Abstract
Background:
Gingivitis is a dysbiotic condition characterized by persistent inflammation caused by a disease-associated multispecies bacterial population that has established itself in the subgingival region.
Aim:
The objectives of this study are to analyze the anti-inflammatory activity of sappan wood extract cream (SWC) in Porphyromonas gingivalis induced-periodontitis rats model.
Methods:
In this study, rats were infected with the gingiva with P. gingivalis as an inducer for gingivitis rats model for 14 days. The SWC were treated topically into the infected tissue. The inflammation and angiogenesis of gingival tissue was analyzed using histological examination. An immunohistochemistry (IHC) assay was used to analyze the nuclear factor kappa-B (NF-kB) protein expression. Masson Trichrome (MT) assay was used to analyze the histopathology of collagen Scores. The tumor necrosis factor-α (TNF-α), Interleukin-1 β (IL-1β), IL-6, and P38 genes expression were measured using quantitative real time-polymerase chain reaction.
Results:
The induction of P. gingivalis-induced gingivitis in rats model was indicated by reddish gingiva and bacterial plaque. MT tests showed that SWC increased collagen density, and Haematoxylin and Eosin (H&E) test showed increased angiogenesis and in contrast, lowered inflammation score. IHC test showed that SWC decreased the expression of NF-kB protein. The TNF-α, IL-1β, IL-6, and P38 gene expression were also decreased due to the SWC treatment.
Conclusion:
SWC proves that it has anti-inflammatory activity which has the potential to prevent or treat gingivitis.
Keywords: Antiinflammation, Caesalpinia sappan, Cream, Gingivitis, Oxidative stress
Introduction
Periodontitis is a complex chronic illness that is characterized by ongoing interactions between bacteria and inflammatory responses, which are influenced by both hereditary and extrinsic factors. Even though oral bacterial plaque has traditionally been considered the major cause of periodontal diseases (Kinane et al., 2017). Normally, the presence of a stable periodontal microbiota and a controlled host immune response leads to a condition of dynamic balance or homeostasis. Inflammation causes a decrease in fibroblast number and collagen degradation, which can lead to attachment loss and the creation of periodontal pockets (Naruishi, 2022). When a wound occurs in gingivitis, the cells that play a role in forming the ground substance and forming collagen fibers in closing the wound are fibroblast cells (Sardi et al., 2023). Fibroblasts are the dominant cells in the proliferation phase, forming new connective tissue and synthesizing collagen which affects tensile strength in tissue rejuvenation (Hanna and Giacopelli, 1997). Dysbiosis can impair periodontal tissue homeostasis by causing an imbalance within the microbial ecosystem, resulting in a significant production of Interleukin (IL)-1 members and initiating an over immune response via host receptors (Jia et al., 2019; Papathanasiou et al., 2020).
Cellular immune responses show the role of various cytokines in the process of inflammation of periodontal tissue (periodontitis). Different mediators influence the equilibrium in chronic inflammation. Pro-inflammatory cytokines such as IL-1β, tumor necrosis factor-α (TNF-α), and IL-6 cause periodontal inflammation and tissue damage (Cardoso et al., 2018). Similarly, cytokine-based therapies have the potential to enhance both periodontitis and overall health (Cheng et al., 2020). Mitogen-activated protein kinases (MAPKs), according to some researchers, are the initial signaling mediators of numerous inflammatory stimuli, including IL-1β and TNF-α. Furthermore, P38 MAPK, which plays an important part in the proinflammatory signaling system, can directly or indirectly regulate cytokines. Indeed, P38 MAPK is important in normal immunological and inflammatory responses; several studies have established its participation in the generation of inflammatory cytokines that contribute to chronic inflammation (Lee and Kim, 2017). Nuclear factor kappa beta (NF-kB) controls the inlammasomes and stimulates the production of a number of pro-inflammatory genes, including those encode cytokines and chemokines. As a result, abnormal NF-kB activation leads to the pathogenesis of numerous inflammatory illnesses (Liu et al., 2017).
Metronidazole is a popular periodontitis/gingivitis treatment. Metronidazole is effective against Porphyromonas gingivalis; however, it can induce impaired taste, nausea, ataxia, neurotoxicity, xerostomia, brain illness, headache, seizures, and stomach cramps (Nastri et al., 2019; Vaithiyam et al., 2019). Therefore, a new agent for gingivitis treatment with minimum side effects might be a solution. Natural plants/plant extracts may be an alternative agent for treating the diseases. The main effect of the active substances in plant extracts has the potential as a treatment agent because these substances have antimicrobial activity, are useful as antioxidants, and are active components that can increase cell proliferation, angiogenesis and collagen production (Ghosh and Gaba, 2013).
Plant-derived chemicals have been identified as possible therapeutic agents due to their powerful biological capabilities. Polyphenols are advantageous constituents found in several plant-based. They are categorized into different groups, including flavonoids, polyphenolic amides, proanthocyanidins, as well as additional polyphenols like resveratrol and curcumin (Jayusman et al., 2022). Caesalpinia sappan L., also known as Brazil or Sappan wood, is a plant native to South East Asia and South India. It is a member of the Legingivainosae family. The heartwood of this plant has been historically utilized as a natural red dye, medicine, and a traditional ingredient in food and beverages (Yang et al., 2010).
The examination of sappan wood’s chemical constituents resulted in numerous structural kinds of phenolic components such as brazilin, chalcones, xanthone, homoisoflavonoids, coumarin, and flavones (Nirmal et al., 2015). Research has demonstrated that sappan wood comprises diverse bioactive chemicals that possess anti-inflammatory properties. In this study, the sappan wood extract was made into a cream form to facilitate gingivitis drug delivery. Because of the ease of handling and the stabilization of the cream, the use of cream-containing extract might be one of the solutions for gingivitis treatment orally. Therefore, in this study, we examine the antiinflammatory of sapan wood extract (Caesalpinia sappan L.) in gingivitis by measuring the activity of antiinflammatory, angiogenesis, NF-kB, collagen, also IL-1β, IL-6, TNF-α, and p38 protein expression.
Material and Methods
Sappan wood extract cream (SWC) preparation and liquid chromatography mass spectrometer (LC-MS/MS) assay
The SWC was processed by the Sekolah Tinggi Farmasi Indonesia. The maceration technique was used in the extraction process of Sappan wood (C. sappan L). Fresh sappan wood is dried, crushed and filtered using a 60 mesh sieve. Sappan wood powder was then extracted using 96% ethanol for 4 days. Every 24 hours, the filtrate is collected and evaporated to obtain sappan wood extract (Widowati, 2011). The detection of bioactive component in sappan wood extarct and determined using a LC-MS/MS (Widowati et al., 2023). The formulation of the SWC was from (Sayuti, 2015) with slight modifications. The cream preparations was made with 3.5 g Sodium Carboxymethylcellulose (Na CMC), 3.5 g Vaseline Flavum, 0.1 g Methyl Paraben, 2g Glycerin, 1g Tretanolamine, and 60 mg of SWC and Aquadest up to 100 ml.
Animal grouping and treatment
The rats were obtained from iRATco Veterinary Laboratory Services in Bogor, Indonesia, and ranged in weight from 150 to 200 g with aged 8–12 weeks. Rats were placed in individually ventilated cages with controlled temperatures for 12 hours day/night. Rats were fed a standard basal metabolism for rats with crude protein 18%, fat 6%, fiber 5,5%, and carbohydrate 44,2% ad libitum. Rats in each group were adapted for 7 days.
