Abstract
Background:
After the first Avian Influenza H5N1 outbreak in Nigerian poultry in 2006, subsequent waves of outbreaks occurred, causing substantial losses. Despite effective control measures by 2008, a resurgence in 2015 led to further losses and required depopulation efforts.
Aim:
The aim of this study was to do pathology and molecular detection of influenza A subtype H9N2 virus in commercial poultry in Nigeria during 2024.
Methods:
In February 2024, a poultry farmer reported high mortality in his mixed commercial poultry flock in Ibadan, Nigeria, submitting carcasses to the University of Ibadan’s V.T.H. and the FAO Regional Laboratory at National Veterinary Research Institute (NVRI), Vom.
Results:
Necropsy of nine Isa Brown layers and three Abor Acre broilers revealed cyanosis of comb and wattles, generalized petechial and ecchymotic hemorrhages including shank hemorrhages with sinusitis, pneumonia, and severe greenish fecal pasting also observed. At histopathology, denudation of the tracheal epithelia and parabronchial epithelial necrosis, obliteration, with airsac edema and emphysema were observed. At NVRI, qPCR detected an Influenza A H9N2 virus in several pooled organ samples of layers, and broilers and eliminating the avian infectious bronchitis and Newcastle disease viruses.
Conclusion:
This is the first report of an H9N2 outbreak in commercial poultry in Southern Nigeria. The high pathogenicity shown in commercial poultry in this outbreak and the risk of dispersal of infected live poultry in Nigeria as previously seen in H5N1 require stakeholders’ intervention.
Keywords: Commercial poultry, LPAI H9N2 virus, Pathology, Nigeria
Introduction
Low Pathogenic Avian influenza (LPAI) is a reportable disease caused by influenza A viruses of the H5, H7, and H9 subtypes that have become a major source of concern to the poultry industry worldwide (Swayne et al., 2011). Infections with LPAI viruses (LPAIVs) are often unnoticed or asymptomatic, while the HPAI viruses cross respiratory and intestinal barriers, and cause viremia, severe systemic infections, pathology birds, high morbidity, and mortality rates (Simancas-Racines et al., 2023). LPAIVs are known to have a low mortality rate and low ability to infect and cause disease but most will cause mild to moderate disease in commercial poultry, especially when complicated by secondary bacterial pathogens, immunosuppression, and stress factors in the environment. The H9N2 subtype has the ability to infect and efficiently spread in domestic bird species despite their low pathogenic phenotype and Infections with these viruses have been known to result in severe economic losses (Joseph et al., 2019). Avian Influenza pathogenicity is determined by the lethality, clinical signs, and molecular characteristics of the virus. In countries in which H9N2 has been shown to cause disease in poultry, high mortality, and severe clinical signs have been observed but these have been attributed to co-infections with other viruses (Roussan et al., 2008; Sid et al., 2015; Hassan et al., 2016; Hassan et al., 2017; Broomand et al., 2018; Arafat et al., 2018) or bacteria (Jaleel et al., 2017). Pathological lesions caused by the H9N2 virus in these reports included generalized petechiation, ecchymoses and shank hemorrhages, tracheal necrosis, and pneumonia. Drops in egg production have also been observed in chicken breeders and layers infected with these viruses (Lu et al., 2004; Pillai et al., 2009). LPAIVs of the H9 subtype have been circulating in avian species resulting in severe economic losses (Kayali et al., 2013) in several countries across the world, and in Africa since the year 2000 (Tombari et al., 2011, Monne et al., 2013, Kammon et al., 2015). H9N2 virus has become endemic in poultry in Lebanon, Jordan, Egypt, Tunisia, Saudi Arabia, and the UAE (Kayali et al., 2013). Recently, there have been reports of infection of poultry with the virus in the West African countries of Burkina Faso (Zecchin et al., 2017), Ghana (Joseph et al., 2019), Benin Republic and Togo, but not in Nigeria. Since the first incursion of HPAI H5N1 virus in commercial poultry in Nigeria in 2006 (Akanbi et al., 2017) and the several waves of outbreaks leading to the effective control in 2008, Nigeria lost a huge number of its poultry birds directly due to the virus, and its depopulation control measures. A resurgent H5N1 outbreak and spread occurred in 2015 (Akanbi et al., 2017) and several subtypes and reassortant strains of AI including H5N1 (Fusaro et al., 2010), H5N2, H5N8 (Fusaro et al., 2019; Laleye, 2021) and lately H5N6 (Shittu et al., 2020) have been detected in live bird markets (LBMs) across the country. In 2019, surveillance and molecular epidemiology revealed the presence of the H9N2 virus in LBMs in Nigeria. Nineteen H9N2 viruses were detected and isolated from apparently healthy poultry species, during active surveillance in nine LBMs across Nigeria (Sulaiman et al., 2021). Earlier, seroconversion to the H9 subtype had been reported in sampled poultry birds at LBMs in a limited study in southwestern Nigeria (Aiki-Raji et al., 2015; Oluwayelu et al., 2017), and more recently, in local chickens at a LBM in the northern Nigerian city of Zaria (Wungak et al., 2021). However, in all these studies and reports, there was no isolation and molecular detection of the H9N2 virus, as the cause of disease or death in commercial poultry in Nigeria. In this study, we report a disease outbreak characterized by unprecedented high morbidity, and mortality in a medium-sized, mixed commercial poultry flock in Ibadan, southwest Nigeria. Detailed pathological, and molecular investigations revealed the etiological agent of this outbreak to be a LPAI H9N2 virus, and in the light of numerous evidence that the H9N2 virus may have a direct or indirect role in the emergence of the next influenza pandemic, despite being rather neglected when compared to the H5 and H7 subtypes (Carnaccini and Perez, 2020), urgent attention is required to stem the tide of this outbreak.
Materials and Methods
Case history
On 25th February 2024, 12 carcasses comprising 9 Isa brown layers, and 3 Abor Acre broilers aged 2, 22, 54, and 95 weeks old were submitted by a poultry farmer who reported high mortality in his medium-sized mixed commercial poultry flock in Ibadan, southwest Nigeria. A total of sixteen thousand eight hundred (16,800) chickens were housed in four (4) pens on the farm. Daily average mortality was 270 and a weekly rate of 1800 chickens. The vaccination history included the oral administration of the bivalent Newcastle Disease virus (NDV) LaSota strain and infectious bronchitis virus (IBV) vaccine on the 17th of February 2024. The mortality was reported to have started five (5) days (22nd February 2024) after the oral vaccination and a day after heavy rain. This mortality started in pen house number 3 (95 weeks layers) with a loss of 5, 17 then 150 birds per day before averaging 220 deaths daily. Subsequently, 500-layer chickens in pen house number 1 were lost in three days, and finally pen house number 2 lost 27-layer chickens in two days. Mortality started in the broilers pen house 4 on the evening of 24th February 2024. The broilers were reported to be on oral administration of anticoccidial, Diclazuril for five days just before the onset of the disease. On the 3rd day post submission of carcasses, the client reported additional mortality, bringing the total mortality to 4,526 across the entire farm. The epidemiological investigation revealed a poultry waste/manure up taker truck visited the farm less than a week before the outbreak, from northern Nigeria.
