Abstract
Aim
The aim of this study was to investigate autonomic nervous system imbalance in schizophrenia by comparing heart rate variability (HRV) between patients with schizophrenia and healthy controls, and to assess changes in HRV in patients before and after electroconvulsive therapy (ECT).
Methods
HRV was compared between patients with schizophrenia (n = 17) and age‐ and gender‐matched healthy controls (n = 34). Changes in HRV were also assessed in patients pre‐ and post‐ECT. Additionally, the relationship between HRV and Positive and Negative Symptom Scale (PANSS) scores in patients with schizophrenia was investigated.
Results
Patients with schizophrenia showed significantly lower high‐frequency (HF) and low‐frequency (LF) power compared with healthy controls, with a trend towards a higher LF/HF ratio. Following ECT, HF power increased significantly while the LF/HF ratio decreased significantly, resulting in no significant differences between patients and controls for these HRV parameters. The associations between HRV and symptoms observed before ECT were largely diminished after ECT, with only changes in the LF component correlating with changes in PANSS scores.
Conclusion
Following ECT, we observed a shift in autonomic balance from sympathetic dominance towards increased parasympathetic activity and a state more closely resembling that in healthy controls.
Keywords: autonomic imbalance, autonomic nervous system dysfunction, electroconvulsive therapy, reduced parasympathetic activity, schizophrenia
This study investigated autonomic nervous system imbalance in schizophrenia by comparing heart rate variability (HRV) between patients with schizophrenia and healthy controls, and assessed HRV changes before and after electroconvulsive therapy (ECT) in patients. Initially, patients showed lower high‐frequency (HF) and low‐frequency (LF) power with a trend towards higher LF/HF ratio compared to healthy controls, while after ECT, HF power increased and LF/HF ratio decreased significantly, eliminating these differences. In conclusion, following ECT, we observed a shift in autonomic balance from sympathetic dominance towards increased parasympathetic activity, resulting in a state more closely resembling that in healthy controls.
INTRODUCTION
Schizophrenia is a persistent and critical mental disorder with frequent relapse that requires life‐long treatment. The syndrome of schizophrenia encompasses a wide range of positive and negative symptoms and/or a broad range of cognitive symptoms. Although much remains unknown about the etiology and pathogenesis of schizophrenia, it is believed to be multifactorial, involving a complicated interconnection of genetic susceptibilities, neurobiological abnormalities, and environmental risk factors. Schizophrenia research employing various approaches, such as neuroimaging and genetic studies, is ongoing and investigation of autonomic nervous system function has become an important focus. Such studies assess electrodermal measures, 1 salivary alpha‐amylase level, 2 and heart rate variability (HRV). HRV is a valuable, non‐invasive indicator for assessing autonomic nervous system function in a variety of diseases, such as myocardial infarction and diabetic neuropathy, as well as schizophrenia. Evidence accumulated in recent years indicates that individuals with schizophrenia exhibit reduced parasympathetic activity. 3 , 4 Some reports indicate that autonomic dysfunction in schizophrenia may be influenced by antipsychotic medication. 5 However, such autonomic abnormalities can also be detected in advance of the disorder's appearance and can be observed in first‐degree relatives. 6 These findings indicate that autonomic dysfunction may represent a disease‐associated abnormality in schizophrenia. Moreover, autonomic dysfunction is linked to multiple features of schizophrenia, including psychopathology, cognitive impairment, and cardiometabolic comorbidities. 3
Research on autonomic nervous system function in various psychiatric disorders, including schizophrenia and bipolar disorder, has generated a remarkable body of evidence regarding autonomic dysfunction, especially in depression, and indicates diminished parasympathetic activity in patients with depression. 7 Additionally, studies of patients with depression have reported changes in autonomic nervous system function after electroconvulsive therapy (ECT), indicating a correlation between symptom improvements following ECT and enhanced parasympathetic function. 8 , 9 ECT has been used as a rapid‐acting and safe treatment modality for major depressive disorder and schizophrenia for many decades. Approximately one‐third of patients with psychosis are classified as resistant to antipsychotic medications and the administration of ECT is restricted. 10 However, ECT combined with antipsychotics, such as clozapine, is regarded as a therapeutic option for medication‐resistant schizophrenia.
