Abstract
In the face of cell damage, cells can initiate a response ranging from survival to death, the balance being crucial for tissue homeostasis and overall health. Cell death, in both accidental and regulated forms, plays a fundamental role in maintaining tissue homeostasis. Among the regulated mechanisms of cell death, ferroptosis has garnered attention for its iron-dependent phospholipid (PL) peroxidation and its implications in aging and age-related disorders, as well as for its therapeutic relevance. In this review, we provide an overview of the mechanisms, regulation, and physiological and pathological roles of ferroptosis. We present new insights into the relationship between ferroptosis, cellular senescence and aging, emphasizing how alterations in ferroptosis pathways contribute to aging-related tissue dysfunction. In addition, we examine the therapeutic potential of ferroptosis in aging-related diseases, offering innovative insights into future interventions aimed at mitigating the effects of aging and promoting longevity.
Keywords: Ferroptosis, Phospholipid (PL) peroxidation, Redox homeostasis, Iron metabolism aging, Aging-related diseases
Highlights
-
•
Ferroptosis is driven by iron metabolism, lipid peroxidation and redox balance.
-
•
Dysregulation of ferroptosis contributes to ageing and diseases such as cancer and neurodegeneration.
-
•
Targeting ferroptosis pathways offers the potential to treat ageing-related diseases.
-
•
Understanding the mechanisms of ferroptosis is key to developing new therapeutic strategies.
1. Introduction
In the 1830s, the “cell theory” was born thanks to the work of Schleiden on plant cells and Schwann on animal cells. It states that all living organisms are made up of one or more cells and that the cell is the structural and functional unit of life. Nowadays, we understand the cell as a dynamic system that integrates both internal and external signals to which it must properly respond. When cell structures are damaged, the cell can activate a response ranging from survival to death, depending on the damage factors (type, intensity, and duration) and the cell (type, state, and number of cells affected), and, ultimately, if the stress stimulus does not exceed a certain threshold [1,2]. Tissue homeostasis is achieved due to a balance between cell division and cell death, and both too much and too little cell death can lead to human disease. Cell death removes nonfunctional, damaged, and harmful cells and is classified into accidental cell death (ACD) or regulated cell death (RCD) [3]. While the former is triggered by uncontrolled damage factors that disassemble the plasma membrane, the latter is driven by the activation of molecularly defined signaling cascades and effector mechanisms and can therefore be modulated either pharmacologically or genetically [4]. Multiple forms of RCD have been described, such as apoptosis, pyroptosis, necroptosis, ferroptosis, autophagy-dependent cell death, and lysosomal cell death. Among them, ferroptosis emerges as a form of RCD characterized by iron-dependent phospholipid peroxidation and regulated by several cellular metabolic pathways [5]. The uncontrolled peroxidation of polyunsaturated fatty acid chains-containing phospholipids (PUFA-PLs), a defining feature of ferroptosis, leads to lethal membrane rupture through altered ion fluxes, water ingress, and biophysical effects [6]. These require the dysregulation of iron metabolism and oxygen to catalyze the PL peroxidation through non-enzymatic, the Fenton reaction, and enzymatic reactions, lipoxygenases (LOXs), and/or cytochrome P450 oxidoreductase (POR) [7,8]. Also, an imbalance in redox homeostasis induces PL peroxidation, considering both the production of reactive oxygen species (ROS) and the antioxidant systems. Aging can be defined as a process of gradual and irreversible disorganization over time leading to the deterioration of physiological functions necessary for survival and fertility. In the last decade, ferroptosis has become of interest because of its implications for aging and the pathogenesis of numerous age-related disorders [9]. Indeed, the modulation of ferroptosis is a promising option in the treatment of aging-related diseases [10,11].
Ferroptosis is tightly regulated by different pathways, highlighting the cellular metabolism, which plays an essential role in cellular fate, and redox homeostasis [12,13]. In this review, we provide an overview of the mechanisms, regulation, and physiological and pathological roles of ferroptosis. Then, we discuss the interconnection of ferroptosis, cellular senescence, and aging. Lastly, we review the important role of ferroptosis in aging-related diseases and possible treatments and interventions.
2. Mechanisms of ferroptosis
The PL peroxidation initiated during ferroptosis relies on ROS generation and iron availability. The term ferroptosis was coined in 2012 [14]. The first evidence of ferroptosis-like cell death appeared in the mid-twentieth century, thanks to the pioneering research of Harry Eagle in amino acid and lipid metabolism [[15], [16], [17]]. Specifically, he demonstrated that cell death was triggered by cysteine deprivation and that endogenous synthesis of cysteine makes cells resistant to such cell death [18]. Further advances were needed in the fields of redox homeostasis, iron metabolism, and cell death, until 2003 when a lethal RAS-selective lethal compound (RSL), erastin, was identified, which induced a non-apoptotic form of cell death [19]. Subsequently, it was demonstrated that this form of cell death was regulated in an iron-dependent manner and highly modulable with molecular perturbations [20,21]. Moreover, the erastin-induced cell death was blocked with iron chelators and lipid ROS scavengers [14]. The relevance of ferroptosis lies in its involvement in various physiological and pathological processes, being an important focus of research in medicine.
The discovery of ferroptosis and its implications in aging and aging-related diseases has attracted great interest in the scientific community. It constitutes an active and rapidly growing field of research aimed at elucidating its complexities and therapeutic potential. Most of the information on the mechanisms of ferroptosis has been obtained from studies with the ferroptosis inducers erastin and RSL3 [20,22]. In addition, lipophilic antioxidants and iron chelating agents suppress cell death, such as vitamin E or DFOM, which establishes the three core hallmarks of ferroptosis: oxidation of PUFA-PLs, redox-active iron, and loss of lipid peroxide repair [23] (Fig. 1).
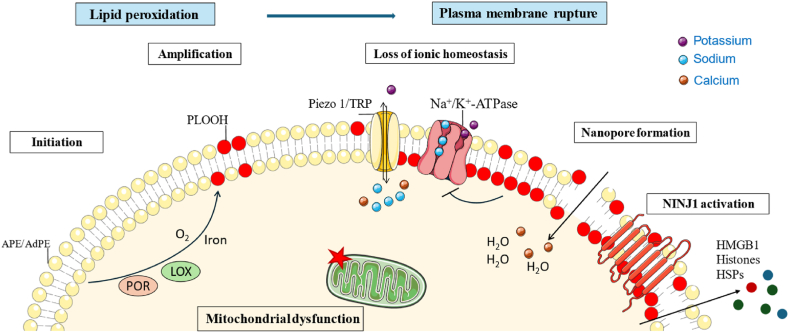
Fig. 1.
Mechanisms of ferroptosis execution. Peroxidation of PUFA-PLs, mainly arachidonic and adrenergic phosphatidylethanolamines (APE and AdPE, respectively), through enzyme- or radical-mediated reactions leads to increased plasma membrane tension, triggering activation of Piezo 1 and TRP channels. Altered ionic homeostasis and nanopore formation contribute to the final membrane rupture and cell lysis, probably due to NINJ1 injury. Mitochondria are characterized by reduced mitochondrial volume, increased mitochondrial membrane density, reduction or disappearance of mitochondrial cristae and rupture of the outer membrane.
2.1. Morphological changes
Morphologically, ferroptosis is distinguished from other types of RCD. Mainly, it is characterized by significant changes in mitochondrial morphology, including reduced mitochondrial volume, increased mitochondrial membrane density, reduction or disappearance of mitochondrial cristae, as well as rupture of the outer membrane [24]. In contrast, the plasma membrane remains intact, the nucleus maintains its size and no chromatin condensation is observed [25]. These various mitochondrial alterations suggest that ferroptosis may be highly dependent on mitochondrial function and integrity, possibly pointing to mitochondria having some relevant role in regulating this cell death process, such as inducing or inhibiting ferroptosis or mitochondrial events as the ultimate step in determining final cell fate [[26], [27], [28]].
2.2. Lipid peroxidation
In the context of the mechanisms underlying ferroptosis, it is crucial to realize the convergence of multiple interrelated cellular factors. These include ROS biology, iron regulation, and metabolism, which ultimately result in irreversible plasma membrane rupture. Importantly, erastin and RSL3 induce ferroptosis through the target of the key elements of the antioxidant system, the system xc− and glutathione peroxidase 4 (GPX4), respectively, leading to the accumulation of PL hydroperoxides (PLOOHs) [29]. The chlorophenoxy group of the erastin molecule, a member of the class of quinazolines, functionally interacts with the Phe254 residue of the TM6b domain of xCT, thereby mediating the inhibitory effects of erastin and regulating erastin-induced ferroptosis [30]. RSL3, a derivative of pyrazole, binds covalently to selenocysteine residue (Sec46) in active site of GPX4 [31]. Also, the cysteine 66 (C66) region serves as an allosteric binding site for RSL3 on GPX4, where covalent attachment of compounds to C66 modulates and inhibits the enzyme's activity, ultimately leading to its degradation [32].
Ferroptosis is the result of PUFA-PLs peroxidation, which leads to the accumulation of PLOOHs and the subsequent cell membrane damage. This damage occurs mainly due to the inactivation of the cellular antioxidant system, especially the system xc−-GPX4 axis. The generation of ROS drives lipid peroxidation, which consists of the removal of hydrogen atoms from the bis-allylic positions of PUFA-PLs in lipid bilayers. Feeding cells with deuterated PUFAs, which are less susceptible to oxidation, suppresses ferroptosis [33]. This process leads to the generation of a PL radical (PL•), which reacts with molecular oxygen to form a PL peroxyl radical (PLOO•). PL peroxyl radicals further react with neighboring PUFAs, forming PLOOHs. Next, PLOOHs, peroxyl radicals, and alkoxyl PL radicals (PLO•) continue the propagation of PLOOHs production and generation of secondary products, such as 4-hydroxynonenal (4HNE) and malondialdehyde (MDA), whose roles in ferroptosis have not yet been identified [34]. Oxidation selectively affects only one class of phospholipids, the phosphatidylethanolamines (PEs), and specifically affects two fatty acyls, arachidonoyl (AA)(20:4) and adrenoyl (AdA)(22:4) [35]. Recently, the Stockwell group has proposed a model for the propagation of lipid peroxidation in ferroptosis, suggesting that it initiates in the endoplasmic reticulum (ER) membranes and other organelles before spreading to the plasma membrane and promoting its rupture [36]. In this scenario, lipid peroxidation could reach the plasma membrane via diffusion of lipid radicals and peroxides, possibly through vesicular transport or lipid exchange mechanisms.
PLOOHs arise also from an enzyme-mediated process catalyzed by (non-heme) iron-dependent lipoxygenases (LOXs) during the initiation of ferroptosis [33]. LOX catalyzes stereospecific dioxygenation of PUFAs, such as AA and linoleic acids, leading to lipid peroxidation, with 15LOX being particularly involved in this process [35]. Phosphatidylethanolamine-binding protein 1 (PEBP1) forms complexes with 15LOX, directing its selectivity towards the peroxidation of eicosatetraenoyl-phosphatidylethanolamine (ETE-PE) [37]. This process generates 15-hydroperoxy-eicosatetraenoyl-phosphatidylethanolamine (15-HpETE-PE), a peroxidation product that serves as a signal to induce ferroptosis and is also used as a biomarker of this process. Indeed, ferrostatin-1 (Fer-1), a common ferroptosis inhibitor, inhibits the 15LOX/PEBP1 complex activity, but not 15LOX alone [38]. Recently, it has been reported that 15LOX also oxidizes doubly PUFA-acylated PLs (di-PUFA-PLs), where, intriguingly, PEBP1 exhibits no regulatory effects on 15LOX activity [39]. Interestingly, Pseudomonas aeruginosa expresses a secreted lipoxygenase (PA-LOX) that induces ferroptosis of human bronchial epithelial cells [40,41].
Alternatively, it has been described the role of POR and cyclooxygenase-2 (COX2) in the enzyme-mediated lipid peroxidation in ferroptosis. Using a series of genome-wide, CRISPR–Cas9 suppressor screens, POR emerged as a key mediator in the initiation of ferroptosis, both intrinsic and induced forms [8]. As suggested by the authors, POR would contribute to lipid peroxidation through the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+) [42]. In addition, POR would transfer electrons to cytochrome P450 and CYB5A and these would remove hydrogens from PUFAs. Cyclooxygenase-2 (COX-2), also known as prostaglandin-endoperoxide synthase 2 (PTGS2), catalyzes the conversion of arachidonic acid to prostaglandins. PTGS2, the gene encoding COX-2 is upregulated during ferroptosis in BJeLR cells upon treatment with either erastin or RSL3 [43]. Although COX-2 is used as a biomarker of ferroptosis initiation, its direct involvement in lipid peroxidation remains unclear [43,44]. This ambiguity arises from observations that the COX-2 inhibitor indomethacin has minimal impact on elastin- or RSL3-induced cytotoxicity.
It is in debate the relative contributions of enzymatic or free radical reactions to lipid peroxidation in ferroptosis [45]. Free radical reactions are influenced by iron metabolism, as iron catalyzes the formation of ROS that attack PUFAs in cellular membranes (reviewed below in section 2.2). While pharmacological inhibition of lipoxygenases (LOX) protects cells from ferroptosis [33,35], ferroptosis inhibitors such as Fer-1 and liproxstatin (Lip-1) show limited efficacy in the inhibition of 15-LOX, but are good reactivity as radical-trapping antioxidants (RTAs), suggesting a more prominent role of lipid autoxidation [46,47]. Probably, both mechanisms are important for the execution of ferroptosis, so they must be interrelated in some yet unknown way. Further research is needed to elucidate the relative importance of enzymatic processes versus free radicals in lipid peroxidation and the interplay between iron regulation, enzymatic pathways, and lipid peroxidation in ferroptosis.
2.3. Plasma membrane rupture
The culmination of ferroptosis, i.e.: the exact molecular event responsible for eventual cell death, involves the rupture of the plasma membrane. Although it remains difficult to understand all the steps in this process, recent discoveries have made it possible to formulate a model to elucidate the sequence of events [48]. Accumulation of lethal levels of PLOOHs, either enzymatically or radical-mediated reactions, causes cell death. This impairs the biophysical properties of the plasma membrane, affecting mechanosensing channels and osmotic processes [49,50]. More specifically, excessive accumulation of PLOOHs in the plasma membrane leads to an increase in membrane tension, triggering the activation of mechanosensitive piezo-type ion channel component 1 (Piezo 1) and transient receptor potential (TRP) channels. Consequently, the cell loses its ionic homeostasis and becomes permeable to cations [49]. This influx of cations, including Na+ and Ca2+, is accompanied by outflow of K+. In addition, Na+/K+-ATPase is inhibited, possibly due to interactions with oxidized PLs, which further increases nonselective cation fluxes. These alterations in ionic balance lead to cell swelling and further increase in membrane tension, initiating a negative feedback loop that culminates in plasma membrane rupture. In addition, lipid peroxidation induces the formation of nanopores, a few nanometers in size, in the plasma membrane, facilitating the water and ion fluxes [51]. The osmoprotectant polyethylene glycol (PEG) can delay the onset of ferroptosis without affecting lipid peroxidation itself. This observation suggests that the degree of membrane damage, including the size and/or number of membrane pores, increases with the duration and intensity of the stimulus. The formation of pores is also characteristic of other forms of RCD, such as necroptosis and pyroptosis [52,53]. On the other hand, the increase in Ca2+ initiates a protective response that limits the extent of the membrane damage through the recruitment of the endosomal sorting complexes required for transport (ESCRT)-III to the plasma membrane [51]. The involvement of ninjurin1 (NINJ1) in the formation of pores in ferroptosis is controversial. Hirata et al. demonstrated that the deletion of NINJ1 did not change significantly the susceptibility of RAW cells to ferroptosis [49]. However, recently Dondelinger et al. and Ramos et al. have demonstrated that cell swelling activates the oligomerization of NINJ1, inducing plasma membrane rupture and release of damage-associated molecular pattern (DAMP) molecules during ferroptosis [54,55]. Notably, NINJ1 activation is trigger-dependent, resulting in plasma membrane rupture induced by GPX4 inhibitors such as RSL3 and ML210, and not by other ferroptosis inducers such as erastin and FINO2 [54]. So, the action of NINJ1 may be context and cell-specific, and targeting NINJ1 could be a therapeutic strategy to reduce the inflammation associated with ferroptosis.