Thirty-two male Sprague Dawley rats were used in the experiment, and they were randomly divided into six groups with four rats in each group. Negative control (NC): normal rats, Positive control (PC): bacterial-induced gingiva, Base cream group (BC): PC + BC, Comparative control group (CC): PC + Kenalog cream, Sappan Wood Cream 1 (SWC1) group: PC + applied with sappan cream 1 times daily in the morning, Sappan Wood Cream 2 (SWC2) group: PC + applied with sappan cream 2 times daily in the morning and afternoon.
In the gingivitis induction, the bacteria used was P. gingivalis with a concentration of 2 × 108 for each rat. The upper gingiva is drilled and then P. gingivalis bacteria are injected. The bacteria are injected into the gingivas using the Nichimate Stepper Injections. The volume of bacteria injected was 50 µl with a ratio of 1:1. Induction was carried out for 14 days and every 3 days were injected until rats suffered positive gingivitis, characterized by bleeding, swelling, and darkly red gingiva tissue (Wang et al., 2022). After that, the rats were sacrificed for sample collection.
Sample collection
The rat was terminated by injection of ketamine (Ikapharmindo) and xylazine (361453, Interchemie) intraperitoneally. Rats were sacrificed after being deeply anesthetized (Widowati et al., 2022). The rat's jaw was then removed and soaked in 10% Formaline fixation solution for 2 days. The rat jaws were then soaked again in a weak acid solution for the decalcification process for 10 days prior to analysis.
Histopathological examination
A histopathological examination was performed using Haematoxylin and Eosin (H&E). The gingival sample was rinsed with 0.9% physiological NaCl and then fixation for 3 days using 10% formalin. The organs were dehydrated with graded alcohol (70%, 80%, 90%, 95%, and 100%) for 2 hours. Subsequently, the organs were cleansed and dipped into the xylol while being continuously agitated. Then, the organs were immersed in liquid paraffin at 60°C and preserved until they formed paraffin blocks. The paraffin block is sectioned using a microtome (Leica RM 2135 BioCut Rotary Microtome). The paraffin slices were placed onto a slide plate and stained for further analysis (Gondokesumo et al., 2019; Widowati et al., 2022).
Collagen proportion examination by Masson Thricome (MT)
The tissue was stained with MT after being submerged in Weigert's Iron Hematoxylin for 10 minutes, washed with distilled water, and then stained with a solution of Biebrich scarlet acid fuschin for 10–15 minutes. The tissue was then washed with distilled water and stained for 10–15 minutes with a phosphomolybdic-phosphotungstic acid solution. The tissue was dyed for 5–10 minutes with aniline blue solution, washed with Aquadest, and then soaked in 1% acetic acid solution. The tissue was washed with distilled water before to being submerged in 95% and 100% ethanol. A coverslip is placed over the preparation, which is then viewed under a microscope. The quantity of collagen proportions was assessed (Suvik and Effendy, 2012).
Immunohistochemistry (IHC) examination
The IHC assay was carried out using the IHC kit (Elabcience, E-IR-R213) as a secondary antibody. The IHC slide contains gingival tissue were incubated in 3% H2O2 (Elabscience, E-IR-R217C) for 10 minutes and added with Normal Goat Blocking Buffer (Elabscience, E-IR-R217A) at temperature 37°C. The NF-kB Monoclonal antibody (E-AB-22066) was added and incubated overnight at room temperature as a primary antibody. The target proteins were then visualized using Polyperoxidase-anti-Mouse/Rabbit IgG (Elabscience, E-IR-R217B) and DAB substrate was added and incubated for 20 minutes at room temperature. As a counterstaining agent H&E was used. The stained tissues were analyzed using a Zeiss primostar microscope and photographed with a Lumenera infinity 1-3c (Hidayat et al., 2016; Pujimulyani et al., 2020).
Real time-polymerase chain reaction (qRT-PCR) examination of TNF-α, IL-1β, IL-6, and P38 gene expression
The isolation and purification of mRNAs were done using the Direct-zol RNA Miniprep Plus (Zymo, R2073). The mRNAs were reversed into cDNAs using SensiFAST cDNA synthesis kit (BIO-65054). SensiFAST SYBR Green for DNA band visualization was used for gene optimation performed based on the manufacturer’s directions. The quantitative polymerase chain reaction reaction mix was performed using SensiFAST SYBR No-ROX Kit (Meridian Bioscience, BIO-98005) and analyzed using Thermo Scientific’s PikoReal Real-time PCR System (Thermo Fisher). The pre-incubation cycle was set at 95°C for 5 minutes followed by 40 cycles of denaturation at 95°C for 30 seconds. Then for each gene, the annealing process was carried out at 55°C, 53°C, 54°C, 58°C, 55°C with 40 cycles, respectively. Pre-elongated and elongated at 72°CC for 1 minute (Hidayat et al., 2016; Widowati et al., 2018; Pujimulyani et al., 2020). The RNA Purity Concentration and primes used in this study was (Macrogen) as shown in Tables 1 and 2 (Widowati et al., 2023).
Table 1. Concentration and purity of RNA.
| Groups | RNA concentration (ng/ml) |
RNA purity (λ260/λ280 nm) |
|---|---|---|
| NC (normal rats) | 47.80 | 2.2111 |
| PC (bacterial-induced rats) | 54.88 | 2.2039 |
| BC (PC + BC) | 60.65 | 2.4406 |
| CC (PC + Kenalog) | 16.23 | 2.0450 |
| SWC1 (PC + sappan cream 1 time daily) | 22.88 | 2.0927 |
| SWC2 (PC + sappan cream 2 times daily) | 44.05 | 2.1380 |
Statistical analysis
The data analysis was based on One-way ANOVA and Tukey Post Hoc (p < 0.05) using SPSS software (Widowati et al., 2016).
Table 2. Primer sequence of rat IL-1β, IL-6, TNF-α, p38 and GADPH.
| Gene | Sequence (5′–3′) | Product size (bp) |
Annealing (°C) |
References |
|---|---|---|---|---|
| IL-1β | 5′- AGAATACCACTTGTTGGCT-3′ 5′- GTGTGATGTTCCCATTAGAC-3′ |
134 | 55 | NM_031512.2 |
| IL-6 | 5′- GAGCATTGGAAGTTGGGGTA-3′ 5′- TGATGGATGCTTCCAAACTG-3′ |
230 | 53 | NM_012589.2 |
| TNF-α | 5′- GAAGACAATAACTGCACCCA-3′ 5′- AACCCAAGTAACCCTTAAAGTC-3′ |
138 | 54 | NM_012675.3 |
| P38 | 5′-AGATAATGCGTCTGACGGG-3′ 5′-AGGGGATTGGCACCAATAAA-3′ |
139 | 58 | NM_031020.3 |
| GADPH | 5′- GAAGGTGAAGGTCGGAGT-3′ 5′- GAAGATGGTGATGGGATTTC–3′ |
172 | 55 | NM_001357943.2 |
Ethical approval
This research was approved by the iRATCo Veterinary Laboratory with ethical number 4.2.012-3/KEHI//I/2023.