Numerous HRV analyses have consistently demonstrated reduced parasympathetic activity in patients with schizophrenia; however, no study has assessed resting HRV after a course of ECT. In this study we, therefore, evaluated autonomic nervous system function in schizophrenia using HRV. High‐frequency (HF) power, one of the frequency‐domain measures of HRV, is particularly considered to reflect parasympathetic nervous system activity. We hypothesized that diminished HF power in patients with schizophrenia would be increased following ECT with parasympathetic dominance. Furthermore, we predicted correlation between baseline clinical severity prior to ECT and HRV measures, and that symptomatic improvement may be associated with alterations in parasympathetic activity.
METHODS
Participants
The study participants consisted of 17 patients with schizophrenia who were hospitalized at Toshima Hospital and who underwent ECT between May 2012 and March 2015. All patients provided informed consent. The entire study population was Asian (Japanese). The study also included 34 age‐ and sex‐matched healthy controls (Table 1). Table 2 provides a comprehensive overview of the medications administered to participants before the initiation of ECT. This includes detailed information on antipsychotic medications, with specification of daily doses, and on other medications that potentially influence HRV. The patients were diagnosed with schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, 11 by a certified psychiatrist of the Japanese Board of Psychiatry. Two patients were diagnosed with catatonic type and 15 with paranoid type.
Table 1.
Demographic and clinical data.
| Patients (n = 17) | Controls (n = 34) | |
|---|---|---|
| Female/male | 11/6 | 19/15 |
| Age (years) | 45.3 ± 16.7 | 45.9 ± 14.1 |
| Duration of illness (years) | 18.4 ± 12.5 | n/a |
| Antipsychotic drugs (CPZ‐eq mg) | 748 ± 625.4 | n/a |
Note: Data are shown as the mean ± standard deviation. The daily dose of antipsychotic drugs was quantified in chlorpromazine equivalents (CPZ‐eq).
Table 2.
Medication data.
| Patient no. | Antipsychotics | Others |
|---|---|---|
| 1 | PAL 3 mg, RIS 6 mg, BLS 16 mg | Free |
| 2 | RIS 3 mg, LP 10 mg, APZ 30 mg | FNZP 2 mg, BPR 1 mg |
| 3 | OLZ 20 mg, RIS 8 mg, QTP 300 mg | BPR 3 mg, FNZP 2 mg, LZP 1.5 mg |
| 4 | CP 25 mg, LP 25 mg, RIS 6 mg | Free |
| 5 | Free | Diazepam 4 mg, distigmine 5 mg |
| 6 | LP 150 mg | FNZP 1 mg, BPR 6 mg |
| 7 | HPD10 mg | Free |
| 8 | RIS 2 mg | Diazepam 10 mg |
| 9 | OLZ 10 mg | Free |
| 10 | Free | VPA 1200 mg, FNZP 2 mg, LZP 1.5 mg, Pemoline, promethazine 50 mg |
| 11 | QTP 200 mg | Mirtazapine 45 mg |
| 12 | QTP 50 mg | Nitrazepam10 mg, zopiclone 7.5 mg |
| 13 | APZ 30 mg, RIS 3 mg | FNZP 2 mg |
| 14 | OLZ 20 mg, APZ 30 mg | Mirtazapine 15 mg |
| 15 | QTP 250 mg, LP 50 mg, CP 150 mg, BLS 8 mg | FNZP 2 mg, BPR 2 mg, LZP 0.5 mg, Brotizolam 0.25 mg |
| 16 | OLZ 15 mg, APZ 30 mg | FNZP 1 mg |
| 17 | OLZ 20 mg, HPD 9 mg | BPR 4 mg, trihexyphenidyl 3 mg |
Note: This table presents the antipsychotic medications (with daily doses) and other medications taken by all patients before the initiation of ECT.
