Abstract
Individuals with pacemakers are at increased risk of left ventricular systolic dysfunction (LVSD). Whether screening for and optimizing the medical management of LVSD in these individuals can improve clinical outcomes is unknown. In the present study, in a multicenter controlled trial (OPT-PACE), we randomized 1,201 patients (717 men) with a pacemaker to echocardiography screening or usual care. In the screening arm, LVSD was detected in 201 of 600 (34%) patients, who then received management in either primary care or a specialist heart failure (HF) and devices clinic. The primary outcome of the trial was the difference in a composite of time to first HF hospitalization or death. Over 31 months (interquartile range = 30–40 months), the primary outcome occurred in 106 of 600 (18%) patients receiving echocardiography screening, which was not significantly different compared with the occurrence of the primary outcome in 115 of 601 (19%) patients receiving the usual care (hazard ratio = 0.89; 95% confidence interval = 0.69, 1.17). In a prespecified, nonrandomized, exploratory analysis, patients with LVSD managed by the specialist clinic experienced the primary outcome event less frequently than those managed in primary care. The results of this trial indicate that echocardiography screening commonly identifies LVSD in individuals with pacemakers but alone does not alter outcomes. ClinicalTrials.gov registration: NCT01819662.
Subject terms: Outcomes research, Heart failure, Cardiac device therapy
For individuals with pacemakers, a care pathway that includes echocardiographic screening to detect signs of heart failure did not improve cardiac outcomes, but patients flagged as having impaired heart function who were managed by a specialized heart failure clinic benefited, as compared to those managed by primary care physicians.
Main
Pacemaker implantation for bradycardia improves quality of life and survival1,2. Over a million devices are implanted globally each year3–5. Long-term right ventricular (RV) pacing is associated with an adverse effect on left ventricular (LV) function and the development of HF, especially in the presence of other cardiovascular morbidities6,7, which augurs a worse prognosis8–10. Routine pacemaker follow-up offers the opportunity to screen for pacemaker-associated HF. However, people with pacemakers were either actively excluded from randomized controlled trials assessing medical therapies for HF with reduced ejection fraction (HFrEF) or subgroup analyses of those with pacemakers were not undertaken. Therefore, as a result of the lack of evidence, no medical treatment strategy has been recommended for this group in current HF or pacing guidelines11, such that the effects of a pathway, which includes screening followed by patient-centered education and optimized medical management for people found to have impaired LV function, are unknown.
OPT-PACE (OPTimizing PACEmaker therapy) was designed to determine the effect on clinical outcomes of screening for impaired LV function in people with pacemakers implanted for bradycardia.
Results
OPT-PACE was a randomized controlled trial that recruited patients, not previously known to have LV dysfunction, implanted with a standard pacemaker for >12 months, who were attending routine pacemaker follow-up at three hospitals in the United Kingdom. Consecutive, unselected patients agreeing to participate were randomly allocated to echocardiography screening or usual care (Extended Data Fig. 1). Depending on the site, patients found to have LV systolic dysfunction (LVSD) defined as an LV ejection fraction (LVEF) < 50% were referred to either their primary care team for management of this or a specialized, multidisciplinary, combined HF and devices service. All patients were followed up for a minimum of 12 months to establish time to a combined primary endpoint of death or HF hospitalization (HFH). Secondary endpoints included attainment of guideline-directed medical therapy for HF and quality of life. A prespecified (nonrandomized) exploratory endpoint compared outcomes in patients managed by their primary care service or the specialized clinic.
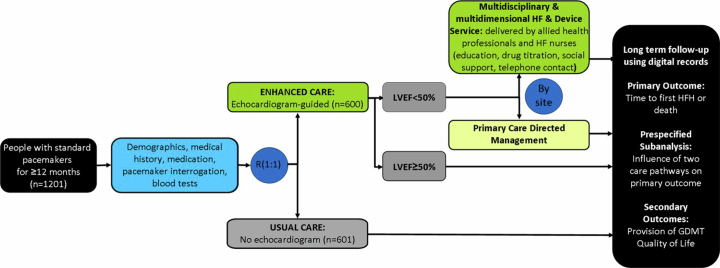
Extended Data Fig. 1. Screening for heart failure and optimizing pathways of care in people with pacemakers: The OPT-PACE randomized controlled trial.
Graphical abstract describing study design, study flow, data collection time points, outcome sources and endpoints.
Patient disposition
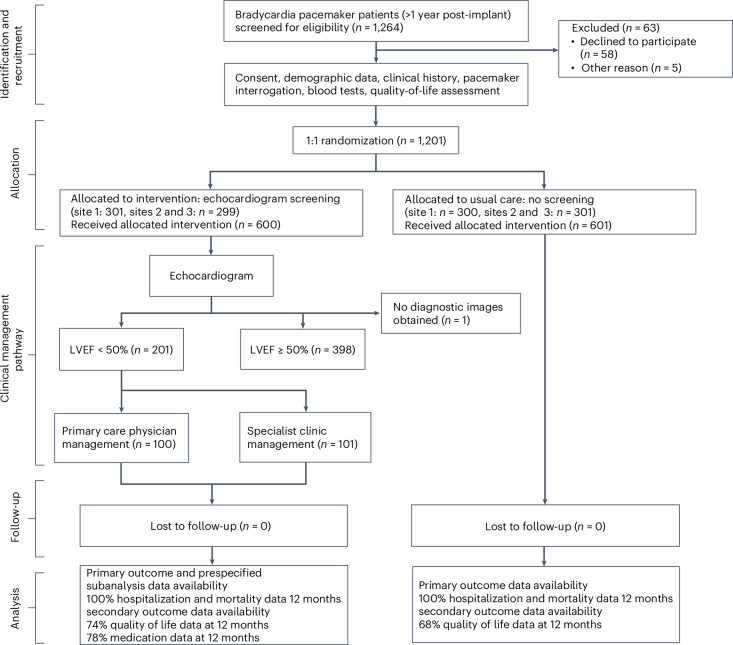
A total of 1,201 people were recruited between 1 June 2013 and 15 November 2016. Of the 1,201, 600 were randomized to echocardiography screening and 601 to the usual care (Fig. 1).
