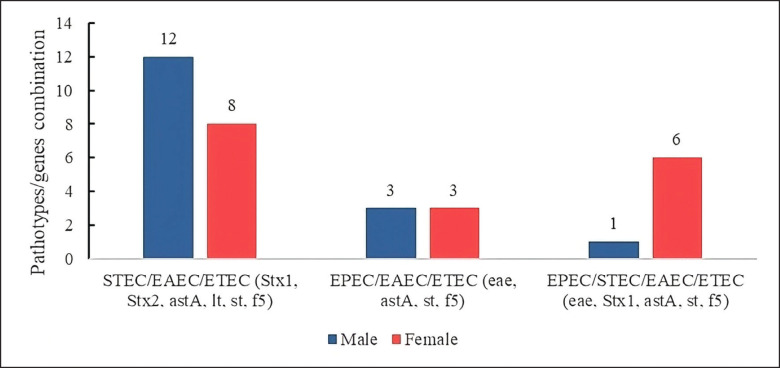
Likewise, E. coli strains were observed to share virulence genes corresponding to several pathotypes, with genes common to three and four different pathotypes. The profiles were 21.05% of the STEC/EAEC/ETEC combination, sharing the genes stx1, stx2, astA, lt, st, and f5, expressed more in males (n = 12/95) than in females (n = 8/95).
The EPEC/EAEC/ETEC combination was present in 6.31%, the most prevalent genes eae, astA, st, f 5, with equal values in males (n = 3/95) and females (n = 3/95).
On the other hand, strains sharing genes from four pathotypes were identified with 7.36% of EPEC/STEC/EAEC/ETEC, with common genes of eae, stx1, astA, st, and f5; this virulence profile is mainly found in females (n = 6/95) compared to males (n = 1/95) (Fig. 9).
Fig. 9. E. coli hybrid strains expressing virulence genes of three and four pathotypes.
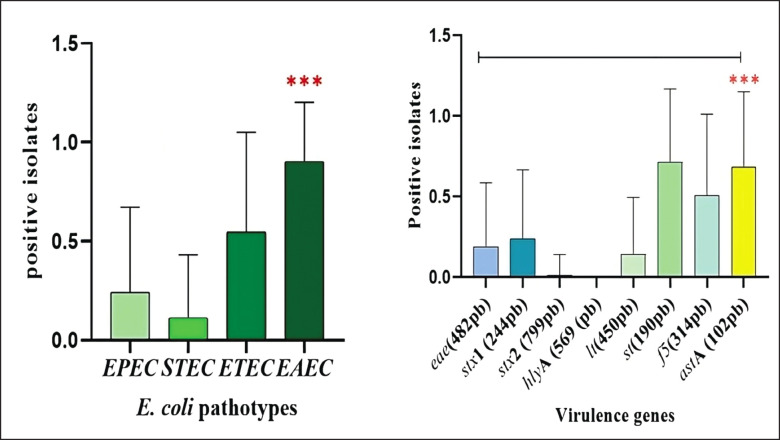
In the statistical analysis, a significant difference (p < 0.0001) could be found for pathotypes and virulence genes of E. coli isolates from calf samples with diarrhea (Fig. 10).
Fig. 10. (a): Pathotypes identified, (b): virulence genes amplified. Connector lines above the bars indicate the presence of statistical difference (p < 0.0001).
Discussion
Escherichia coli is established as the primary health obstacle in dairy herds (Mohammed et al., 2019). Some data, such as breeding system, calving, colostrum consumption of neonates, and perinatal antibiotic treatments to cows in milk production, could not be used due to the absence of records of producers in the Cajamarca region; it is also suggested to include other research factors to reduce the possible risk causes associated with the presentation of E. coli diarrhea in calves such as genetic characteristics of animals, dietary supplementation with probiotics, environmental factors (Bi et al., 2017), the study was conducted in a pasture-based rearing system without any technical criteria for the prevention of gastroenteric diseases; therefore, it should be interpreted with caution as the sampling was based on animals with the presence of diarrhea.