Case location
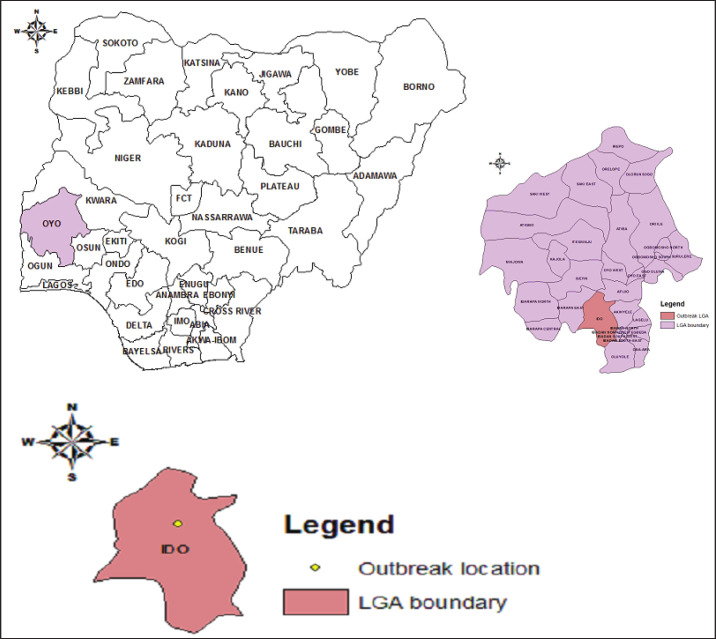
The disease outbreak occurred in Ibadan city, the capital of Oyo state within the Ido local government area of the state (Fig. 1). Oyo state is bounded in the north by Kwara State, in the east by Osun State, in the south by Ogun State and in the west partly by Ogun State and partly by the Republic of Benin. The state is ranked 14th by size with an area of 28,454 square kilometers. The average daily temperature ranges from 25°C to 35°C almost throughout the year with a relatively high humidity. The common occupation of the people of the state is agriculture. Ibadan is a beehive of commercial poultry activities housing many large poultry farms. Furthermore, the largest hatcheries in the country are in Oyo state. The outbreak of the H9N2 poses a serious threat to commercial poultry production in Nigeria as there is constant free movement of birds from the state to other parts of the country.
Fig. 1. Map of the location of the detected H9N2 virus in Ido LGA, Ibadan, Oyo State, Nigeria.
Postmortem examination and histopathology
Submitted carcasses were examined grossly for pathological changes at the Department of Veterinary Pathology, Veterinary Teaching Hospital, University of Ibadan, and at the Central Diagnostic Laboratory of the National Veterinary Research Institute (NVRI), Vom, and tissues samples including comb, trachea, lung, heart, spleen, proventriculus, liver and intestine were harvested for histopathology, while lungs, trachea, liver, and spleen samples were harvested for virological examination and qPCR for virus detection at the Regional Laboratory for Animal Influenza and Transboundary Animal Disease, NVRI Vom. Tissues with pathological changes were fixed in 10% buffered formaldehyde, and processed in different concentrations of alcohol, followed by paraffin embedding and sectioned at 5 μm using a Micron micrometer. Tissue sections were mounted on frosted glass slides and stained with hematoxylin and eosin (H&E) stains for histopathological examination using low and high-powered field Carl Zeiss binocular microscope as previously described (Akanbi et al., 2017).
Tissue homogenate preparation
In the laboratory, the tissues were pooled and homogenized in an antibiotic mixture containing Penicillin (2,000 units/ml), Streptomycin (2 mg/ml), Gentamycin (50 μg/ml), and Amphotericin B (5 mg/ml) as contained in the WOAH Manual of Diagnostics. The homogenate was clarified by centrifugation at 3,000 rpm for 10 min, tissue supernatant was collected and stored at −80°C until used for viral extraction.
Viral RNA extraction
Total viral RNA was extracted from the homogenized tissue sample using the QIAamp Viral RNA Mini Kit (QIAGEN, Germany), following the manufacturer’s protocol as follows. A total of 140 μl of the sample was lysed by adding a mixture of 560 μl of buffer AVL and carrier RNA, after 10 minutes of incubation, 560 μl of 100% ethanol was added. Successive aliquots of 630 μl were added to a QIAamp spin column and centrifuged at 8,000 rpm for 1 minute. The bound RNA on the QIAamp spin column was washed with 500 μl of buffer AW1 and centrifuged at 8,000 rpm for 1 minute. This was followed by 500 μl of buffer AW2 and then centrifuged at 14,000 rpm for 3 minutes. AIV H5N1 (IZSVe, Padua, Italy) and nuclease-free water were extracted as positive and negative controls. The RNAs extracts (sample and controls) were then eluted in 60 μl of buffer AVE and stored at −80°C until qPCR analysis.
AIV Real-time RT-PCR amplification matrix gene
Qiagen QuantiTect® Multiplex qPCR Kit was used to amplify the different regions within the matrix (M) gene which is well-conserved among all avian influenza A virus subtypes (Spackman et al., 2002). The qPCR reaction consisted of 4.8 μl nuclease-free water, 12.5 μl 2× QuantiTect® Multiplex qPCR buffer, 1.5 μl of each primer (5 μM), 2.5 μl probe (1 μM), 1 μl of the enzyme mix and 5 μl of RNA template. The total reaction mixture was 25-μl. The qPCR reaction was performed at 50°C for 20 minutes of RT reaction, then 95°C for 15 minutes of initial denaturation, followed by 40 cycles of denaturation at 94°C for 45 seconds, and 60°C for 45 seconds for annealing and data collection. The sample that was positive for the Matrix gene was then screened for different haemagglutinin (H) and neuraminidase genes using protocols that were previously reported: H5N1 Duplex, H7 (Spackman et al., 2002; Slomka et al., 2007), H9, N2, N6, and N8 characterization. The reactions were performed on MIC PCR (Biomolecular Systems, Australian). Other suspected avian respiratory viruses such as avian infectious bronchitis and NDVs were also screened for using specific primers and probes (Fuller et al., 2010; Shittu et al., 2019).