Fig. 1. OPT-PACE CONSORT diagram.
Disposition and flow of participants enrolled to OPT-PACE.
Demographic and clinical characteristics were balanced across randomized groups (Tables 1 and 2). More than half (60%) were male and the mean (s.d.) age of participants was 75 (12) years. Comorbidities included type 2 diabetes mellitus (21%), a history of myocardial infarction (18%), coronary artery bypass grafting (9%) and percutaneous coronary intervention (9%). Mean (s.d.) time since first pacemaker implantation was 7.2 (6.2) years and mean (s.d.) atrial and ventricular pacing percentages were 33% (35%) and 40% (42%), respectively.
Table 1.
Patient demographics and characteristics at baseline
| Echocardiography screening (n = 600) | No echocardiography screening (n = 601) | Total (n = 1,201) | |
|---|---|---|---|
| Patient distribution by site | |||
| Site 1, n (%) | 301 (50) | 300 (50) | 601 (50) |
| Site 2, n (%) | 148 (25) | 152 (25) | 300 (25) |
| Site 3, n (%) | 150 (25) | 150 (25) | 300 (25) |
| Patient demographics | |||
| Male sex, n (%) | 358 (60) | 359 (60) | 717 (60) |
| Age (years) | 74.9 (12.2) | 75.5 (11.9) | 75.2 (12.0) |
| Height (cm) | 167 (13) | 166 (14) | 167 (14) |
| Weight (kg) | 78 (16) | 77 (17) | 78 (17) |
| Atrial rhythm | |||
| Atrial fibrillation, n (%) | 194 (32) | 162 (27) | 356 (30) |
| Paced, n (%) | 46 (8) | 62 (10) | 108 (9) |
| Sinus rhythm, n (%) | 359 (60) | 62.79 (63) | 737 (61) |
| Clinical history data | |||
| IHD, n (%) | 206 (34) | 208 (35) | 414 (35) |
| Diabetes mellitus | |||
| Type 2, n (%) | 119 (20) | 128 (21) | 247 (21) |
| Type 1, n (%) | 3 (0.5) | 3 (0.5) | 6 (0.5) |
| CVA, n (%) | 100 (17) | 90 (15) | 190 (16) |
| Haemodynamic data | |||
| Resting heart rate (beats per min) | 69 (12) | 69 (12) | 69 (12) |
| Resting systolic BP (mmHg) | 138 (22) | 138 (24) | 138 (23) |
| Pacing data | |||
| Pacing indication | |||
| Atrioventricular block, n (%) | 212 (35.6) | 207 (34.3) | 419 (34.9) |
| Sinus node disease, n (%) | 321 (53.5) | 322 (53.5) | 643 (53.5) |
| Other, n (%) | 67 (11.1) | 76 (12.6) | 143 (11.9) |
| Longevity of pacing (years) | 7.2 (6.0) | 7.2 (6.4) | 7.2 (6.2) |
| Atrial fibrillation burden (%) | 30 (45) | 28 (43) | 29 (44) |
| Atrial pacing burden (%) | 32 (35) | 33 (35) | 32 (35) |
| Ventricular pacing burden (%) | 41 (43) | 38 (42) | 40 (42) |
| Base rate (beats per min) | 56 (8) | 56 (8) | 56 (7) |
| Echocardiographic data | |||
| LVEF (%) | 50 (10) | ||
| LVEDD (mm) | 47 (7) | ||
Continuous data are expressed as mean (s.d.) and categorical data as n (%) as indicated.
CVA, cerebrovascular attack; LVEDD, left ventricular end-diastolic diameter.
Table 2.
Drug and device treatment of surviving patients by screening arm at baseline and after 12 months of follow-up
| Medical therapy | Echocardiography screening (n = 600) | No echocardiography screening (n = 601) | ||
|---|---|---|---|---|
| Baseline (n = 600) | Follow-up (n = 468) | Baseline (n = 601) | Follow-up (n = 435) | |
| β-Blocker, n (%) | 263 (44) | 248 (53) | 264 (44) | 210 (49) |
| ACEi or ARB, n (%) | 297 (50) | 256 (54) | 301 (50) | 213 (49) |
| Loop diuretic, n (%) | 136 (23) | 118 (25) | 125 (21) | 29 (18,) |
| MRA, n (%) | 97 (16) | 25 (5) | 99 (16) | 11 (2.5) |
| Statin, n (%) | 308 (51) | 239 (51) | 300 (50) | 205 (47) |
| Anti-platelet, n (%) | 199 (33) | 127 (27) | 202 (34) | 125 (29) |
| Anticoagulation, n (%) | 210 (35) | 185 (43) | 203 (34) | 148 (34) |
| Digoxin, n (%) | 48 (8) | 37 (8) | 38 (6) | 30 (7) |
| CRT upgrade, n (%) | 0 (0) | 7 (1.2) | 0 () | 4 (0.6) |
ARB, angiotensin II receptor blocker.
One participant randomized to echocardiographic screening had no diagnostic images obtainable but was nevertheless included in their allocated group. In the 600 people allocated to echocardiography screening, the mean LVEF was 50% (10%), with 201 (34%) individuals identified as having LVEF < 50%. The prevalence of LVEF < 50% was similar across sites such that 101 (34% of those screened) were seen in the combined HF and devices clinic at site 1 and 100 (33% of those screened) received primary-care-led management (sites 2 and 3) (Fig. 1).