Abbreviations: APZ, aripiprazole; BLS, blonanserin; BPR, biperiden; CP, chlorpromazine; FNZP, flunitrazepam; HPD, haloperidol; LP, levomepromazine; LZP, lorazepam; OLZ, olanzapine; PAL, paliperidone; QTP, quetiapine; RIS, risperidone; VPA, valproic acid.
We excluded patients with a history of major medical conditions, including cardiovascular, respiratory, neurological, or endocrine disorders, epilepsy, head trauma, and substance abuse. We also excluded healthy controls who were current smokers or had heart disease, hypertension, diabetes, or a history of psychiatric disorder.
Electroconvulsive therapy
We performed ECT one to three times per week using either a pulse wave device or a sine wave device. For a pulse wave device, a Thymatron System IV ECT apparatus (Somatics LLC) that included inbuilt two‐channel electroencephalography (Fp1‐M1, Fp2‐M2, international 10–20 system) was used at bifrontotemporal locations. The pulse wave device delivered brief pulses with a width of 0.5 ms. Pulse wave frequency was 70 Hz. In cases where adequate seizures were not achieved with 100% output on the pulse wave device, we switched to a sine wave device (CS‐1; Sakai Iryo Co). This device was used at a voltage of 110 to 115 and a duration of 5 s per session, with careful monitoring for cognitive side‐effects and other potential adverse events. Of the 17 patients, six required the use of the sine wave device. The number of ECT sessions administered was 9.3 ± 2.9 (mean ± SD) with a total of 159 ECT sessions across all patients. The sine wave apparatus was used in 40 sessions, while the pulsed wave apparatus was employed for the remaining 119 sessions. Treatment continued until sustained remission was achieved or until improvement plateaued. Patients received intravenous thiopental (2–4 mg/kg) as an anesthetic and rocuronium (0.6–1.3 mg/kg) or suxamethonium (0.7–1.2 mg/kg) as a muscle relaxant prior to ECT. Other medications that could potentially affect HRV were used, including esmolol hydrochloride (a β‐blocker) in three sessions, atropine sulfate hydrate (an anticholinergic) in one session, and butylscopolamine bromide (an anticholinergic) in 15 sessions.
ELECTROCARDIOGRAPHY
We measured electrocardiography (ECG) signals using a Marquette Holter recorder (LRR‐03; GMS) for 5 min after 10 min of rest in the supine position. These measurements were taken within the 7‐day periods before the first and after the final ECT sessions. In practice, pre‐ECT measurements were obtained with a median of 0 days (0–7 days, where 0 indicates the same day) prior to the initiation of ECT treatment. For post‐ECT recordings, all measurements were conducted at least 24 h after the final ECT session to minimize the acute effects of both the electrical stimulation and anesthesia, with a median of 4 days (1–7 days) post‐ECT completion. The ECG signals were recorded using bipolar leads with two separate adhesive monitoring electrodes: the positive input electrode was placed at V5 corresponding to the V5 position in a 12‐Lead ECG, while the reference electrode was placed under the right clavicle, corresponding to the CS5 lead. ECG waveforms were digitized at a sampling rate of 1 kHz to calculate R–R intervals.