Results
Case location analysis
Ibadan, Oyo state is a beehive of commercial poultry activities housing many large farms with a very high poultry population. In addition, the largest hatcheries in the country are in Oyo state. The outbreak of the H9N2 poses a serious threat to commercial poultry production in Nigeria as there is constant free movement of birds from the state to other parts of the country. The epidemiological history of events on the farm revealed the potential source of the introduction of the H9N2 virus into this commercial poultry farm to be most likely through the poultry waste/manure uptaker truck that visited the farm less than a week before the outbreak, from northern Nigeria.
Gross pathologic and histopathological findings
Layers
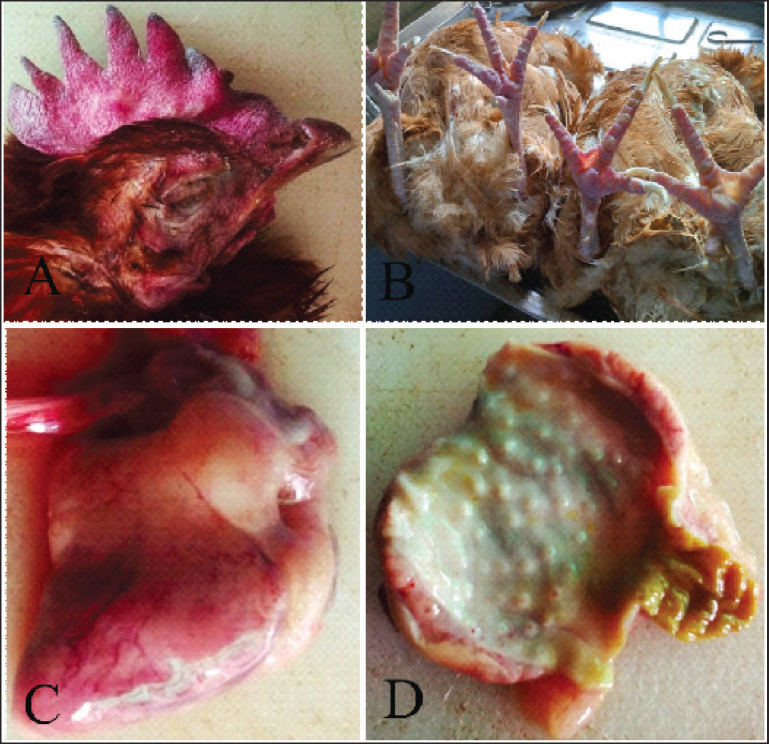
All twelve carcasses were in moderately good body condition. The combs were mildly cyanotic with necrosis of the tips in four carcasses. The shanks were dried and had petechial hemorrhages. The sinuses contained excess mucus with bloody discharge in three carcasses. The tracheal mucosa was markedly hyperemic with marked bilateral pulmonary congestion. The liver was pale brown, enlarged, and friable with occasional hematoma in three carcasses. Other lesions were petechiation and ecchymoses on coronary and abdominal fat. There was proventricular glandular tip necrosis (Fig. 2D). In summary dehydration, cyanosis of combs, acute catarrhal rhinitis and sinusitis, hemorrhages, acute tracheitis, pulmonary congestion, fatty liver with rupture, enteritis, acute salpingitis and oophoritis and widespread hemorrhages were the gross lesions seen. Table 1 classified the lesions seen in each category of chicken carcasses.
Fig. 2. Layer chicken. (A) Hyperemic comb. (B) Hyperemic shank and feet. (C) Heart, epicardial petechial hemorrhages. (D) Proventricular glandular tip petechial hemorrhages.
Table 1. Gross and histopathology of H9N2 virus in poultry in Nigeria.
| Chicken/breed | Age in weeks | Number dead | Gros Pathology | Histopathology |
|---|---|---|---|---|
| Broiler/Abor Acre | 2 | 27 | Congestion of trachea, lung, liver 3/3, friable liver 3/3 and enlarged spleen 3/3, yellowish faecal pasting of Cloaca 3/3 and edematous bursa of Fabricius 3/3. | Trachea, mucosal epithelial necrosis with poly and mononuclear cellular infiltration; liver, diffused hepatic fatty infiltration, micro and macro-vesicular; Spleen and Bursa of Fabricius, diffused lymphoid necrosis. |
| Pullet /Isa Brown | 22 | 441 | Nasal sinus contained excess mucus 1/3, with bloody discharge 1/3; congestion of trachea, lung, myocardium and cloudy air sacs 2/3, tracheal mucosa hyperemia 1/3, myocardial coronary fat petechial and ecchymotic haemorrhages 1/3, with hematoma in 1/3; abdominal fat petechiation 1/3; edematous and haemorrhagic proventriculus with tip necrosis, and cecal tonsillar haemorrhage and necrosis; congestion of duodenum, friable, and swollen liver, and spleen; cloudy mesentery 1/3, with follicular pedunculation 1/3, shell-less eggs in oviduct 1/3, and greenish faecal pasting of Cloacal 1/3; and hyperemic shanks 2/3. | Tracheal mucosal epithelial necrosis with poly and mononuclear cellular infiltration; lung, parabronchial epithelial necrosis, denudation and accumulation of oedema fluid in the parabronchi and airsacs, with interstitial infiltration of mononuclear cells; emphysematous and oftentimes obliterated airsacs; heart, myocardial necrosis and multifocal infiltration of mononuclear inflammatory cells within the cardiomyocytes fibers; and myocarditis; proventriculus, mucosal epithelial necrosis and sloughing, glandular necrosis; diffused hepatic fatty infiltration, micro and macro-vesicular; diffused splenic lymphoid necrosis. |