Primary outcome measure
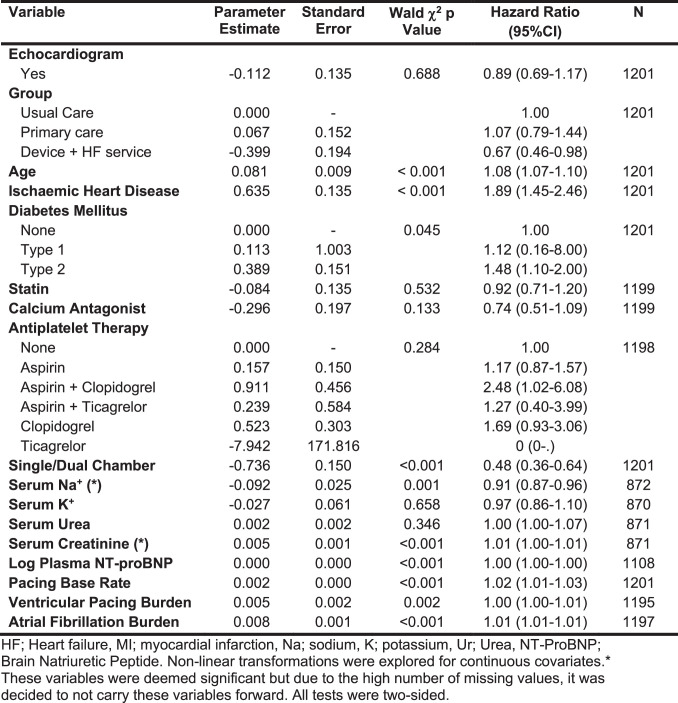
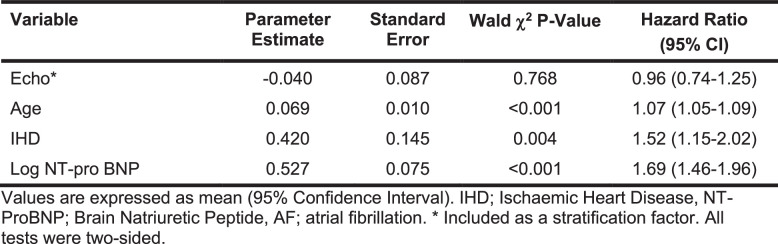
Participants were followed for a median of 31 months (interquartile range (IQR) = 30, 40 months) with a minimum follow-up for all patients of 12 months. Univariate and multivariable predictors of the primary outcome are demonstrated in Extended Data Tables 1 and 2 and included age (odds ratio (OR) = 1.07, 95% confidence interval (CI) = 1.05, 1.09), overt ischemic heart disease (IHD; OR = 1.52, 95% CI = 1.15, 2.02) and log(N-terminal pro-brain natriuretic peptide (NT-proBNP)) (OR = 1.69, 95% CI = 1.46, 1.96).
Extended Data Table 1.
Univariate estimates to event-free survival
Extended Data Table 2.
Multivariable analysis (Cox’s regression, adjusted for randomization to echocardiography) for time to survival from HFH or death
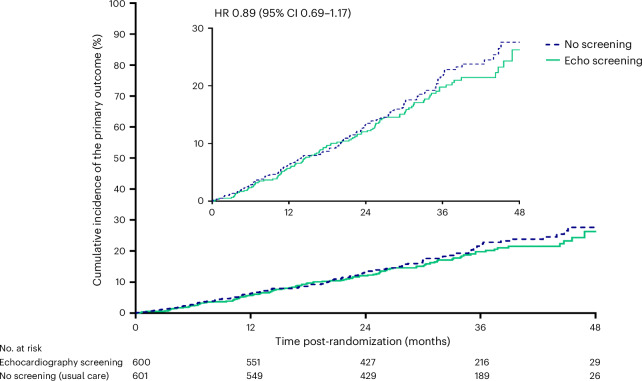
The primary outcome occurred in 106 of 600 (18%) people randomized to echocardiographic screening and 115 of 601 (19%) in the usual care group (hazard ratio (HR) = 0.89; 95% CI = 0.69, 1.17) (Fig. 2). The estimated treatment effect adjusted by statistically significant predictors of outcome (Extended Data Table 1) did not alter the results (HRadjusted = 0.95; 95% CI = 0.72, 1.24).
Fig. 2. Time free of all-cause mortality or HFH by randomization group.
Kaplan–Meier curve demonstrating the primary outcome in those allocated screening by echocardiography (intervention) or no screening (standard care).
Secondary outcome measures
Out of 903 participants with complete 12-month medical therapy data (468 echocardiographic screening, 435 usual care), participants screened with echocardiography received more medical therapy optimization (118 diuretic, 247 β-blocker, 255 angiotensin-converting enzyme inhibitor (ACEi) initiation/titration episodes) than participants in the usual care group (79 diuretic, 211 β-blocker, 214 ACEi initiation/titration episodes). Device system upgrades to cardiac resynchronization therapy (CRT) were performed in seven (1.2%) patients in the echocardiography group and four (0.6%) of those allocated to usual care.
Of 1,201 participants, 1,198 completed quality-of-life questionnaires at baseline (pre-randomization) with 878 (73%) also completing questionnaires at the 12-month follow-up. The EuroQoL-5D (EQ-5D) scores were similar at baseline (n = 1,184, 99%) for transthoracic echocardiogram-guided care (n = 591) and usual care (n = 593) (0.77 ± 0.25 and 0.76 ± 0.24, respectively). Conditional on survival, the quality of life described by EQ-5D scores at follow-up (n = 855, 71%) did not change for either echocardiography screening (n = 444) or usual care (n = 411) groups (0.76 ± 0.24 and 0.73 ± 0.31). Analysis of covariance (ANCOVA), allowing adjustment for baseline EQ-5D visual analog score (VAS), showed that the mean difference in VAS of those receiving echo (n = 436) to those receiving no echo (n = 406) was 0.85 (95% CI = −1.38, 3.08).
