Abstract
Recently approved anti-amyloid immunotherapies for Alzheimer’s disease (AD) require evidence of amyloid-β pathology from positron emission tomography (PET) or cerebrospinal fluid (CSF) before initiating treatment. Blood-based biomarkers promise to reduce the need for PET or CSF testing; however, their interpretation at the individual level and the circumstances requiring confirmatory testing are poorly understood. Individual-level interpretation of diagnostic test results requires knowledge of disease prevalence in relation to clinical presentation (clinical pretest probability). Here, in a study of 6,896 individuals evaluated from 11 cohort studies from six countries, we determined the positive and negative predictive value of five plasma biomarkers for amyloid-β pathology in cognitively impaired individuals in relation to clinical pretest probability. We observed that p-tau217 could rule in amyloid-β pathology in individuals with probable AD dementia (positive predictive value above 95%). In mild cognitive impairment, p-tau217 interpretation depended on patient age. Negative p-tau217 results could rule out amyloid-β pathology in individuals with non-AD dementia syndromes (negative predictive value between 90% and 99%). Our findings provide a framework for the individual-level interpretation of plasma biomarkers, suggesting that p-tau217 combined with clinical phenotyping can identify patients where amyloid-β pathology can be ruled in or out without the need for PET or CSF confirmatory testing.
Subject terms: Alzheimer's disease, Alzheimer's disease, Ageing
Therriault et al. provide a framework for the individual-level interpretation of plasma biomarkers by determining their positive and negative predictive values for amyloid positron emission tomography status in relation to patient age and clinical symptoms.
Main
With the recent Food and Drug Administration approval of disease-modifying therapies for Alzheimer’s disease (AD)1, determining eligibility for anti-amyloid-β therapy is an important need for cognitively impaired individuals where AD is a suspected etiology. Anti-amyloid-β immunotherapies currently require evidence of amyloid-β pathology from either positron emission tomography (PET) or cerebrospinal fluid (CSF) to initiate treatment2. PET and CSF assessments are limited by cost, accessibility and invasiveness. Minimally invasive, scalable and cost-effective methods to determine the presence of AD pathology are urgently needed3.
Several recent studies have reported that plasma biomarkers have excellent diagnostic accuracy for AD, with sensitivity or specificity often exceeding 90% (refs. 4–10). However, sensitivity and specificity provide limited information when making decisions about individual patients11–13. In contrast, predictive values are critical for interpreting individual-level test results11,12,14,15. Sufficiently high positive predictive values (PPVs) or negative predictive values (NPVs) of plasma biomarkers for AD pathology could circumvent the need for the majority of PET or CSF testing, with confirmatory testing used in remaining situations with lower predictive values3,16.
Evaluation of the PPVs and NPVs of diagnostic tests in large, unselected populations requires knowledge the prevalence of the disease of interest11,12,15,17. As the prevalence of amyloid-β pathology is closely linked to age and clinical syndrome18–20, clinical and demographic information can be used to infer the clinical pretest probability of amyloid-β positivity (Aβ+) based on standard clinical assessments17,21,22. Here, using the prevalence of amyloid-β pathology from meta-analyses of memory clinic and research settings, we determined the age- and clinical dementia syndrome-associated PPV and NPV of different plasma biomarkers for amyloid-β pathology.
Results
This study examined a total of 6,896 individuals from Canada, France, South Korea, Spain, Sweden and the United States who were assessed with standardized cognitive assessments, plasma AD biomarkers and established reference standard AD biomarkers (PET, CSF or neuropathological assessments). The mean (s.d.) age of all participants was 69.7 (9.2) years, and 3,698 (53.6%) were female. The mean (s.d.) years of education of the sample was 13.3 (3.6) years. A summary of clinical and demographic characteristics of the entire sample is presented in Table 1, with cohort-specific data presented in Supplementary Tables 3–15. MMSE, Mini-Mental State Examination.
Table 1.
Demographic and clinical characteristics of the study participants
| Cognitively unimpaired | Cognitively impaired | |
|---|---|---|
| No. | 3,393 | 3,503 |
| Mean age in years (s.d.) | 66.9 (10.6) | 72.4 (7.81) |
| No. of females (%) | 1,865 (55.0%) | 1,833 (52.3%) |
| Mean years of education (s.d.) | 14.4 (3.18) | 12.2 (3.99) |
| No. of APOE ε4 carriers (%) | 983 (29%) | 1,436 (41%) |
| Mean MMSE (s.d.) | 28.7 (1.35) | 24.1 (4.54) |
| Amyloid-β positive (%) | 776 (22.6%) | 1,940 (55.4%) |
Data are represented as mean and s.d. for continuous variables and number and percentage for categorical variables.