| Layer/Isa Brown | 54 | 500 | Cyanosis of comb 2/3; nasal sinus contained excess mucus 1/3 and congestion of trachea, lung, myocardium and cloudy air sacs 2/3 with tracheal mucosal hyperemia 2/3; myocardial coronary fat, petechial and ecchymotic haemorrhages 2/3; abdominal fat petechiation 2/3; edematous and haemorrhagic proventriculus with tip necrosis, and cecal tonsillar haemorrhage and necrosis; congestion of duodenum, friable and swollen liver, swollen spleen, cloudy mesentery 1/3, with follicular pedunculation 1/3, shelless eggs in oviduct 1/3 and cloacal greenish fecal pasting 1/3; and hyperemic shanks 2/3. | Comb, intradermal edema with vascular congestion, tracheal mucosal epithelial necrosis with poly and mononuclear cellular infiltration; parabronchial epithelial necrosis, denudation, and accumulation of oedema fluid in the parabronchi and airsacs, with interstitial infiltration of poly and mononuclear cells, and emphysema of airsacs with obliteration; myocardial necrosis, and multifocal infiltration of mononuclear inflammatory cells within the cardiomyocyte fibres (myocarditis); proventricular mucosal epithelial necrosis, sloughing and glandular necrosis; diffused hepatic fatty infiltration, micro and macro-vesicular; diffused splenic lymphoid necrosis; |
| Layer/Isa Brown | 95 | 832 | Cyanosis of comb 2/3 with tip necrosis 1/3; nasal sinuses contained excess mucus 3/3, congestion of tracheal, lung and myocardium; cloudy air sacs 2/3, tracheal mucosa hyperemia 1/3; myocardial coronary fat, petechial and ecchymotic hemorrhages 2/3, abdominal fat petechiation 2/3; edematous and haemorrhagic proventriculus with tip necrosis, and cecal tonsillar hemorrhage and necrosis; congestion of duodenum, friable and swollen liver, swollen spleen, cloudy mesentery 2/3, with follicular pedunculation 2/3, shelless eggs in oviduct 2/3 and cloacal greenish fecal pasting 2/3; and hyperemic shanks 1/3. | Comb, intradermal edema and numerous vascular congestions, Tracheal mucosal epithelial necrosis with poly and mononuclear cellular infiltration, and serosal large vessels thrombosis; lung parabronchial epithelial necrosis, denudation, and accumulation of oedema fluid in the parabronchi and airsacs, with interstitial infiltration of poly and mononuclear cells, severe pulmonary vascular congestion, and airsacs emphysema and obliteration; myocardial necrosis, and multifocal infiltration of mononuclear inflammatory cells within the cardiomyocyte fibers (myocarditis); proventricular mucosal epithelial necrosis and sloughing, and glandular necrosis; liver, diffused hepatic fatty infiltration, micro and macro-vesicular; diffused splenic lymphoid necrosis. |
Broilers
All three carcasses were in excellent body condition. The lungs were congested, and the livers were moderately congested, friable, and enlarged in three carcasses. The bursae of Fabricius were edematous in three carcasses. There was a pasting of the vent with yellowish feces in all three carcasses.
Histopathological findings
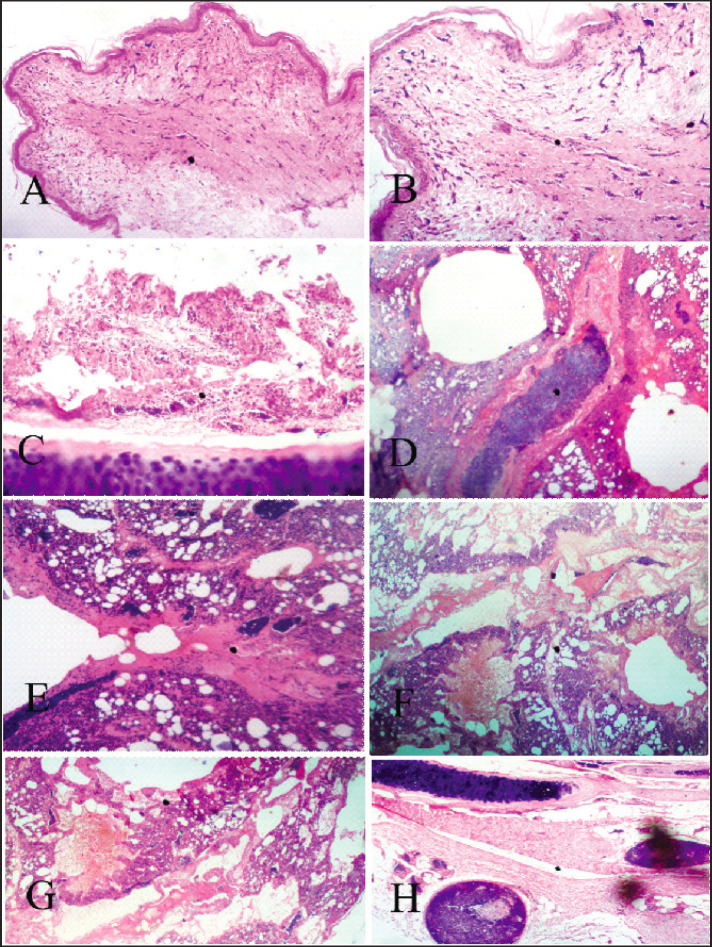
Comb, trachea, lung, proventriculus, spleen, and liver from qPCR-positive chickens’ tissues were examined. The comb dermal tissues showed edema (spongiosis) with loss of dermal tissues. Trachea showed severe necrosis and denudation of the epithelia lining of the mucosa and diffuse infiltration by mononuclear cells (Fig. 3C). The lungs revealed parabronchial epithelia necrosis (Fig. 3D) and denudation with an accumulation of edema fluid in the parabronchi and airsacs, and interstitial infiltration of poly and mononuclear cells. There was severe vascular congestion, and the airsacs were emphysematous and often obliterated.
Fig. 3. Layer (A) Comb, intradermal edema, and numerous vascular congestions H&E stain X 100. (B) Comb, intradermal edema and numerous vascular congestion higher magnification, H&E stain X 400. (C) Trachea, mucosal epithelial necrosis with poly and mononuclear cellular infiltration, H&E stain X 400. (D) Lung, parabronchial epithelial necrosis, denudation, and accumulation of edema fluid in the parabronchi and airsacs, with interstitial infiltration of poly and mononuclear cells, H&E stain X 400. (E) Lung, parabronchi, and airsacs infiltration by poly and mononuclear cells and severe vascular congestion, airsacs are emphysematous and oftentimes obliterated, H&E stain X 400. (F) and (G) Lung, parabronchial epithelia necrosis and denudation and accumulation of edema fluid in the parabronchi and airsacs, with interstitial infiltration of poly and mononuclear cells, airsacs are emphysematous and oftentimes obliterated H&E stain X 400 and 100. (H) tracheal serosal large vessels are thrombotic, H&E stain X 100.