Exploratory outcomes
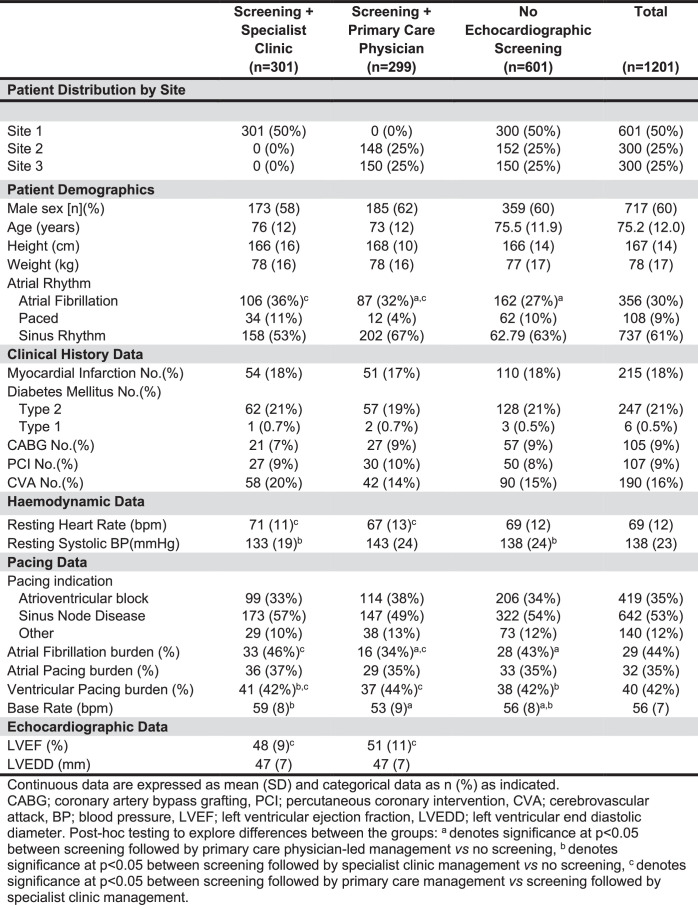
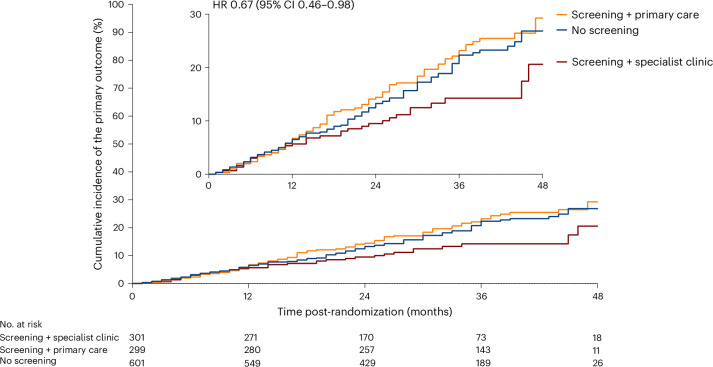
The prespecified exploratory analysis of the echocardiography screening group, according to the follow-up pathway, suggested that the groups, although nonrandomized for this comparison, were well balanced (Extended Data Table 3). Patients with LVEF < 50% were not randomly allocated to the follow-up care because this was a site-level pathway and only available at site 1. The rate of the primary outcome appeared lower in the site with access to the specialist clinic than in the two sites where the pathway of care included primary-care-led management (specialist clinic versus primary-care-led management: 12% versus 24%; HR = 0.67, 95% CI = 0.46, 0.98) (Fig. 3). This nonrandomized comparison is hypothesis generating and requires further validation. The difference between these two groups remained significant, even after adjustment for differences in the baseline variables, which, as shown in Extended Data Table 3, were atrial rhythm, systolic blood pressure (BP), resting heart rate, atrial fibrillation (AF) burden, ventricular pacing proportion and LVEF (HR = 0.62; 95% CI = 0.40, 0.97). On the other hand, there was no difference in outcome between those allocated to the usual care (no echocardiography) and those who received echocardiography but were not seen in the specialist clinic (HR = 1.01; 95% CI = 0.72, 1.40), including when the analysis was adjusted for baseline differences shown in Extended Data Table 3 (atrial rhythm, AF burden, base rate; HR = 0.88; 95% CI = 0.61, 1.27).
Extended Data Table 3.
Patient demographics at baseline by care pathway
Fig. 3. Time free of all-cause mortality or HFH by the clinical management pathway.
Kaplan–Meier curve demonstrating the exploratory analysis of the primary outcome in those allocated screening by echocardiography (intervention) divided by management pathway (primary-care-led management or specialist clinic-led management) or no screening (standard care).
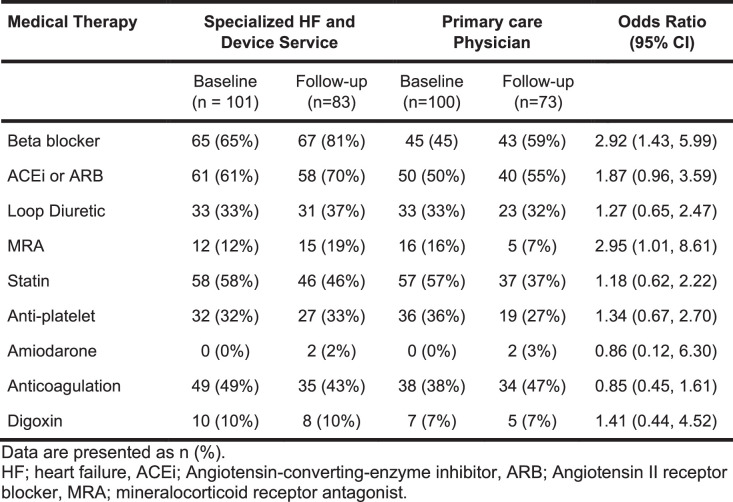
There were apparent differences in the medical management of those with impaired LV function at 12 months in participants randomized to echocardiographic screening and subsequently managed in a multidisciplinary HF and devices service compared with those receiving primary-care-based management. People with LVEF < 50% managed through the specialist service were almost 3× more likely at the 12-month follow-up to have undergone initiation or titration of β-antagonists (OR = 2.92, 95% CI = 1.43, 5.99) or a mineralocorticoid receptor antagonist (MRA; OR = 2.95, 95% CI = 1.01, 8.61) than people with LVEF < 50% in the echocardiography arm with primary-care-coordinated management. There were no differences in initiation or titration of ACEi therapy (OR = 1.64, 95% CI = 0.86, 3.14) (Extended Data Table 4).
Extended Data Table 4.