PPVs and NPVs of plasma biomarkers for Aβ+ in MCI
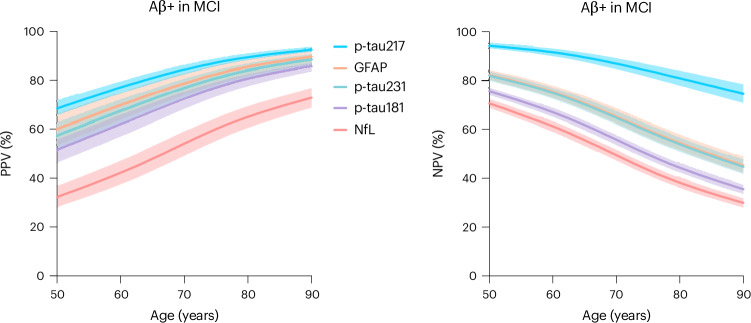
Age-related PPVs and NPVs of five plasma biomarkers for Aβ+ in mild cognitive impairment (MCI) are illustrated in Fig. 1. The ability of plasma biomarkers to rule in or rule out amyloid-β was closely associated with the age-related prevalence of AD pathology in MCI. For individuals with MCI, PPVs of plasma biomarkers increased with age, with p-tau217 reaching 80.9% (95% confidence interval (CI) 78.7–83.1%) at age 65 years and reaching 92.5% (95% CI 91.6–93.5%) for individuals aged 90 years. NPVs for Aβ+ in MCI decreased with age, with NPVs above 90% for individuals younger than 65 years, 80.8% (95% CI 77.8–83.9%) at age 80 years and 74.6% (95% CI 70.9–78.4%) at age 90 years. P-tau181, p-tau231, glial fibrillary acidic protein (GFAP) and neurofilament light chain (NfL) all had lower performance than plasma p-tau217. In APOE ε4 carriers with MCI, the PPV of plasma p-tau217 for amyloid-β was higher, reaching 90.8% (95% CI 89.6–91.9%) by age 70 years and 95.6% (95% CI 95.0–96.1%) by age 80 years. Furthermore, in APOE ε4 noncarriers with MCI, the NPV of plasma p-tau217 was also higher, being above 95% (95% CI 94.1–96.0%) for individuals aged under 65 years and 89.8% (95% CI 87.9–91.6%) for individuals aged under 80 years. A summary of the PPVs and NPVs of plasma p-tau217 for amyloid PET positivity in all ages and clinical syndromes is presented in Table 2, and a summary of age- and APOE ε4-adjusted PPVs and NPVs for individuals with MCI is presented in Supplementary Tables 16–18.
Fig. 1. PPVs and NPVs of plasma AD biomarkers in individuals with MCI.
Age-associated PPV (left) and NPV (right) of five plasma biomarkers for amyloid PET positivity in MCI. The solid lines represent the point estimate, and error bars represent 95% CIs.
Table 2.
PPVs and NPVs of plasma p-tau217 for amyloid-β pathology in different clinical syndromes
| MCI | Probable AD dementia | Frontotemporal dementia | Vascular dementia | Corticobasal syndrome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age in years | PPV % (95% CI) | NPV % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | PPV % (95% CI) | NPV % (95% CI) |
| 50–54 | 68.4% (65.5–71.4%) | 94.4% (93.3–95.4%) | 97.5% (96.5–98.1%) | 44.6% (40.1–50%) | 27.6% (25–30.5%) | 99% (98.8–99.2%) | 45.2% (41.9–48.7%) | 97.8% (97.3–98.2%) | Prevalence data unavailable | Prevalence data unavailable |
| 55–59 | 73.1% (70.5–75.9%) | 93% (91.7–94.3%) | 97.4% (96.3–98%) | 45.7% (41.2–51.1%) | 30.5% (27.8–33.7%) | 98.8% (98.6–99%) | 49.1% (45.8–52.7%) | 97.4% (96.9–97.9%) | 82.9% (81–84.9%) | 88.2% (86.1–90.3%) |
| 60–64 | 77.1% (74.7–79.6%) | 91.5% (89.9–93%) | 97.3% (96.1–97.9%) | 47% (42.4–52.4%) | 33.3% (30.5–36.6%) | 98.6% (98.4–98.9%) | 52.6% (49.3–56.2%) | 97% (96.4–97.6%) | 81.2% (79.1–83.3%) | 89.4% (87.5–91.3%) |
| 65–69 | 80.9% (78.7–83%) | 89.6% (87.7–91.4%) | 97% (95.7–97.7%) | 49.5% (44.8–54.8%) | 40.8% (37.7–44.3%) | 98.1% (97.8–98.5%) | 57.3% (54.1–60.8%) | 96.4% (95.7–97.1%) | 76.4% (73.9–78.9%) | 91.8% (90.3–93.3%) |
| 70–74 | 84% (82.2–85.9%) | 87.3% (85.1–89.5%) | 96.7% (95.4–97.5%) | 51.7% (47.1–57.1%) | 47.2% (43.9–50.7%) | 97.6% (97.1–98.1%) | 64% (60.9–67.2%) | 95.3% (94.4–96.2%) | 74% (71.4–76.7%) | 92.7% (91.4–94.1%) |
| 75–79 | 87.5% (86–89%) | 83.9% (81.2–86.6%) | 96.5% (95–97.3%) | 53.9% (49.3–59.2%) | 52.6% (49.3–56.2%) | 97% (96.4–97.6%) | 69.4% (66.6–72.4%) | 94.1% (93–95.2%) | 69.4% (66.6–72.4%) | 94.1% (93–95.2%) |
| 80–84 | 89.6% (88.3–90.9%) | 80.8% (77.8–83.9%) | 96.2% (94.6–97.1%) | 55.9% (51.3–61.1%) | 57.3% (54.1–60.8%) | 96.4% (95.7–97.1%) | 74% (71.4–76.7%) | 92.7% (91.4–94.1%) | 64% (60.9–67.2%) | 95.3% (94.4–96.2%) |
| 85–89 | 91.1% (90–92.2%) | 77.9% (74.6–81.4%) | 95.7% (93.9–96.7%) | 58.6% (54.1–63.7%) | 61.5% (58.3–64.8%) | 95.8% (95–96.6%) | 77.8% (75.5–80.2%) | 91.2% (89.6–92.8%) | 60.2% (57–63.6%) | 96% (95.2–96.8%) |
| 90–95 | 92.5% (91.6–93.5%) | 74.6% (70.9–78.4%) | 95.3% (93.3–96.4%) | 61.2% (56.7–66.1%) | 65.2% (62.1–68.3%) | 95.1% (94.2–96%) | 81.2% (79.1–83.3%) | 89.4% (87.5–91.3%) | 55.8% (52.6–59.3%) | 96.6% (96–97.3%) |
PPVs and NPVs of plasma biomarkers for Aβ+ in probable AD dementia
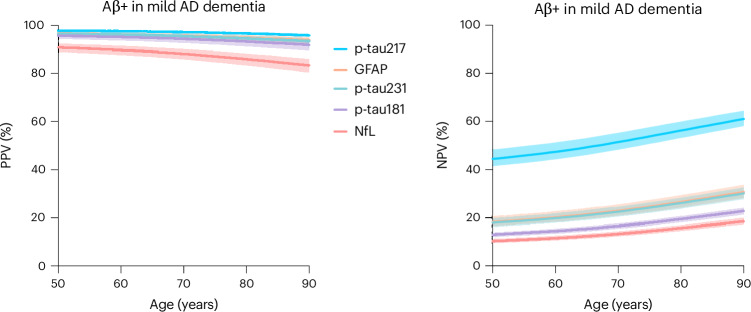