Heart rate variability
After collecting resting‐state data for 5 min, we performed power spectrum analysis using MemCalc BonalyLight real‐time analysis software (GMS). This software employs the maximum entropy method for spectral analysis. We calculated HRV parameters using a 30‐s window, with calculations updated every 2 s and then obtained the average of these parameters for 5 min. The following components from the quantification of different frequency bands of HRV were used as indicators of cardiac autonomic nervous activity: HF range (0.15–0.4 Hz), which reflects respiratory variability caused by cardiac parasympathetic activity; low‐frequency (LF) range (0.04–0.15 Hz), which includes both the sympathetic and parasympathetic nervous systems; and LF/HF, which indicates the cardiac sympathetic nervous system. 3 , 12
Clinical evaluation
A certified psychiatrist of the Japanese Board of Psychiatry assessed the clinical symptoms of the schizophrenia patients using a Japanese translation of the Positive and Negative Syndrome Scale (PANSS) 13 within the 7‐day periods before the first and after the final ECT sessions.
Statistical analysis
All statistical analyses were performed using SPSS for Windows Version 29.0 (SPSS). The LF, HF, and LF/HF values were log‐transformed to achieve a normal distribution and the Shapiro–Wilk test was used to assess the normality of all variables. Demographic comparisons between groups were performed using an independent t‐test for age and a chi‐square test for sex. A chi‐square test was performed to assess the distribution of ECT device types (sine wave and pulse wave) used in the treatment sessions. To compare HRV before and after ECT between healthy controls and patients with schizophrenia, a one‐way analysis of variance (ANOVA) was conducted. To examine the effect of ECT on three HRV indices (LF, HF, and LF/HF) in patients before and after ECT, we performed a repeated‐measures analysis of covariance (ANCOVA) on three indices with time (pre‐ECT, post‐ECT) and frequency (LF, HF, LF/HF) as between‐subject factors. Post hoc comparisons were conducted using Bonferroni correction. Partial correlation analyses with Pearson's correlation were conducted to examine the relationships between HRV before and after ECT and PANSS scores, and the changes in both HRV and PANSS scores during the course of ECT. T‐tests were employed to compare heart rate (HR). Positive symptom PANSS scores were non‐normally distributed; therefore, we employed the Wilcoxon signed‐rank test. Repeated‐measures ANCOVA was conducted with age as a covariate and the pre‐ECT daily dose of antipsychotic drugs, converted to chlorpromazine equivalents (CPZ‐eq), 14 as a between‐subjects factor. However, no significant between‐subjects effect was observed for CPZ‐eq [F(1, 14) = 0.005, p = 0.994]; therefore, we excluded this factor from our final model. Age was used as a covariate in ANCOVA and partial correlation analyses with Pearson's correlation. The level of statistical significance was set at p = 0.05.
RESULTS
Demographic and clinical data and treatment characteristics
Demographic and clinical data for all subjects are listed in Table 1.
There was no significant difference between patients and healthy controls for age and sex [age; t(49) = −0.139, p = 0.890] [sex; χ2(1, N = 51) = 0.364, p = 0.546].
The mean and standard deviation for CPZ‐eq at baseline was 748.8 ± 625.4 mg. The CPZ‐eq after ECT was 806.2 ± 568.5 mg, which was not significantly different from the CPZ‐eq at baseline [t(16) = −0.896, p = 0.384]. ECT sessions were conducted using both sine wave (n = 40) and pulse wave (n = 119) apparatuses, with no significant difference in their distribution [apparatus; χ2(1, N = 2) = 2.000, p = 0.157].
Comparison of autonomic parameters between schizophrenic patients pre‐ and post‐ECT and healthy controls
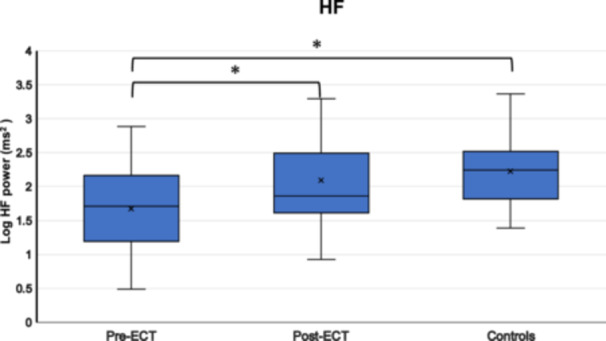
Table 3 presents the autonomic parameters and PANSS scores, while Figure 1 illustrates HRV comparisons between patients (pre‐ and post‐ECT) and healthy controls.