As assessed by molecular dynamics simulation of lipid membranes, the impact of lipid peroxidation on the integrity of the plasma membrane would arise from the conical shape of PE hydroperoxides [56]. These cannot pack in a lipid bilayer, leading to an increased membrane curvature and accessibility of oxidants, starting a negative feedback loop. Ultimately, despite repair efforts, the accumulated membrane damage is likely to be irreparable, leading to cell lysis and rupture of the plasma membrane.
3. Regulation
The sensitivity of cells to ferroptosis is modulated by various molecules and pathways that exert both positive and negative regulatory effects, such as lipid metabolism, iron homeostasis, redox regulation, and associated processes (Fig. 1).
3.1. Redox homeostasis
Cysteine availability is the rate-limiting step in the biosynthesis of reduced glutathione (GSH). The system xc− is a cystine/glutamate antiporter that is part of the SoLute Carrier 7 (SLC7) family. It functions as a heterodimer consisting of a light chain, known as xCT or SLC7A11, and a heavy chain 4F2hc, known as SLC3A2 or CD98hc [57]. The system xc− imports cystine, the oxidized form of cysteine, in exchange for intracellular glutamate. Cyst(e)ine levels regulate the GPX4 protein synthesis, at least partially through the Rag-mTORC1-4EBP signaling pathway [58]. The transsulfuration pathway of methionine and glucose can also synthesize cysteine [59,60]. Adequate GSH levels are necessary for the normal function of GPX4, an antioxidant selenoenzyme that reduces any PL hydroperoxides (PLOOHs) to the corresponding alcohols (PLOHs) in the cell membrane, acting as a ferroptosis gatekeeper. GPX4 presents a catalytic selenocysteine residue and employs the pair of electrons, most commonly provided by GSH as a cofactor, but also by other low-molecular thiols or even protein thiols in conditions of extreme GSH limitation [61]. GPX4 knockout mice revealed that GPX4 is essential for development and inducible knockout mice also result in adult death, accompanied by mitochondrial dysfunction, increased apoptosis, and neurodegeneration in the brain. Studies with GPX4 knockout mice have shown that GPX4 is essential in development, whereas inducible knockout mice exhibit adult mortality, accompanied by mitochondrial dysfunction, increased apoptosis, and neurodegeneration in the brain [62,63]. These findings demonstrate the role of GPX4 in cellular homeostasis and suggest that its function extends beyond its antioxidant activity to roles in mitochondrial integrity and neuronal survival. The consequences of GPX4 deficiency highlight its potential as a therapeutic target in diseases associated with oxidative stress, neurodegeneration and mitochondrial dysfunction, warranting an exploration of the mechanistic pathways regulated by this enzyme.
Although GPX4 is an essential regulator counteracting lipid peroxidation, it has been observed that GPX4 inhibition fails to completely arrest ferroptosis in specific pathological contexts. Furthermore, some cells show resistance to ferroptosis-inducing agents directed against GPX4, indicating the existence of alternative regulatory pathways of ferroptosis that operate independently of GPX4 [64]. The first discovery was the ferroptosis suppressor protein 1- coenzyme Q10 (FSP1-CoQ10) pathway [65,66]. FSP1, also known as AIFM2, catalyzes the reduction of CoQ10 (ubiquinone) to CoQ10H2 (ubiquinol) and vitamin K, using NAD(P)H and FAD, independently of GSH [67]. CoQ10H2 effectively traps PL peroxyl radicals (lipophilic radical-trapping antioxidant (RTA)), thus stopping the process of PL peroxidation. FSP1 requires myristoylation for its antiferroptotic function, as this post-translational modification enables the recruitment of FSP1 to the plasma membrane, Golgi apparatus, and perinuclear structures [65]. Moreover, FSP1 regenerates oxidized α-tocopheryl radical (vitamin E) directly in vitro or indirectly by CoQ10H2 [66]. In addition, it has been reported that FSP1 activates the ESCRT-III-dependent membrane repair pathway, which is responsible for ferroptosis resistance [68]. Since the discovery of FSP1, numerous novel ferroptosis pathways have been identified that function independently of GPX4. These pathways include p53-mediated lipoxygenase, and iPLA2β pathway, iNOs/NO pathway, DHODH pathway, GCH1/BH4 pathway, Ferritin, and prominin2 pathway, and SREBP1-SCD1-MUFA pathway [64,69,70]. Further research is needed to elucidate the different functions of these pathways and how they interact with each other. Understanding these relationships could open new avenues for therapeutic intervention, especially in diseases where ferroptosis plays a critical role, offering the possibility of more targeted and effective treatments.
3.2. Lipid metabolism
Two membrane-remodeling enzymes (Lands cycle), acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3), have been identified as essential genes for ferroptosis through genome-wide CRISPR-based genetic screening and microarray analysis of ferroptosis-resistant cell lines methods [71,72]. These enzymes are involved in the incorporation of PUFAs into PLs and contribute to the maintenance of lipid membrane integrity, thus influencing the susceptibility of cells to ferroptotic cell death. ACSL4 mediates the ligation of PUFAs to CoA, with a high preference for arachidonoyl (AA-CoA). Then, LPCAT3 catalyzes the esterification of AA-CoA into lysophospholipids, specifically targeting phosphatidylcholine and phosphatidylethanolamine, primarily present in the ER [73]. The inhibition of LPCAT3 does not completely suppress ferroptosis, partially explained by an increase in C22:4 PLs [74]. Collectively, ACSL4, LPCAT3, and 15-LOX work in coordination, converting AA and AdA to PE-AA/PE-AdA hydroperoxides, and promoting and regulating ferroptosis. Moreover, a novel ACSL4-independent ferroptosis pathway has been identified which requires ALOX12 for p53-mediated tumor suppression [75].
3.3. Iron metabolism
As the name suggests, ferroptosis is a type of cell death that depends on the presence of iron. Free iron or iron-containing enzymes are responsible for the peroxidation of PL-PUFAs by free Fenton reactions or enzymatically regulated reactions, respectively. Thus, the mechanisms governing iron homeostasis and bioavailability are essential in the control of the sensitivity of cells toward ferroptosis [76]. These include iron uptake, export, chaperoning and storage, and different regulatory pathways of iron metabolism, such as the iron-regulatory protein (IRP)–iron-responsive element (IRE) network, the nuclear receptor co-activator 4 (NCOA4)-mediated ferritinophagy pathway, the prolyl hydroxylase domain (PHD)–hypoxia-inducible factor (HIF) axis or the nuclear factor erythroid 2-related factor 2 (NRF2) regulatory hub [29,76]. Iron is transported through the bloodstream bound to transferrin and enters cells through transferrin receptor-mediated endocytosis. Once inside the cells, ferritin stores iron in the form of ferric iron. Iron chelators dexrazoxane and deferoxamine have protective effects on ferroptosis. The former reverses doxorubicin-induced ferroptosis and cardiotoxicity in rats by regulating high mobility group box 1 (HMGB1) [77]. The latter upregulates the system xc−-GPX4 axis, with neuroprotective after experimental traumatic brain injury and in primary cortical neurons from mouse embryos [78,79].
On the other hand, autophagic degradation of ferritin promotes ferroptosis, as it leads to an increase in cellular labile iron content [80]. NCOA4 mediates ferritinophagy through the recognition of ferritin and delivery to autophagosomes [81,82]. The recent development of the non-classical inhibitor of ferroptosis, YL-939, revealed the role of prohibitin 2 (PHB2) in ferroptosis [83]. PHB2 regulates ferroptosis by modulating both the ferritin expression and ferritinophagy and therefore, the iron bioavailability. YL-939 upregulates the expression of ferritin, decreasing the iron content and the ferroptosis susceptibility. Thus, in a sense, ferroptosis appears to be a form of autophagy-dependent cell death.
3.4. Amino acid metabolism
The transsulfuration and glutaminolysis pathways play significant roles in ferroptosis. As we have reviewed, cysteine is essential in the control of ferroptosis. In addition to the system xc−, the transsulfuration pathway also provides cysteine from methionine [84]. The transsulfuration pathway involves the consecutive action of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH). Knockdown of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits erastin-induced ferroptosis, probably because of the observed increase in intracellular cysteine [85]. Moreover, erastin-resistant cells present NRF2 constitutively activated, which upregulates the expression of CBS [84]. Thus, the transsulfuration pathway emerges as a critical source of cysteine, a crucial precursor for GSH synthesis, thereby increasing the cell's antioxidant capacity and reducing susceptibility to lipid peroxidation.
Glutaminolysis is also required for the execution of ferroptosis [86]. In glutaminolysis, the glutamine is converted into glutamate and α-ketoglutarate, an important tricarboxylic acid (TCA) cycle intermediate. Indeed, it is remarkable that the mitochondrial glutaminase (GLS) GLS2, rather than cytosolic GLS1, plays a crucial role in the induction of ferroptosis, highlighting the importance of mitochondria in this process [86,87]. Glutamate serves a dual function: it facilitates cysteine uptake through the system xc-for the synthesis of GSH and constitutes a component of GSH itself. In addition, endogenous glutamate levels regulate ferroptosis sensitivity through adenylyl cyclase 10 (ADCY10)-dependent yes-associated protein (YAP) suppression in lung adenocarcinoma cells. Elevated extracellular glutamate inhibits the function of the xc-system, reducing cystine entry into the cell. In particular, glutamate acts as an excitatory neurotransmitter, with neurotoxic and excitatory effects.
4. Functions of ferroptosis
Although ferroptosis was discovered in the context of screening for new anticancer molecules, this process has physiological functions and is also found in phylogenetically separate species such as protozoa, algae, fungi, and plants [[88], [89], [90], [91], [92]]. Emerging evidence suggests that ferroptosis plays physiological roles in a variety of biological processes, including tumor suppression, immune functions, bone homeostasis, development, and aging [[93], [94], [95], [96], [97], [98]].
4.1. Tumor suppressor and immune-associated functions
First reports of ferroptosis as a tumor suppressor revealed that p53 downregulates the expression of SLC7A11, the cystine/glutamate antiporter, limiting the production of intracellular glutathione (GSH) and sensitizing cells to ferroptosis [93]. Ferroptosis influences the cycle, proliferation, and progression of cancer, including both solid and blood-related tumors, and it is mediated through the epigenetic regulator MLL4 [99]. Its induction in cancer cells has been associated with impairments in cell migration, invasion, and metastasis [100]. Moreover, the CD8+ T cells induce ferroptosis of tumor cells through the secretion of interferon-γ (IFN-γ) to downregulate the expression of SLC3A2 and SLC7A11 and through the incorporation of arachidonic acid into C16 and C18 acyl chain-containing PLs via ACSL4 [101,102]. However, recent research indicates that, under specific circumstances, ferroptosis may act as a potential carcinogenic factor. For instance, the ferroptosis of pathologically activated neutrophils (PMNs) promotes tumor growth as it mediates immune suppression [103]. Moreover, a cholesterol-rich diet upregulates the CD36 expression and the uptake of fatty acids through CD36 by CD8+ T cells in the tumor microenvironment (TME) induces the ferroptotic death of this, resulting in a decreased production of cytotoxic cytokines [104]. So, how can we take advantage of the beneficial aspects of ferroptosis in cancer therapy while mitigating its potential to promote immunosuppression and tumor growth?
Therefore, the dual role of ferroptosis in cancer appears to depend on the specific type of cell undergoing cell death. Although inducing ferroptosis in tumor cells may be advantageous, its impact on immune cells is detrimental. In this context, Nishizawa et al. demonstrated that lipid peroxidation occurring in ferroptotic cells is transmitted to neighboring cells through paracrine signaling, through the secretome, thus facilitating the spread of ferroptosis [105]. Furthermore, Alarcón-Veleiro et al. demonstrated the ability of ovarian cancer cells to propagate ferroptosis via paracrine signaling through the release of small extracellular vesicles [106]. In addition, cell-to-cell contact of cancer cells with other cell populations, such as cancer-associated fibroblasts (CAF), endothelial cells, and immune cells in the TME, regulates the sensitivity of cells to ferroptosis, which is of particular importance in the context of antineoplastic drug resistance [107]. This underlines the importance of ferroptosis in the context of cancer progression and highlights the intercellular communication mechanisms involved in this process. Could these intercellular signals be targeted as a new strategy to selectively induce ferroptosis in tumor cells while protecting immune cells?
Ferroptosis is also related to antiviral immunity and vaccination. Selenium supplementation upregulates the GPX4 expression in follicular helper T (TFH) cells, which inhibits the ferroptosis in TFH cells and promotes the production of long-lasting immunological B cell responses [108]. As research progresses, these questions will be crucial in guiding the development of targeted therapies that can exploit ferroptosis for effective cancer treatment while minimizing its potential drawbacks.
4.2. Roles in aging, homeostasis, and development
Ferroptosis plays diverse roles in bone homeostasis, aging, and development. During the aging of Caenorhabditis elegans, glutathione depletion is associated with ferrous iron elevation, making the cells more susceptible to ferroptosis [98]. Interestingly, blocking ferroptosis, either by inhibiting lipid peroxidation or limiting iron retention, prolongs life and health expectancy, but not on a temporal scale, suggesting a role for ferroptosis in organism frailty at specific life stages rather than in constant aging. Likewise, Fischer-344 rats show an age-dependent increase in ferroptosis in the kidney, spleen, liver, ovary, uterus, cerebellum, and bone marrow, assessed with an HNEJ-1 mouse monoclonal antibody which recognizes 4-hydroxy-2-nonenal (HNE)-modified proteins [97]. In addition, ferroptosis is involved in embryonic erythropoiesis, where its inhibition delays erythrocyte enucleation.
Finally, Shen et al. demonstrated that ferroptosis is crucial for the development and pathogenicity of Magnaporthe oryzae, the rice blast fungus [91]. This process is dependent on ferric ions and is characterized by the accumulation of lipid peroxides, which was confirmed by an optimized fluorescence imaging technique. The study demonstrated that reduced glutathione and NADPH oxidases regulate the production of reactive oxygen species required for ferroptosis. This form of cell death is essential for the maturation of conidia into appressoria, the structures that allow the fungus to infect rice plants. Disruption of ferroptosis, either by iron chelation or chemical inhibition, reduces the ability of the fungus to invade its host. Furthermore, ferroptosis and autophagy cooperate to regulate this cell death during fungal development, highlighting their interconnected roles in fungal pathogenesis.
5. Interconnection of ferroptosis, cellular senescence, and aging
Contrary to cell death, cellular senescence is a cell state induced by stress signals that lead to cell-cycle arrest, senescence-associated secretory phenotype (SASP), metabolic dysregulation, and macromolecular damage [109]. Cellular senescence is a hallmark of aging, as senescent cells accumulate in aged tissues, notably fibroblasts, endothelial cells, and immune cells, but almost any cell, attributable in large part to telomere shortening [110,111]. However, great variations are found across tissue type, tissue section, and methods of assessing senescence [112]. While cellular senescence is recognized primarily by its contribution to aging, its main function is to prevent the propagation of cellular damage and to induce its elimination by the immune system, especially in the context of tumorigenesis [113]. Consequently, senotherapeutic strategies aimed at selectively eliminating senescent cells (senolytics) or modulating SASP (senomorphs) hold great promise for promoting healthy aging and mitigating age-related conditions such as osteoarthritis, atherosclerosis and cancer [114,115].