Age-associated PPVs and NPVs of five AD plasma biomarkers in probable AD dementia are reported in Fig. 2. In individuals with probable AD dementia, plasma biomarkers, particularly p-tau217, had very high PPVs (above 95%) for Aβ+ at all ages. Owing to the high prevalence of Aβ+ in individuals with probable AD dementia, NPVs of plasma biomarkers was comparatively lower. Again, p-tau217 had the highest NPV at all age ranges for individuals with probable AD dementia, reaching 60% by age 90 years. Other plasma biomarkers had lower NPVs at all ages. A summary of age- and APOE ε4-adjusted PPVs and NPVs for individuals with probable AD dementia is presented in Supplementary Tables 19–21.
Fig. 2. PPVs and NPVs of plasma biomarkers of AD in individuals with probable AD dementia.
Age-associated PPV (left) and NPV (right) of five plasma biomarkers for amyloid PET positivity in probable AD dementia. The solid lines represent the point estimate, and error bars represent 95% CIs.
PPVs and NPVs of plasma biomarkers for Aβ+ in non-AD clinical syndromes
In non-AD dementia syndromes, plasma biomarkers, in particular p-tau217, could rule out the presence of AD pathology with NPVs above 90% in nearly all circumstances. Two exceptions to this were ruling out amyloid-β pathology in individuals with vascular dementia above age 90 years (NPV 89.4%, 95% CI 87.5–91.3%) and ruling out amyloid-β pathology in individuals with corticobasal syndrome younger than age 65 years (NPV 88.2%, 95% CI 86.1–90.3%). A summary of the PPVs and NPVs of plasma p-tau217, the best-performing biomarker, for amyloid PET positivity in all ages and clinical syndromes is presented in Table 2. A summary of PPVs and NPVs of plasma biomarkers for amyloid-β pathology additionally adjusted for the APOE ε4 genotype in non-AD dementia syndromes is presented in Supplementary Tables 22–33.
Discussion
This study evaluated the PPVs and NPVs of plasma biomarkers for amyloid-β pathology in relation to patient age and clinical syndrome. We report that, in older adults with MCI (ages 80+ years) or in individuals with clinically diagnosed probable AD dementia, plasma p-tau217 can rule in amyloid-β pathology with PPVs above 90%. Furthermore, in non-AD dementia syndromes such as frontotemporal dementia, vascular dementia and corticobasal syndrome, plasma p-tau217 could rule out AD pathology with NPVs above 90%. Owing to the high prevalence of amyloid-β pathology in individuals with clinically diagnosed AD dementia, negative plasma biomarkers will warrant confirmatory testing to rule out AD pathology in individuals with these symptoms. Similarly, in older adults with MCI where the prevalence of AD pathology is high, confirmatory testing is needed to rule out AD pathology. Taken together, our study provides a framework for the individual-level interpretation of plasma biomarkers for AD according to patient age and clinical syndrome23.
The PPVs and NPVs reported in the present study are to be understood within the context of the prevalence of amyloid-β pathology within MCI, probable AD dementia and other non-AD dementia syndromes. MCI is a highly heterogeneous clinical syndrome that can be caused by several different neurodegenerative and nonneurodegenerative conditions24. Estimates from memory clinic and community-based studies suggests the prevalence of amyloid-β pathology in individuals with MCI is relatively low for individuals in their 60s but reaches 75–80% by age 90 years19,20. Correspondingly, the PPV of plasma p-tau217 for the detection of amyloid-β pathology in MCI rose with age, exceeding 95% by age 90 years. Owing to the high pretest probability that amyloid-β is present in older adults with MCI, the NPV of even highly accurate plasma biomarkers fell below 80% with more advanced age.
The clinical syndrome of probable AD dementia is more closely associated with amyloid-β pathology than MCI at all ages19,20,25 Therefore, in clinically diagnosed probable AD dementia, the PPV of plasma biomarkers, particularly p-tau217, is very high and probably sufficient to rule in amyloid-β pathology. The corollary is that the NPV of plasma biomarkers for AD was lower owing to the high prevalence of AD pathology in this clinical syndrome. Studies in other areas of medicine have also found lower NPVs of even highly sensitive and specific tests in situations where the pretest probability of a disease is high23,26,27. The risk of a false negative in probable AD dementia may be high enough to warrant confirmatory CSF or PET testing for individuals with clinically diagnosed probable AD dementia with a negative plasma biomarker test result, even for highly accurate biomarkers such as plasma p-tau217.