Drug therapy of patients with LVSD at baseline and after a 12-month follow-up by care pathway
Sensitivity analysis
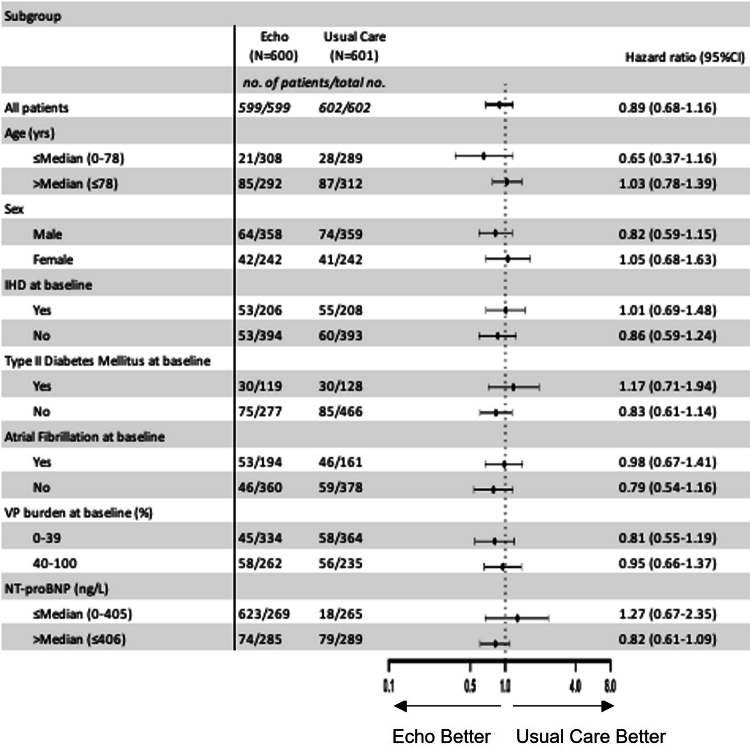
Exploratory analysis of the primary endpoint within subgroups relevant to outcomes in this population (Extended Data Table 2), and associated with adverse outcomes in people with HF (diabetes mellitus and IHD)12,13, revealed that even in those at higher risk, the effect of echocardiography screening alone was neutral (Extended Data Fig. 2).
Extended Data Fig. 2. Time Free of All-Cause Mortality or Heart Failure Hospitalisation According to Prespecified Subgroup.
Forest plot describing the primary outcome by relevant prespecified clinical subgroups showing no difference in primary outcome between those allocated screening by echocardiography and those allocated standard care.
Discussion
OPT-PACE tested whether a screening test, in this case an echocardiogram to identify LVSD, in people with a risk factor, in this case a pacemaker, is sufficient to improve outcomes. The present study was pragmatically delivered, collating additional information on two possible pathways of care of people with a positive result. As patient pathways for enhanced care differed at the site level, it was not possible to randomize patients between the specialist clinic and primary care.
OPT-PACE provides three important findings. First, in a contemporary population with pacemakers implanted for bradycardia but not yet known to have HF, routine echocardiographic screening identifies impaired LV function in around a third. Second, simply embedding echocardiography into a pacemaker service does not, in itself, lead to improved clinical outcomes. Third, a nonrandomized, planned exploratory analysis suggests that a pathway of care that includes coordinated optimization of medical therapy for HF within a multidisciplinary clinic, screening for and treating pacemaker-associated LV dysfunction might improve clinical outcomes, although this analysis is hypothesis generating and the results require further validation.
Consistent with our data, previous studies have shown that people with pacemakers implanted for bradycardia have a risk of impaired LV function (LVEF < 50%) or overt HF far higher than the general population14. Those with impaired LV function have a lower quality of life, higher hospitalization rate and poorer prognosis than those with a pacemaker and normal LV function7. Observational studies in people with pacemakers have consistently shown that the degree of LV dysfunction is related to the RV pacing burden7,15 with a linear relationship to risk of HF events and cardiovascular death16 and that LV impairment is more frequent in patients with pacemakers who also have underlying IHD or myocardial fibrosis7,17.
Although the adverse effects of RV pacing on LV function, and the fact that many people with standard pacemakers have LV dysfunction, have been appreciated for many years14,16, the medical management of pacemaker-associated HF is under-investigated. Optimal care in patients with impaired LV function includes renin–angiotensin–aldosterone system inhibitors18 and β-adrenoceptor antagonists19,20, with proven favorable effects on LV remodeling21 and patient-oriented long-term outcomes22. Moreover, a program with a protocol of initiation and titration of medical therapy for people with HFrEF provided in a specialized setting is associated with better outcomes23. Most of the phase III trials of medical therapy for HFrEF did not actively exclude people with standard pacemakers, but, although around 9% of people with HFrEF have a standard pacemaker24,25, none of the trials reported analyses for outcomes in this subgroup. Hence, as a result of a lack of data, guidelines make no particular mention of the investigation and management of people with pacemakers at risk of, or with proven, HFrEF except to comment on the use of algorithms to limit RV pacing26, and the potential benefits of upgrading standard pacemakers to CRT in the presence of symptoms, LV dysfunction and a high requirement for ventricular pacing11,27.
OPT-PACE has confirmed that a coordinated screening program involving routine echocardiography will detect previously undiagnosed impaired LV function in around a third of people with pacemakers implanted for bradycardia. The present study was not designed to determine the etiology of the LV dysfunction or whether it was primarily the result of RV pacing, but rather to determine whether screening for LV dysfunction led to improved outcomes in patients with standard pacemakers. The primary combined outcome of time to first HFH or death did not differ between those randomized to the usual care and those randomized to echocardiographic screening.
A prespecified exploratory analysis was included to explore whether a care pathway, involving a specialized HF and devices clinic tasked with patient-centered care, education, and initiation and titration of medical therapy for patients found to have an LVEF < 50%, as proposed in HF guidelines and a recent position paper, could be of additional benefit28–30. The present data revealed that those randomized to the screening arm who received management via the specialized service had a significantly lower event rate than either of the other two groups. This outcome could be the result of a combination of education around HF and the improved provision of medical therapy for HF, especially titration of β-antagonists. Higher doses of neurohormonal blockade are associated with improved outcomes, especially in people with comorbidities12,31. The trial was not, however, designed to definitively answer this question, which will require further validation.