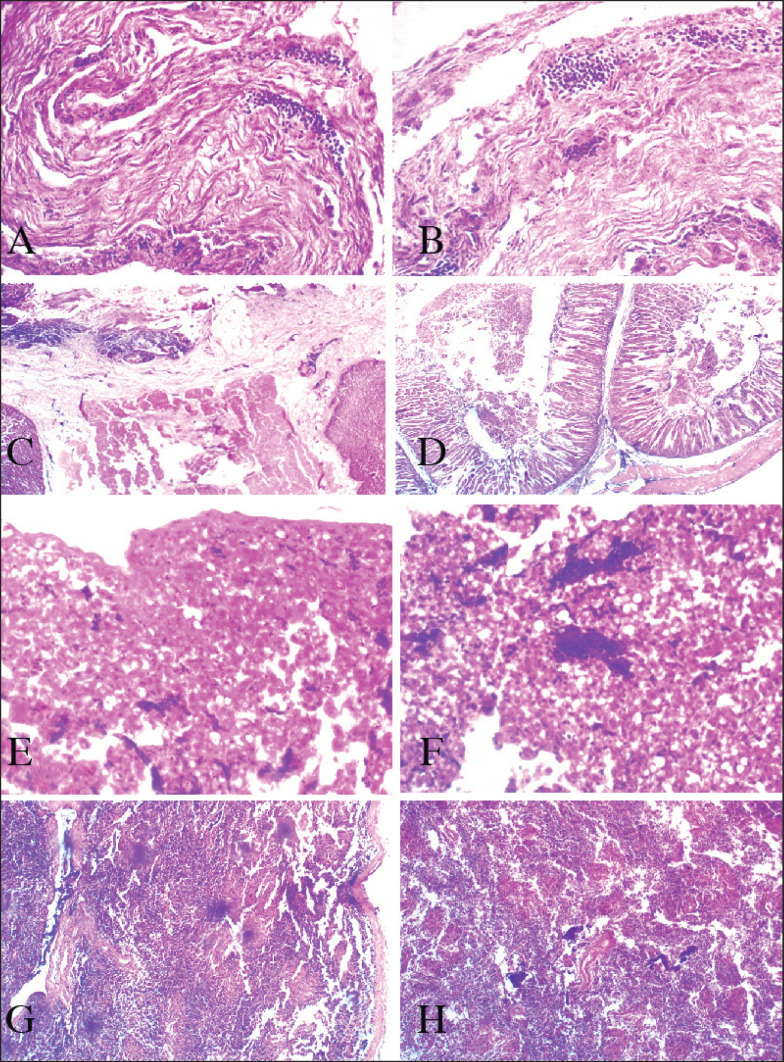
Table 1 classified the lesions seen in each category of chicken carcasses. The heart myocardial necrosis, and multifocal infiltration of mononuclear inflammatory cells within the cardiomyocyte fibers (Fig. 4I–J). Other histopathology lesions are presented in Figure 4.
Fig. 4. Layer (A) Heart, myocardial necrosis, and multifocal infiltration of mononuclear inflammatory cells within the cardiomyocyte fibers H&E stain X 100. (B) Heart, higher magnification of A, myocardial necrosis, and myocarditis H&E stain X 400. (C) Proventriculus, mucosal epithelial necrosis and sloughing, H&E stain X 100. (D) Proventriculus, glandular necrosis, H&E stain X 400. (E) Liver diffused hepatic lipidosis with micro and macrovesicular fat vacuoles, H&E stain X 100. (F) Liver, higher magnification of E, H&E stain X 400. (G) Spleen diffused lymphoid necrosis, H&E stain X 400. (H) Spleen diffused lymphoid necrosis, H&E stain X 400.
Molecular analysis
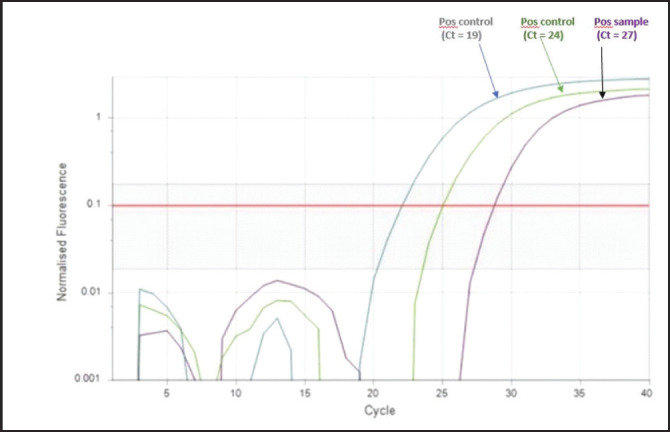
LPAI H9N2 virus was detected and isolated from the tissues of the broilers and layer chickens by qPCR amplification using specific primers for hemagglutinin gene (HA), and the amplification product was obtained at 1,700 bp. One of the positive sample qPCR images is shown in Figure 5.
Fig. 5. Graph of the qPCR detection of H9N2 virus in chickens in Nigeria.
Discussion
Molecular analysis of the samples from this outbreak revealed a LPAIV H9N2 subtype was the cause of the high mortality seen in this flock. This high mortality of 1,800 dead chickens at submission for postmortem examination was recorded over five days and increased to 4,526 across the entire farm before depopulation (per. comm from the farmer). The pattern of mortality and severity of the disease mimics highly pathogenic avian influenza viruses of the H5 subtypes. Outbreaks of HPAI that resulted from circulating LPAI H5, H7, and H9 viruses have previously been reported in poultry worldwide (Iqbal et al., 2009; Snoeck et al., 2011).
The increase in mortality seen in this outbreak may be associated with the H9N2 virus infection, lowered immunity because of stress associated with Newcastle disease vaccination, and the low temperature and high humidity due to the heavy rains. Similar H9N2 outbreaks, characterized by high morbidity and mortality in poultry in the Middle East, turned out to be caused by viruses clustering for the HA gene in a lineage described as G1 (Lau et al., 2016). Having ruled out co-infections with NDV and IBV or even HPAI H5NI by RT-PCR and qPCR, in the poultry farm in southern Nigeria, the molecular investigation revealed the severity of the clinical disease was ascribable to the H9N2 virus alone and therefore the outbreak was due to a LPAI A subtype H9N2 virus.