Owing to the substantially higher prevalence of Aβ+ in APOE ε4 carriers18,19,28, plasma biomarkers had higher PPVs for brain amyloid-β, particularly in individuals with MCI. Conversely, the NPV of plasma biomarkers, particularly p-tau217, was substantially higher in APOE ε4 noncarriers. Therefore, genotyping for APOE (also available with a blood sample) will lead to higher predictive values for Aβ+.
Across all cohorts and assays investigated, a consistent finding in this study is that plasma p-tau217 had the highest PPVs and NPVs for amyloid-β pathology. These results are consistent with a number of recent studies demonstrating excellent performance of multiple p-tau217 assays in the differential diagnosis of cognitive impairment8–10,29–32, its close association with amyloid-β and tau pathologies33,34 and longitudinal increases over time in Aβ+ individuals35. Plasma GFAP had slightly lower performance than p-tau217, with notably lower specificity. Despite the role of GFAP in AD pathogenesis36 and in predicting future dementia incidence37, the lower specificity of GFAP may limit its role as a diagnostic biomarker for AD38. For example, GFAP elevations have been reported in frontotemporal dementia39, traumatic brain injury40, multiple sclerosis41 and inflammatory central nervous system diseases42. Despite these limitations, GFAP nonetheless performed better overall than other plasma biomarkers such as p-tau181. However, it is important to emphasize that head-to-head studies indicate that different assays for p-tau181 vary substantially in their diagnostic performance9,10 and may not all perform inferiorly to GFAP in all contexts34,43. As expected, plasma NfL had relatively lower PPV and NPV for AD, as NfL is a nonspecific biomarker of neurodegeneration, elevated in multiple different neurodegenerative diseases44. Taken together, these results highlight the utility of plasma p-tau217 for the differential diagnosis of cognitive impairment and for determining eligibility for anti-amyloid-β disease-modifying therapies.
Currently, anti-amyloid monoclonal antibodies require the confirmation of amyloid-β pathology from PET or CSF before initiating therapy45,46. On the basis of the present results, plasma biomarkers, particularly plasma p-tau217, may be suitable to rule in amyloid-β pathology in individuals with probable AD dementia or in older adults with MCI, which stands to circumvent a large number of PET scans or lumbar punctures. In contrast, in non-AD clinical syndromes such as frontotemporal dementia, vascular dementia and corticobasal syndrome, which are less frequently associated with AD pathology18, plasma biomarkers can rule out AD pathology at almost all ages. As the prevalence of AD pathology is associated with age in non-AD syndromes18, the PPV and NPV of plasma biomarkers also varies slightly with age. For example, because of the relatively higher prevalence of AD pathology in younger individuals with corticobasal syndrome18, caution is warranted in using plasma biomarkers to rule out AD in these individuals. Overall, however, plasma biomarkers are more limited in ruling in AD pathology in non-AD clinical syndromes and follow-up testing with either PET or CSF may be warranted; in these instances, the topographical information provided by tau-PET47,48 may be useful. Plasma biomarkers may therefore have an important role in reducing the patient burden associated with the initiation of anti-amyloid-β therapies for AD, which at present require biomarker confirmation with PET or CSF, as well as serial magnetic resonance imaging to monitor for adverse events45,46. However, it is also important to consider that multiple neuropathological processes are often present in older individuals with cognitive symptoms, and plasma biomarkers alone cannot determine whether AD is the driving force behind a specific clinical syndrome. This is especially true of biomarkers that plateau in later disease stages49. In the future, plasma biomarker panels that measure p-tau217 in addition to biomarkers that become abnormal at later stages such as p-tau205 (refs. 50,51) or MTBR-tau243 (ref. 52) may prove beneficial in this regard53. Furthermore, more work is needed to determine what is an acceptable PPV for Aβ+ for the initiation of anti-amyloid therapy, as it is possible that PPVs below 85–90% may not be sufficient and more invasive/expensive testing may be warranted.
The results of our study used amyloid-β pathology prevalence estimates derived from the Amyloid Biomarker Study Group, an international multicenter study of more than 19,000 individuals19,28. These prevalence estimates informed the age-associated pretest probability of amyloid-β pathology in MCI, probable AD dementia and non-AD dementia clinical syndromes, which permit PPVs and NPVs to be estimated12,17. These prevalence estimates are largely based on subjects recruited from clinical and research settings that feature some enrollment biases and are not representative in terms of race or ethnicity of the populations at risk for dementia globally. Furthermore, research-level phenotyping may result in stronger clinico-pathological correlations in individuals with MCI, AD dementia and non-AD syndromes than can be reasonably achieved in nonspecialist centers. However, very similar results were observed when using prevalence estimates from the Mayo Clinic Study of Aging, a population-based cohort study20.
Performance of specific plasma AD biomarkers was overall highly comparable across different centers, settings and populations. For example, the sensitivity and specificity of p-tau181 in the Health and Aging Brain Study–Health Disparities (HABS-HD) cohort, a multiethnic and multiracial community-based research study, which features a high proportion of Mexican–American and African–American individuals, was nearly identical to p-tau181 performance in highly specialized memory clinic settings. While p-tau217 was not available in some cohorts, previous studies have provided evidence that this biomarker also has excellent performance in different racial and ethnic groups54,55. Our study contributes to this finding by providing evidence of excellent diagnostic performance of plasma p-tau217 for AD in a large multicenter memory clinic cohort from South Korea.