Hence, OPT-PACE demonstrates that simply embedding echocardiographic screening into routine pacemaker follow-up services is not sufficient to improve patient outcomes, possibly contributed to by a highly heterogeneous response to the echocardiography result. It is possible that, to achieve better outcomes, echocardiography should form an integral part of a coordinated pathway of care that includes access to specialist HF expertise, education and coordinated patient-tailored initiation and up-titration of medical therapy for people with a standard pacemaker found to have impaired LV function, as for patients who undergo CRT28, although this hypothesis remains unanswered.
OPT-PACE is noteworthy in that studies testing the effects of diagnostic and therapeutic interventions in a single trial are uncommon. One example in the field of HF of a randomized controlled trial that combines screening with treatment is the randomized controlled STOP-HF study, which tested the utility of screening for HF in an asymptomatic population using natriuretic peptides, followed by optimization of medical therapy in a specialized clinic, on clinical outcomes32. However, OPT-PACE differs markedly from previous trials in that it targets an at-risk patient population and then explores two pathways of care on patient-oriented outcomes for those with the condition, thereby offering information not only on the effects of screening itself but also exploratory information on the response to it.
Guidelines recommend natriuretic peptides as an appropriate method of screening for HF in people with symptoms of breathlessness or fatigue29,30. We have previously explored the use of BNPs to identify HF in a pacemaker population33, but found modest specificity for LVSD. Hence, despite data that echocardiographic screening of the asymptomatic general population has a low positive rate and does not lead to improvements in outcomes34, the higher rate of LV impairment in the pacemaker population and the adverse outcomes associated with this, along with the therapeutic information provided by echocardiography, underpinned our decision to use this as our screening test. Further analysis of the OPT-PACE dataset, combined with ongoing observational studies including our previous work7, might serve to identify a cohort at highest risk of prevalent LV dysfunction in whom a tailored echocardiographic screening program might be particularly beneficial.
There are several limitations to the present study. OPT-PACE recruited people from three hospitals within a single region in the United Kingdom. Although this may limit generalizability compared with international models of care, the baseline demographic data suggest that our study is representative of a population treated with pacemakers for bradycardia. We utilized digital data extraction for follow-up. Hospitalization data were not available through a single system at the time of the study, such that patient admissions to other hospitals may be incomplete. However, the distribution of hospital facilities across the United Kingdom means that care is usually delivered by a single organization in a particular locality, making it unlikely that participants would be hospitalized elsewhere. Moreover, we do not envisage any ascertainment bias for one or other group owing to this. UK national mortality data are updated daily across the entire country, so the mortality data are reliable. There is an appreciable delay to the clinical effects of medical therapy for LV dysfunction that is longer for people who are less symptomatic35,36. Hence, longer follow-up might have shown greater effects on patient-oriented outcomes. OPT-PACE was designed and largely completed before the routine use of sacubitril–valsartan and sodium glucose transport protein 2 inhibitors for HFrEF. It is reasonable to propose that the effects of the specialized clinic are likely to have been greater with these agents as additional standard therapy.
Although allocation to echocardiography was randomized, it was not possible to randomize allocation to the follow-up pathway within the present study, given that this was a site-level pathway available in only one site. Hence, the results of the prespecified exploratory analysis describing the effect of the specialist clinic are hypothesis generating and require further research to provide independent validation.
In conclusion, the present study has shown that a third of people with a pacemaker implanted for bradycardia have impaired LV function. Implementing a pathway of care that includes only echocardiographic screening does not improve clinical outcomes. Further research will be required to determine whether a specialist clinic combining device and medical management improves outcomes.
Methods
Trial design and ethical approval
The study design and protocol have been published previously. OPT-PACE was a multicenter, randomized, open-label, parallel group trial conducted in three hospitals in the United Kingdom37. The trial design set out to test the clinical effects of screening echocardiography for impaired LV function in a population at risk, using a randomized controlled methodology. A prespecified, nonrandomized exploratory analysis to explore the effects of two different pathways of care for those identified as having impaired LV function was included. All participants provided written, informed consent and the trial was conducted according to principles outlined in the Declaration of Helsinki, having received full ethical approval from the Health Research Authority (South Yorkshire Research Ethics Committee: no. 12/YH/0487).
Trial participants
Participants were eligible to take part if they had a pacemaker implanted for bradycardia at least 12 months previously due to any indication, according to the clinical guidelines in place at the time38, and were attending routine follow-up at three centers in the United Kingdom. People were ineligible if they were known to have HFrEF, had implantable cardioverter defibrillator or cardiac resynchronization devices, were <18 years old, pregnant, already under the care of HF services or awaiting heart transplantation, or had an anticipated life expectancy of <1 year due to comorbidity or significant cognitive impairment.
Trial procedures
All participants were approached by their clinical team at a routine appointment and all provided written, informed consent before any trial activities. At baseline (pre-randomization) each patient underwent a standard pacemaker interrogation, the medical history was recorded, blood was tested for full blood count, renal function and NT-proBNP, and quality of life was assessed using the EQ-5D questionnaire. Quality-of-life questionnaires during the follow-up period were sent by mail.
The trial design included two phases. First, after the completion of baseline procedures, participants were randomly allocated on a one-to-one basis to echocardiographic screening for LV dysfunction or the usual care (Fig. 1 and Extended Data Fig. 1), using a randomization schedule derived by an independent statistical service and accessed through a web-based system. Those allocated to echocardiographic screening underwent an assessment of LV function according to European Society of Cardiology criteria using Simpson’s biplane measures to determine the LVEF39.
Intervention
Participants allocated the usual care and those in the echocardiographic screening arm with an LVEF ≥ 50% continued with standard pacemaker follow-up. For those found to have impaired LV function (LVEF < 50%), two pathways of care were applied in a nonrandomized fashion, according to center-level practice. In two of the three centers, the results of the echocardiogram were forwarded to the patient’s primary care physician. Subsequent treatment, including onward referral, was at their discretion, accepting that this approach would include considerable heterogeneity. In the third center, patients with an LVEF < 50% were referred directly to a specialized multidisciplinary clinic combining HF and devices therapy, where medical therapy optimization was led by a team of HF nurse specialists and cardiac physiologists in a coordinated program of patient-centered care, education and titration visits, as outlined in HF guidelines and a recent position paper28–30. In all three centers, the usual care included programming to avoid unnecessary RV pacing where appropriate, as previously published40.