Although the LPAI subtype H9N2 virus has occasionally been reported to cause severe clinical signs in poultry, such as respiratory distress, intestinal lesions, and a drop in egg production (Houadfi et al., 2016), which is consistent with our observation of this outbreak. H9N2 has been associated with increased pathogenicity, especially during co-infections with other bacterial (Jaleel et al., 2017) and viral pathogens, such as IBV (Roussan et al., 2008; Sid et al., 2015; Hassan et al., 2016; Hassan et al., 2017; Broomand et al., 2018) and infectious laryngotracheitis virus (Arafat et al., 2018). In this outbreak, the co-infection with NDV and IBV or even HPAI H5NI were ruled out using qPCR, while lesions of acute salpingitis and oophoritis were suggestive of bacterial complications, no significant bacteria were isolated from the tissues of the carcasses. Other predominant lesions of general petechiations and ecchymosis, pneumonia, enteritis, caeciatis, and comb cyanosis are associated lesions of Avian influenza (Akanbi et al., 2007, 2017), especially H5N1.
In 2019, LBMs surveillance and molecular epidemiology revealed the presence of H9N2 in LBM. Nineteen H9N2 viruses were detected from apparently healthy poultry species, during an active surveillance in nine LBMs across Nigeria (Sulaiman et al., 2021).
Although there have been previous reports of serological detection of Influenza A H5, H7, and H9 subtypes in poultry from commercial farms and LBM in the northern city of Kaduna (Aiki-Raji et al., 2015; Oluwayelu et al., 2017; Wungak et al., 2021), this is the first report of H9N2 outbreak in a commercial poultry farm in southern Nigeria. This finding is significant as it underscores the possibility of future outbreaks of HPAI in poultry flocks across Nigeria, especially considering previous reports of the possibility of field LPAIV strains mutating into highly pathogenic strains after circulating in susceptible poultry (Swayne and Halvorson, 2003; Werner and Harder, 2006; Briand et al., 2010).
The detection of influenza A H9N2, poses a threat to poultry species, humans, and the environment considering the potential zoonosis, the high pathogenicity seen in commercial poultry, and the risk of dispersal of infected live poultry in Nigeria as previously reported for H5N1 outbreaks is a concern (Akanbi et al., 2017). Notably, zoonotic occurrences of mild respiratory conditions have been associated with specific strains of H9N2 viruses. The World Health Organization (WHO) has identified the H9N2 viruses as a pandemic concern due to the threat they present to human health and poultry (Carnaccini and Perez, 2020). The epidemiological investigation suggests a potential source of the introduction of the H9N2 virus into this commercial poultry farm to be most likely through the poultry waste/manure up takers truck that visited the farm less than a week before the outbreak, from northern Nigeria where this virus has been detected in LBM and commercial farms where reassortment between H5N1 and H9N2 has been reported (Wungak et al., 2021; Meseko et al., 2023). Due to H9N2 viruses’ ability to cross the species barrier and infect humans (Butt et al., 2005), we recommend that poultry waste/manure uptaker trucks should be discouraged from visiting farm premises, and the Ministries of Agriculture and Animal Health should as a matter of urgency monitor the spread of the virus for an effective control strategy.
Conclusion
The detection of H9N2 in this commercial poultry flock raises a serious concern in the light of one health because reassortment between H5N1 and H9N2 has been reported (Wungak et al., 2021; Meseko et al., 2023) earlier in LBM in Nigeria and recently, Uyeki et al. (2024), reported a mutation in H5N1 detected in cattle in Texas, US to be related to the viral adaptation to mammalian hosts, detected previously in humans and other mammals infected with HPAI A(H5N1), A(H7N9), and A(H9N2) viruses. It is therefore advocated, that concerted efforts be channeled towards enhanced surveillance to control the spread of H9N2 in poultry and its spillover effect in other mammalian species including humans.
Acknowledgments
Not applicable.
Authors’ contributions
Akanbi O.B. was involved in Manuscript writing, conceptualizing, postmortem, and histopathological diagnosis and taking of photomicrographs. Alaka O.O. was involved in postmortem case review, sending the samples to FAO regional laboratory, NVRI, and manuscript writing and review. Olaifa O.S. was involved in postmortem case review, sample collection, histopathological diagnosis and taking of photomicrographs, sending the samples to FAO regional laboratory, NVRI, and manuscript writing and review. Meseko C.A. was involved in molecular diagnosis with qPCR for confirmation of the influenza type and elimination of ND and IB viruses and providing the methodology for the molecular technique used in addition to manuscript writing and review. Inuwa B. was involved in molecular diagnosis with qPCR for confirmation of the Influenza type and elimination of ND and IB viruses in addition to manuscript writing and review. Tijani M, Ola O.O, and Ohore O. were involved in postmortem case diagnosis and review in VTH, University of ibadan in addition to manuscript writing and review. Jarikre O. was involved in postmortem case and histopathological diagnosis and review in VTH, University of ibadan in addition to manuscript writing and review. Odita C.I. provided the maps for in Figure 1 in addition to the manuscript writing and review. Ahmed J.S. and Muhammed M. performed the postmortem diagnosis and sample collection for viral isolation in NVRI, Vom in addition to manuscript writing and review. Fagbohun, O., Oluwayelu, D., Daodu O.B., Olapade J.O., and Oladele, O., reviewed the manuscript structure and content, provided expert references on the relevant aspects in virology and molecular diagnosis in addition to manuscript writing and review. Taiwo V.O. coordinated disease reporting, postmortem case review in addition to manuscript writing and review.
Funding
Not applicable.
Data availability
All data are provided in the manuscript. I, Taofeek Olanrewaju, hereby consent to the submission of the described case history regarding poultry mortality on Date: 25th February 2024. I understand that this information will be used for investigation and analysis by relevant professionals.
Conflict of interest
The authors have no conflict of interest in the publication of this paper.