Ferroptosis, on the other hand, is a regulated form of cell death driven by iron-dependent lipid peroxidation. Despite their distinct roles, both ferroptosis and cellular senescence play distinct but complementary roles in the maintenance of cellular homeostasis and aging. Although numerous studies have highlighted the interconnection between ferroptosis and cellular senescence, the precise underlying mechanisms are not fully understood. Senescent cells, both cultured in vitro and observed ex vivo, show impaired ferritinophagy together with high iron accumulation, making them resistant to ferroptosis [116,117]. The induction of ferroptosis selectively targets and eliminates senescent cells, both primary and paracrine, establishing itself as a senolytic approach [[118], [119], [120], [121]]. For instance, the BRD4 bromodomain protein inhibitor JQ1 facilitates the induction of ferroptosis by promoting the activation of ferritinophagy and suppressing the expression of ferroptosis-associated genes such as GPX4, SLC7A11, and SLC3A2 [120,122].
However, the relationship between ferroptosis and cellular senescence is complex. Recent findings of Ma et al. point in another direction. They have described that cryptochrome 1 is downregulated in aged human ovarian granulosa cells, promoting ferritinophagy, through reduction of the ubiquitination and degradation of NCOA4, and inducing cell senescence [123]. Also, pro-ferroptosis signaling induces vascular smooth muscle senescence (VSMCs), promoting arterial aging [124,125]. In hepatic stellate cells, curcumol activates the HIF-1α-NCOA4-FTH1 signaling axis, inducing ferroptosis and cellular senescence that protects from hepatic fibrosis [126]. On the other hand, induction of senescence by palbociclib, an inhibitor of cyclin-dependent kinase 4 and 6 (CDK4/6) in B16F10 melanoma cells, increases their susceptibility to auranofin-induced ferroptosis [127]. These various findings underscore the complex relationship between ferroptosis and cellular senescence, raising the question of causality between the two processes. Further research is required to elucidate their underlying mechanisms and their potential therapeutic implications in aging and aging-related disorders.
To this whole network between senescence and ferroptosis must be added oxidative stress and iron overload. Persistent low-grade inflammation, known as inflammaging, not only triggers oxidative stress but also acts as a driver of the aging process [128,129]. This chronic inflammaging state promotes ROS generation and exacerbates oxidative damage, further perpetuating cellular senescence and ferroptosis, contributing to age-related pathologies [130]. More research is needed to further investigate the complex interplay between various factors associated with aging, such as cellular senescence, inflammation, and oxidative stress, and their relationship with ferroptosis (Fig. 2).
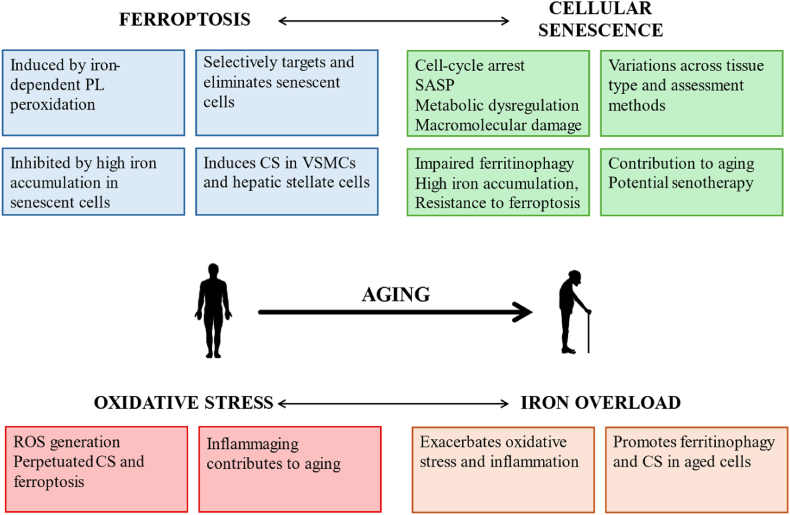
Fig. 2.
Schematic representation of the interaction between ferroptosis, cellular senescence, oxidative stress and iron overload in aging. Ferroptosis and cellular senescence play distinct but complementary roles in the maintenance of cellular homeostasis and aging. Oxidative stress and iron overload exacerbate cellular senescence and ferroptosis, contributing to aging. The state of chronic inflammation further perpetuates cellular aging processes.
6. Role of ferroptosis in aging-related diseases
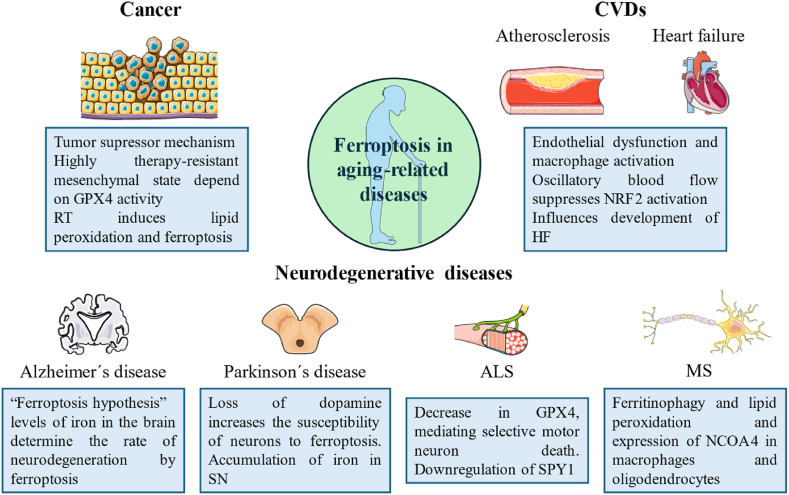
Due to the strong connection between ferroptosis and aging, the contribution of ferroptosis to the progression of aging-related disorders has been extensively researched, including cancer, neurodegenerative, and cardiovascular diseases (Fig. 3). By specifically modulating the ferroptotic pathways, for example, using iron chelators or inhibitors of GPX4, it may be possible to intervene in the progression of these diseases and mitigate their detrimental effects in aging individuals. Also, ferroptosis is emerging as a degenerative mechanism involved in the pathogenesis and progression of several aging-related diseases that pose a major global burden. These include type 2 diabetes mellitus (T2DM) [131,132], osteoarthritis and osteoporosis [133,134], chronic obstructive pulmonary disease (COPD) [135,136], non-alcoholic fatty liver disease (NAFLD) [137,138], age-related macular degeneration (AMD) [139,140] and presbycusis [141,142].
Fig. 3.
Role of ferroptosis in different aging-related diseases.
6.1. Cancer
As we have previously reviewed, ferroptosis is a tumor suppressor mechanism. Indeed, cancer cells require higher concentrations of iron, which makes them more vulnerable to death by ferroptosis [143]. Cancer cells, which often exhibit increased iron metabolism, are particularly vulnerable to ferroptosis, making this pathway a promising target for cancer therapy.
Some examples of cancer types where ferroptosis has been validated are hepatocellular carcinoma (HCC), gastric cancer, pancreatic ductal adenocarcinoma, triple-negative breast cancer (TNBC), and renal cell carcinoma [[144], [145], [146], [147], [148]]. Tumor suppressor genes p53, BRCA1-associated protein 1 (BAP1), fumarate hydratase, Kelch-like ECH-associated protein 1 (KEAP1), and the epigenetic regulator MLL4 have been shown to exert their antitumor effects, in part, by promoting ferroptosis in cancer cells [149]. For instance, p53 downregulates the SLC7A11 expression, critical for ROS-dependent or low-dose paclitaxel-induced ferroptosis [93,150], and requires LOX12 [75], increasing sensitivity to ferroptosis. Moreover, the S47 single-nucleotide polymorphism of TP53, prevalent in many premenopausal African-American women, is defective in promoting ferroptosis and correlates with increased susceptibility to breast cancer [151]. The interplay between ferroptosis and tumor suppressor genes underscores a common mechanism in cancer where disrupted iron metabolism and increased oxidative stress led to cell death.
Ferroptosis is also employed as a biomarker of patient outcomes. He et al. developed a ferroptosis score (FPS) model to assess the ferroptosis status of melanoma patients [152]. This model was based on 32 ferroptosis-related genes (FRGs) identified from the FerrDB4 database, which are linked to patient outcomes and can distinguish patient states [153].
The clinical relevance of ferroptosis is further highlighted by its role in therapy resistance. Cancer cells in a mesenchymal state, often associated with resistance to conventional therapies, rely on GPX4 activity for survival [[154], [155], [156], [157]]. This dependence extends to persister cells of various cancer types, which also show increased sensitivity to GPX4 inhibition. GPX4 inhibition has been shown to induce selective ferroptotic death of these treatment-resistant cells, preventing tumor recurrence in vivo [158]. This reveals a broader theme in which modulation of ferroptosis pathways could overcome drug resistance, a major challenge in cancer treatment.
Ferroptosis is also intricately linked to the epithelial-to-mesenchymal transition (EMT), a process that contributes to cancer metastasis and drug resistance. Mesenchymal cancer cells tend to be more susceptible to ferroptosis than their epithelial counterparts, and drug-resistant cancer cells undergoing EMT are more easily killed by ferroptosis inducers. Epigenetic reprogramming of EMT has been shown to contribute to promoting ferroptosis in head and neck cancer cells [159]. Inhibition of EMT through histone deacetylase inhibition decreased ferroptosis susceptibility, while EMT induction through miR-200 family inhibitors increased susceptibility to ferroptosis inducers. This connection between EMT and ferroptosis highlights a broader mechanism where changes in cell state influence sensitivity to oxidative stress and iron metabolism dysregulation. Thus, targeting EMT-associated pathways represents a promising therapeutic approach to enhance ferroptosis responsiveness and overcome drug resistance in cancer therapy, such as small molecules, nanomaterials, exosomes, or genic therapy [11,160,161] (Table 1).
Table 1.
Pharmacological modulation of ferroptosis induction in cancer therapy.
| Compound | Molecular target(s) | Mechanism of action | Cancer | References (PMID) |
|---|---|---|---|---|
| RSL3 | GPX4 | Inhibits GPX4 through the alkylation of selenocysteine | Fibrosarcoma, NSCLC, pancreatic cancer, leukemia, TNBC, HCC, thyroid cancer, CRC |
24439385 30545638 36257316 30524291 36895980 37596261 |
| FINO2 | GPX4 and iron | Iron and PUFAs oxidation, ROS production and deactivation of GPX4 | Fibrosarcoma | 29610484 |
| Artesunate | GPX4 | Oxidation of iron, depletion of GSH and induction of ferritinophagy | RCC, HCC, non-Hodgkin lymphoma, DLBCL, PDAC |
35028002 38560249 33121039 37326033 26097885 |
| Erastin | SLC7A11 | Inhibition of SLC7A11 and GSH depletion | HCC, melanoma, NSCLC, LUAD, ovarian cancer, thyroid cancer, glioma, breast cancer, RCC, prostate cancer |
31974380 37277863 31897145 33483372 |
| JQ1 | SLC7A11, GPX4 and BRD4 | BRD4 inhibition, induction of ferritinophagy and downregulation of GPX4, SLC7A11 and SLC3A2 | Breast cancer, lung cancer |
30988278 34003467 |
| Sorafenib | SLC7A11and GPX4 | Inhibition of SLC7A11 and TRIM54-mediated FSP1 ubiquitination | AML, HCC, neuroblastoma, NSCLC, RCC, liver cancer |
25368241 37460053 37351514 37695069 27264843 22725255 34768109 |
| Cisplatin | GSH | Reaction with GSH to inhibit GPX4 | Ovarian cancer, pancreatic cancer, NSCLC, urothelial cancer |
27477897 28012440 35534546 35784745 |
| Cyst(e)inase | GSH | Degradation of cysteine and cystine, leading to a decrease in GSH levels | RCC, prostate cancer, chronic lymphocytic leukemia, pancreatic cancer, ovarian cancer |
27869804 35086957 31043744 32241947 |
| Heme | Iron | Upregulation of HMOX1 and increase in LIP | Glioblastoma, leukemia, lung cancer |
36228518 36395526 |
| Lovastatin | HMGCR | Increases the efficacy of ICB by downregulating PD-L1 expression | NSCLC, prostate cancer, ovarian cancer | 35943796 |
| Simvastatin | HMGCR | Downregulation of the mevalonate pathway and GPX4 | TNBC | 34627266 |
| Atorvastatin | HMGCR | Induction of mitochondria-dependent ferroptosis by downregulating GPX4 | CRC |
35309902 34564973 |
| Haloperidol | DRD2 and S1R | Association with autophagy-mediated ferroptosis | Glioblastoma, HCC |
37249604 28756230 37249604 |
| Curcumin derivatives | FTH1 and TFEB | TFEB-mediated lysosome degradation of GPX4 Downregulation of lncRNA H19 |
Lung cancer |
37689240 35224289 |
Ferroptosis is also connected to radiotherapy (RT), which employs ionizing radiation to eliminate cancer cells by inducing DNA damage and radiolysis of water, which generates ROS [162]. RT induces lipid peroxidation and ferroptosis in several types of cancer, as evidenced by increased staining of lipid peroxidation markers, such as C11-BODIPY and lipid peroxidation markers MDA and 4-HNE, and expression of ferroptosis-associated genes, such as PTGS2 or ACSL4 [[162], [163], [164]]. RT also activates ATM to suppress SLC7A11 expression [165]. In addition, varying RT doses and fractionation schedules induce differential levels of ferroptosis. RT also induces GSH depletion [163]. Novel strategies, such as the use of magnetic fields polarized with iron oxide nanoparticles or the use of photosens-photodynamic therapy, induce ferroptosis through the generation of ROS, which confers immunogenic characteristics to this form of cell death [166,167]. Taken together, these findings establish a synergistic relationship between ferroptosis and RT, suggesting that the use of ferroptosis inducers in conjunction with RT could be a promising strategy to increase ferroptosis sensitivity and thus improve RT efficacy.
RSL3 Ras-selective lethal, GPX4 Glutathione peroxidase 4, NSCLC Non-small cell lung cancer, TNBC Triple-negative breast cancer, HCC Hepatocellular carcinoma, CRC Colorectal cancer, PUFAs Polyunsaturated fatty acids, ROS Reactive oxygen species, GSH Glutathione, RCC Renal cell carcinoma, DLBCL Diffuse large B-cell lymphoma, PDAC Pancreatic ductal adenocarcinoma, SLC7A11 Solute carrier family 7 member 11, LUAD Lung adenocarcinoma, BRD4 Bromodomain-containing protein 4, TRIM54 Tripartite motif-containing protein 54, FSP1 Ferroptosis suppressor protein 1, AML Acute myeloid leukemia, HMOX1 Heme oxygenase 1, LIP Labile iron pool, HMGCR 3-hydroxy-3-methylglutaryl-CoA reductase, ICB Immune checkpoint blockade, PD-L1 Programmed death-ligand 1, DRD2 Dopamine receptor D2, S1R Sigma-1 receptor, FTH1 Ferritin heavy chain 1, TFEB Transcription factor EB.
The complex role of ferroptosis in cancer points to both its potential as a therapeutic target and the challenges associated with its manipulation. While the induction of ferroptosis in cancer cells holds promise as a strategy to overcome drug resistance and improve the efficacy of traditional therapies such as radiotherapy, the dual role of ferroptosis in different cellular contexts must be carefully considered. Could the same pathways that make ferroptosis a powerful anticancer tool also contribute to unintended consequences, such as immunosuppression or increased tumor aggressiveness in certain contexts? Furthermore, emerging evidence that ferroptosis can spread through paracrine signaling within the tumor microenvironment raises questions about how we can selectively target cancer cells to induce ferroptosis without triggering detrimental effects on surrounding normal or immune cells. These questions highlight the need to better understand the effects of ferroptosis in a specific context.
6.2. Neurodegenerative diseases
Ferroptosis is crucial in the onset and progression of neurodegenerative disorders, such as Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis, amyotrophic lateral sclerosis (ALS), Huntington's disease (HD) and stroke [[168], [169], [170], [171], [172], [173], [174]]. These conditions share common pathological mechanisms such as iron accumulation, oxidative stress, and disruptions in cell death pathways. By examining these mechanisms across different diseases, we can better understand the unifying role of ferroptosis in neurodegeneration and its potential as a therapeutic target.