Our study has important limitations. First, the binary classification of individuals into categories based on the presence/absence of disease is a limitation; it is anticipated that plasma biomarker accuracy is higher in later-stage disease when burden of pathology is greater. Second, while our study used a standardized method of determining plasma biomarker abnormality across centers, future work may be able to further optimize this method, in turn providing higher PPVs and NPVs. For example, recent evidence suggests that a three-range method leads to higher accuracy to identify amyloid PET positivity in individuals with MCI56. Third, the use of plasma biomarker ratios may further improve accuracy by circumventing associations between chronic kidney disease and elevated plasma biomarker concentrations57. Fourth, refinements to the clinical pretest probability estimates (for example, through polygenetic risk scores58 or through basic algorithms incorporating age, APOE genotype and cognitive testing59) will probably further improve plasma biomarker diagnostic performance and interpretation. Fifth, our study is a cross-sectional diagnostic study and is not designed to predict who will develop AD dementia in the future. Blood biomarkers of amyloid-β misfolding have shown promise in this regard60,61. Sixth, the amyloid PET positivity prevalence estimates employed in our study are derived from meta-analyses of predominantly memory clinic and research settings18,19. Correspondingly, the PPV and NPV estimates from our study should not be extrapolated to other clinical settings where the prevalence of AD is substantially different11,12,14,15.
In conclusion, our study provides information about the interpretation of plasma biomarkers for AD at the individual level, adjusted to clinical pretest probability. Our study provides evidence that, in individuals with probable AD dementia and in older individuals with MCI, plasma biomarkers can be used to rule in amyloid-β pathology, required for the initiation of disease-modifying therapies. In individuals with non-AD dementia syndromes, a negative plasma p-tau217 result can rule out AD pathology, with follow-up testing required for non-AD dementia syndrome cases with a positive AD plasma biomarker.
Methods
Study patients
This study evaluated individuals assessed with standardized cognitive assessments, plasma biomarkers of AD and reference standard AD biomarker assessments (either PET, CSF or neuropathological assessments). Patients were enrolled from prospective cohort studies in Canada, France, South Korea, Spain, Sweden and the United States. AD biomarker abnormality was not required for enrollment in any of the participating sites. All study participants provided written informed consent, and local institutional review boards approved the studies. A detailed description of inclusion and exclusion criteria for all prospective cohort studies is provided in the Supplementary Appendix.
Plasma biomarker assessments
The plasma biomarkers evaluated in this study were p-tau181, p-tau217, p-tau231, GFAP and NfL. Assays for p-tau181 included the in-house assay from the University of Gothenburg and from Quanterix. Assays of p-tau217 included assays from Lilly, Janssen and ALZPath. Plasma p-tau231 was assessed using the in-house assay developed at the University of Gothenburg. GFAP and NfL concentrations were measured using the Quanterix assay. The details of all assays can be found in the Supplementary Information.
Reference standard biomarker assessments
The reference standards used in this study to determine the presence of AD pathology were PET, CSF and neuropathological assessments. Abnormality criteria for all reference standard biomarkers have been published previously and are described in the Supplementary Information for all cohorts.
Statistics and reproducibility
Abnormality for plasma biomarkers was determined in a standardized manner across all cohorts using z-scores created based on the means and s.d. of cognitively unimpaired individuals without elevated amyloid-β pathology, as previously done in several studies8,62,63. These z-scores were applied to the cognitively impaired individuals with reference standard biomarkers assessed by dementia specialists. In the TRIAD cohort and McGill memory clinic cohorts, a z-score of 1.5 had high discriminative accuracy for biological AD versus other neurodegenerative diseases. Therefore, plasma biomarker abnormality was defined by a z-score of 1.5 and above, and this was applied consistently to all cohorts. Prevalence-adjusted (that is, pretest probability-adjusted) PPVs and NPVs were calculated using the Bayesian formula provided by Altman and Bland15,64,65 using age-associated prevalence of Aβ+ in MCI, probable AD dementia and non-AD dementia syndromes (frontotemporal dementia, vascular dementia and corticobasal syndrome) from published meta-analyses18,19 using the following formulas:
We furthermore conducted three sets of sensitivity analyses. First, owing to the strong association of APOE ε4 genotype with amyloid-β pathology18,19, we estimated age- and clinical syndrome-associated plasma biomarker PPVs and NPVs adjusted for APOE ε4 carriership. In the second, we estimated PPVs and NPVs using the upper and lower estimates of the reported prevalence of amyloid-β pathology18,19. In the third, we used prevalence estimates of amyloid PET positivity from the Mayo Clinic Study of Aging, a population-based cohort study20. No statistical methods were used to predetermine sample sizes. No data were excluded from any of the analyses. Data were visualized using GraphPad Prism (version 10). This study complied with Standards for Reporting Diagnostic Accuracy Studies guidelines.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Supplementary Methods and Tables 1–45.
Source data
Point estimates and confidence intervals for PPVs and NPVs for individuals with MCI.
Point estimates and confidence intervals for PPVs and NPVs for individuals with probable AD dementia.