Results
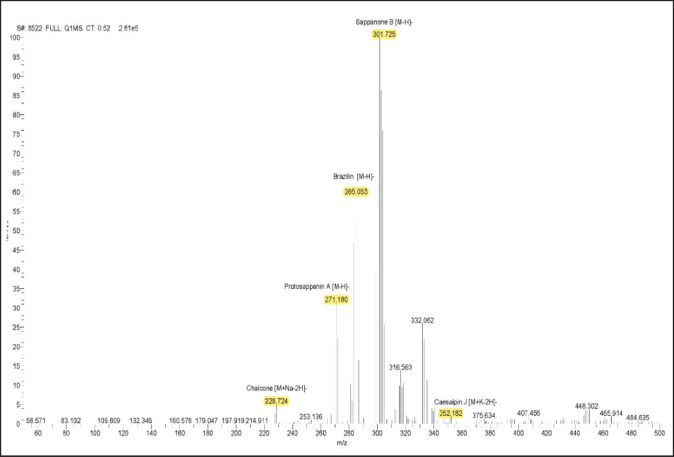
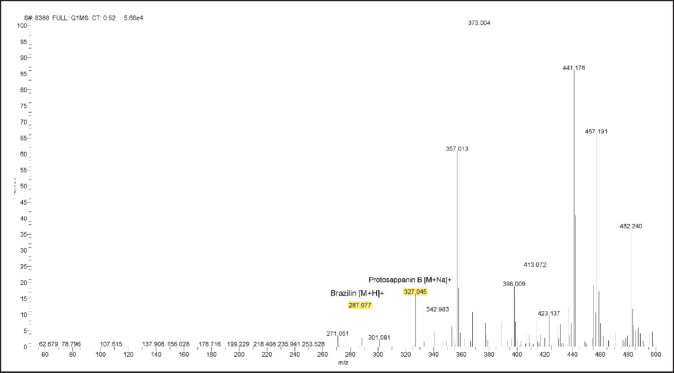
The bioactive compound of sappan wood extract by LC-MS/MS
The chromatogram peak was used to define the different chemicals based on their respective molecular weights (Figs. 1 and 2). Based on our study, there are 6 compounds present in sappan wood extract. The compounds detected in sappan wood extract were Chalcone (208.25 g/mol), ProtosappaninA (272.25 g/mol), Brazilin (286.28 g/mol), Sappanone B (302.28 g/mol), CaesalpinJ (316.30 g/mol), and Protosappanin B (304.29 g/mol).
Fig. 1. LC-MS/MS spectrum of sappan wood extract, mass spectra 50–500 m/z with negative ionisaton.
Fig. 2. LC-MS/MS spectrum of sappan wood extract, mass spectra 50–500 m/z with positive ionization.
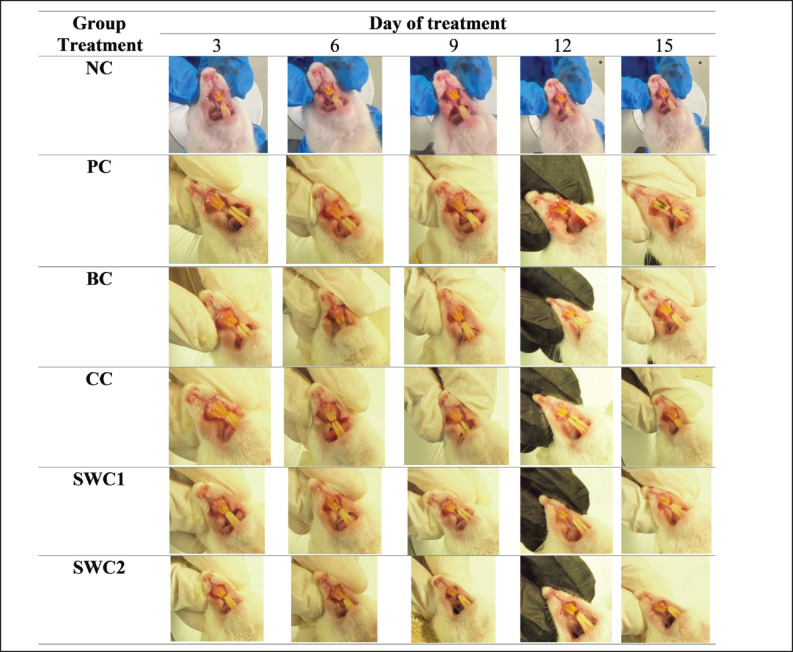
The effect of SWC on macro gingival rat model
Figure 3 shows the effect of SWC on macro gingival morphology after bacterial induction. Based on the results showed that bacterial induction caused gingivitis in the rat model indicated by gingival bleeding and bacterial dental plaque in rat teeth. However, the group treated with SWC 1 and 2 showed macro improvements in the condition of the gingival. There was tissue repairement every day marked by the reduction of the reddish gingiva. It was able to treat the condition of the gingival tissue in rats, although plaque remained but did not cause dental calculus. These results demonstrate that treatment with SWC cream has anti-inflammatory activity that is able to treat the condition of the gingivas and teeth caused by gingivitis.
Fig. 3. Macrogingival morphology in gingivitis rat model treated with SWC. *NC: Negative Control, PC: Positive control (Bacterial induced rats), BC: Base cream (1 × oral BC applied), CC: Comparative control (1× kenalog cream applied), SWC1: Sappan cream 1 (PC + 1 times cream applied), SWC2: Sappan Cream 2 (PC + 2 times cream applied).
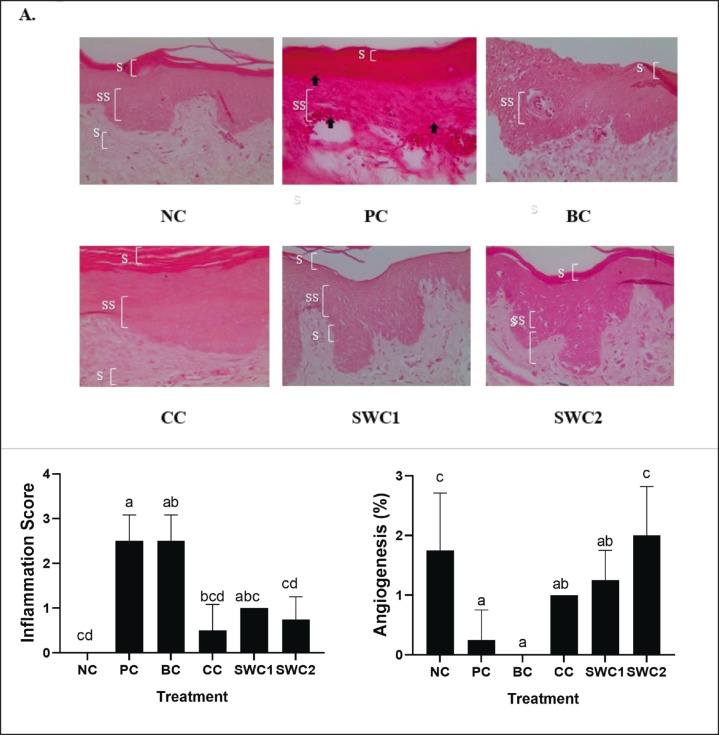
The effect of SWC towards inflammation and angiogenesis of gingival tissue in gingivitis rat model
Figure 4 shows the histopathology of inflammation and angiogenesis of gingival tissue in gingivitis model mice using H&E staining. The bacterial induction caused the damage of the gingival tissue marked by the redness of gingiva (Fig. 4A). The Inflammation and angiogenesis is shown in Figure 4B. The results of angiogenesis showed that in the PC and BC, the inflammation score is increased and the angiogenesis score has no significant differences (p < 0.05). Meanwhile, the sappan cream treatment group has a significantly differences (p < 0.05) compared to PC and BC. Sappan crean treatment was effective in decreasing the inflammation score and in contrast increasing the angiogenesis score. Therefore, the number of fibroblast cells, increasing new blood vessels and reducing the inflammatory process so it was able to help the rat gingival wound healing process. The best result was found in SWC2 groups with 2 times smears of sappan cream.