References
- Aiki-Raji C.O., Adebiyi A.I., Agbajelola V.I., Adetunji S.A., Lameed Q., Adesina M., Adekanye G., Omidokun F., Fagbohun O., Oluwayelu D.O. Surveillance for low pathogenic avian influenza viruses in live-bird markets in Oyo and Ogun States, Nigeria. Asian Pac. J. Trop. Dis. 2015;5:369–373. [Google Scholar]
- Akanbi B.O., Teifke J.P., Ekong P.S., Ighodalo E.T., Ogunsan E.A., Makinde A.A. Warri Nigeria. Remi-Adewunmi B.D., Hassan A.Z., Ogo I.N. Nigerian Veterinary Medical Association 44th Annual Conference/Congress, 15/10/2007 to 19/10/2007, Abuja, Nigeria: Nigerian Veterinary Medical Association; 2007. The epidemiology, pathology and immunohistochemistry of Nigerian highly pathogenic avian influenza (HPAI) H5N1 virus infections in chickens. Available via www.nvma.ng . [Google Scholar]
- Akanbi B.O., Fereidouni S., Taiwo V.O., Starick E., Okewole P.A., Binder A., Heenemann K., Teifke J.P. Formalin fixed and paraffin embedded tissues of chickens are useful for retrospective studies on pathology of H5N1 highly pathogenic avian influenza virus outbreaks in Nigeria. Nigerian Vet. J. 2017;38(3):1–10. [Google Scholar]
- Arafat N., Eladl A.H., Marghani B.H., Saif M.A., El-shafei R.A. Enhanced infection of avian influenza virus H9N2 with infectious laryngeotracheitis vaccination in chickens. Vet. Microbiol. 2018;219:8–16. doi: 10.1016/j.vetmic.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Briand F.X., Le Gall-Recule G., Guillou-Cloarec C., Ogor K., Jestin V. Phylogeny and genotyping of recent avian low-pathogenic H5 subtype influenza viruses from French ducks. J. Gen. Virol. 2010;91:960–970. doi: 10.1099/vir.0.016733-0. [DOI] [PubMed] [Google Scholar]
- Broomand Z., Jafari R.A., Mayahi M. Detection of Newcastle disease, H9N2 avian influenza, and infectious bronchitis viruses in respiratory diseases in backyard chickens in Ahvaz, Iran, in 2014–2015. Arch. Razi Institute. 2018;73:19–25. doi: 10.22092/ARI.2018.114056. [DOI] [PubMed] [Google Scholar]
- Butt K.M., Smith G.J., Chen H. Human infection with an avian H9N2 influenza a virus in Hong Kong in 2003. J. Clin. Microbiol. 2005;43(11):5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnaccini S., Perez D.R. H9 influenza viruses: an emerging challenge. Cold Spring Harb. Perspect. Med. 2020;10(6):a038588. doi: 10.1101/cshperspect.a038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller C.M., Brodd L., Irvine R.M., Alexander D.J., Aldous E.W. Development of an L gene real-time reverse-transcription PCR assay for the detection of avian paramyxovirus type 1 RNA in clinical samples. Arch. Virol. 2010;155(6):817–823. doi: 10.1007/s00705-010-0632-1. [DOI] [PubMed] [Google Scholar]
- Fusaro A., Monne I., Salviato A., Valastro V., Schivo A., Amarin N.M., Gonzalez C., Ismail M.M., Al-Ankari A.R., Al-Blowi M.H., Khan O.A., Maken Ali A.S., Hedayati A., Garcia Garcia J., Ziay G.M., Shoushtari A., Al Qahtani K.N., Capua I., Holmes E.C., Cattoli G. Phylogeography and evolutionary history of reassortant H9N2 viruses with potential human health implications. J. Virol. 2011;85(16):8413–8421. doi: 10.1128/JVI.00219-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan K.E., Ali A., Shany S.A.S., El-Kady M.F. Experimental co-infection of infectious bronchitis and low pathogenic avian influenza H9N2 viruses in commercial broiler chickens. Res. Vet. Sci. 2017;115:356–362. doi: 10.1016/j.rvsc.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan K.E., Shany S.A.S., Ali A., Dahshan, A.-H.M., El-Sawah A.A., El-Kady M.F. Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poult. Sci. 2016;95:1271–1280. doi: 10.3382/ps/pew068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M., Yaqub T., Reddy K., McCauley J.W. Novel genotypes of H9N2 influenza A viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 viruses. PLoS One. 2009;4:e5788. doi: 10.1371/journal.pone.0005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaleel S., Younus M., Idrees A., Arshad M., Khan A.U., Ehtisham-Ul-Haque S., Zaheer M., I., Tanweer M., Towakal F., Munibullah, Tipu M.Y., Sohail M.L., Umar S. Pathological alterations in respiratory system during co-infection with low pathogenic Avian influenza virus (H9N2) and Escherichia Coli in Broiler Chickens. J. Vet. Res. 2017;61(3):253–258. doi: 10.1515/jvetres-2017-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A., Awunia A., Biancob O.J., Dogbeya A.F., Daniel T.Y., Annalisa S., Patrick T.A., Adelaide M., Francesco B., Monne I. Avian influenza H9N2 subtype in Ghana: virus characterization and evidence of co-infection. Avian Pathol. 2019;48(5):470–476. doi: 10.1080/03079457.2019.1624687. [DOI] [PubMed] [Google Scholar]
- Houadfi M.E., Fellahi S., Nassik S., Guérin, J.-L. and Ducatez M.F. First outbreaks and phylogenetic analyses of avian influenza H9N2 viruses isolated from poultry flocks in Morocco. Virol. J. 2016;13:140. doi: 10.1186/s12985-016-0596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammon A., Heidari A., Dayhum A., Eldaghayes I., Sharif M., Monne I., Kraim E. Characterization of avian influenza and Newcastle disease viruses from poultry in Libya. Avian Dis. 2015;59:422–430. doi: 10.1637/11068-032215-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Kayali G., Webby R.J., Samhouri D., Mafi A.R., Bassili A. Influenza research in the Eastern Mediterranean region: the current state and the way forward. Influenza Other Respir. Viruses. 2013;7(6):914–921. doi: 10.1111/irv.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laleye A.T., Bianco A., Shittu I., Sulaiman L., Fusaro A., Inuwa B., Oyetunde J., Zecchin B., Bakam J., Pastori A., Olawuyi K., Schivo A., Meseko C., Vakuru C., Fortin A., Monne I., Joannis T. Genetic characterization of highly pathogenic Avian influenza H5Nx clade 2.3.4.4b reveals independent introductions in Nigeria. Transbound. Emerg. Dis. 2022;69(2):423–433. doi: 10.1111/tbed.14000. [DOI] [PubMed] [Google Scholar]