Iron accumulation in several cortical areas (hippocampus, cortex, and basal ganglia), PL peroxidation, and ferroptosis have been described in AD [169,175,176]. The progression of Alzheimer's disease (AD) is due to the accumulation of β-amyloid (Aβ) plaques and neurofibrillary tangles, and increasing evidence suggests a link between ferroptosis and AD progression, presenting ferroptosis as a potential therapeutic target. Indeed, Ayton et al. proposed the “ferroptosis hypothesis”, independent of the “Aβ hypothesis” and “tau protein hypothesis”, where levels of iron in the brain determine the rate of neurodegeneration by ferroptosis [177]. This hypothesis challenges long-standing models of AD and raises important questions: Could interventions that reduce brain iron levels or inhibit ferroptosis significantly alter the course of AD? Multiple factors, including dysfunctions in iron metabolism and antioxidant systems, neuroinflammation, and oxidative stress play a role in AD pathology and contribute to neuronal cell death and cognitive decline [178,179]. Presenilin mutations, the cause of autosomal dominant familial AD, sensitize multiple cell types to ferroptosis due to the downregulation of GPX4 expression and selenium uptake [180]. The observed connections between ferroptosis and mutations associated with familial AD underscore the need to explore whether ferroptosis could be a common pathway in both genetic and sporadic forms of the disease. Increased extracellular glutamate levels due to reduced levels of vesicular glutamate transporter (VGLUT1) expression in the cortex inhibit system xc−, leading to ferroptosis [181,182]. NCOA4-mediated ferritinophagy has been suggested to play a role in the pathogenesis of AD, although there are no studies of NCOA4 expression levels or function in pathological specimens from AD patients [183]. In AD patients there is a decrease in brain levels of NRF2, making the brains of AD patients more susceptible to ferroptosis [184]. So, NRF2 activation represents a promising avenue for the treatment of AD, offering the potential to counteract oxidative stress, neuroinflammation, and ferroptosis.
The relationship between Parkinson's disease (PD) and ferroptosis remains relatively unexplored. Recently, Liu et al. demonstrated that three differentially expressed genes-HMGB1, ceruloplasmin, and transferrin-encode secreted proteins that could influence susceptibility to ferroptosis of dopaminergic neurons in the substantia nigra of PD patients [185]. On the one hand, the formation of oligomeric α-synuclein (α-syn) generates ROS-promoting PL peroxidation [186]. On the other hand, the loss of dopamine increases the susceptibility of neurons to ferroptosis, as dopamine is a strong inhibitor of ferroptotic cell death that increases GPX4 stability [187]. Lastly, it has been demonstrated by different techniques the accumulation of iron, a major hallmark of ferroptosis, in the substantia nigra of PD patients, which would contribute to neuronal cell loss by generating ROS via Fenton reaction [188]. Also, it is crucial the regulation of ferroptosis by non-neural cells, such as the heme oxygenase 1 (HO-1) activity in the microglia, and alterations in the neuron-glia crosstalk can contribute to PD pathogenesis [189,190]. The involvement of non-neuronal cells, such as microglia, in the regulation of ferroptosis further complicates the landscape, indicating that therapeutic strategies may have to target not only neurons, but also the broader neuroinflammatory environment.
Ferroptosis is implicated in ALS, with a decrease in GPX4 observed in patients and mouse models, mediating selective motor neuron death [172]. In ALS, SPY1, a member of the Speedy/RINGO family of cell cycle regulators, is downregulated, leading to increased vulnerability to ferroptosis through dysregulation of the GCH1/BH4 axis and transferrin receptor protein 1 (TFR1)-induced iron accumulation [191]. Therefore, SPY1 proves to be an interesting therapeutic target for ALS through suppression of ferroptosis. In the case of HD, the expression of the N-terminal fragment containing the expanded polyglutamine (HTTQ94) of mutant huntingtin can promote ferroptosis, and ALOX5 is a major factor required for the ACSL4-independent ferroptosis [192]. Like other neurodegenerative diseases, iron dyshomeostasis, increased ROS, lipid peroxides, decreased activity of GPX4, and depletion of GSH are found in HD models [173].
In both multiple sclerosis (MS) and its animal model, experimental autoimmune encephalomyelitis (EAE), there are features of the activation of ferroptosis [193,194]. There is expression of NCOA4 in macrophages and oligodendrocytes located along the lesions of autopsy specimens, where ferritinophagy and lipid peroxidation are observed [171,195]. The treatment of EAE with UAMC-3203 delays relapse and ameliorates disease progression [193].
Pharmacological inhibition of ferroptosis by small molecule compounds aims to preserve neuronal integrity and function, thus slowing the progression of neurodegenerative diseases [10,196]. These inhibitors mainly target iron metabolism, such as transferrin receptor 1 or ferritin, lipid peroxidation, such as ACSL4 or lipoxygenases, and antioxidant defenses, such as GSH or CoQ10 [[197], [198], [199]] (Table 2). Preclinical studies with animal models of neurodegeneration have achieved promising results, demonstrating the efficacy of ferroptosis inhibitors in protecting neurons and improving neurological outcomes. However, translating these findings into clinical practice requires further research to identify safe and effective ferroptosis inhibitors and to pursue targeted drug delivery strategies in the central nervous system.
Table 2.
Pharmacological inhibition of ferroptosis in the therapy of neurodegenerative diseases (NGDs).
| Compound | Target | Mode of action | NGD | References (PMID) |
|---|---|---|---|---|
| Selenium | GPX4 | GPX4 synthesis | AD |
29974078 27447428 25567069 |
| GSH | GPX4 | Increase GPX4 activity | PD | 28436395 |
| N-acetyl cysteine | GSH and GPX4 | Increases GSH and GPX4 activity | PD, AD |
28499986 30574085 |
| Liproxstatin-1 | Lipid peroxides and GPX4 | Inhibition of lipid peroxidation and restoration of the expression of GPX4 and FSP1 | AD, ALS |
33398092 32985488 |
| Ferrostatin-1 | Lipid peroxides and GPX4 | Inhibition of the production of ROS | PD, ALS |
33398092 34145375 36443440 |
| Vitamin E | Lipid peroxides | Prevents lipid peroxidation and iron accumulation | PD, ALS |
35122944 26400084 34111668 |
| Salidroside | NRF2 | Activation of NRF2/HO1/GPX4 signaling pathway | AD |
36965372 35787281 |
| Quercetin | NRF2 | Activation of NRF2 | PD |
36105483 36501161 |
| β-Hydroxybutyric acid | NRF2 | Activation of NRF2 pathway | PD, AD |
36435477 36118706 37164173 |
| Deferoxamine | Iron | Iron chelation, upregulation of GPX4 and FTH1, and prevention of oxidative stress | AD, ALS |
33646533 35313783 34111668 |
| Deferiprone | Iron | Inhibition of lipid peroxidation | PD, ALS |
31912279 21791473 31202468 18331585 36449420 28469157 |
| Clioquinol | Iron | Iron chelation and activation of AKT/mTOR survival pathway | PD, AD |
32424108 22286308 |
| Salicylaldehyde isonicotinoyl hydrazone | Iron | Iron chelation | ALS | 19158288 |
| Edaravone | FR | FR scavenger that eliminates the generation of hydroxyl radicals and peroxynitrite radicals | AD, ALS |
36333599 32077821 |
| Senegenin | RhoGDIα | Upregulation of GPX4 and downregulation of PEBP1 and ACSL4 | AD |
36068400 35080740 |
| Coenzyme Q10 | FSP1 | Increase FSP1 activity | AD, PD |
29154270 24664227 |
| Eriodictyol | VDR | Activation of the Nrf2/HO-1 signaling pathway | AD | 35093024 |
| (−)-Clausenamide | ALOX5 | Inhibition of the nuclear translocation of ALOX5 | PD, AD |
37121496 20674676 |
NGD Neurodegenerative disease, GPX4 Glutathione peroxidase 4, AD Alzheimer's disease, GSH Glutathione, PD Parkinson's disease, FSP1 Ferroptosis suppressor protein 1, ALS Amyotrophic lateral sclerosis, ROS Reactive oxygen species, NRF2 Nuclear factor erythroid 2-related factor 2, HO1 Heme oxygenase 1, FTH1 Ferritin heavy chain 1, mTOR - Mechanistic target of rapamycin, FR Free radical, RhoGDIα - Rho GDP dissociation inhibitor alpha, PEBP1 Phosphatidylethanolamine-binding protein 1, ACSL4 Acyl-CoA synthetase long-chain family member 4, VDR Vitamin D receptor, ALOX5 5-lipoxygenase.
Despite these promising prospects, significant challenges remain. Although preclinical studies have demonstrated the potential of ferroptosis inhibitors to protect neurons and improve outcomes in animal models, translating these findings to human patients is fraught with difficulties. The blood-brain barrier represents a formidable obstacle to drug delivery, and the long-term safety and efficacy of ferroptosis inhibitors in humans remains unknown. Furthermore, the multifactorial nature of neurodegenerative diseases means that ferroptosis alone is not sufficient to halt disease progression. How can we design therapies that effectively inhibit ferroptosis without disrupting other critical cellular processes? Is it possible to develop combination therapies that simultaneously act on multiple pathways and offer a more comprehensive approach to treating these complex disorders?
6.3. Cardiovascular diseases
In recent years, new research has uncovered a strong implication of ferroptosis to the pathogenesis of various cardiovascular disorders, including atherosclerosis, stroke, ischemia-reperfusion injury, and heart failure [[200], [201], [202], [203]]. Atherosclerosis and heart failure are closely linked with aging and highlight the broader pathological mechanisms of iron metabolism, oxidative stress, and disrupted cell death pathways that underpin ferroptosis.
Atherosclerosis is a chronic vascular disease characterized by the buildup of lipid-rich plaques in the arterial walls, leading to endothelial dysfunction and cardiovascular events. Different RCDs, such as apoptosis, pyroptosis, necroptosis, and ferroptosis, are significant contributors to atherosclerosis [204,205]. Ferroptosis is a major cause of endothelial dysfunction and macrophage activation and foam cell formation, leading to atherosclerosis [206,207]. Iron promotes the oxidation of low-density lipoprotein (Ox-LDL) within the artery wall, which is an inductor of both ferroptosis and atherosclerosis [206]. Fer-1 could alleviate atherosclerosis lesions in high-fat diet-fed ApoE−/− mice by downregulating the expression of adhesion molecules and upregulating the nitric oxide synthases (eNOS). Moreover, the overexpression of GPX4 inhibits the development of atherosclerosis in ApoE−/− mice by the scavenging of ROS and lipid peroxides [208]. Oscillatory blood flow suppresses NRF2 activation, increasing oxidative stress and inflammaging and favoring a proatherogenic environment [209,210]. It is important to note that the first strategy for treating atherosclerosis begins with lifestyle modification, in which diet plays a key role [211]. Low-iron diet and iron chelation therapy improve the course of atherosclerosis in a murine model of type IV haemochromatosis [212]. Could ferroptosis inhibitors be integrated into existing treatment regimens for atherosclerosis? Moreover, how might lifestyle interventions, particularly those focused on dietary iron intake, synergize with pharmacological strategies to reduce the burden of this disease?
Heart failure is characterized by the inability of the heart to pump sufficient blood to meet the nutritional and oxygen needs of the tissues. In the face of various hemodynamic stressors, such as valvular heart disease, hypertension, or myocardial infarction, cardiomyocytes initiate a compensatory response that results in hypertrophic cardiomyopathy, which can progress to dilated cardiomyopathy [213]. Ferroptosis has emerged as an important factor in the pathophysiology of heart failure, as it influences its development and progression in different clinical settings, such as myocardial infarction and cardiomyopathy [201,[214], [215], [216], [217]]. Various signaling pathways, such as those of MLK3, angiotensin II, and CD147, are involved in the aggravation of myocardial hypertrophy and fibrosis through the induction of ferroptosis [[218], [219], [220]]. Catecholamine-induced cardiotoxicity and pathological remodeling present ferroptosis cell death in cardiomyocytes through GPX4 and Bach1-HO-1-dependent signaling pathways [221]. TLR4 and NOX4 promote myocyte death through autophagy and ferroptosis in HF rats [222]. Excessive iron accumulation, a process that occurs in conditions such as hemochromatosis, can promote ferroptosis in cardiomyocytes and other cardiac cells, leading to myocardial injury and dysfunction [223]. Moreover, recent studies have demonstrated the involvement of ferroptosis in myocardial ischemia-reperfusion injury [224]. Furthermore, interventions targeting ferroptosis, such as the use of ferrostatin-1 or natural plant products, show promise in improving HF by decreasing myocardial cell death [225,226]. Puerarin treatment reduced NOX4 expression and increased GPX4 expression, increased striated muscle arrangement, and reduced mitochondrial atrophy. Exploring the role of ferroptosis in heart failure is essential for identifying specific drug targets and developing novel therapeutic strategies.
However, the translation of these findings to clinical practice presents difficulties. The regulatory mechanisms of the heart imply that inhibition of ferroptosis could have unintended consequences, such as interference with other cell death pathways essential for normal cardiac function. In addition, the systemic nature of ferroptosis-related interventions, particularly those involving iron chelation or dietary modifications, requires careful consideration of potential side effects and patient-specific factors. How do we ensure the specificity and safety of ferroptosis inhibitors in the clinical setting? What are the long-term effects of modulating iron metabolism in patients with chronic cardiovascular disease? And, most importantly, how can we integrate these new approaches with existing therapies to achieve the best outcomes for patients?
7. Conclusions and future perspectives
In conclusion, ferroptosis stands out as a distinctive form of RCD dependent on the metabolism of iron and lipid peroxidation. Unlike other forms of cell death, such as apoptosis or necrosis, excessive accumulation of lipid hydroperoxides in cell membranes drives the cell to death by rupture of the plasma membrane. The regulation of ferroptosis is under the control of multiple metabolic pathways, such as iron metabolism, redox homeostasis, and lipid and amino acid metabolism. Evidence has demonstrated that ferroptosis plays very important functions at the organism level, highlighting the antitumor and immune-associated functions and their role in aging, development, and overall homeostasis.
As we age, a cascade of physiological changes occurs leading to a gradual decline of various cellular functions. Among these, the dysregulation of ferroptosis has gained significant attention due to its implications in aging and age-related diseases. The aging process is characterized by progressive iron accumulation, increased oxidative stress, and a chronic low-grade inflammation known as inflammaging. These factors collectively contribute to the dysregulation of ferroptosis, leading to impaired tissue homeostasis and the onset of age-associated pathologies. Furthermore, the interaction between ferroptosis and cellular senescence, a hallmark of aging, adds another layer of complexity to our understanding of the mechanisms of aging. While some evidence suggests that senescent cells may be more resistant to ferroptosis, other studies indicate that certain inducers of ferroptosis can selectively target and eliminate senescent cells. This dichotomy underscores the need for further research to unravel the precise relationship between these two processes and their implications for aging and disease.
In multiple aging-related diseases, it has been demonstrated the occurrence of ferroptosis, including cancer, neurodegenerative diseases, and cardiovascular diseases. In cancer, ferroptosis acts as a tumor suppressor mechanism, and cancer cells often show increased sensitivity to ferroptosis due to their increased iron requirements. In neurodegenerative diseases, ferroptosis contributes to neuronal cell death, which aggravates disease progression. In cardiovascular diseases, dysregulation of ferroptosis is implicated in endothelial dysfunction, atherosclerosis and heart failure, highlighting its importance in the pathophysiology of these diseases. Looking ahead, a deeper understanding of the molecular and cellular mechanisms governing ferroptosis will be essential to unveil new insights into its physiological and pathological functions. This knowledge will not only improve our understanding of ferroptosis but will also open new avenues for therapeutic intervention. Targeting the pathways that regulate ferroptosis holds immense potential for the development of new treatments aimed at restoring tissue homeostasis and ameliorating aging-related diseases. Given the increasing global burden of population aging, these therapeutic strategies could have a profound impact on public health by addressing the underlying mechanisms of aging and promoting healthy longevity. Ferroptosis inhibitors and inducers show potential in treating diseases like cancer and neurological disorders, but they are not yet clinically applied due to several challenges. These include incomplete understanding of ferroptosis mechanisms, concerns about safety and unintended effects, complexities in drug delivery, and the dual roles of some compounds. Further research is needed to overcome these hurdles and ensure the safe and effective clinical use of ferroptosis-targeting therapies. Moreover, these compounds must undergo rigorous testing in clinical trials to establish their efficacy and safety, which is a time-consuming and costly process.