Fig. 4. Effect of SWC towards histopathology of inflammation and angiogenesis of gingival tissue in gingivitis rat model (40× magnification). *Data are presented as mean ± standard deviation. For each treatment, testing was carried out in four repetitions. *NC: Negative control, PC: Positive control (Bacterial induced rats), BC: Base cream control (1 × oral BC applied), CC: Comparative control (1× kenalog cream applied), SWC1: Sappan cream 1 (PC + 1 times cream applied), SWC2: Sappan cream 2 (PC + 2 times cream applied). Different superscripts (a,ab,abc,bcd,c,cd) mark significant differences among treatment for based on Tukey HSD post hoc test (p < 0.05). The black arrows (indicate the inflammation site on the gingival tissue). SC: stratun corneum; SS: stratum spinosum; SB: stratum basalis.
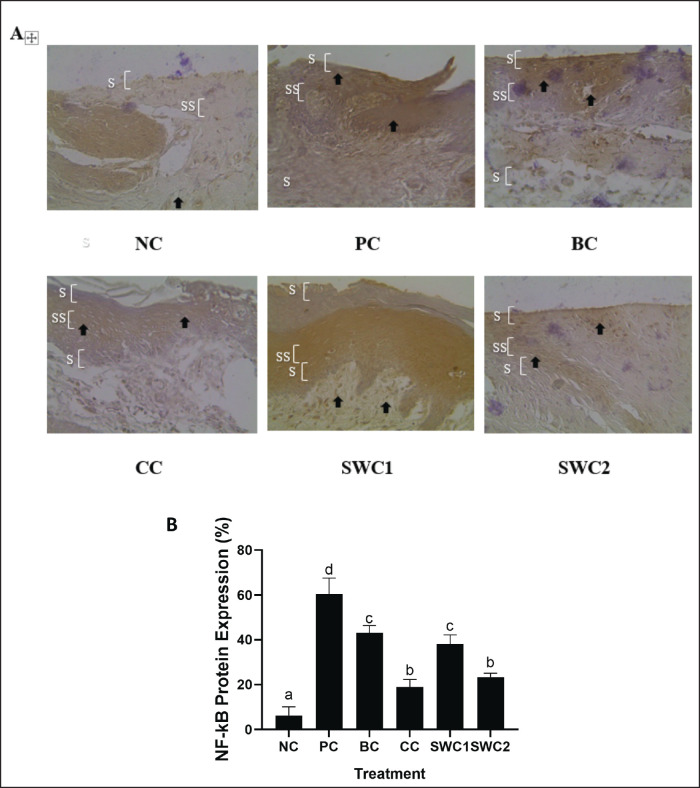
The effect of SWC towards NF-kB protein expression in gingivitis rat model
Figure 5 shows the effect of SWC towards NF-kB protein expression in the gingivitis rat model. The results showed that the gingival tissue treated with SWC is rejuvenated. When compared to SWC1 and SWC2, a darker color indicates more NF-kB expression, which is a symptom of inflammation. The brown color intensity (light brown) is lower in the SWC2 treatments with 2 times smear (Fig. 5A). Based on Figure 5B, the SWC2 treatment group, showed significant differences compared to the PC group (p < 0.05). The NF-kB protein expression in SWC2 was 23.43 ± 1.70 (Fig. 5B). Indicating that sapan wood extract cream may downregulate NF-kB expression.
Fig. 5. Expression of NF-kB protein in gingiva using immunohistochemical methods. *Data are presented as mean ± standard deviation. For each treatment, testing was carried out in four repetitions. * NC: Negative control, PC: Positive control (Bacterial induced rats), BC: Base cream control (1 × oral BC applied), CC: Comparative control (1× kenalog cream applied), SWC1: Sappan cream 1 (PC + 1 times cream applied), SWC2: Sappan cream 2 (PC + 2 times cream applied). Different superscripts (a,b,c,d) mark significant differences among treatments based on Tukey HSD post hoc test (p < 0.05). The black arrows indicate the cell positive with NF-kB protein. SC: stratum corneum; SS: stratum spinosum; SB: stratum basalis.
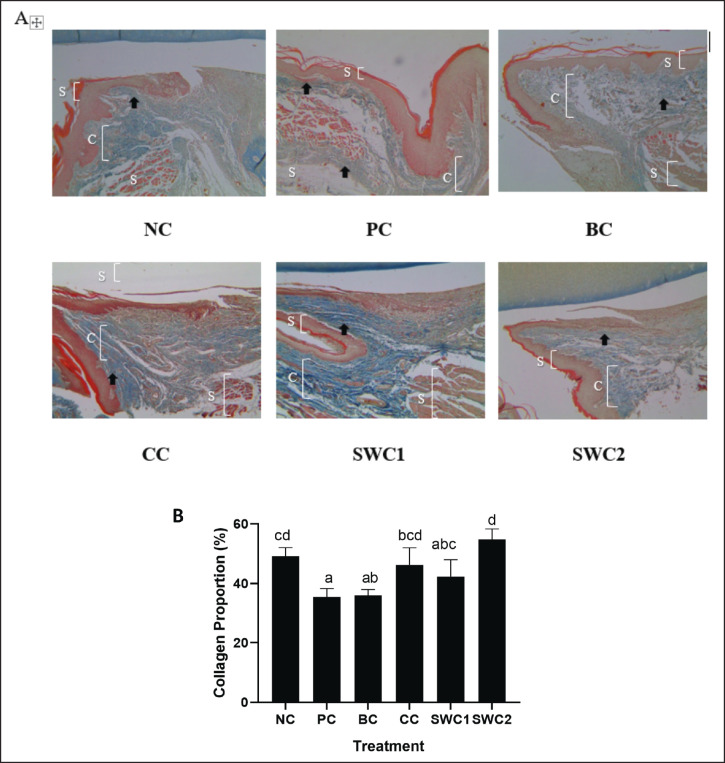
The effect of SWC towards collagen density in gingivitis rat model
Based on Figure 6B, it shows that the collagen score has been calculated based on the results of gingival histopathology images using the MT method. The collagen proportion was indicated in blue colour (Fig. 6A). The graph shows that the SWC2 treatment group had the highest collagen score and was not significantly different from the NC group (normal) (p < 0.05). This indicates that SWC2 treatment is effective in increasing collagen in rat gingival tissue and has the potential to act as an anti-inflammatory agent.
Fig. 6. Collagen score on gingival histopathology using the MT method. *Data are presented as mean ± standard deviation. For each treatment, testing was carried out in four repetitions. * NC: Negative control, PC: Positive control (Bacterial induced rats), BC: BC control (1 × oral BC applied), CC: Comparative Control (1× kenalog cream applied), SWC1: Sappan Cream 1 (PC + 1 times cream applied), SWC2: Sappan Cream 2 (PC + 2 times cream applied). Different superscripts (a,b,c,d) mark significant differences among treatment based on Tukey HSD post hoc test (p < 0.05). The black arrows indicate the collagen site on the gingival tissue. SC: stratun corneum; C: collagen; SB: stratum basalis.