The presence of the astA gene (89.47%) detected in E. coli strains as the most prevalent, which encodes a thermostable toxin, was reported by other researchers and is very frequent in many pathogenic pathotypes (EAEC, ETEC, STEC, and EHEC) (Zajacova et al., 2012); this gene has higher pathogenicity and virulence than other associated genes that cause severe diarrhea in calves, compromising the health viability of herds and the economics of producers (Awad et al., 2020). The presence of the st (83.15%) and f5 (57.89%) genes are a cause for concern in Cajamarca livestock because the f5-fimbria gene is used for colonization and establishment in small intestine cells at the surface protein level; it also constitutes the initial phase of colibacillosis and is associated with thermostable st and thermolabile lt enterotoxins (17.89%) that increase its pathogenicity (Awad et al., 2020), similarly, it could be observed that the f 5 gene was found in a high number of calves, contrary to what other authors claim with a prevalence of 5.3% (Shams et al., 2012), this may be because, in the breeding area, vaccines are not used for the prevention of colibacillosis in calves (Ryu et al., 2020).
It was also observed that Shiga toxin virulence genes Stx 2 (1.05%) were minimally present and manifested in males (n = 1/95) compared to females. On the other hand, the presence of the hly A gene (0%) was not identified, which is of concern because ruminants are considered potential reservoirs (Kagambèga et al., 2012), which can contaminate water food and increase the potential risk of infection by exposure (Sethulekshmi et al., 2018).
The presence of virulence genes encoding pathotypes of E. coli was observed in isolates of pure pathotypes, which do not share genes expressing other pathotypes. Thus, isolates of enteroaggregative E. coli—EAEC (6.25%) were isolated, which correlates with other research reporting calf samples with diarrhea from 2% (Belete et al., 2022) to a detection rate of 39% (Coura et al., 2019). In addition, they are common pathotypes that cause mild to moderate infections in animals (Pakbin et al., 2021); there is concern about the risks it could cause to the population through contamination of milk, water consumption, and food contaminated with feces (Jeamsripong et al., 2019). The virulence factors observed in isolation in these pathotypes were 87.5% of the astA gene EAEC, which is higher than the rate found in calves of 15.6%, 32.6% (Yuste et al., 2006), possibly due to differences in the type of rearing, the calf diarrhea prevention measures used by farmers, the species, the breed and the altitudinal floor where the cattle are raised.
Another important pathotype that did not present virulence genes from other pathotypes was STEC 1.05%, identifying the virulence gene stx 1, which is zoonotic and can contaminate feed with animal manure during the handling process (Sapountzis et al., 2020). In addition, relatively low prevalences of the virulence gene stx 1 (9.3%), expressed in pre-weaned calves with diarrhea and 0.3% stx (Ryu et al., 2020), coinciding with our results where stx was expressed more than stx, with this virulence gene being observed more in animals with diarrhea (Fakih et al., 2017), which is considered a potential problem that these strains adapt to the environment and transmit these resistance characteristics to various bacteria found in the environment (Metlay et al., 2006 ).
Hybrid strains of E. coli that exhibit virulence factors of various pathotypes are more virulent and essential, so understanding their pathogenicity and their association cause disease in humans and animals is very challenging (Lee et al., 2023); these strains have been reported worldwide and are associated with public health problems (Santos et al., 2020), these virulence factors are contained in phages and plasmids that allow horizontal gene transfer leading to the generation of hybrid pathotypes (Jesser and Levy, 2020), observe strain profiles of E. coli strains consisting of two pathotypes EAEC/ETEC; CEEA/EPEC; EPEC/ETEC; STEC/ETEC; three pathotypes EAEC/ETEC,/STEC; EAEC/ETEC/EPEC and four pathotypes EPEC/STEC/ETEC/ETEC/ETEC/EAEC; isolated from feces of calves with diarrhea.
Among the most critical hybrid pathotypes of E. coli, we have the STEC/ETEC association, which represents a potential threat due to its higher virulence compared to other pathotypes and can be associated with causing diarrhea and hemolytic uraemic syndrome in humans (Bai et al., 2019), representing a potential threat due to its ability to be transmitted to dairy farmers in the Cajamarca region.