Acknowledgements
This research is supported by the Weston Brain Institute, Canadian Institutes of Health Research (MOP-11-51-31; RFN 152985, 159815 and 162303), Canadian Consortium of Neurodegeneration and Aging (MOP-11-51-31-team 1), the Alzheimer’s Association (NIRG-12-92090 and NIRP-12-259245), Brain Canada Foundation (CFI Project 34874; 33397), the Fonds de Recherche du Québec–Santé (Chercheur Boursier, 2020-VICO-279314) and the Colin J. Adair Charitable Foundation. Research reported in this publication from the HABS study was supported by the National Institute on Aging of the National Institutes of Health under award numbers R01AG054073, R01AG058533, P41EB015922 and U19AG078109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.T. is funded by the Colin J. Adair Charitable Foundation scholarship and the McGill Faculty of Medicine bursary award. H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (nos. 2022-01018 and 2019-02397), the European Union’s Horizon Europe research and innovation program under grant agreement no. 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation, USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie grant agreement no. 860197 (MIRIADE), the European Union Joint Programme–Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre and the UK Dementia Research Institute at UCL (UKDRI-1003). The BioFINDER-2 study was supported by the Alzheimer’s Association (SG-23-1061717), Swedish Research Council (2022-00775), ERA PerMed (ERAPERMED2021-184), the Knut and Alice Wallenberg foundation (2017-0383), the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation (AF-980907), the Swedish Brain Foundation (FO2021-0293), the Parkinson foundation of Sweden (1412/22), the Cure Alzheimer’s fund, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Skåne University Hospital Foundation (2020-O000028), Regionalt Forskningsstöd (2022-1259) and the Swedish federal government under the ALF agreement (2022-Projekt0080). The precursor of 18F-flutemetamol was sponsored by GE Healthcare. The Mayo Clinic Study of Aging is supported by the National Institutes of Aging (U01 AG006786), the GHR foundation and the Mayo Medical Foundation for Education and Research, as well as NIA grants R37 AG011378 and R01 AG041851. M.S.-C. receives funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 948677), project PI19/00155, funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union, and from a fellowship from La Caixa Foundation (ID 100010434) and from the European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie grant agreement no. 847648 (LCF/BQ/PR21/11840004). The SPIN cohort has received funding from CIBERNED; Instituto de Salud Carlos III; jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, ‘Una manera de hacer Europa’; Generalitat de Catalunya; Fundació ‘La Marató TV3’ Fundació Bancària Obra Social La Caixa; Fundación BBVA; Fundación Española para el Fomento de la Investigación de la Esclerosis Lateral Amiotrófica; Global Brain Health Institute; Fundació Catalana Síndrome de Down; Fundació Víctor Grífols i Lucas; Jérôme Lejeune Foundation. This study was conducted with data obtained from the consortium of the Biobank Innovations for Chronic cerebrovascular disease With ALZheimer’s disease Study (BICWALZS), which was funded by the Korea Disease Control and Prevention Agency for the Korea Biobank Project (#6637-303). Data used in preparation of this article were obtained from the HABS-HD database (https://apps.unthsc.edu/itr/researchers). HABS-HD MPIs include S.E. O’Bryant, K. Yaffe, A. Toga, R. Rissman and L. Johnson; and the HABS-HD Investigators: M. Braskie, K. King, J.R. Hall, M. Petersen, R. Parlmer, R. Barber, Y. Shi, F. Zhang, R. Nandy, R. McColl, M. Rivera Mindt, A. Cheema, L. Barnes, M. Mapstone, A. Cohen, A. Kind, O. Okonkwo, R. Vintimilla, Z. Zhou, M. Donohue, R. Raman, M. Borzage, M. Miekle, B. Ances, G. Babulal, J. Llibre-Guerra, C. Hill and R. Vig. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (https://adni.loni.usc.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
Study concept and design: J.T., H.Z., K.B., O.H. and P.R.-N. Statistical analysis: J.T. and P.R.-N. Figure and manuscript draft: J.T., H.Z., K.B., O.H. and P.R.-N. Obtaining and preparation of data and obtaining study funding: all authors. Critical review of manuscript for important intellectual content: all authors.
Peer review
Peer review information
Nature Aging thanks Takeshi Iwatsubo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
Data from the ADNI cohort can be accessed from https://ida.loni.usc.edu. Data from the HABS-HB study can be accessed from https://apps.unthsc.edu/itr/researchers. Raw and analyzed de-identified data from the Mayo Clinic Study of Aging can be requested at https://ras-rdrs.mayo.edu/Request/IndexRequest. The request will be reviewed by the Mayo Clinic Study of Aging investigators and Mayo Clinic to verify whether the request is subject to any intellectual property or confidentiality obligations. A data sharing agreement must be obtained before release. Anonymized data from the BICWALZS, BioCogBank, BIODEGMAR, BioFINDER, SPIN, TRIAD and UCSD-ADRC cohort studies will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in this Letter and as long as the data transfer is in agreement with all local legislation on general data protection regulation and will be regulated by a material transfer agreement. Source data are provided with this paper.
Code availability
No custom code or mathematical algorithm that was central to the conclusions was used for this study.
Competing interests
J.T. has served as a consultant for the Neurotorium educational platform, outside of the scope of the submitted work. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, which is a part of the GU Ventures Incubator Program (outside submitted work). O.H. has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. R.C.P. has consulted for Roche, Genentech, Eli Lilly, Eisai and Nestle, all outside the scope of the current work. M.S.-C. has served as a consultant and at advisory boards for Roche Diagnostics International Ltd and Grifols S.L.; has given lectures in symposia sponsored by Roche Diagnostics, S.L.U and Roche Farma, S.A.; and was granted with a project funded by Roche Diagnostics International Ltd; payments were made to the institution (BBRC). A.P.P. has served at advisory boards for Schwabe Farma Iberica. D.A. participated in advisory boards from Fujirebio-Europe, Roche Diagnostics, Grifols S.A. and Lilly, and received speaker honoraria from Fujirebio-Europe, Roche Diagnostics, Nutricia, Krka Farmacéutica S.L., Zambon S.A.U. and Esteve Pharmaceuticals S.A. D.A. declares a filed patent application (WO2019175379 A1 Markers of synaptopathy in neurodegenerative disease). S. Johnson has served at scientific advisory boards or as a consultant for ALZpath, Prothena, Roche Diagnostics, and Enigma. M.M.M. has served as a consultant and at advisory boards for Biogen, Eisai, Lilly, Merck, Roche, and Siemens Healthineers. P.R.-N. has served at scientific advisory boards and/or as a consultant for Roche, Novo Nordisk, Eisai, and Cerveau radiopharmaceuticals. A.A.-S. has participated in advisory boards for Roche Diagnostics, Fujirebio Diagnostics and Siemens Healthineers. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Oskar Hansson, Pedro Rosa-Neto.