Future research should focus on elucidating the intricate interplay between ferroptosis and other cellular processes, such as senescence, autophagy, and immune responses. In addition, the identification of specific biomarkers of ferroptosis in different tissues and diseases will be crucial for the development of targeted therapies. Moreover, exploring both positive and negative modulation of ferroptosis, depending on the context, offers a promising strategy to combat a broad spectrum of diseases, from cancer to neurodegeneration and cardiovascular disorders. Ultimately, the goal is to harness the therapeutic potential of ferroptosis modulation to promote overall health and prolong healthy life.
CRediT authorship contribution statement
Diego De Leon-Oliva: Investigation, Conceptualization. Diego Liviu Boaru: Investigation. Ana M. Minaya-Bravo: Investigation. Patricia De Castro-Martinez: Investigation, Funding acquisition. Oscar Fraile-Martinez: Investigation. Cielo Garcia-Montero: Investigation. David Cobo-Prieto: Investigation. Silvestra Barrena-Blázquez: Investigation. Laura Lopez-Gonzalez: Investigation. Agustín Albillos: Investigation, Conceptualization. Melchor Alvarez-Mon: Investigation, Conceptualization. Miguel A. Saez: Conceptualization. Raul Diaz-Pedrero: Investigation, Conceptualization. Miguel A. Ortega: Investigation, Funding acquisition, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study (FIS-PI21/01244) was supported by the Instituto de Salud Carlos III (grant no. Estatal de I + D + I 2020–2027) and co-financed by the European Development Regional Fund “A way to achieve Europe”, as well as P2022/BMD-7321 (Comunidad de Madrid) and ProACapital, Halekulani S. L. and MJR. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Fulda S., Gorman A.M., Hori O., Samali A. Cellular stress responses: cell survival and cell death. Int. J. Cell Biol. 2010;2010 doi: 10.1155/2010/214074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil G.S., Davis R.J. Cell signaling and stress responses. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park W., Wei S., Kim B.S., Kim B., Bae S.J., Chae Y.C., Ryu D., Ha K.T. Diversity and complexity of cell death: a historical review. Exp. Mol. Med. 2023;55:1573–1594. doi: 10.1038/s12276-023-01078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., Annicchiarico-Petruzzelli M., Antonov A.V., Arama E., Baehrecke E.H., Barlev N.A., Bazan N.G., Bernassola F., Bertrand M.J.M., Bianchi K., Blagosklonny M.V., Blomgren K., Borner C., Boya P., Brenner C., Campanella M., Candi E., Carmona-Gutierrez D., Cecconi F., Chan F.K.M., Chandel N.S., Cheng E.H., Chipuk J.E., Cidlowski J.A., Ciechanover A., Cohen G.M., Conrad M., Cubillos-Ruiz J.R., Czabotar P.E., D'Angiolella V., Dawson T.M., Dawson V.L., De Laurenzi V., De Maria R., Debatin K.M., Deberardinis R.J., Deshmukh M., Di Daniele N., Di Virgilio F., Dixit V.M., Dixon S.J., Duckett C.S., Dynlacht B.D., El-Deiry W.S., Elrod J.W., Fimia G.M., Fulda S., García-Sáez A.J., Garg A.D., Garrido C., Gavathiotis E., Golstein P., Gottlieb E., Green D.R., Greene L.A., Gronemeyer H., Gross A., Hajnoczky G., Hardwick J.M., Harris I.S., Hengartner M.O., Hetz C., Ichijo H., Jäättelä M., Joseph B., Jost P.J., Juin P.P., Kaiser W.J., Karin M., Kaufmann T., Kepp O., Kimchi A., Kitsis R.N., Klionsky D.J., Knight R.A., Kumar S., Lee S.W., Lemasters J.J., Levine B., Linkermann A., Lipton S.A., Lockshin R.A., López-Otín C., Lowe S.W., Luedde T., Lugli E., MacFarlane M., Madeo F., Malewicz M., Malorni W., Manic G., Marine J.C., Martin S.J., Martinou J.C., Medema J.P., Mehlen P., Meier P., Melino S., Miao E.A., Molkentin J.D., Moll U.M., Muñoz-Pinedo C., Nagata S., Nuñez G., Oberst A., Oren M., Overholtzer M., Pagano M., Panaretakis T., Pasparakis M., Penninger J.M., Pereira D.M., Pervaiz S., Peter M.E., Piacentini M., Pinton P., Prehn J.H.M., Puthalakath H., Rabinovich G.A., Rehm M., Rizzuto R., Rodrigues C.M.P., Rubinsztein D.C., Rudel T., Ryan K.M., Sayan E., Scorrano L., Shao F., Shi Y., Silke J., Simon H.U., Sistigu A., Stockwell B.R., Strasser A., Szabadkai G., Tait S.W.G., Tang D., Tavernarakis N., Thorburn A., Tsujimoto Y., Turk B., Vanden Berghe T., Vandenabeele P., Vander Heiden M.G., Villunger A., Virgin H.W., Vousden K.H., Vucic D., Wagner E.F., Walczak H., Wallach D., Wang Y., Wells J.A., Wood W., Yuan J., Zakeri Z., Zhivotovsky B., Zitvogel L., Melino G., Kroemer G. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X., Stockwell B.R., Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon S.J., Olzmann J.A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 2024 doi: 10.1038/s41580-024-00703-5. [DOI] [PubMed] [Google Scholar]
- 7.Conrad M., Pratt D.A. The chemical basis of ferroptosis. Nat. Chem. Biol. 2019;15:1137–1147. doi: 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- 8.Zou Y., Li H., Graham E.T., Deik A.A., Eaton J.K., Wang W., Sandoval-Gomez G., Clish C.B., Doench J.G., Schreiber S.L. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol. 2020;16:302–309. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazhar M., Din A.U., Ali H., Yang G., Ren W., Wang L., Fan X., Yang S. Implication of ferroptosis in aging. Cell Death Discov. 2021;7:1–9. doi: 10.1038/s41420-021-00553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Wu S., Li Q., Sun H., Wang H. Pharmacological inhibition of ferroptosis as a therapeutic target for neurodegenerative diseases and strokes. Adv. Sci. 2023;10 doi: 10.1002/advs.202300325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S., Shen J., Jiang J., Wang F., Min J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct. Target. Ther. 2023;8 doi: 10.1038/s41392-023-01606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-Even A., Flamholz A., Noor E., Milo R. Rethinking glycolysis: on the biochemical logic of metabolic pathways. Nat. Chem. Biol. 2012;8:509–517. doi: 10.1038/nchembio.971. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh-Choudhary S., Liu J., Finkel T. Metabolic regulation of cell fate and function. Trends Cell Biol. 2020;30:201–212. doi: 10.1016/j.tcb.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., Morrison B., Stockwell B.R. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–504. doi: 10.1111/j.1753-4887.1958.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 16.Eagle H. The specific amino acid requirements of a human carcinoma cell (strain HeLa) in tissue culture. J. Exp. Med. 1955;102:37–48. doi: 10.1084/jem.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagle H. Amino acid metabolism in human cell cultures. Science. 1959;130:432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- 18.Eagle H., Piez K.A., Oyama V.I. The biosynthesis of cystine in human cell cultures. J. Biol. Chem. 1961;236:1425–1428. doi: 10.1016/s0021-9258(18)64190-0. [DOI] [PubMed] [Google Scholar]
- 19.Stockwell B.R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolpaw A.J., Shimada K., Skouta R., Welsch M.E., Akavia U.D., Péer D., Shaika F., Bulinski J.C., Stockwell B.R. Modulatory profiling identifies mechanisms of small molecule-induced cell death. Proc. Natl. Acad. Sci. U. S. A. 2011;108:771–780. doi: 10.1073/pnas.1106149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolma S., Lessnick S.L., Hahn W.C., Stockwell B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/S1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 23.Dixon S.J., Stockwell B.R. The hallmarks of ferroptosis. Annu. Rev. Cancer Biol. 2019;3:35–54. doi: 10.1146/annurev-cancerbio-030518-055844. [DOI] [Google Scholar]
- 24.Yagoda N., Von Rechenberg M., Zaganjor E., Bauer A.J., Yang W.S., Fridman D.J., Wolpaw A.J., Smukste I., Peltier J.M., Boniface J.J., Smith R., Lessnick S.L., Sahasrabudhe S., Stockwell B.R. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., Kang R., Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y., Lu S., lei Wu L., Yang L., Yang L., Wang J. The diversified role of mitochondria in ferroptosis in cancer. Cell Death Dis. 2023;14:1–12. doi: 10.1038/s41419-023-06045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh S.J., Ikeda M., Ide T., Hur K.Y., Lee M.S. Mitochondrial event as an ultimate step in ferroptosis. Cell Death Discov. 2022;8 doi: 10.1038/s41420-022-01199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu C., Cao N., Zeng S., Zhu W., Fu X., Liu W., Fan S. Role of mitochondria in the regulation of ferroptosis and disease. Front. Med. 2023;10:1–11. doi: 10.3389/fmed.2023.1301822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X., Li J., Kang R., Klionsky D.J., Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan R., Xie E., Li Y., Li J., Zhang Y., Chi X., Hu X., Xu L., Hou T., Stockwell B.R., Min J., Zhou Q., Wang F. The structure of erastin-bound xCT–4F2hc complex reveals molecular mechanisms underlying erastin-induced ferroptosis. Cell Res. 2022;32:687–690. doi: 10.1038/s41422-022-00642-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng C., Wang C., Sun D., Wang H., Li B., Liu G., Liu Z., Zhang L., Xu P. Structure-activity relationship study of RSL3-based GPX4 degraders and its potential noncovalent optimization. Eur. J. Med. Chem. 2023;255 doi: 10.1016/j.ejmech.2023.115393. [DOI] [PubMed] [Google Scholar]
- 32.Liu H., Forouhar F., Lin A.J., Wang Q., Polychronidou V., Soni R.K., Xia X., Stockwell B.R. Small-molecule allosteric inhibitors of GPX4. Cell Chem. Biol. 2022;29:1680–1693.e9. doi: 10.1016/j.chembiol.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W.S., Kim K.J., Gaschler M.M., Patel M., Shchepinov M.S., Stockwell B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng H., Stockwell B.R. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:1–15. doi: 10.1371/journal.pbio.2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagan V.E., Mao G., Qu F., Angeli J.P.F., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., Kapralov A.A., Amoscato A.A., Jiang J., Anthonymuthu T., Mohammadyani D., Yang Q., Proneth B., Klein-Seetharaman J., Watkins S., Bahar I., Greenberger J., Mallampalli R.K., Stockwell B.R., Tyurina Y.Y., Conrad M., Baylr H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Krusenstiern A.N., Robson R.N., Qian N., Qiu B., Hu F., Reznik E., Smith N., Zandkarimi F., Estes V.M., Dupont M., Hirschhorn T., Shchepinov M.S., Min W., Woerpel K.A., Stockwell B.R. Identification of essential sites of lipid peroxidation in ferroptosis. Nat. Chem. Biol. 2023;19:719–730. doi: 10.1038/s41589-022-01249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenzel S.E., Tyurina Y.Y., Zhao J., Croix C.M. St, Dar H.H., Mao G., Tyurin V.A., Anthonymuthu T.S., Kapralov A.A., Amoscato A.A., Mikulska-Ruminska K., Shrivastava I.H., Kenny E.M., Yang Q., Rosenbaum J.C., Sparvero L.J., Emlet D.R., Wen X., Minami Y., Qu F., Watkins S.C., Holman T.R., VanDemark A.P., Kellum J.A., Bahar I., Bayır H., Kagan V.E. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171:628–641.e26. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anthonymuthu T.S., Tyurina Y.Y., Sun W.Y., Mikulska-Ruminska K., Shrivastava I.H., Tyurin V.A., Cinemre F.B., Dar H.H., VanDemark A.P., Holman T.R., Sadovsky Y., Stockwell B.R., He R.R., Bahar I., Bayır H., Kagan V.E. Resolving the paradox of ferroptotic cell death: ferrostatin-1 binds to 15LOX/PEBP1 complex, suppresses generation of peroxidized ETE-PE, and protects against ferroptosis. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samovich S.N., Mikulska-Ruminska K., Dar H.H., Tyurina Y.Y., Tyurin V.A., Souryavong A.B., Kapralov A.A., Amoscato A.A., Beharier O., Karumanchi S.A., St Croix C.M., Yang X., Holman T.R., VanDemark A.P., Sadovsky Y., Mallampalli R.K., Wenzel S.E., Gu W., Bunimovich Y.L., Bahar I., Kagan V.E., Bayir H. Strikingly high activity of 15-lipoxygenase towards di-polyunsaturated arachidonoyl/adrenoyl-phosphatidylethanolamines generates peroxidation signals of ferroptotic cell death. Angew. Chemie - Int. Ed. 2024;63 doi: 10.1002/anie.202314710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banthiya S., Kalms J., Galemou Yoga E., Ivanov I., Carpena X., Hamberg M., Kuhn H., Scheerer P. Structural and functional basis of phospholipid oxygenase activity of bacterial lipoxygenase from Pseudomonas aeruginosa. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2016;1861:1681–1692. doi: 10.1016/j.bbalip.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Dar H.H., Tyurina Y.Y., Mikulska-Ruminska K., Shrivastava I., Ting H.C., Tyurin V.A., Krieger J., Croix C.M.S., Watkins S., Bayir E., Mao G., Armbruster C.R., Kapralov A., Wang H., Parsek M.R., Anthonymuthu T.S., Ogunsola A.F., Flitter B.A., Freedman C.J., Gaston J.R., Holman T.R., Pilewski J.M., Greenberger J.S., Mallampalli R.K., Doi Y., Lee J.S., Bahar I., Bomberger J.M., Bayır H., Kagan V.E. Pseudomonas aeruginosa utilizes host polyunsaturated phosphatidylethanolamines to trigger theft-ferroptosis in bronchial epithelium. J. Clin. Invest. 2018;128:4639–4653. doi: 10.1172/JCI99490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan B., Ai Y., Sun Q., Ma Y., Cao Y., Wang J., Zhang Z., Wang X. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol. Cell. 2021;81:355–369.e10. doi: 10.1016/j.molcel.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 43.Yang W.S., Sriramaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., Brown L.M., Girotti A.W., Cornish V.W., Schreiber S.L., Stockwell B.R. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y., Xue Z., Huang T., Che X., Wu G. Lipid metabolism in ferroptosis and ferroptosis-based cancer therapy. Front. Oncol. 2022;12:1–11. doi: 10.3389/fonc.2022.941618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah R., Shchepinov M.S., Pratt D.A. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zilka O., Shah R., Li B., Friedmann Angeli J.P., Griesser M., Conrad M., Pratt D.A. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent. Sci. 2017;3:232–243. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scarpellini C., Klejborowska G., Lanthier C., Hassannia B., Vanden Berghe T., Augustyns K. Beyond ferrostatin-1: a comprehensive review of ferroptosis inhibitors. Trends Pharmacol. Sci. 2023;44:902–916. doi: 10.1016/j.tips.2023.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Hirata Y., Mishima E. Membrane dynamics and cation handling in ferroptosis. Physiology. 2024;39:73–87. doi: 10.1152/physiol.00029.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirata Y., Cai R., Volchuk A., Steinberg B.E., Saito Y., Matsuzawa A., Grinstein S., Freeman S.A. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol. 2023;33:1282–1294.e5. doi: 10.1016/j.cub.2023.02.060. [DOI] [PubMed] [Google Scholar]