Another significant hybrid pathotype of E. coli observed was the EPEC/STEC combination, which is also zoonotic for causing diarrhea and mortality in children, suggesting that this combination is highly virulent because it contains the eae and est genes encoding binding lesions, effacement, and thermostable enterotoxins of E. coli (Lee et al., 2023) which could increase the potential threat of bovine reservoirs of E. coli in the transmission of these pathogenic strains to producers. On the other hand, combinations of three and four pathotypes of E. coli where the presence of the astA, eae, and f 5 genes was more prevalent, suggesting that this combination may confer greater virulence to these strains because the eae gene encodes intimin, which causes lesions in the gut (Trabulsi et al., 2002) and confers adhesins (fimbriae or pili) caused by virulence factor f 5 (Dubreuil, 2017) and the astA gene, present in most of the pathotypes analyzed, can cause outbreaks of diarrhea (Yatsuyanagi et al., 2003), in addition to the fact that in the Cajamarca region there is resistance to the drugs used, it was reported that 96.15% of the E. coli strains were resistant to tetracycline. The virulence gene astA may confer more excellent resistance to the strains identified. Therefore, it is necessary to know and identify the main pathotypes and virulence genes of E. coli, establish control and monitoring programs, use antibiotics appropriately, and avoid contact of feces with food, especially during manual milking and dairy food processing.
Conclusion
The most frequent pathotypes of E. coli in calves with diarrhea were EAEC and ETEC, which caused disease pathogenesis in calves, and the most prevalent virulence genes were astA, st, and f 5.
Acknowledgments
The “Proyecto de Mejoramiento Genético Nacional-PROMEG CUI 2432072” is gratefully acknowledged.
Authors’ contributions
Conceptualization, methodology, and writing of the original manuscript, MCG Project management and fund acquisition CQP Overall revision of the manuscript, formal analysis HC English revision and editing, formal analysis WYAG, FAT Standardisation and validation of laboratory protocols, research ATS Laboratory Sample processing DRV Conceptualisation and Methodology MCR All authors have read the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This research was partially funded by the “Proyecto de Mejoramiento Genético Nacional-PROMEG CUI 2432072”.
Data availability
Data supporting the findings of this study are available from the corresponding author, MCG and MCR upon reasonable request.
References
- Algammal A.M., El- Kholy A.W., Riad EM., Mohamed H.E., Elhaig M.M., Al Yousef S.A., Hozzein WN, Ghobashy M.O.I. Genes encoding virulence and antimicrobial resistance in enterotoxigenic and shigatoxigenic E. coli isolated from diarrhoeic calves. Toxins (Basel). 2020;12(6):383. doi: 10.3390/toxins12060383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aref N.E.M., Abdel-Raheem A.R.A., Kamaly HF, Hussien S.Z. Clinical and seromolecular characterisation of Escherichia coli; emphasising hybrid strain in healthy and diarrheic neonatal calves in Egypt. Open Vet. J. 2018;8(4):351–359. doi: 10.4314/ovj.v8i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.S., El-Sayed A.A, Mohammed F.F., Bakry. N.M, Nadra-Elwgoud., Abdou M.I, Kamel. M.S. Molecular characterisation of pathogenic Escherichia coli isolated from diarrhoeic and in-contact cattle and buffalo. Trop. Anim. Health Prod. 2020;52(6):3173–3185. doi: 10.1007/s11250-020-02343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X., Zhang J., Ambikan A., Jernberg C., Ehricht R., Scheutz F., Xiong Y., Matussek A. In Sweden, molecular and comparative genomic characterisation of clinical hybrid shiga Toxin-producing and enterotoxigenic Escherichia coli strains (steC / eteC) Sci. Rep. 2019;9(1):5619. doi: 10.1038/s41598-019-42122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belete M.A., Demlie TB, Chekole W.S., Tessema T.S. Molecular identification of diarrhoeogenic Escherichia coli pathotypes and antibiotic resistance patterns among diarrhoeic children and in-contact calves in Bahir Dar town, northwestern Ethiopia. PLoS One. 2022;17(9):e0275229. doi: 10.1371/journal.pone.0275229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Reynaga R., Thompson-Bonilla R., Lopez-Saucedo C., Pech-Armenta M., Estrada-Parra S., Estrada-Garcia T. C57-CD40 ligand-deficient mice: a potential model for enterotoxigenic colonization of Escherichia coli (H10407) colonization. Vet. Immunol. Immunopathol. 