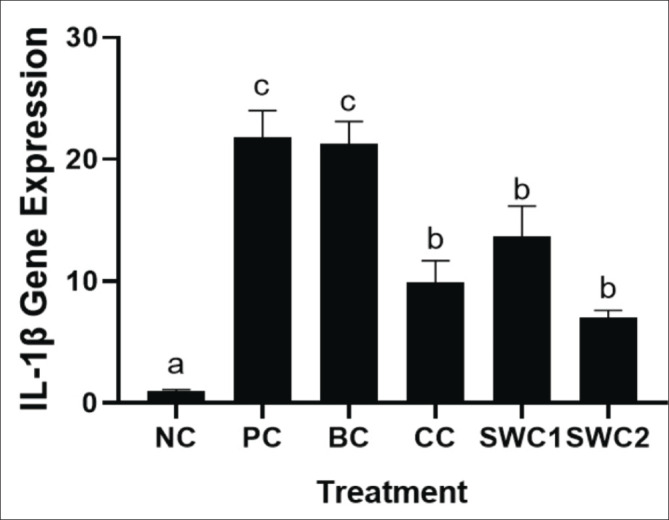
The effect of SWC towards IL-1β gene expression
The IL-1β gene expression in gingivitis rats is shown in Figure 7. The bacterial induced only and BC treatment groups showed a significant increase in IL-1β expression than NC and SWC groups (SWC1 and 2). Treatment with SWC with two topicals can significantly decrease the expression of the IL-1β gene expressions in gingivitis rat model (p < 0.05). Treatment with SWC with two times smear was also the more effective compared to the one-time treatment significantly (p < 0.05).
Fig. 7. Collagen score on gingival histopathology using the MT method. *Data are presented as mean ± standard deviation. For each treatment, testing was carried out in four repetitions. * NC: Negative control, PC: Positive control (Bacterial induced rats), BC: BC control (1 × oral BC applied), CC: Comparative Control (1× kenalog cream applied), SWC1: Sappan Cream 1 (PC + 1 times cream applied), SWC2: Sappan Cream 2 (PC + 2 times cream applied). Different superscripts (a,b,c,d) mark significant differences among treatment based on Tukey HSD post hoc test (p < 0.05). The black arrows indicate the collagen site on the gingival tissue. SC: stratun corneum; C: collagen; SB: stratum basalis.
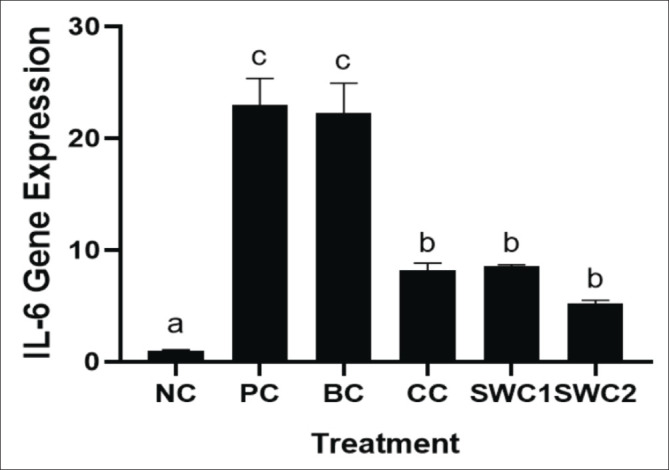
The effect of SWC towards IL-6 gene expression
The IL-6 gene expression after treated using SWC is displayed in Figure 8. Sappan cream can supressed the IL-6 gene expression compared to the PC group significantly (p < 0.05). The SWC2 treatment has shown the lowest IL-6 gene expression (5.18 ± 0.33), that indicated have higher anti-inflammatory activity. In contrast, the positive and BC group has the highest IL-6 gene expressions.
Fig. 8. IL-6 gene expression in rat gingival tissue. *Data are presented as mean ± standard deviation. For each treatment, testing was carried out in four repetitions. * NC: Negative control, PC: Positive control (Bacterial induced rats), BC: Base cream control (1 × oral BC applied), CC: Comparative control (1× kenalog cream applied), SWC1: Sappan cream 1 (PC + 1 times cream applied), SWC2: Sappan cream 2 (PC + 2 times cream applied). Different superscripts (a,b,c,d) mark significant differences among treatment based on Tukey HSD post hoc test (p < 0.05).
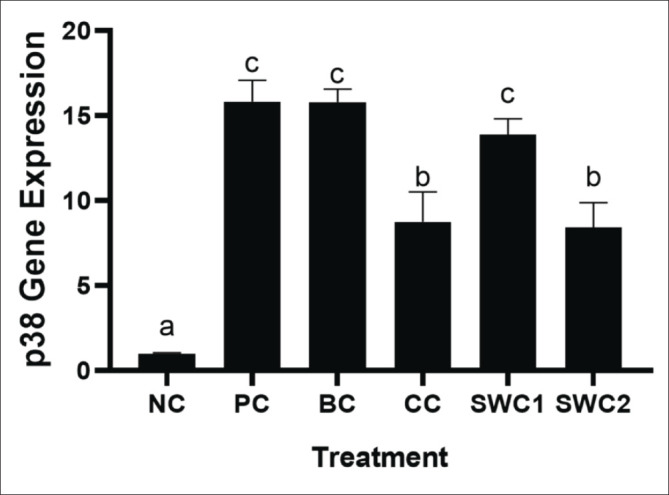
The effect of SWC towards p38 gene expression
Based on qRT-PCR results showed that sappan cream (SWC) can decrease the expression of p38 gene (Fig. 9). The SWC treatment with 2 times applied shows the lowest expressions of p38 (8.41 ± 1.46) significantly compared to the PC and BC groups (p < 0.05). The sappan cream treatment with 2 times smear of SWC was also more effective compared to the one-time smear treatment (p < 0.05).
The effect of SWC towards TNF-α gene expression
The effect of sappan cream (SWC) towards TNF-α gene expression in the gingivitis rat model is presented in Figure 10. The positive groups increased significantly the TNF-α gene expression compared to NC. The SWC groups treatment was found to statistically significant reduce the TNF-α expression compared to PC (p < 0.05). Based on Figure 9, it shows that the gene expression for TNF-α in the SWC2 treatment group (SWC cream 2 times smear) showed the lowest TNF-α gene expression, with a value of 7.86 ± 0.43.
Fig. 10. TNF-α gene expression in rat gingival tissue. *Data are presented as mean ± standard deviation. For each treatment, testing was carried out in four repetitions. * NC: Negative control, PC: Positive control (Bacterial induced rats), BC: Base cream control (1 × oral BC applied), CC: Comparative control (1× kenalog cream applied), SWC1: Sappan cream 1 (PC + 1 times cream applied), SWC2: Sappan cream 2 (PC + 2 times cream applied). Different superscripts (a,b,c,d) mark significant differences among treatment based Dunnet T3 test.
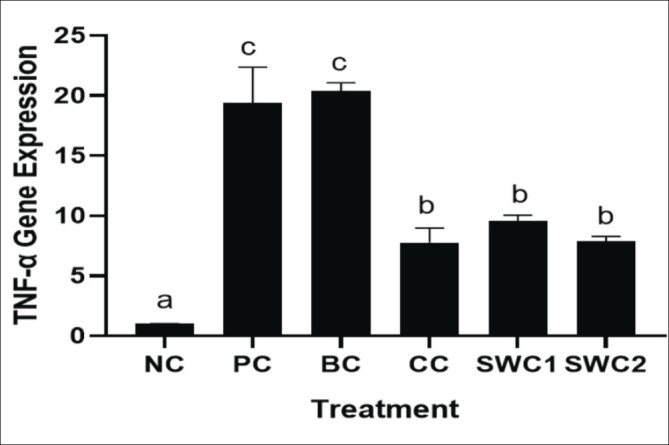
Fig. 9. p38 gene expression in rat gingival tissue. *Data are presented as mean ± standard deviation. For each treatment, testing was carried out in four repetitions. * NC: Negative control, PC: Positive control (Bacterial induced rats), BC: Base cream control (1 × oral BC applied), CC: Comparative control (1× kenalog cream applied), SWC1: Sappan cream 1 (PC + 1 times cream applied), SWC2: Sappan cream 2 (PC + 2 times cream applied). Different superscripts (a,b,c,d) mark significant differences among treatments based on Dunnet T3 test (p < 0.05).