Contributor Information
Joseph Therriault, Email: joseph.therriault@mail.mcgill.ca.
Pedro Rosa-Neto, Email: pedro.rosa@mcgill.ca.
Supplementary information
The online version contains supplementary material available at 10.1038/s43587-024-00731-y.
References
- 1.van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 10.1056/NEJMoa2212948 (2022).
- 2.Lecanemab Prescribing Information (US Food and Drug Administration, 2023).
- 3.Hansson, O., Blennow, K., Zetterberg, H. & Dage, J. Blood biomarkers for Alzheimer’s disease in clinical practice and trials. Nat. Aging3, 506–519 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thijssen, E. H. et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med.26, 387–397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janelidze, S. et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med.26, 379–386 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol.19, 422–433 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Thijssen, E. H. et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol.20, 739–752 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmqvist, S. et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease versus other neurodegenerative disorders. JAMA324, 772–781 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashton, N. J. et al. Plasma and CSF biomarkers in a memory clinic: head-to-head comparison of phosphorylated tau immunoassays. Alzheimers Dement. 10.1002/alz.12841 (2022). [DOI] [PMC free article] [PubMed]
- 10.Janelidze, S. et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer’s disease. Brain146, 1592–1601 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimes, D. A. & Schulz, K. F. Uses and abuses of screening tests. Lancet359, 881–884 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Vecchio, T. J. Predictive value of a single diagnostic test in unselected populations. N. Engl. J. Med.274, 1171–1173 (1966). [DOI] [PubMed] [Google Scholar]
- 13.Pewsner, D. et al. Ruling a diagnosis in or out with ‘SpPIn’ and ‘SnNOut’: a note of caution. Br. Med. J.329, 209–213 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manrai, A. K., Bhatia, G., Strymish, J., Kohane, I. S. & Jain, S. H. Medicine’s uncomfortable relationship with math: calculating positive predictive value. JAMA Intern. Med.174, 991–993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman, D. G. & Bland, M. J. Diagnostic tests 2: predictive values. Br. Med. J.309, 102 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teunissen, C. E. et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol.21, 66–77 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Katz, M. A. A probability graph describing the predictive value of a highly sensitive diagnostic test. N. Engl. J. Med.291, 1115–1116 (1974). [DOI] [PubMed] [Google Scholar]
- 18.Ossenkoppele, R. et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA313, 1939–1949 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen, W. J. et al. Prevalence estimates of amyloid abnormality across the Alzheimer disease clinical spectrum. JAMA Neurol.79, 228–243 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Jack, C. R. et al. Prevalence of biologically versus clinically defined Alzheimer spectrum entities using the National Institute on Aging–Alzheimer’s Association research framework. JAMA Neurol.76, 1174–1183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearon, C. et al. Diagnosis of pulmonary embolism with d-dimer adjusted to clinical probability. N. Engl. J. Med.381, 2125–2134 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Diamond, G. A. & Forrester, J. S. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N. Engl. J. Med.300, 1350–1358 (1979). [DOI] [PubMed] [Google Scholar]
- 23.Goodman, K. E., Rodman, A. M. & Morgan, D. J. Preparing physicians for the clinical algorithm era. N. Engl. J. Med. 10.1056/NEJMp2304839 (2023). [DOI] [PubMed]
- 24.Schneider, J. A., Arvanitakis, Z., Leurgans, S. E. & Bennett, D. A. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol.66, 200–208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therriault, J. et al. Frequency of biologically-defined AD in relation to age, sex, APOEε4 and cognitive impairment. Neurology96, e975–e985 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The CRyPTIC Consortium and the 100,000 Genomes Project. Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N. Engl. J. Med. 379, 1403–1415 (2018). [DOI] [PMC free article] [PubMed]
- 27.Neumann, J. T. et al. Application of high-sensitivity troponin in suspected myocardial infarction. N. Engl. J. Med.380, 2529–2540 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Jansen, W. J. et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA313, 1924–1938 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Therriault, J. et al. Equivalence of plasma p-tau217 with cerebrospinal fluid in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 10.1002/alz.13026 (2023). [DOI] [PMC free article] [PubMed]
- 30.Brum, W. S. et al. A blood-based biomarker workflow for optimal tau-PET referral in memory clinic settings. Nat. Commun.15, 2311 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therriault, J. et al. Comparison of two plasma p-tau217 assays to detect and monitor Alzheimer’s pathology. eBioMedicine102, 105046 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barthélemy, N. R. et al. Highly accurate blood test for Alzheimer’s disease comparable or superior to clinical CSF tests. Nat. Med.2024, 1085–1095 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Therriault, J. et al. Association of phosphorylated tau biomarkers with amyloid-PET versus with tau-PET. JAMA Neurol.10.1001/jamaneurol.2022.4485 (2022). [Google Scholar]
- 34.Salvadó, G. et al. Specific associations between plasma biomarkers and postmortem amyloid plaque and tau tangle loads. EMBO Mol. Med.15, e17123 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashton, N. J. et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat. Med.28, 2555–2562 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellaver, B. et al. Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer’s disease. Nat. Med.29, 1775–1781 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo, Y. et al. Plasma proteomic profiles predict future dementia in healthy adults. Nat. Aging4, 247–260 (2024). [DOI] [PubMed] [Google Scholar]
- 38.Abdelhak, A. et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol.18, 158–172 (2022). [DOI] [PubMed] [Google Scholar]