2013;152(1-2):50–56. doi: 10.1016/j.vetimm.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Bi Y., Yang C., Diao Q., Tu Y. Effects of dietary supplementation with two alternatives to antibiotics on the gut microbiota of weaned calves exposed to Escherichia coli K99. Sci. Rep. 2017;7:5439. doi: 10.1038/s41598-017-05376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabal A., García-Castillo M., Cantón R., Gortázar C., Domínguez L., Álvarez J. Prevalence of Escherichia coli virulence genes in patients with diarrhea and a subpopulation of healthy volunteers in Madrid, Spain. Front. Microbiol. 2016;7:1–6. doi: 10.3389/fmicb.2016.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-González M.A.C., Pérez H.V.V., Quilcate-Pairazamán C., Bazán-Arce J., Cueva-Rodríguez M. Assessment of antibiotic resistance in fecal samples from calves withdiarrhea in the Cajamarca region, Peru. Rev. Mex. Cienc. Pecu. 2023;14(4):782–795. [Google Scholar]
- Coura F., De Araújo Diniz S., Xavier Silva M., Sousa de Oliveira C., Suhet Mussi J., Fonseca de Oliveira C., Pereira Lage A., Heinemann M. Virulence factors and phylotyping of Escherichia coli isolated from non-diarrheic and diarrheic water buffalo calves. Ciência Rural. 2019;49(5):e20180998. [Google Scholar]
- Dubreuil JD. Enterotoxigenic Escherichia coli and probiotics in swine: what the bleep do we know? Biosci. Microbiota. Food Health. 2017;36(3):75–90. doi: 10.12938/bmfh.16-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih I., Thiry D., Duprez J.N, Saulmont M., Iguchi A., Piérard D., Jouant L., Daube G., Ogura Y., Hayashi T., Taminiau B., Mainil J.G. Identification of Shiga toxin-producing (STEC) and enteropathogenic (EPEC) Escherichia coli in diarrhoeic calves and comparative genomics of O5 bovine and human STEC. Vet. Microbiol. 2017;202:16–22. doi: 10.1016/j.vetmic.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Havt A., Lima I.F., Medeiros P.H., Clementino M.A, Santos A.K., Amaral M.S., Veras H.N., Prata. M.M., Lima N.L., Di Moura A., Leite, Á.M., Soares AM, Filho J.Q., Houpt E.R., Nataro J.P., Guerrant R.L., Lima A.A. Prevalence and virulence gene profiling of enteroaggregative Escherichia coli in malnourished and malnourished Brazilian children. Diagn. Microbiol. Infect. Dis. 2017;89(2):98–105. doi: 10.1016/j.diagmicrobio.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeamsripong S., Chase J.A., Jay-Russell M.T., Buchanan R.L., Atwill E.R. Experimental field transfer and survival of Escherichia coli from animal feces to romaine lettuce in Salinas Valley, California. Microorganisms. 2019;7(10):408. doi: 10.3390/microorganisms7100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesser K.J., Levy K. Updates on defining and detecting diarrheagenic Escherichia coli pathotypes. Curr. Opin. Infect. Dis. 2020;33(5):372–380. doi: 10.1097/QCO.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagambèga A., Martikainen O., Siitonen A., Traoré A.S, Barro N., Haukka K. Prevalence of diarrheagenic Escherichia coli virulence genes in the feces of slaughtered cattle, chickens, and pigs in Burkina Faso. Microbiologyopen. 