Discussion
Periodontitis is a highly prevalent inflammatory disorder affecting people of all ages; however, it is more common in the elderly (Xu et al., 2020). Periodontitis affects around 50% of adults globally and is most frequent in maturity. The frequency of severe forms is around 10%, with a large rise during the third and fourth decades (Könönen et al., 2019). Categorized as an infectious, long-lasting inflammatory condition affecting the tooth-supporting tissues (Kinane et al., 2017).
The origins and pathophysiology of periodontal disease are explained by the polymicrobial synergy and dysbiosis concept. In a healthy periodontium, bacterial populations are governed by the host's inflammatory response. However, following the development of resistant microorganism such as P. gingivalis, there is a significant increase in the bacterial population and leading to dysbiosis. This, in turn, results in the host's immune response becoming ineffective and damaging to the host's tissue (Hajishengallis and Lamont, 2016). In the healthy oral environtment, P. gingivalis is found in small quantities and activates itself by consuming iron from the blood in swollen gingiva. Porphyromonas gingivalis, once established, can actively contribute to the aggravation of periodontal disease. Because of the expensive expense of synthetic medications, researchers are more identifying bioactive chemicals of natural origin as therapeutics, one of the plant that has the potential to prevent periodontal disease is Caesalpinia sappan. Here, our study highlights the potential antiinflamatory activity of sapan wood extract.
The in vivo assessment was done by administering SWC for 14 days to rats that had been induced by P. gingivalis bacteria. H&E was done for asses the inflammation and angiogenesis score, IHC was done for detecting protein expressions of Nf-KB, and MT staining was used to analyze the collagen proportions in gingivitis rat models.
The gingiva is one of the oral mucossa most susceptible to injury or inflammation. According to our findings, the P. gingivalis bacterial induction causes the gingivitis. The occurrence of gingivitis is characterized by bleeding, edema and/or redness of the gingivas. This is in line with our findings that the rat macrogingiva experience reddish gingivas and bacterial plaque and there is repairment of the gingiva together with SWC treatment (Fig. 3). The redness gingiva tissue is a sign of inflammation that is mediated to excessive injury (Zhang et al., 2018).
According to our results, the rat treated with SWC with 2 times smear had a lower score of inflammatory, angiogenesis, and expression of NF-kB protein. This finding is correspondent with previous study found that, Sappan wood extract is shown to have pain-relieving effects, which can also assist in alleviating damaged caused by inflammation (Sa’diyah, 2018). In this study, sappan wood extract is proven to suppress NF-kB, the factor transcription that control the expression of genes associated with inflammation. The IHC assay revealed that sappan cream also can decrease the NF-kB protein expressions. This means that the transcription pathway for pro-inflammatory protein related to gingivitis could be suppressed.
As known before that sappan wood extract contains flavonoid polyphenolic compounds. These flavonoid compounds are thought to play a role in the ability to inhibit angiogenesis. The angiogenesis inhibitory action of flavonoids in the methanolic extract of sappan wood is thought to be through several mechanisms (Kolamala and Chava, 2023). In previous research it has been proven that flavonoid compounds, resveratrol and quercetin are able to inhibit aspects of angiogenesis (proliferation, migration of endothelial cells and formation of blood vessel pipes). The angiogenesis process is blocked by the COX-2 which plays a role in the angiogenesis process through the synthesis of prostaglandin (Kolamala and Chava, 2023). This is are in line with our study result showed that the angiogenesis of cell were increased following SWC treatment.
The results of this investigation indicate that SWC increased angiogenesis and collagen proportion in the gingivitis rats induced by P. gingivalis induction. The evidence was shown in the collagen density in the gingiva of periodontitis rats treated with SWC, which were higher than that in periodontitis rats given Kenalog cream. Collagen breakdown is a critical physiologic mechanism involved in wound healing and tissue remodeling (Golub and Lee, 2020). The progression of disease is influenced by a number of associated molecular pathways, including growth factors, cytokines, and Matix Mettaloprotease (MMPs), as well as the regulators and inhibitors of these pathways (Binderman et al., 2017). The MMPs generated by inflammatory cells are the main host factor responsible for degradation of marginal gingiva collagen. In chronic periodontitis, the breakdown of the collagenous fibrous tissue of the marginal gingiva speeds up the resorption of alveolar bone (Tewtrakul et al., 2015). A study reported that sapan wood extract acts as a collagen receptor and involved in wound healing. An in vitro study reported that ethanolic sappan wood extract could increased type-I collagen production significantly by the L929 cells (Sobocki et al., 2022).
Gingivitis is a prolonged inflammatory response that is primarily caused by gram-negative bacteria. The increase in the synthesis and release of different proinflammatory factors results in excessive damage to gingivitis (Zhang et al., 2018). Proinflammatory factors including TNF α, IL-1, IL-6, and INF-γ have been found to be strongly associated with hemostasis, particularly in the development of dental plaques (Sobocki et al., 2022). Porphyromonas gingivalis' virulent component, a cysteine protease called gingipain, can cleave pro-MMP9 into its active form MMP9 (Zhou et al., 2015). Metalloproteinase (MMP) families degrade the extracellular matrix, basement membrane, and collagen degradation which promotes neoplastic cell invasion. Porphyromonas gingivalis gingipains can also activate PAR2 and PAR4, resulting in IκB phosphorylation, NF-κB nuclear translocation, p38 activation, and enhanced pro-MMP9 synthesis (Masi et al., 2011). Besides that, oxidative stress is taking a part in the emergence of periodontal diseases (Tamaki et al., 2014). Oxidative stress can lead to gingival damage due to the excessive production of ROS. This can occur through various pathways, including lipid peroxidation, DNA damage, protein degradation, oxidation of crucial enzymes, and the activation of proinflammatory cytokines (Vij et al., 2023; Zheng et al., 2023).
IL-1β is a pro-inflammatory cytokine that is taking a part in inflammatory mechanisms, this cytokine must be suppressed so that it can reduce the inflammatory process in the body (Rehman and Akash, 2016). TNF-α is regarded as the most important inflammatory cytokine, TNF-α inhibitors are now utilized as treatment medicines for certain disorders (Jang et al., 2021). IL-6 is a vital role in inflammation and its inhibitors are currently being employed as therapeutic medicines for a variety of disorders, with the potential to expand to more in the future, inhibiting IL-6 activity is useful against inflammatory disorders (Hirano, 2021).