Outcomes
Long-term survival, hospitalization and medical therapy were assessed using hospital and primary care digital patient records, including National Health Service (NHS) national and local systems.
The primary outcome was a composite of the time to first HFH or death comparing those randomized to echocardiographic screening or the usual care. A prespecified analysis of the primary outcome was included to enable an exploratory assessment of the effects of a care pathway that included screening for impaired LV function and medical optimization delivered through a multidisciplinary specialized HF and devices service or the patient’s primary care physician. Secondary outcome measures were the provision of guideline-directed medical therapy for HFrEF and quality of life, measured at 12 months.
Statistical analysis
OPT-PACE was powered to detect an absolute reduction in the primary outcome events from 15% in the usual care group to 9% in the echocardiography screening group. A primary event rate of 15% was anticipated in people randomized to usual care12,22,37,41,42 and it was assumed that a third of people in both arms would have LVEF < 50% (ref. 7). Based on contemporary data of combined medical therapy in people with impaired LV function12,22, a larger reduction in clinical events, from 15% to 7.5%, was assumed in people with LVEF < 50%, which would be diluted by people with LVEF > 50%. To detect a reduction in events from 15% to 9% (equivalent to an HR of 0.58, based on the effects of combination therapy for HFrEF12,21,31,43) using log(rank analysis) with an overall type 1 error rate of 0.05 (two-sided analysis) and a power of 0.90, a total of 146 events were required to be observed in at least 1,070 participants (nQuery Advisor v.3.0, assuming 18-month recruitment and 12-month follow-up). The target recruitment was increased to 1,200 participants in anticipation of a drop-out rate of 10%.
Time to first HFH or death was calculated from the date of randomization to the date of the first event or the date of censor set at October 2017, when all participants had had a minimum of 12 months of follow-up. Event-free survival estimates were calculated using the Kaplan–Meier method compared across randomized groups using log(rank testing) in an intention-to-treat analysis.
Exploratory descriptive analysis in those allocated echocardiography, specified a priori in the statistical analysis plan, reported outcomes in the group with access to the specialist clinic and those treated by their primary care physician.
Multivariable analysis of the primary outcome assessed the influence of patient baseline characteristics using Cox’s proportional hazards regression modeling. Variables considered for selection were those previously reported in this population7 and included age, previous history of overt coronary artery disease, atrial rhythm, log(NT-proBNP), ventricular pacing burden, sex and the presence of diabetes mellitus. Adjusted treatment effects are reported.
Primary data storage was on Microsoft Excel (v.15) and primary analyses were performed using SAS. Exploratory analyses used SPSS statistical software. The attainment of medical therapy was assessed using Pearson’s χ2 analysis. Quality-of-life data between the randomized groups were reported descriptively and analyzed using ANCOVA. The statistical analysis plan is available as a Supplementary Note.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-024-03265-3.
Supplementary information
Statistical analysis plan.
Acknowledgements
Funding was provided by the National Institute for Health Research (NIHR) in the form of a clinician scientist award for K.K.W. (grant no. NIHR-CS-2012-032). We express our appreciation for the valuable contributions of participants with a pacemaker who took part in this trial, the patient–public involvement and engagement advisory groups for their practical and insightful suggestions and the consistent support of the HF administrative team. The research was carried out at the Leeds NIHR Biomedical Research Centre (NIHR203331), specifically the Leeds Clinical Research Facility at Leeds Teaching Hospitals NHS Trust. The views expressed are ours and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The NIHR had no involvement in study design, data collection, analysis, reporting or decision to submit. OPT-PACE is registered at ClinicalTrials.gov (NCT01819662).
Extended data
Author contributions
All authors contributed to data interpretation and paper preparation and all have approved the submitted version of this paper and agreed to be personally accountable for its contents, including those parts of the study and analysis with which they were not directly involved. K.K.W., M.F.P., J.G., M.T.K. and R.M.C. researched the topic and devised the study. M.F.P. and K.K.W. provided the first draft of the paper. D.D.S. provided statistical oversight. A.F. and M.F.P. conducted the statistical analysis. R.G.G. managed data storage and aided analysis. M.F.P., J.G., H.A.J., J.E.L., S.S., R.B., T.A.S., H.C., R.M.C., M.T.K. and K.K.W. contributed equally to data collection.
Peer review
Peer review information
Nature Medicine thanks Stuart Connolly, Jeroen Hendriks, Kevin Vernooy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michael Basson, in collaboration with the Nature Medicine team.
Data availability
Individual participant data that underlie the results reported in this article will be available after de-identification (text, tables, figures and appendices) beginning 9 months and ending 36 months after article publication. Investigators requesting access will require a methodologically sound proposal approved by an independent review committee identified for this purpose to achieve the aims in their approved proposal. Data will be provided within 3 months of a request. Proposals should be directed to the corresponding author (K.K.W.).
Competing interests
M.F.P. holds an NIHR clinical post-doctoral fellowship outside of this work. J.G. holds an NIHR post-doctoral fellowship outside of this work. S.S. holds an NIHR academic clinical lectureship outside of this work. R.M.C. held an intermediate fellowship with the British Heart Foundation (BHF). D.D.S. holds an NIHR research professor award outside of this work. M.T.K. holds a BHF chair. K.K.W. held an NIHR clinician scientist award for the duration of the present study (no. NIHR-CS-012-032). The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41591-024-03265-3.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-024-03265-3.