2012;1(3):276–284. doi: 10.1002/mbo3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Kim H.Y., Choi E.W, Kim D. Causative agents and epidemiology of diarrhea in Korean native calves. J. Vet. Sci. 2019;20(6):e64. doi: 10.4142/jvs.2019.20.e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Sung S., Ha J., Kim E., An E.S., Kim S.H., Kim S.H, Kim H.Y. Molecular and genomic analysis of the virulence factors and potential transmission of hybrid enteropathogenic and enterotoxigenic Escherichia coli (EPEC/ETEC) strains isolated in South Korea. Int. J. Mol. Sci. 2023;24(16):12729. doi: 10.3390/ijms241612729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J.P., Powers J.H., Dudley M.N., Christiansen K., Finch RG. Antimicrobial drug resistance, regulation, and research. Emerg. Infect. Dis. 2006;12(2):183–190. doi: 10.3201/eid1202.050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed S.A.E.M., Marouf S.A.E.M., Erfana A.M., El- Jakee J.K.A.E.H., Hessain A.M., Dawoud T.M., Kabli S.A., Moussa I.M. Risk factors associated with E. coli causing neonatal calf diarrhea. Saudi J. Biol. Sci. 2019;26(5):1084–1088. doi: 10.1016/j.sjbs.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakbin B., Brück W.M., Rossen J.W.A. Virulence factors of enteric pathogenic Escherichia coli: a review. Int. J. Mol. Sci. 2021;22(18):9922. doi: 10.3390/ijms22189922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J.H., Kim S.H., Park J., Choi K.S. Characterization of virulence genes in Escherichia coli strains isolated from pre-weaned calves in the Republic of Korea. Acta Vet. Scand. 2020;2020:62–45. doi: 10.1186/s13028-020-00543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A., Santos FF, Silva RM, Gomes T. Diversity of hybrid- and hetero-pathogenic Escherichia coli and their potential implication in more severe diseases. Front. Cell. Infect. Microbiol. 2020;10:339. doi: 10.3389/fcimb.2020.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapountzis P., Segura A., Desvaux M., Forano E. An overview of the elusive passenger in the gastrointestinal tract of cattle: the shiga toxin producing Escherichia coli. Microorganisms. 2020;8(6):877. doi: 10.3390/microorganisms8060877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethulekshmi C., Latha C., Anu C.J. Occurrence and quantification of Shiga toxin-producing Escherichia coli from food matrices. Vet. World. 2018;11(2):104–111. doi: 10.14202/vetworld.2018.104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams Z., Tahamtan Y., Pourbakhsh A., Hosseiny MH, Kargar M., Hayati M. Detection of enterotoxigenic K99 (F5) and F41 from fecal sample of calves by molecular and serological methods. Comp. Clin. Pathol. 2012;21(4):475–478. doi: 10.1007/s00580-010-1122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabulsi L.R., Keller R., Tardelli Gomes T.A. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 2002;8(5):508–513. doi: 10.3201/eid0805.010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutija J.F, Ramos C.A., Lemos R.A., Santos A.A., Reckziegel G.H., Freitas M.G., Leal C.R. Molecular and phenotypic characterization of Escherichia coli from calves in an important meat-producing region in Brazil. J. Infect. Dev. Ctries. 2022;16(6):1030–1036. doi: 10.3855/jidc.13377. [DOI] [PubMed] [Google Scholar]
- Yatsuyanagi J., Saito S., Miyajima Y., Amano K.I., Enomoto K. Characterization of atypical enteropathogenic Escherichia coli strains harboring the astA gene that were associated with a waterborne outbreak of diarrhea in Japan. J. Clin. Microbiol. 2003;41(5):2033–2039. doi: 10.1128/JCM.41.5.2033-2039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste M., De La Fuente R., Ruiz-Santa- Quiteria J.A., Cid D., Orden J.A. Detection of the astA (EAST1) gene in attaching and effacing Escherichia coli from ruminants. J. Vet. Med. B. Infect. Dis. Vet. Public Health. 2006;53(2):75–77. doi: 10.1111/j.1439-0450.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- Zajacova Z.S., Konstantinova L., Alexa P. Detection of virulence factors of Escherichia coli focused on prevalence of EAST1 toxin in stool of diarrheic and non-diarrheic piglets and presence of adhesion involving virulence factors in astA positive strains. Vet. Microbiol. 154(3-4), 369–375. 2012 doi: 10.1016/j.vetmic.2011.07.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author, MCG and MCR upon reasonable request.