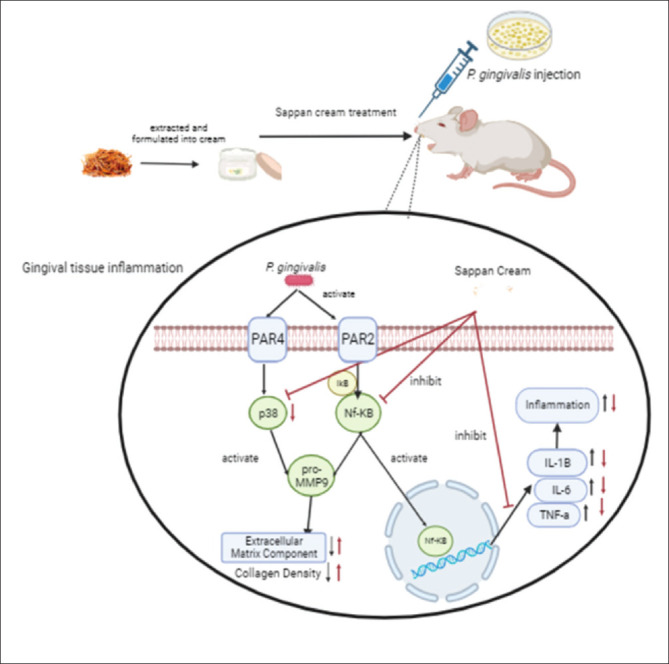
SWC has the ability to suppress gene expression cytokines in the inflammatory process. According to Sa’diyah (2018), secang wood extract reduces levels of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and p38 in serum. These cytokines have a central role in chronic inflammation and tissue damage. Secang wood extract exhibits anti-inflammatory properties through numerous mechanisms such as inhibition of p38 MAPK pathway and regulation of NF-kB which also inhibits the pro-inflammatory cytokines (Nirmal et al., 2015). This is in line with this study, which revealed that sappan cream could decrease the TNF-α, IL-1β, IL-6, and p38 gene expressions compared to the PC. This result are in line with the IHC assay showed that Nf-KB protein expression was decreased following by the SWC treatment. The suppression of Nf-KB expression will affect the transcriptional pathway of TNF-α, IL-1β, IL-6, and p38 gene to be expressed in the cells. The proposed mechanism of sappan cream as anti-inflammatory agent is explained in Figure 11. However, another method should be added for validation and further experiments to explore the precise mechanism.
Fig. 11. Proposed Mechanism of sappan cream in reducing the anti-inflammatory markers of gingivitis rat models. *P. gingivalis injection caused gingival inflammation. It is activated Nf-KB and p38 pathway and increase the pro-MMP9 production. Nf-KB pathway also induce the inflammation by producing the of pro-inflamamtory cytokines expressions. The sappan cream treatment could reduce the inflammatory markers evaluated by RT-PCR and IHC.
Overall, the sappan cream has the best effect on the animal tests compared to the basis cream even the comparative product. This is due to the biologically active compound of SWC. The active compound contained in cream have a significant effect in the collagen density and angiogenesis, as shown by basis cream who don’t have any active compound inside. In contrast, sappan cream contain bioactive compound such as flavonoid that enhanced the regeneration of gingival tissues, reduced inflammation in gingivitis. Further clinical study must be done to confirm its effect on human.
In conclusion, SWC has anti-inflammatory activity with 2 times smear has the best results to suppress inflammation,, and decrease the expression of IL-1β, IL-6, TNF-α, and p38 pro-inflammatory cytokines. In contrast, it also has the ability to increase the collagen density and angiogenesis.
Acknowledgments
The authors are thankful to the Maranatha Christian University for providing funds for this research (Skema Percepatan Guru Besar) with research grant Number 007/PRJ-GB/UKM/I/2023. The authors are also grateful to Aretha Medika Utama, Bandung, West Java, Indonesia, and iRATCo Veterinary Laboratory which supplied the laboratory facilities and their assistance.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This research is funded by Maranatha Christian University (Skema Percepatan Guru Besar) with research grant Number 007/PRJ-GB/UKM/I/2023.
Authors’ contributions
VKS, JJ, WW, and RF designed the experiments; RF, DMM, and DSH performed experiments and collected data; VKS, NSMD, DMM, and DSH analyzed and interpretation of the data; VKS, WW, NSMD, JJ, and RF drafted the manuscript; VKS, WW, RF Analyzed the data, Supervised and directed the study; VKS, JJ, WW, NSMD, RF, DMM, and DSH Final approved of the version to be published.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
- Binderman I., Gadban N., Yaffe A. Extracellular ATP is a key modulator of alveolar bone loss in periodontitis. Arch. Oral Biol. 2017;81:131–135. doi: 10.1016/j.archoralbio.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Cardoso E.M., Reis C., Manzanares-Céspedes M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018;130(1):98–104. doi: 10.1080/00325481.2018.1396876. [DOI] [PubMed] [Google Scholar]
- Cheng R., Wu Z., Li M., Shao M., Hu T. Interleukin-1β is a potential therapeutic target for periodontitis: a narrative review. Int. J. Oral Sci. 2020;12(1):1–9. doi: 10.1038/s41368-019-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P.K., Gaba A. Phyto-extracts in wound healing. J. Pharm. Pharm. Sci. 2013;16(5):760–820. doi: 10.18433/j3831v. [DOI] [PubMed] [Google Scholar]
- Golub L.M., Lee H.M. Periodontal therapeutics current host-modulation agents and future directions. Periodontol. 2020;2000(82):186–204. doi: 10.1111/prd.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondokesumo M.E., Pardjianto B., Handono K., Sumitro S.B., Widowati W., Wismandanu O., Hartady T., Rosdianto A.M., Goenawan H., Lesmana R., Wathoni N., Supratman U. Garcinia mangostana extract enhances skin epithelialization in rat induced burn injury. Pak. Vet. J. 2019;39(3):365–370. [Google Scholar]
- Hajishengallis G., Lamont R.J. Nibali L., Henderson B. In The human microbiota and chronic disease: dysbiosis as a cause of human pathology. Hoboken, NJ: Willey Publisher; 2016. The polymicrobial synergy and dysbiosis model of periodontal disease pathogenesis; pp. 227–242. [Google Scholar]
- Hanna J.R., Giacopelli J.A. A review of wound healing and wound dressing products. J. Foot. Ankle. Surg. 1997;36(1):2–14. doi: 10.1016/s1067-2516(97)80003-8. [DOI] [PubMed] [Google Scholar]
- Hidayat M., Prahastuti S., Fauziah N., Maesaroh M., Balqis B., Widowati W. Modulation of adipogenesis-related gene expression by ethanol extracts of detam 1 soybean and jati belanda leaf in 3T3-L1 cells. Bangladesh J. Pharmacol. 2016;11(3):697–702. [Google Scholar]
- Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021;33(3):127–148. doi: 10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang D.I., Lee A.H., Shin H.Y., Song H.R., Park J.H., Kang T.B., Lee S.R., Yang S.H. The role of tumor necrosis factor alpha (Tnf-α) in autoimmune disease and current tnf-α inhibitors in therapeutics. Int. J. Mol. Sci. 2021;22(5):1–16. doi: 10.3390/ijms22052719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayusman P.A., Nasruddin N.S., Mahamad Apandi N.I., Ibrahim N., Budin S.B. Therapeutic potential of polyphenol and nanoparticles mediated delivery in periodontal inflammation: a review of current trends and future perspectives. Front. Pharmacol. 2022;13(7):1–17. doi: 10.3389/fphar.2022.847702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Han N., Du J., Guo L., Luo Z., Liu Y. Pathogenesis of important virulence factors of Porphyromonas gingivalis via toll-like receptors. Front. Cell. Infect. Microbiol. 2019;9(7):1–14. doi: 10.3389/fcimb.2019.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane D.F., Stathopoulou P.G., Papapanou P.N. Periodontal diseases. Nat. Rev. Dis. 2017;3(6):1–14. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- Kolamala N., Chava V.K. Collagen degradation in periodontal health and disease: a brief review. Essent. Dent. 2023;2(1):39–44. [Google Scholar]