- 50.Riegman M., Sagie L., Galed C., Levin T., Steinberg N., Dixon S.J., Wiesner U., Bradbury M.S., Niethammer P., Zaritsky A., Overholtzer M. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat. Cell Biol. 2020;22:1042–1048. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedrera L., Espiritu R.A., Ros U., Weber J., Schmitt A., Stroh J., Hailfinger S., von Karstedt S., García-Sáez A.J. Ferroptotic pores induce Ca2+ fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 2021;28:1644–1657. doi: 10.1038/s41418-020-00691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ros U., Peña-Blanco A., Hänggi K., Kunzendorf U., Krautwald S., Wong W.W.L., García-Sáez A.J. Necroptosis execution is mediated by plasma membrane nanopores independent of calcium. Cell Rep. 2017;19:175–187. doi: 10.1016/j.celrep.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C., Ruan J. Mechanistic insights into gasdermin pore formation and regulation in pyroptosis. J. Mol. Biol. 2022;434 doi: 10.1016/j.jmb.2021.167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dondelinger Y., Priem D., Huyghe J., Delanghe T., Vandenabeele P., Bertrand M.J.M. NINJ1 is activated by cell swelling to regulate plasma membrane permeabilization during regulated necrosis. Cell Death Dis. 2023;14:755. doi: 10.1038/s41419-023-06284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos S., Hartenian E., Santos J.C., Walch P., Broz P. NINJ1 induces plasma membrane rupture and release of damage-associated molecular pattern molecules during ferroptosis. EMBO J. 2024;43:1164–1186. doi: 10.1038/s44318-024-00055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agmon E., Solon J., Bassereau P., Stockwell B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-23408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parker J.L., Deme J.C., Kolokouris D., Kuteyi G., Biggin P.C., Lea S.M., Newstead S. Molecular basis for redox control by the human cystine/glutamate antiporter system xc. Nat. Commun. 2021;12:1–11. doi: 10.1038/s41467-021-27414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y., Swanda R.V., Nie L., Liu X., Wang C., Lee H., Lei G., Mao C., Koppula P., Cheng W., Zhang J., Xiao Z., Zhuang L., Fang B., Chen J., Qian S.B., Gan B. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-21841-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sbodio J.I., Snyder S.H., Paul B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019;176:583–593. doi: 10.1111/bph.14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ursini F., Bosello Travain V., Cozza G., Miotto G., Roveri A., Toppo S., Maiorino M. A white paper on Phospholipid Hydroperoxide Glutathione Peroxidase (GPx4) forty years later. Free Radic. Biol. Med. 2022;188:117–133. doi: 10.1016/j.freeradbiomed.2022.06.227. [DOI] [PubMed] [Google Scholar]
- 62.Imai H., Hirao F., Sakamoto T., Sekine K., Mizukura Y., Saito M., Kitamoto T., Hayasaka M., Hanaoka K., Nakagawa Y. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 2003;305:278–286. doi: 10.1016/S0006-291X(03)00734-4. [DOI] [PubMed] [Google Scholar]
- 63.Yoo S.E., Chen L., Na R., Liu Y., Rios C., Van Remmen H., Richardson A., Ran Q. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 2012;52:1820–1827. doi: 10.1016/j.freeradbiomed.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma T., Du J., Zhang Y., Wang Y., Wang B., Zhang T. GPX4-independent ferroptosis—a new strategy in disease's therapy. Cell Death Discov. 2022;8 doi: 10.1038/s41420-022-01212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bersuker K., Hendricks J.M., Li Z., Magtanong L., Ford B., Tang P.H., Roberts M.A., Tong B., Maimone T.J., Zoncu R., Bassik M.C., Nomura D.K., Dixon S.J., Olzmann J.A. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doll S., Freitas F.P., Shah R., Aldrovandi M., da Silva M.C., Ingold I., Grocin A.G., Xavier da Silva T.N., Panzilius E., Scheel C.H., Mourão A., Buday K., Sato M., Wanninger J., Vignane T., Mohana V., Rehberg M., Flatley A., Schepers A., Kurz A., White D., Sauer M., Sattler M., Tate E.W., Schmitz W., Schulze A., O'Donnell V., Proneth B., Popowicz G.M., Pratt D.A., Angeli J.P.F., Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 67.Mishima E., Ito J., Wu Z., Nakamura T., Wahida A., Doll S., Tonnus W., Nepachalovich P., Eggenhofer E., Aldrovandi M., Henkelmann B., ichi Yamada K., Wanninger J., Zilka O., Sato E., Feederle R., Hass D., Maida A., Mourão A.S.D., Linkermann A., Geissler E.K., Nakagawa K., Abe T., Fedorova M., Proneth B., Pratt D.A., Conrad M. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature. 2022;608:778–783. doi: 10.1038/s41586-022-05022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai E., Zhang W., Cong D., Kang R., Wang J., Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem. Biophys. Res. Commun. 2020;523:966–971. doi: 10.1016/j.bbrc.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 69.Mishima E., Nakamura T., Zheng J., Zhang W., Mourão A.S.D., Sennhenn P., Conrad M. DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature. 2023;619:E9–E18. doi: 10.1038/s41586-023-06269-0. [DOI] [PubMed] [Google Scholar]
- 70.Kraft V.A.N., Bezjian C.T., Pfeiffer S., Ringelstetter L., Müller C., Zandkarimi F., Merl-Pham J., Bao X., Anastasov N., Kössl J., Brandner S., Daniels J.D., Schmitt-Kopplin P., Hauck S.M., Stockwell B.R., Hadian K., Schick J.A. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doll S., Proneth B., Tyurina Y.Y., Panzilius E., Kobayashi S., Ingold I., Irmler M., Beckers J., Aichler M., Walch A., Prokisch H., Trümbach D., Mao G., Qu F., Bayir H., Füllekrug J., Scheel C.H., Wurst W., Schick J.A., Kagan V.E., Angeli J.P.F., Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao Y., Chen Y.Q., Bonacci T.M., Bredt D.S., Li S., Bensch W.R., Moller D.E., Kowala M., Konrad R.J., Cao G. Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J. Biol. Chem. 2008;283:8258–8265. doi: 10.1074/jbc.M710422200. [DOI] [PubMed] [Google Scholar]
- 73.Hishikawa D., Shindou H., Kobayashi S., Nakanishi H., Taguchi R., Shimizu T. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2830–2835. doi: 10.1073/pnas.0712245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reed A., Ichu T.A., Milosevich N., Melillo B., Schafroth M.A., Otsuka Y., Scampavia L., Spicer T.P., Cravatt B.F. LPCAT3 inhibitors remodel the polyunsaturated phospholipid content of human cells and protect from ferroptosis. ACS Chem. Biol. 2022;17:1607–1618. doi: 10.1021/acschembio.2c00317. [DOI] [PubMed] [Google Scholar]
- 75.Chu B., Kon N., Chen D., Li T., Liu T., Jiang L., Song S., Tavana O., Gu W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019;21:579–591. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galy B., Conrad M., Muckenthaler M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 2023 doi: 10.1038/s41580-023-00648-1. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H., Wang Z., Liu Z., Du K., Lu X. Protective effects of dexazoxane on rat ferroptosis in doxorubicin-induced cardiomyopathy through regulating HMGB1. Front. Cardiovasc. Med. 2021;8:1–16. doi: 10.3389/fcvm.2021.685434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Fan B.Y., Pang Y.L., Shen W.Y., Wang X., Zhao C.X., Li W.X., Liu C., Kong X.H., Ning G.Z., Feng S.Q., Yao X. Neuroprotective effect of deferoxamine on erastin induced ferroptosis in primary cortical neurons. Neural Regen. Res. 2020;15:1539–1545. doi: 10.4103/1673-5374.274344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang Y., Wang Y., Sun C., Xiang Y., Deng Y. Deferoxamine reduces endothelial ferroptosis and protects cerebrovascular function after experimental traumatic brain injury. Brain Res. Bull. 2024;207 doi: 10.1016/j.brainresbull.2024.110878. [DOI] [PubMed] [Google Scholar]
- 80.Gao M., Monian P., Pan Q., Zhang W., Xiang J., Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;508:105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gryzik M., Asperti M., Denardo A., Arosio P., Poli M. NCOA4-mediated ferritinophagy promotes ferroptosis induced by erastin, but not by RSL3 in HeLa cells. Biochim. Biophys. Acta - Mol. Cell Res. 2021;1868 doi: 10.1016/j.bbamcr.2020.118913. [DOI] [PubMed] [Google Scholar]
- 83.Yang W., Mu B., You J., Tian C., Bin H., Xu Z., Zhang L., Ma R., Wu M., Zhang G., Huang C., Li L., Shao Z., Dai L., Désaubry L., Yang S. Non-classical ferroptosis inhibition by a small molecule targeting PHB2. Nat. Commun. 2022;13:1–16. doi: 10.1038/s41467-022-35294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu N., Lin X., Huang C. Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance. Br. J. Cancer. 2020;122:279–292. doi: 10.1038/s41416-019-0660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hayano M., Yang W.S., Corn C.K., Pagano N.C., Stockwell B.R. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao M., Yi J., Zhu J., Minikes A.M., Monian P., Thompson C.B., Jiang X. Role of mitochondria in ferroptosis. Mol. Cell. 2019;73:354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin X., Tang J., Qiu X., Nie X., Ou S., Wu G., Zhang R., Zhu J. Ferroptosis: emerging mechanisms, biological function, and therapeutic potential in cancer and inflammation. Cell Death Discov. 2024;10:1–10. doi: 10.1038/s41420-024-01825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bogacz M., Krauth-Siegel R.L. Tryparedoxin peroxidase-deficiency commits trypanosomes to ferroptosis-type cell death. Elife. 2018;7:1–29. doi: 10.7554/eLife.37503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carbó M., Chaturvedi P., Álvarez A., Pineda-Cevallos D., Ghatak A., González P.R., Cañal M.J., Weckwerth W., Valledor L. Ferroptosis is the key cellular process mediating Bisphenol A responses in Chlamydomonas and a promising target for enhancing microalgae-based bioremediation. J. Hazard Mater. 2023;448 doi: 10.1016/j.jhazmat.2023.130997. [DOI] [PubMed] [Google Scholar]
- 91.Shen Q., Liang M., Yang F., Deng Y.Z., Naqvi N.I. Ferroptosis contributes to developmental cell death in rice blast. New Phytol. 2020;227:1831–1846. doi: 10.1111/nph.16636. [DOI] [PubMed] [Google Scholar]
- 92.Distéfano A.M., López G.A., Setzes N., Marchetti F., Cainzos M., Cascallares M., Zabaleta E., Pagnussat G.C. Ferroptosis in plants: triggers, proposed mechanisms, and the role of iron in modulating cell death. J. Exp. Bot. 2021;72:2125–2135. doi: 10.1093/jxb/eraa425. [DOI] [PubMed] [Google Scholar]
- 93.Jiang L., Kon N., Li T., Wang S.J., Su T., Hibshoosh H., Baer R., Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Fang Z.M., Yi X., Wei X., Jiang D.S. The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. 2023;14:1–13. doi: 10.1038/s41419-023-05716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dang Q., Sun Z., Wang Y., Wang L., Liu Z., Han X. Ferroptosis: a double-edged sword mediating immune tolerance of cancer. Cell Death Dis. 2022;13 doi: 10.1038/s41419-022-05384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiong Y., Chen L., Lin Z., Hu Y., Panayi A.C., Zhou W., Sun Y., Cao F., Liu G., Dai G., Mi B., Liu G. The regulatory role of ferroptosis in bone homeostasis. Stem Cells Int. 2022;2022 doi: 10.1155/2022/3568597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng H., Jiang L., Tsuduki T., Conrad M., Toyokuni S. Embryonal erythropoiesis and aging exploit ferroptosis. Redox Biol. 2021;48 doi: 10.1016/j.redox.2021.102175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jenkins N.L., James S.A., Salim A., Sumardy F., Speed T.P., Conrad M., Richardson D.R., Bush A.I., McColl G. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans. Elife. 2020;9:1–28. doi: 10.7554/eLife.56580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Egolf S., Zou J., Anderson A., Simpson C.L., Aubert Y., Prouty S., Ge K., Seykora J.T., Capell B.C. MLL4 mediates differentiation and tumor suppression through ferroptosis. Sci. Adv. 2021;7:1–12. doi: 10.1126/sciadv.abj9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Z., Wang W., Abdul Razak S.R., Han T., Ahmad N.H., Li X. Ferroptosis as a potential target for cancer therapy. Cell Death Dis. 2023;14 doi: 10.1038/s41419-023-05930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang W., Green M., Choi J.E., Gijón M., Kennedy P.D., Johnson J.K., Liao P., Lang X., Kryczek I., Sell A., Xia H., Zhou J., Li G., Li J., Li W., Wei S., Vatan L., Zhang H., Szeliga W., Gu W., Liu R., Lawrence T.S., Lamb C., Tanno Y., Cieslik M., Stone E., Georgiou G., Chan T.A., Chinnaiyan A., Zou W. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liao P., Wang W., Wang W., Kryczek I., Li X., Bian Y., Sell A., Wei S., Grove S., Johnson J.K., Kennedy P.D., Gijón M., Shah Y.M., Zou W. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40:365–378.e6. doi: 10.1016/j.ccell.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim R., Hashimoto A., Markosyan N., Tyurin V.A., Tyurina Y.Y., Kar G., Fu S., Sehgal M., Garcia-Gerique L., Kossenkov A., Gebregziabher B.A., Tobias J.W., Hicks K., Halpin R.A., Cvetesic N., Deng H., Donthireddy L., Greenberg A., Nam B., Vonderheide R.H., Nefedova Y., Kagan V.E., Gabrilovich D.I. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature. 2022;612:338–346. doi: 10.1038/s41586-022-05443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma X., Xiao L., Liu L., Ye L., Su P., Bi E., Wang Q., Yang M., Qian J., Yi Q. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–1012.e5. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishizawa H., Matsumoto M., Chen G., Ishii Y., Tada K., Onodera M., Kato H., Muto A., Tanaka K., Igarashi K. Lipid peroxidation and the subsequent cell death transmitting from ferroptotic cells to neighboring cells. Cell Death Dis. 2021;12 doi: 10.1038/s41419-021-03613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alarcón-Veleiro C., Mato-Basalo R., Lucio-Gallego S., Vidal-Pampín A., Quindós-Varela M., Al-Qatarneh T., Berrecoso G., Vizoso-Vázquez Á., Arufe M.C., Fafián-Labora J. Study of ferroptosis transmission by small extracellular vesicles in epithelial ovarian cancer cells. Antioxidants. 2023;12 doi: 10.3390/antiox12010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vucetic M., Daher B., Cassim S., Meira W., Pouyssegur J. Together we stand, apart we fall: how cell-to-cell contact/interplay provides resistance to ferroptosis. Cell Death Dis. 2020;11:789. doi: 10.1038/s41419-020-02994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao Y., Chen Z., Zhang H., Chen C., Zeng M., Yunis J., Wei Y., Wan Y., Wang N., Zhou M., Qiu C., Zeng Q., Ong H.S., Wang H., Makota F.V., Yang Y., Yang Z., Wang N., Deng J., Shen C., Xia Y., Yuan L., Lian Z., Deng Y., Guo C., Huang A., Zhou P., Shi H., Zhang W., Yi H., Li D., Xia M., Fu J., Wu N., de Haan J.B., Shen N., Zhang W., Liu Z., Yu D. Selenium–GPX4 axis protects follicular helper T cells from ferroptosis. Nat. Immunol. 2021;22:1127–1139. doi: 10.1038/s41590-021-00996-0. [DOI] [PubMed] [Google Scholar]