References
- 1.Shaw, D. B., Kekwick, C. A., Veale, D., Gowers, J. & Whistance, T. Survival in second degree atrioventricular block. Heart53, 587–593 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamas, G. A. et al. Quality of life and clinical outcomes in elderly patients treated with ventricular pacing as compared with dual-chamber pacing. Pacemaker selection in the elderly investigators. N. Engl. J. Med.338, 1097–1104 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Raatikainen, M. P. et al. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace17, i1–i75 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Mond, H. G. & Proclemer, A. The 11th world survey of cardiac pacing and implantable cardioverter‐defibrillators: calendar year 2009—a World Society of Arrhythmias project. Pacing Clin. Electrophysiol.34, 1013–1027 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw, P. J., Stobie, P., Knuiman, M. W., Briffa, T. G. & Hobbs, M. S. Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Heart1, e000177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee, M. A. et al. Effects of long-term right ventricular apical pacing on left ventricular perfusion, innervation, function and histology. J. Am. Coll. Cardiol.24, 225–232 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Gierula, J. et al. Patients with long-term permanent pacemakers have a high prevalence of left ventricular dysfunction. J. Cardiovasc. Med.16, 743–750 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Shen, W. K. et al. Survival and functional independence after implantation of a permanent pacemaker in octogenarians and nonagenarians: a population-based study. Ann. Intern. Med.125, 476–480 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Brunner, M., Olschewski, M., Geibel, A., Bode, C. & Zehender, M. Long-term survival after pacemaker implantation: prognostic importance of gender and baseline patient characteristics. Eur. Heart J.25, 88–95 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Jahangir, A. et al. Relation between mode of pacing and long-term survival in the very elderly. J. Am. Coll. Cardiol.33, 1208–1216 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Glikson, M. et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: developed by the task force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) with the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J.42, 3427–3520 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Witte, K. K. et al. Mortality reduction associated with β-adrenoceptor inhibition in chronic heart failure is greater in patients with diabetes. Diabetes Care41, 136–142 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Drozd, M. et al. Association of heart failure and its comorbidities with loss of life expectancy. Heart107, 1417–1421 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thackray, S. D. et al. The prevalence of heart failure and asymptomatic left ventricular systolic dysfunction in a typical regional pacemaker population. Eur. Heart J.24, 1143–1152 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Nielsen, J. C. et al. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J. Am. Coll. Cardiol.42, 614–623 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Udo, E. O., van Hemel, N. M., Zuithoff, N. P., Doevendans, P. A. & Moons, K. G. Risk of heart failure and cardiac death gradually increases with more right ventricular pacing. Int. J. Cardiol.185, 95–100 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Saunderson, C. E. D. et al. Detrimental immediate- and medium-term clinical effects of right ventricular pacing in patients with myocardial fibrosis. Circ. Cardiovasc. Imaging14, e012256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdulla, J. et al. A systematic review: effect of angiotensin converting enzyme inhibition on left ventricular volumes and ejection fraction in patients with a myocardial infarction and in patients with left ventricular dysfunction. Eur. J. Heart Fail.9, 129–135 (2007). [DOI] [PubMed] [Google Scholar]
- 19.van de Ven, L. L. et al. The effect of treatment with bisoprolol-first versus enalapril-first on cardiac structure and function in heart failure. Int. J. Cardiol.144, 59–63 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee, S. et al. Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. Br. Med. J.346, f55 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Packer, M. et al. Comparative effects of carvedilol and metoprolol on left ventricular ejection fraction in heart failure: results of a meta-analysis. Am. Heart J.141, 899–907 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Cubbon, R. M. et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: a study across therapeutic eras. Circ. Heart Fail.4, 396–403 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Mebazaa, A. et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomized, trial. Lancet400, 1938–1952 (2022). [DOI] [PubMed] [Google Scholar]
- 24.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet353, 9–13 (1999). [PubMed]
- 25.McMurray, J. J. et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med.371, 993–1004 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Chung, M. K. et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm20, e17–e91 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epstein, A. E. et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation127, e283–e352 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Mullens, W. et al. Integration of implantable device therapy in patients with heart failure. A clinical consensus statement from the Heart Failure Association (HFA) and European Heart Rhythm Association (EHRA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail.26, 483–501 (2024). [DOI] [PubMed] [Google Scholar]
- 29.McDonagh, T. A. et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J.44, 3627–3639 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation145, e895–e1032 (2022). [DOI] [PubMed] [Google Scholar]
- 31.Straw, S. et al. Effect of disease-modifying agents and their association with mortality in multi-morbid patients with heart failure with reduced ejection fraction. ESC Heart Fail.7, 3859–3870 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ledwidge, M. et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA310, 66–74 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Thackray, S. D. et al. N-terminal brain natriuretic peptide as a screening tool for heart failure in the pacemaker population. Eur. Heart J.27, 447–453 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Lindekleiv, H. et al. Echocardiographic screening of the general population and long-term survival: a randomized clinical study. JAMA Intern. Med.173, 1592–1598 (2013). [DOI] [PubMed] [Google Scholar]
- 35.CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N. Engl. J. Med.316, 1429–1435 (1987). [DOI] [PubMed] [Google Scholar]
- 36.SOLVD Investigators; Yusuf, S., Pitt, B., Davis, C. E., Hood, W. B. & Cohn, J. N. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med.325, 293–302 (1991). [DOI] [PubMed]
- 37.Paton, M. F. et al. Optimising pacemaker therapy and medical therapy in patients for heart failure: protocol for the OPT-PACE randomized controlled trial. BMJ Open9, e028613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dual-chamber Pacemakers for Symptomatic Bradycardia due to Sick Sinus Syndrome without Atrioventricular Block (TA324) (NICE, 2014). [DOI] [PMC free article] [PubMed]
- 39.Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr.28, 1–39.e14 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Gierula, J. et al. Pacing-associated left ventricular dysfunction? Think reprogramming first! Heart100, 765–769 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Sweeney, M. O. et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation107, 2932–2937 (2003). [DOI] [PubMed] [Google Scholar]
- 42.Zhang, X. H. et al. New-onset heart failure after permanent right ventricular apical pacing in patients with acquired high-grade atrioventricular block and normal left ventricular function. J. Cardiovasc. Electrophysiol.19, 136–141 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Straw, S. et al. Guideline-directed medical therapy is similarly effective in heart failure with mildly reduced ejection fraction. Clin. Res. Cardiol.112, 111–122 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical analysis plan.
Data Availability Statement
Individual participant data that underlie the results reported in this article will be available after de-identification (text, tables, figures and appendices) beginning 9 months and ending 36 months after article publication. Investigators requesting access will require a methodologically sound proposal approved by an independent review committee identified for this purpose to achieve the aims in their approved proposal. Data will be provided within 3 months of a request. Proposals should be directed to the corresponding author (K.K.W.).