- 39.Heller, C. et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry91, 263–270 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Bazarian, J. J. et al. Serum GFAP and UCH-L1 for prediction of absence of intracranial injuries on head CT (ALERT-TBI): a multicentre observational study. Lancet Neurol.17, 782–789 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Meier, S. et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as a biomarker for disease progression in multiple sclerosis. JAMA Neurol.80, 287–297 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe, M. et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology93, E1299–E1311 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Benedet, A. L. et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol.78, 1471–1483 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashton, N. J. et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 12, 3400 (2021). [DOI] [PMC free article] [PubMed]
- 45.Cummings, J. L. et al. Lecanemab: appropriate use recommendations. J. Prev. Alzheimers Dis.10.14283/jpad.2023.34 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummings, J. L. et al. Aducanumab: appropriate use recommendations. J. Prev. Alzheimers Dis.8, 398–410 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ossenkoppele, R. et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain139, 1551–1567 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Therriault, J. et al. Intrinsic connectivity of the human brain provides scaffold for tau aggregation in clinical variants of Alzheimer’s disease. Sci. Transl. Med.14, eabc8693 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Therriault, J. et al. Biomarker modeling of Alzheimer’s disease using PET-based Braak staging. Nat. Aging10.1038/s43587-022-00204-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lantero-Rodríguez, J. et al. CSF p-tau205: a biomarker of tau pathology in Alzheimer’s disease. Acta Neuropathol.147, 12 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montoliu-Gaya, L. et al. Mass spectrometric simultaneous quantification of tau species in plasma shows differential associations with amyloid and tau pathologies. Nat. Aging10.1038/s43587-023-00405-1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horie, K. et al. CSF MTBR-tau243 is a specific biomarker of tau pathology in Alzheimer’s disease. Nat. Med.10.1038/s41591-023-02443-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Therriault, J. et al. Biomarker-based staging of Alzheimer disease: rationale and clinical applications. Nat. Rev. Neurol.10.1038/s41582-024-00942-2 (2024). [DOI] [PubMed] [Google Scholar]
- 54.Brickman, A. M. et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement.17, 1353–1364 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohs, R. C. et al. The Bio-Hermes Study: biomarker database developed to investigate blood-1 based and digital biomarkers in community-based, diverse populations clinically screened for Alzheimer’s disease. Alzheimers Dement. 10.1002/alz.13722 (2024). [DOI] [PMC free article] [PubMed]
- 56.Brum, W. S. et al. A two-step workflow based on plasma p-tau217 to screen for amyloid β positivity with further confirmatory testing only in uncertain cases. Nat. Aging3, 1079–1090 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janelidze, S., Barthélemy, N. R., He, Y., Bateman, R. J. & Hansson, O. Mitigating the associations of kidney dysfunction with blood biomarkers of Alzheimer disease by using phosphorylated tau to total tau ratios. JAMA Neurol. 10.1001/jamaneurol.2023.0199 (2023). [DOI] [PMC free article] [PubMed]
- 58.Ramanan, V. K. et al. Genetic risk scores enhance the diagnostic value of plasma biomarkers of brain amyloidosis. Brain146, 4508–4519 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palmqvist, S. et al. Accurate risk estimation of β-amyloid positivity to identify prodromal Alzheimer’s disease: cross-validation study of practical algorithms. Alzheimers Dement.15, 194–204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beyer, L. et al. Amyloid-beta misfolding and GFAP predict risk of clinical Alzheimer’s disease diagnosis within 17 years. Alzheimers Dement.19, 1020–1028 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Nabers, A. et al. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol. Med.10, e8763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmqvist, S. et al. Prediction of future Alzheimer’s disease dementia using plasma phospho-tau combined with other accessible measures. Nat. Med.27, 1034–1042 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Milà-Alomà, M. et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat. Med.10.1038/s41591-022-01925-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell, M. J., Machin, D. & Walters, S. J. Medical Statistics: a Textbook for the Health Sciences (Wiley, 2021).
- 65.Calculator for positive predictive value (PPV) and negative predictive value (NPV) for individual tests and combined. US Food and Drug Administrationhttps://www.fda.gov/media/137612/download (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods and Tables 1–45.
Point estimates and confidence intervals for PPVs and NPVs for individuals with MCI.
Point estimates and confidence intervals for PPVs and NPVs for individuals with probable AD dementia.
Data Availability Statement
Data from the ADNI cohort can be accessed from https://ida.loni.usc.edu. Data from the HABS-HB study can be accessed from https://apps.unthsc.edu/itr/researchers. Raw and analyzed de-identified data from the Mayo Clinic Study of Aging can be requested at https://ras-rdrs.mayo.edu/Request/IndexRequest. The request will be reviewed by the Mayo Clinic Study of Aging investigators and Mayo Clinic to verify whether the request is subject to any intellectual property or confidentiality obligations. A data sharing agreement must be obtained before release. Anonymized data from the BICWALZS, BioCogBank, BIODEGMAR, BioFINDER, SPIN, TRIAD and UCSD-ADRC cohort studies will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in this Letter and as long as the data transfer is in agreement with all local legislation on general data protection regulation and will be regulated by a material transfer agreement. Source data are provided with this paper.
No custom code or mathematical algorithm that was central to the conclusions was used for this study.