- 109.Gorgoulis V., Adams P.D., Alimonti A., Bennett D.C., Bischof O., Bishop C., Campisi J., Collado M., Evangelou K., Ferbeyre G., Gil J., Hara E., Krizhanovsky V., Jurk D., Maier A.B., Narita M., Niedernhofer L., Passos J.F., Robbins P.D., Schmitt C.A., Sedivy J., Vougas K., von Zglinicki T., Zhou D., Serrano M., Demaria M. Cellular senescence: defining a path forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 110.Xu P., Wang M., min Song W., Wang Q., Yuan G.C., Sudmant P.H., Zare H., Tu Z., Orr M.E., Zhang B. The landscape of human tissue and cell type specific expression and co-regulation of senescence genes. Mol. Neurodegener. 2022;17:1–20. doi: 10.1186/s13024-021-00507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 112.Tuttle C.S.L., Waaijer M.E.C., Slee-Valentijn M.S., Stijnen T., Westendorp R., Maier A.B. Cellular senescence and chronological age in various human tissues: a systematic review and meta-analysis. Aging Cell. 2020;19:1–11. doi: 10.1111/acel.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmitt C.A., Wang B., Demaria M. Senescence and cancer — role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022;19:619–636. doi: 10.1038/s41571-022-00668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Childs B.G., Gluscevic M., Baker D.J., Laberge R.M., Marquess D., Dananberg J., Van Deursen J.M. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niedernhofer L.J., Robbins P.D. Senotherapeutics for healthy ageing. Nat. Rev. Drug Discov. 2018;17:377. doi: 10.1038/nrd.2018.44. [DOI] [PubMed] [Google Scholar]
- 116.Wei X., Liu M., Zheng Z., Yu S., Huang L., Ma J., Gao Y., Peng Y., Chen L., Tan R., She Z., Yang L. Defective NCOA4-dependent ferroptosis in senescent fibroblasts retards diabetic wound healing. Cell Death Discov. 2023;9:1–10. doi: 10.1038/s41420-023-01437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masaldan S., Clatworthy S.A.S., Gamell C., Meggyesy P.M., Rigopoulos A.T., Haupt S., Haupt Y., Denoyer D., Adlard P.A., Bush A.I., Cater M.A. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018;14:100–115. doi: 10.1016/j.redox.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Admasu T.D., Kim K., Rae M., Avelar R., Gonciarz R.L., Rebbaa A., Pedro de Magalhães J., Renslo A.R., Stolzing A., Sharma A. Selective ablation of primary and paracrine senescent cells by targeting iron dyshomeostasis. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112058. [DOI] [PubMed] [Google Scholar]
- 119.Liao C.M., Wulfmeyer V.C., Chen R., Erlangga Z., Sinning J., von Mässenhausen A., Sörensen-Zender I., Beer K., von Vietinghoff S., Haller H., Linkermann A., Melk A., Schmitt R. Induction of ferroptosis selectively eliminates senescent tubular cells. Am. J. Transplant. 2022;22:2158–2168. doi: 10.1111/ajt.17102. [DOI] [PubMed] [Google Scholar]
- 120.Go S., Kang M., Kwon S.P., Jung M., Jeon O.H., Kim B.S. The senolytic drug JQ1 removes senescent cells via ferroptosis. Tissue Eng. Regen. Med. 2021;18:841–850. doi: 10.1007/s13770-021-00346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wan X., Li C., Tan Y.H., Zuo S.Q., Deng F.M., Sun J., Liu Y.L. Dihydroartemisinin eliminates senescent cells by promoting autophagy-dependent ferroptosis via AMPK/mTOR signaling pathway. Cell Biol. Int. 2024 doi: 10.1002/CBIN.12143. [DOI] [PubMed] [Google Scholar]
- 122.Sui S., Zhang J., Xu S., Wang Q., Wang P., Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 2019;10:331. doi: 10.1038/s41419-019-1564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma J., Chen S., Liu J., Liao Y., Li L., Wang C.C., Song S., Feng R., Hu H., Quan S. Cryptochrome 1 regulates ovarian granulosa cell senescence through NCOA4-mediated ferritinophagy. Free Radic. Biol. Med. 2024;217:1–14. doi: 10.1016/j.freeradbiomed.2024.03.015. [DOI] [PubMed] [Google Scholar]
- 124.Sun D.Y., Bin Wu W., Wu J.J., Shi Y., Xu J.J., Ouyang S.X., Chi C., Shi Y., Ji Q.X., Miao J.H., Fu J.T., Tong J., Zhang P.P., Zhang J.B., Li Z.Y., Qu L.F., Shen F.M., Li D.J., Wang P. Pro-ferroptotic signaling promotes arterial aging via vascular smooth muscle cell senescence. Nat. Commun. 2024;15 doi: 10.1038/s41467-024-45823-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fraile-Martinez O., De Leon-Oliva D., Garcia-Montero C., Barrena-Blázquez S., Alvarez-Mon M., Lopez-Gonzalez L., Ortega M.A. Connecting epigenetics and inflammation in vascular senescence: state of the art, biomarkers and senotherapeutics. Front. Genet. 2024;15:1–18. doi: 10.3389/fgene.2024.1345459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zheng Y., Wang L., Wang J., Zhao T., Wang J. Modulation of the HIF-1α-NCOA4-FTH1 signaling Axis regulating ferroptosis-induced hepatic stellate cell senescence to explore the anti-hepatic fibrosis mechanism of curcumol. Curr. Med. Chem. 2024;31:2821–2837. doi: 10.2174/0109298673271261231213051410. [DOI] [PubMed] [Google Scholar]
- 127.Fan L., Du P., Li Y., Chen X., Liu F., Liu Y., Petrov A.M., Li X., Wang Z., Zhao Y. Targeted liposomes sensitize plastic melanoma to ferroptosis via senescence induction and coenzyme depletion. ACS Nano. 2024;18:7011–7023. doi: 10.1021/acsnano.3c10142. [DOI] [PubMed] [Google Scholar]
- 128.Li X., Li C., Zhang W., Wang Y., Qian P., Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023;8 doi: 10.1038/s41392-023-01502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu X., Chen Z., Shen W., Huang G., Sedivy J.M., Wang H., Ju Z. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduct. Target. Ther. 2021;6:1–29. doi: 10.1038/s41392-021-00646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Olivieri F., Prattichizzo F., Grillari J., Balistreri C.R. Cellular senescence and inflammaging in age-Related diseases. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/9076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang S., Lu Y., Chi T., Zhang Y., Zhao Y., Guo H., Feng L. Identification of ferroptosis-related genes in type 2 diabetes mellitus based on machine learning. Immunity, Inflamm. Dis. 2023;11 doi: 10.1002/iid3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miao R., Fang X., Zhang Y., Wei J., Zhang Y., Tian J. Iron metabolism and ferroptosis in type 2 diabetes mellitus and complications: mechanisms and therapeutic opportunities. Cell Death Dis. 2023;14:1–9. doi: 10.1038/s41419-023-05708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ru Q., Li Y., Xie W., Ding Y., Chen L., Xu G., Wu Y., Wang F. Fighting age-related orthopedic diseases: focusing on ferroptosis. Bone Res. 2023;11 doi: 10.1038/s41413-023-00247-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu Y., Yang Z., Dai T., Xue X., Xia D., Feng Z., Huang J., Chen X., Sun S., Zhou J., Dai Y., Zong J., Li S., Meng Q. Characteristics and time points to inhibit ferroptosis in human osteoarthritis. Sci. Rep. 2023;13:1–11. doi: 10.1038/s41598-023-49089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meng D., Zhu C., Jia R., Li Z., Wang W., Song S. The molecular mechanism of ferroptosis and its role in COPD. Front. Med. 2023;9 doi: 10.3389/FMED.2022.1052540/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yoshida M., Minagawa S., Araya J., Sakamoto T., Hara H., Tsubouchi K., Hosaka Y., Ichikawa A., Saito N., Kadota T., Sato N., Kurita Y., Kobayashi K., Ito S., Utsumi H., Wakui H., Numata T., Kaneko Y., Mori S., Asano H., Yamashita M., Odaka M., Morikawa T., Nakayama K., Iwamoto T., Imai H., Kuwano K. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-10991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cheng Z., Chu H., Zhu Q., Yang L. Ferroptosis in non-alcoholic liver disease: molecular mechanisms and therapeutic implications. Front. Nutr. 2023;10 doi: 10.3389/FNUT.2023.1090338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu J., Wang Y., Jiang R., Xue R., Yin X., Wu M., Meng Q. Ferroptosis in liver disease: new insights into disease mechanisms. Cell Death Discov. 2021;7:1–9. doi: 10.1038/s41420-021-00660-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao T., Guo X., Sun Y. Iron Accumulation and lipid peroxidation in the aging retina: implication of ferroptosis in age-related macular degeneration. Aging Dis. 2021;12:529–551. doi: 10.14336/AD.2020.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu D., Liu Z., Liao H., Chen Z.S., Qin B. Ferroptosis as a potential therapeutic target for age-related macular degeneration. Drug Discov. Today. 2024;29 doi: 10.1016/j.drudis.2024.103920. [DOI] [PubMed] [Google Scholar]
- 141.Zeng C., Gu X., Chen Y., Lin Y., Chen J., Chen Z., Chen C., Yao G., Lin C. Identification and experimental validation of ferroptosis-related gene lactotransferrin in age-related hearing loss. Front. Aging Neurosci. 2024;16:1–11. doi: 10.3389/fnagi.2024.1309115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen X., Li D., Sun H.Y., Wang W.W., Wu H., Kong W., Kong W.J. Relieving ferroptosis may partially reverse neurodegeneration of the auditory cortex. FEBS J. 2020;287:4747–4766. doi: 10.1111/febs.15266. [DOI] [PubMed] [Google Scholar]
- 143.Hassannia B., Vandenabeele P., Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 144.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kuang Y., Yang K., Meng L., Mao Y., Xu F., Liu H. Identification and validation of ferroptosis-related biomarkers and the related pathogenesis in precancerous lesions of gastric cancer. Sci. Rep. 2023;13:1–13. doi: 10.1038/s41598-023-43198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Badgley M.A., Kremer D.M., Carlo Maurer H., DelGiorno K.E., Lee H.J., Purohit V., Sagalovskiy I.R., Ma A., Kapilian J., Firl C.E.M., Decker A.R., Sastra S.A., Palermo C.F., Andrade L.R., Sajjakulnukit P., Zhang L., Tolstyka Z.P., Hirschhorn T., Lamb C., Liu T., Gu W., Scott Seeley E., Stone E., Georgiou G., Manor U., Iuga A., Wahl G.M., Stockwell B.R., Lyssiotis C.A., Olive K.P. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–89. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen M.S., Wang S.F., Hsu C.Y., Yin P.H., Yeh T.S., Lee H.C., Tseng L.M. CHAC1 degradation of glutathione enhances cystine-starvation induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget. 2017;8:114588–114602. doi: 10.18632/oncotarget.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang W.H., Ding C.K.C., Sun T., Rupprecht G., Lin C.C., Hsu D., Chi J.T. The hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28:2501–2508.e4. doi: 10.1016/j.celrep.2019.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou Q., Meng Y., Li D., Yao L., Le J., Liu Y., Sun Y., Zeng F., Chen X., Deng G. Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024;9 doi: 10.1038/s41392-024-01769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ye J., Jiang X., Dong Z., Hu S., Xiao M. Low-concentration PTX and RSL3 inhibits tumor cell growth synergistically by inducing ferroptosis in mutant p53 hypopharyngeal squamous carcinoma. Cancer Manag. Res. 2019;11:9783–9792. doi: 10.2147/CMAR.S217944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leu J.I.J., Murphy M.E., George D.L. Mechanistic basis for impaired ferroptosis in cells expressing the African-centric S47 variant of p53. Proc. Natl. Acad. Sci. U. S. A. 2019;116:8390–8396. doi: 10.1073/pnas.1821277116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.He Y., Dong Y., Chen Y., Zhang G., Zhang H., Lei G., Du Y., Chen X., Ye Y., Liu H. Multi-omics characterization and therapeutic liability of ferroptosis in melanoma. Signal Transduct. Target. Ther. 2022;7 doi: 10.1038/s41392-022-01067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou N., Bao J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database. 2020;2020:1–8. doi: 10.1093/database/baaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Viswanathan V.S., Ryan M.J., Dhruv H.D., Gill S., Eichhoff O.M., Seashore-Ludlow B., Kaffenberger S.D., Eaton J.K., Shimada K., Aguirre A.J., Viswanathan S.R., Chattopadhyay S., Tamayo P., Yang W.S., Rees M.G., Chen S., Boskovic Z.V., Javaid S., Huang C., Wu X., Tseng Y.Y., Roider E.M., Gao D., Cleary J.M., Wolpin B.M., Mesirov J.P., Haber D.A., Engelman J.A., Boehm J.S., Kotz J.D., Hon C.S., Chen Y., Hahn W.C., Levesque M.P., Doench J.G., Berens M.E., Shamji A.F., Clemons P.A., Stockwell B.R., Schreiber S.L. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Friedmann Angeli J.P., Krysko D.V., Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer. 2019;19:405–414. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 156.Tsoi J., Robert L., Paraiso K., Galvan C., Sheu K.M., Lay J., Wong D.J.L., Atefi M., Shirazi R., Wang X., Braas D., Grasso C.S., Palaskas N., Ribas A., Graeber T.G. Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell. 2018;33:890–904.e5. doi: 10.1016/j.ccell.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nie Z., Chen M., Gao Y., Huang D., Cao H., Peng Y., Guo N., Wang F., Zhang S. Ferroptosis and tumor drug resistance: current status and major challenges. Front. Pharmacol. 2022;13:1–15. doi: 10.3389/fphar.2022.879317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hangauer M.J., Viswanathan V.S., Ryan M.J., Bole D., Eaton J.K., Matov A., Galeas J., Dhruv H.D., Berens M.E., Schreiber S.L., McCormick F., McManus M.T. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee J., You J.H., Kim M.S., Roh J.L. Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Y., Wei Z., Pan K., Li J., Chen Q. The function and mechanism of ferroptosis in cancer. Apoptosis. 2020;25:786–798. doi: 10.1007/s10495-020-01638-w. [DOI] [PubMed] [Google Scholar]
- 161.Ren Y., Mao X., Xu H., Dang Q., Weng S., Zhang Y., Chen S., Liu S., Ba Y., Zhou Z., Han X., Liu Z., Zhang G. Ferroptosis and EMT: key targets for combating cancer progression and therapy resistance. Cell. Mol. Life Sci. 2023;80:1–15. doi: 10.1007/s00018-023-04907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lei G., Zhang Y., Koppula P., Liu X., Zhang J., Lin S.H., Ajani J.A., Xiao Q., Liao Z., Wang H., Gan B. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ye L.F., Chaudhary K.R., Zandkarimi F., Harken A.D., Kinslow C.J., Upadhyayula P.S., Dovas A., Higgins D.M., Tan H., Zhang Y., Buonanno M., Wang T.J.C., Hei T.K., Bruce J.N., Canoll P.D., Cheng S.K., Stockwell B.R. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol. 2020;15:469–484. doi: 10.1021/acschembio.9b00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ortega M.A., Fraile-Martinez O., García-Montero C., Funes Moñux R.M., Rodriguez-Martín S., Bravo C., De Leon-Luis J.A., Saz J.V., Saez M.A., Guijarro L.G., Lahera G., Mora F., Fernandez-Rojo S., Quintero J., Monserrat J., García-Honduvilla N., Bujan J., Alvarez-Mon M., Alvarez-Mon M.A. The placentas of women who suffer an episode of psychosis during pregnancy have increased lipid peroxidation with evidence of ferroptosis. Biomolecules. 2023;13 doi: 10.3390/biom13010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lang X., Green M.D., Wang W., Yu J., Choi J.E., Jiang L., Liao P., Zhou J., Zhang Q., Dow A., Saripalli A.L., Kryczek I., Wei S., Szeliga W., Vatan L., Stone E.M., Georgiou G., Cieslik M., Wahl D.R., Morgan M.A., Chinnaiyan A.M., Lawrence T.S., Zou W. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9:1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu B., Choi B., Li W., Kim D.H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-17380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Turubanova V.D., Balalaeva I.V., Mishchenko T.A., Catanzaro E., Alzeibak R., Peskova N.N., Efimova I., Bachert C., Mitroshina E.V., Krysko O., Vedunova M.V., Krysko D.V. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer. 2019;7:1–13. doi: 10.1186/s40425-019-0826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Y., Li H.J., He Q.X., Zou R., Cai J.R., Zhang L. Ferroptosis: underlying mechanisms and involvement in neurodegenerative diseases. Apoptosis. 2024;29:3–21. doi: 10.1007/s10495-023-01902-9. [DOI] [PubMed] [Google Scholar]