- Könönen E., Gursoy M., Gursoy U.K. Periodontitis: a multifaceted disease of tooth-supporting tissues. J. Clin. Med. 2019;8(8):1135. doi: 10.3390/jcm8081135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Kim N.J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules. 2017;22(8):1287. doi: 10.3390/molecules22081287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2(3):1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi S., Salpea K.D., Li K., Parkar M., Nibali L., Donos N., Patel K., Taddei S., Deanfield J.E., DAiuto F., Humphries S.E. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic. Biol. Med. 2011;50(6):730–735. doi: 10.1016/j.freeradbiomed.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Naruishi K. Biological roles of fibroblasts in periodontal diseases. Cells. 2022;11(21):3345. doi: 10.3390/cells11213345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastri L., De Rosa A., De Gregorio V., Grassia V., Donnarumma G. A new controlled-release material containing metronidazole and doxycycline for the treatment of periodontal and peri-implant diseases: formulation and in vitro testing. Int. J. Dent. 2019;2019(1):9374607. doi: 10.1155/2019/9374607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmal N.P., Rajput M.S., Prasad R.G.S.V., Ahmad M. Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: a review. Asian Pac. J. Trop. Med. 2015;8(6):421–430. doi: 10.1016/j.apjtm.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Papathanasiou E., Conti P., Carinci F., Lauritano D., Theoharides T.C. IL-1 superfamily members and periodontal diseases. J. Dent. Res. 2020;99(13):1425–1434. doi: 10.1177/0022034520945209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujimulyani D., Yulianto W.A., Setyowati A., Arumwardana S., Kusuma H.S.W., Sholihah I.A., Rizal R., Widowati W., Maruf A. Hypoglycemic activity of curcuma mangga val. Extract via modulation of GLUT4 and ppar-γ mrna expression in 3T3-L1 adipocytes. J. Exp. Pharmacol. 2020;12:363–369. doi: 10.2147/JEP.S267912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K., Akash M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J. Biomed. Sci. 2016;23(1):1–18. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa’diyah I.F. Efek Anti-Angiogenesis Ekstrak Kayu Secang Sebagai Terapi Adjuvant pada Diabetes Retinopati. Saint. Med. 2018;14(2):114–118. [Google Scholar]
- Sardi N.W.A., Adnyasari N.L.P.S.M., Ekasari N.P.R.B. Gel extraction of earthworms (Lumbricus rubellus) to the number of fibrobal cells in male Wistar rats (Rattus norvegiccus) gingival wound healing. IJKG. 2023;19(1):34–42. [Google Scholar]
- Sayuti N.A. Formulasi dan Uji Stabilitas Fisik Sediaan Gel Ekstrak Daun Ketepeng Cina (Cassia alata L.) JKI. 2015;5(2):74–82. [Google Scholar]
- Sobocki B.K., Basset C.A., Ka K. Molecular mechanisms leading from periodontal disease to cancer. Int. J. Mol. Sci. 2022;23(2):970. doi: 10.3390/ijms23020970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvik A., Effendy A.W.M. The use of modified Masson’ S trichrome staining in collagen evaluation in wound healing study. Malaysian J. Vet. Res. 2012;3(1):39–47. [Google Scholar]
- Tamaki N., Cristina Orihuela-Campos R., Inagaki Y., Fukui M., Nagata T., Ito H.O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014;75:222–229. doi: 10.1016/j.freeradbiomed.2014.07.034. [DOI] [PubMed] [Google Scholar]
- Tewtrakul S., Tungcharoen P., Sudsai T., Karalai C., Ponglimanont C., Yodsaoue O. Antiinflammatory and wound healing effects of Caesalpinia sappan L. Phytother. Res. 2015;29(6):850–856. doi: 10.1002/ptr.5321. [DOI] [PubMed] [Google Scholar]
- Vaithiyam V., Jadon R.S., Ray A., Manchanda S., Meena V.P., Ranjan P., Vikram N.K. Metronidazole induced encephalopathy: a rare side effect with a common drug. Indian J. Radiol. Imaging. 2019;29:431–434. doi: 10.4103/ijri.IJRI_330_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij T., Anil P.P., Shams R., Dash K.K., Kalsi R., Pandey V.K., Harsányi E., Kovács B., Shaikh A.M. A comprehensive review on bioactive compounds found in Caesalpinia sappan. Molecules. 2023;28(17):6247. doi: 10.3390/molecules28176247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fan Q., Xu Y., Zeng F., Liu X., Zhao D., Zhang L., Bai G. Effect of Eucommia water extract on gingivitis and periodontitis in experimental rats. BMC Oral Health. 2022;22(1):1–9. doi: 10.1186/s12903-022-02353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widowati W. Uji Fitokimia dan Potensi Antioksidan Ekstrak Etanol Kayu Secang (Caesalpinia sappan L.) JMH. 2011;11(65):23–31. [Google Scholar]
- Widowati W., Afifah E., Mozef T., Sandra F., Rizal R., Amalia A., Arinta Y., Bachtiar I., Murti H. Effects of insulin-like growth factor-induced Wharton jelly mesenchymal stem cells toward chondrogenesis in an osteoarthritis model. Iran. J. Basic Med. Sci. 2018;21(7):745–752. doi: 10.22038/IJBMS.2018.28205.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widowati W., Darsono L., Lucianus J., Setiabudi E., Susang Obeng S., Stefani S., Wahyudianingsih R., Reynaldo Tandibua K., Gunawan R., Riski Wijayanti C., Novianto A., Sari Widya Kusuma H., Rizal R. Butterfly pea flower (Clitoria ternatea L.) extract displayed antidiabetic effect through antioxidant, anti-inflammatory, lower hepatic GSK-3β, and pancreatic glycogen on diabetes mellitus and dyslipidemia rat. J. King Saud Univ. Sci. 2023;35(4):102579. [Google Scholar]
- Widowati W., Darsono L., Suherman J., Fauziah N., Maesaroh M., Erawijantari P.P. Anti-inflammatory effect of mangosteen (Garcinia mangostana l.) peel extract and its compounds in lps-induced raw264.7 cells. Nat. Prod. Sci. 2016;22(3):147–153. [Google Scholar]
- Widowati W., Wargasetia T., Rahardja F., Gunanegara R., Priyandoko D., Gondokesumo M., Afifah E., Wijayanti C., Rizal R. Human Wharton’s jelly mesenchymal stem cells inhibit cytokine storm in acute respiratory distress syndrome in a rat model. Asian Pac. J. Trop. Biomed. 2022;12(8):343–350. [Google Scholar]
- Xu W., Zhou W., Wang H., Liang S. Roles of Porphyromonas gingivalis and its virulence factors in periodontitis. Adv. Protein Chem. Struc. Biol. 2020;120:45–84. doi: 10.1016/bs.apcsb.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Zhongzhi Q., Yanze L., Guoping Z., Yong P., Peigen X. New collection of crude drugs in Chinese Pharmacopoeia 2010 I. Callicarpa Linn. And related items. Chin. Herb. Med. 2010;2(4):272–288. [Google Scholar]
- Zhang F., Geng Y., Zhao H., Wang H., Zhang Y., Li D., Bian B., Yang H. Effects of Huanglian Jiedu decoration in rat gingivitis. Evid. Based. Complement. Alternat. Med. 2018;2018(1):8249013. doi: 10.1155/2018/8249013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Chen J., Rao N., Yang C., Liu J., Zhang J., Li Y. The effect of an extract of sappanwood, protosappanin A and protosappanin B on osteogenesis in periodontitis. Front. Biosci. (Landmark Ed). 2023;28(8):172. doi: 10.31083/j.fbl2808172. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Sztukowska M., Wang Q., Inaba H., Potempa J., Scott D.A., Wang H., Lamont R.J. Noncanonical activation of β-catenin by Phorpyromonas gingivalis. Am. Soc. Microbiol. 2015;83(25):3195–3203. doi: 10.1128/IAI.00302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.