- Lau, S.-Y.S.K.P., Joseph S., Chan, K.-H., Chen H., Annie N., Patteril G., Woo P.C.Y. Complete genome sequence of influenza virus H9N2 associated with a fatal outbreak among chickens in Dubai. Genome Announc. 2016;4:752–768. doi: 10.1128/genomeA.00752-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Dunn P.A., Wallner-Pendleton E.A., Henzler D.J., Kradel D.C., Liu J. Investigation of H7N2 avian influenza outbreaks in two broiler breeder flocks in Pennsylvania, 2001–2002. Avian Dis. 2004;48:26–33. doi: 10.1637/6063. [DOI] [PubMed] [Google Scholar]
- Meseko C., Milani A., Inuwa B., Chinyere C., Shittu I., Ahmed J., Muhammad M. The evolution of highly pathogenic Avian influenza A (H5) in poultry in Nigeria, 2021–2022. Viruses. 2023;15(6):1387. doi: 10.3390/v15061387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monne I., Hussein H.A., Fusaro A., Valastro V., Hamoud M.M., Khalefa R.A., Cattoli G. H9N2 influenza A virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respir. Viruses. 2013;7:240–243. doi: 10.1111/j.1750-2659.2012.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwayelu D.O., Omolanwa A., Adebiyi A.I., Aiki-Raji O.C. Flock-based surveillance for low pathogenic Avian influenza virus in commercial breeders and layers, Southwest Nigeria. Afr. J. Infect. Dis. 2017;11:44–49. doi: 10.21010/ajid.v11i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S.P., Pantin-Jackwood M., Jadhao S.J., Suarez D.L., Swayne D.E. Pathobiology of triple reassortant H3N2 influenza viruses in breeder turkeys and its potential implication for vaccine studies in turkeys. Vaccine. 2009;27:819–824. doi: 10.1016/j.vaccine.2008.11.076. [DOI] [PubMed] [Google Scholar]
- Roussan D.A., Haddad R., Khawaldeh G. Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult. Sci. 2008;87:444–448. doi: 10.3382/ps.2007-00415. [DOI] [PubMed] [Google Scholar]
- Shittu I., Bianco A., Gado D., Mkpuma N., Sulaiman L., Laleye A., Joannis T. First detection of highly pathogenic H5N6 avian influenza virus on the African continent. Emerg. Microb. Infect. 2020;9(1):886–888. doi: 10.1080/22221751.2020.1757999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shittu I., Gado D.A., Meseko C.A., Nyam D.C., Olawuyi K.A., Moses G.D., Chinyere C.N., Joannis T.M. Occurrence of infectious bronchitis in layer birds in Plateau state, North Central Nigeria. Open Vet. J. 2019;9(1):74–80. doi: 10.4314/ovj.v9i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sid H., Benachour K., Rautenschlein S. Co-infection with multiple respiratory pathogens contributes to increased mortality rates in Algerian poultry flocks. Avian Dis. 2015;59:440–446. doi: 10.1637/11063-031615-Case.1. [DOI] [PubMed] [Google Scholar]
- Simancas-Racines A., Cadena-Ullauri S., Guevara-Ramírez P., Zambrano A.K., Simancas-Racines D. Avian Influenza: Strategies to Manage an Outbreak. Pathogens. 2023;12(4):610. doi: 10.3390/pathogens12040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomka M.J., Coward V.J., Banks J., Löndt B.Z., Brown I.H, Voermans J., Koch G., Handberg K.J., Jørgensen P.H., Cherbonnel-Pansart M., Jestin V., Cattoli G., Capua I., Ejdersund A., Thorén P., Czifra G. Identification of sensitive and specific avian influenza PCR methods through blind ring trials in the European Union. Avian Dis. 2007;51:227–234. doi: 10.1637/7674-063006R1.1. [DOI] [PubMed] [Google Scholar]
- Snoeck C.J., Adeyanju A.T., De Landtsheer S. Reassortant low-pathogenic Avian influenza H5N2 viruses in African wild birds. J. Gen. Virol. 2011;92:1172–slo1183. doi: 10.1099/vir.0.029728-0. [DOI] [PubMed] [Google Scholar]
- Spackman E., Senne D.A., Myers T.J., Bulaga L.L., Garber L.P., Perdue M.L., Lohman K., Daum L.T., Suarez D.L. Development of a real time reverse trancriptase PCR for type A influenza virus and the Avian H5 and H7 hemagglutination subtypes. J. Clin. Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman L., Shittu I., Fusaro A., Inuwa B., Zecchin B., Gado D., Schivo A., Bianco A., Laleye A., Gobbo F., Vakuru Joannis T., Monne I., Meseko C. Live bird markets in Nigeria: a potential reservoir for H9N2 Avian influenza viruses. Viruses. 2021;13:1445. doi: 10.3390/v13081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne D.E., Halvorson D.A. Saif Y.M., Barnes H.J., Fadly A.M., Glisson J.R., McDougald L.R., Swayne D.E. In Diseases of poultry. 11th. Ames, IA: Iowa State University Press; 2003. Influenza; pp. 135–160. [Google Scholar]
- Swayne D.E., Pavade G., Hamilton K., Vallat B., Miyagishima K. Assessment of national strategies for control of high-pathogenicity avian influenza and low-pathogenicity notifiable Avian influenza, with emphasis on vaccines and vaccination. Rev. Sci. Tech. 2011;30(3):839–870. doi: 10.20506/rst.30.3.2081. [DOI] [PubMed] [Google Scholar]
- Tombari W., Nsiri J., Larbi I., Guerin J.L., Ghram A. Genetic evolution of low pathogenecity H9N2 avian influenza viruses in Tunisia: acquisition of new mutations. Virol J. 2011;8:467. doi: 10.1186/1743-422X-8-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeki T.M., Milton S., Abdul Hamid C., Reinoso Webb C., Presley S.M., Shetty V., Rollo S.N., Martinez D.L., Rai S., Gonzales E.R., Kniss K.L., Jang Y., Frederick J.C., De La Cruz J.A., Liddell J., Di H., Kirby M.K., Barnes J.R., Davis C.T. Highly pathogenic Avian influenza A(H5N1) virus infection in a dairy farm worker. N. Engl. J. Med. 2024;390(21):2028–2029. doi: 10.1056/NEJMc2405371. [DOI] [PubMed] [Google Scholar]
- Werner O., Harder T.C. Kamps B.S., Hoffman C., Preiser W. Influenza report. Paris, France: Flying Publisher; 2006. Avian influenza; pp. 48–86. [Google Scholar]
- Wungak Y.S., Orakpoghenor O., Bitrus I., Olawuyi K.A., Osemeke O.H., Ularamu H.G., Shittu I., Meseko C.A. Detection of antibodies to H5 and H9 subtypes of influenza viruses in wild birds in Zaria, Nigeria. Sokoto J. Vet. Sci. 2021;19(4):160–165. [Google Scholar]
- Zecchin B., Minoungou G., Fusaro A., Moctar S., Ouedraogo-Kaboré A., Schivo A., Monne I. Influenza A(H9N2) virus, Burkina Faso. Emerg. Infect. Dis. 2017;23:2118–2119. doi: 10.3201/eid2312.171294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided in the manuscript. I, Taofeek Olanrewaju, hereby consent to the submission of the described case history regarding poultry mortality on Date: 25th February 2024. I understand that this information will be used for investigation and analysis by relevant professionals.