- 169.Jakaria M., Belaidi A.A., Bush A.I., Ayton S. Ferroptosis as a mechanism of neurodegeneration in Alzheimer's disease. J. Neurochem. 2021;159:804–825. doi: 10.1111/jnc.15519. [DOI] [PubMed] [Google Scholar]
- 170.Ding X., Gao L., Han Z., Eleuteri S., Shi W., Shen Y., yao Song Z., Su M., Yang Q., Qu Y., Simon D.K., lian Wang X., Wang B. Ferroptosis in Parkinson's disease: molecular mechanisms and therapeutic potential. Ageing Res. Rev. 2023;91 doi: 10.1016/j.arr.2023.102077. [DOI] [PubMed] [Google Scholar]
- 171.Jhelum P., Zandee S., Ryan F., Zarruk J.G., Michalke B., Venkataramani V., Curran L., Klement W., Prat A., David S. Ferroptosis induces detrimental effects in chronic EAE and its implications for progressive MS. Acta Neuropathol. Commun. 2023;11:1–22. doi: 10.1186/s40478-023-01617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang T., Tomas D., Perera N.D., Cuic B., Luikinga S., Viden A., Barton S.K., McLean C.A., Samson A.L., Southon A., Bush A.I., Murphy J.M., Turner B.J. Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis. Cell Death Differ. 2022;29:1187–1198. doi: 10.1038/s41418-021-00910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mi Y., Gao X., Xu H., Cui Y., Zhang Y., Gou X. The emerging roles of ferroptosis in Huntington's disease. NeuroMolecular Med. 2019;21:110–119. doi: 10.1007/s12017-018-8518-6. [DOI] [PubMed] [Google Scholar]
- 174.Tian X., Li X., Pan M., Yang L.Z., Li Y., Fang W. Progress of ferroptosis in ischemic stroke and therapeutic targets. Cell. Mol. Neurobiol. 2024;44 doi: 10.1007/s10571-024-01457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen K., Jiang X., Wu M., Cao X., Bao W., Zhu L.Q. Ferroptosis, a potential therapeutic target in alzheimer's disease. Front. Cell Dev. Biol. 2021;9:1–9. doi: 10.3389/fcell.2021.704298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Park M.W., Cha H.W., Kim J., Kim J.H., Yang H., Yoon S., Boonpraman N., Yi S.S., Yoo I.D., Moon J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ayton S., Portbury S., Kalinowski P., Agarwal P., Diouf I., Schneider J.A., Morris M.C., Bush A.I. Regional brain iron associated with deterioration in Alzheimer's disease: a large cohort study and theoretical significance. Alzheimer’s Dement. 2021;17:1244–1256. doi: 10.1002/alz.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhao D., Yang K., Guo H., Zeng J., Wang S., Xu H., Ge A., Zeng L., Chen S., Ge J. Mechanisms of ferroptosis in Alzheimer's disease and therapeutic effects of natural plant products: a review. Biomed. Pharmacother. 2023;164 doi: 10.1016/j.biopha.2023.114312. [DOI] [PubMed] [Google Scholar]
- 179.Ashraf A., Jeandriens J., Parkes H.G., So P.W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer's disease: evidence of ferroptosis. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Greenough M.A., Lane D.J.R., Balez R., Anastacio H.T.D., Zeng Z., Ganio K., McDevitt C.A., Acevedo K., Belaidi A.A., Koistinaho J., Ooi L., Ayton S., Bush A.I. Selective ferroptosis vulnerability due to familial Alzheimer's disease presenilin mutations. Cell Death Differ. 2022;29:2123–2136. doi: 10.1038/s41418-022-01003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ma H., Dong Y., Chu Y., Guo Y., Li L. The mechanisms of ferroptosis and its role in alzheimer's disease. Front. Mol. Biosci. 2022;9:1–17. doi: 10.3389/fmolb.2022.965064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kashani A., Lepicard È., Poirel O., Videau C., David J.P., Fallet-Bianco C., Simon A., Delacourte A., Giros B., Epelbaum J., Betancur C., El Mestikawy S. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol. Aging. 2008;29:1619–1630. doi: 10.1016/j.neurobiolaging.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 183.Del Rey M.Q., Mancias J.D. NCOA4-mediated ferritinophagy: a potential link to neurodegeneration. Front. Neurosci. 2019;13:1–7. doi: 10.3389/fnins.2019.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Osama A., Zhang J., Yao J., Yao X., Fang J. Nrf2: a dark horse in Alzheimer's disease treatment. Ageing Res. Rev. 2020;64 doi: 10.1016/j.arr.2020.101206. [DOI] [PubMed] [Google Scholar]
- 185.Liu L., Cui Y., Chang Y.Z. Ferroptosis - related factors in the substantia nigra are associated with Parkinson ’ s disease. Sci. Rep. 2023;13:1–11. doi: 10.1038/s41598-023-42574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Angelova P.R., Choi M.L., V Berezhnov A., Horrocks M.H., Hughes C.D., De S., Rodrigues M., Yapom R., Little D., Dolt K.S., Kunath T. Alpha synuclein aggregation drives ferroptosis: an interplay of iron, calcium and lipid peroxidation. Cell Death Differ. 2020;27:2781–2796. doi: 10.1038/s41418-020-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang D., Peng Y., Xie Y., Zhou B., Sun X., Kang R., Tang D. Antiferroptotic activity of non-oxidative dopamine. Biochem. Biophys. Res. Commun. 2016;480:602–607. doi: 10.1016/j.bbrc.2016.10.099. [DOI] [PubMed] [Google Scholar]
- 188.Shahmoradian S.H., Lewis A.J., Genoud C., Hench J., Moors T.E., Navarro P.P., Castaño-díez D., Schweighauser G., Graff-meyer A., Goldie K.N., Sütterlin R., Huisman E., Ingrassia A., De Gier Y., Rozemuller A.J.M., Wang J., De Paepe A., Erny J., Staempfli A., Hoernschemeyer J., Großerüschkamp F., Niedieker D., El-mashtoly S.F., Quadri M., Van Ijcken W.F.J., Bonifati V. Crowded organelles and lipid membranes. Nat. Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 189.Wang Z., Yuan L., Li W., Li J. Ferroptosis in Parkinson's disease: glia – neuron crosstalk. Trends Mol. Med. 2022;28:258–269. doi: 10.1016/j.molmed.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 190.Sadeghi M., Shankara S., Guo L., Li C., Pontarelli F., Jensen E.H., Comer A.L., Kumar D., Zhang M., Gans J., Zhang B., Proto J.D., Saleh J., Dodge J.C., Savova V., Rajpal D., Ofengeim D., Hammond T.R. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 2023;26:12–26. doi: 10.1038/s41593-022-01221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang D., Liang W., Huo D., Wang H., Wang Y., Cong C., Zhang C., Yan S., Gao M., Su X. SPY1 inhibits neuronal ferroptosis in amyotrophic lateral sclerosis by reducing lipid peroxidation through regulation of GCH1 and TFR1. Cell Death Differ. 2023;30:369–382. doi: 10.1038/s41418-022-01089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Song S., Su Z., Kon N., Chu B., Li H., Jiang X., Luo J., Stockwell B.R., Gu W. ALOX5-mediated ferroptosis acts as a distinct cell death pathway upon oxidative stress in Huntington ’ s disease. Genes Dev. 2023;37:204–217. doi: 10.1101/gad.350211.122.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Van San E., Debruyne A.C., Veeckmans G., Tyurina Y.Y., Tyurin V.A., Zheng H., Choi S.M. Ferroptosis contributes to multiple sclerosis and its pharmacological targeting suppresses experimental disease progression. Cell Death Differ. 2023;30:2092–2103. doi: 10.1038/s41418-023-01195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.White A.R. Ferroptosis drives immune-mediated neurodegeneration in multiple sclerosis. Cell. Mol. Immunol. 2023;20:112–113. doi: 10.1038/s41423-022-00941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li X., Chu Y., Ma R., Dou M., Li S., Song Y., Lv Y., Zhu L. Ferroptosis as a mechanism of oligodendrocyte loss and demyelination in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2022;373 doi: 10.1016/j.jneuroim.2022.577995. [DOI] [PubMed] [Google Scholar]
- 196.Ryan S.K., Ugalde C.L., Rolland A., Skidmore J., Devos D., Hammond T.R. Therapeutic inhibition of ferroptosis in neurodegenerative disease. Trends Pharmacol. Sci. 2016;44:674–688. doi: 10.1016/j.tips.2023.07.007. [DOI] [PubMed] [Google Scholar]
- 197.Zhang Y., Wang D., Xu S., Zhang S., Fan Y. Redox Biology α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018;14:535–548. doi: 10.1016/j.redox.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lin Z., Liu Y., Xue N., Zheng R., Yan Y., Wang Z., Li Y. Quercetin protects against MPP+/MPTP-Induced dopaminergic neuron death in Parkinson's disease by inhibiting ferroptosis. Oxid. Med. Cell. Longev. 2022;2022:1–17. doi: 10.1155/2022/7769355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu Y., Chen Z., Li B., Yao H., Zarka M., Welch J., Sachdev P., Bridge W., Braidy N. Neurochemistry International Supplementation with γ -glutamylcysteine (γ -GC) lessens oxidative stress , brain inflammation and amyloid pathology and improves spatial memory in a murine model of AD. Neurochem. Int. 2021;144 doi: 10.1016/j.neuint.2020.104931. [DOI] [PubMed] [Google Scholar]
- 200.Yu Y., Yan Y., Niu F., Wang Y., Chen X., Su G., Liu Y., Zhao X., Qian L., Liu P., Xiong Y. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Differ. 2021;7:1–10. doi: 10.1038/s41420-021-00579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fang X., Wang H., Han D., Xie E., Yang X., Wei J., Gu S., Gao F., Zhu N. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dai Y., Wei X., Jiang T., Wang Q., Li Y., Ruan N. Ferroptosis in age-related vascular diseases: molecular mechanisms and innovative therapeutic strategies. Biomed. Pharmacother. 2024;173 doi: 10.1016/j.biopha.2024.116356. [DOI] [PubMed] [Google Scholar]
- 203.Wang K., Chen X.Z., Wang Y.H., Cheng X.L., Zhao Y., Zhou L.Y., Wang K. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death Discov. 2022;8 doi: 10.1038/s41420-022-01183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li M., Wang Z.W., Fang L.J., Cheng S.Q., Wang X., Liu N.F. Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis. 2022;13:1–13. doi: 10.1038/s41419-022-04923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ortega M.A., De Leon-Oliva D., García-Montero C., Fraile-Martinez O., Boaru D.L., de Castro A.V., Saez M.A., Lopez-Gonzalez L., Bujan J., Alvarez-Mon M.A., García-Honduvilla N., Diaz-Pedrero R., Alvarez-Mon M. Reframing the link between metabolism and NLRP3 inflammasome: therapeutic opportunities. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1232629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bai T., Li M., Liu Y., Qiao Z., Wang Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020;160:92–102. doi: 10.1016/j.freeradbiomed.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 207.Yuan W., Xia H., Xu Y., Xu C., Chen N., Shao C., Dai Z., Chen R., Tao A. The role of ferroptosis in endothelial cell dysfunction. Cell Cycle. 2022;21:1897–1914. doi: 10.1080/15384101.2022.2079054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guo Z.M., Ran Q., Roberts L.J., Zhou L., Richardson A., Sharan C., Wu D.F., Yang H. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free Radic. Biol. Med. 2008;44:343–352. doi: 10.1016/j.freeradbiomed.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Howden R. Nrf2 and cardiovascular defense. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/104308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cornelissen A., Guo L., Sakamoto A., Virmani R., Finn A.V. New insights into the role of iron in inflammation and atherosclerosis. EBioMedicine. 2019;47:598–606. doi: 10.1016/j.ebiom.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Torres N., Guevara-Cruz M., Velázquez-Villegas L.A., Tovar A.R. Nutrition and atherosclerosis. Arch. Med. Res. 2015;46:408–426. doi: 10.1016/j.arcmed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 212.Vinchi F., Porto G., Simmelbauer A., Altamura S., Passos S.T., Garbowski M., Silva A.M.N., Spaich S., Seide S.E., Sparla R., Hentze M.W., Muckenthaler M.U. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 2020;41:2681–2695. doi: 10.1093/eurheartj/ehz112. [DOI] [PubMed] [Google Scholar]
- 213.Arrigo M., Jessup M., Mullens W., Reza N., Shah A.M., Sliwa K., Mebazaa A. Acute heart failure. Nat. Rev. Dis. Prim. 2020;6 doi: 10.1038/s41572-020-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wu X., Li Y., Zhang S., Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11:3052–3059. doi: 10.7150/THNO.54113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Huang L., Wang X., Hu B., Rong S. Expression levels and clinical significance of ferroptosis-related genes in patients with myocardial infarction. Sci. Rep. 2024;14:1–9. doi: 10.1038/s41598-023-49336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang X., Kawasaki N.K., Min J., Matsui T., Wang F. Ferroptosis in heart failure. J. Mol. Cell. Cardiol. 2022;173:141–153. doi: 10.1016/j.yjmcc.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li D., Pi W., Sun Z., Liu X., Jiang J. Ferroptosis and its role in cardiomyopathy. Biomed. Pharmacother. 2022;153 doi: 10.1016/J.BIOPHA.2022.113279. [DOI] [PubMed] [Google Scholar]
- 218.Wang J., Deng B., Liu Q., Huang Y., Chen W., Li J., Zhou Z., Zhang L., Liang B., He J., Chen Z., Yan C., Yang Z., Xian S., Wang L. Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis. 2020;11:1–19. doi: 10.1038/s41419-020-02777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang X., Zheng C., Gao Z., Chen H., Li K., Wang L., Zheng Y., Li C., Zhang H., Gong M., Zhang H., Meng Y. SLC7A11/xCT prevents cardiac hypertrophy by inhibiting ferroptosis. Cardiovasc. Drugs Ther. 2022;36:437–447. doi: 10.1007/S10557-021-07220-Z/FIGURES/6. [DOI] [PubMed] [Google Scholar]
- 220.Zhong F.Y., Zhao Y.C., Zhao C.X., Gu Z.C., Lu X.Y., Jiang W.L., Gao L.C., Li W.L., Qin Z.H., Ge H., Pu J. The role of CD147 in pathological cardiac hypertrophy is regulated by glycosylation. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/6603296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen Y., Guo X., Zeng Y., Mo X., Hong S., He H., Li J., Steinmetz R., Liu Q. Ferroptosis contributes to catecholamine-induced cardiotoxicity and pathological remodeling. Free Radic. Biol. Med. 2023;207:227–238. doi: 10.1016/J.FREERADBIOMED.2023.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen X., Xu S., Zhao C., Liu B. Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem. Biophys. Res. Commun. 2019;516:37–43. doi: 10.1016/j.bbrc.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 223.Sukumaran A., Chang J., Han M., Mintri S., Khaw B.A., Kim J. Iron overload exacerbates age-associated cardiac hypertrophy in a mouse model of hemochromatosis. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-05810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhao W.K., Zhou Y., Xu T.T., Wu Q. Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/9929687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liu B., Zhao C., Li H., Chen X., Ding Y., Xu S. Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem. Biophys. Res. Commun. 2018;497:233–240. doi: 10.1016/j.bbrc.2018.02.061. [DOI] [PubMed] [Google Scholar]
- 226.Zhang T., Deng W., Deng Y., Liu Y., Xiao S., Luo Y., Xiang W., He Q. Mechanisms of ferroptosis regulating oxidative stress and energy metabolism in myocardial ischemia-reperfusion injury and a novel perspective of natural plant active ingredients for its treatment. Biomed. Pharmacother. 2023;165 doi: 10.1016/J.BIOPHA.2023.114706. [DOI] [PubMed] [Google Scholar]