Table 3.
Autonomic parameters and PANSS scores.
| Patients | Patients | Controls | |
|---|---|---|---|
| HRV parameters | Before ECT n = 17 | After ECT n = 17 | n = 34 |
| LF (ms2) | 1.96 ± 0.67 | 2.14 ± 0.57 | 2.32 ± 0.42 |
| HF (ms2) | 1.60 ± 0.70 | 2.09 ± 0.64 | 2.22 ± 0.50 |
| LF/HF (ms2) | 0.36 ± 0.38 | 0.05 ± 0.50 | 0.1 ± 0.40 |
| HR (bpm) | 82.83 ± 16.75 | 70.30 ± 9.98 | 68.43 ± 9.40 |
| PANSS scores | |||
| Total | 111.6 ± 25.4 | 70.8 ± 21.8 | n/a |
| Positive Symptoms | 30.7 ± 8.7 | 14.1 ± 6.9 | n/a |
| Negative Symptoms | 26.5 ± 8.8 | 21.3 ± 6.7 | n/a |
| General Psychopathology | 54.4 ± 15.0 | 35.3 ± 12.3 | n/a |
Note: Data are shown as the mean ± standard deviation.
Abbreviations: ECT, electroconvulsive therapy; HF, high‐frequency, HR, heart rate; HRV, heart rate variability, LF, low‐frequency; PANSS, Positive and Negative Symptom Scale.
Figure 1.
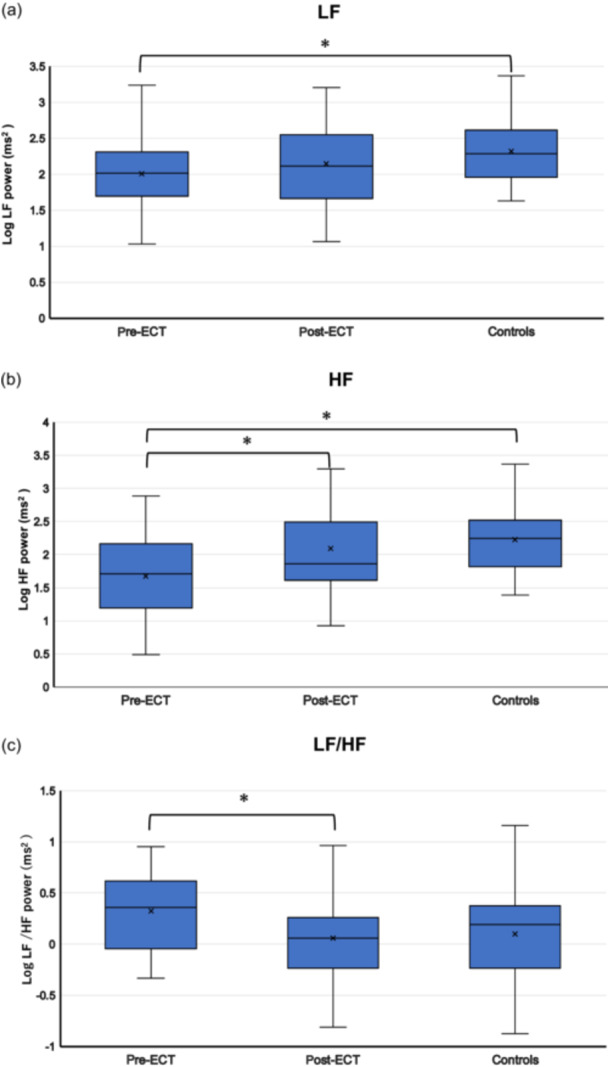
Comparison of heart rate variability (HRV) in patients with schizophrenia pre‐ and post‐electroconvulsive therapy (ECT) and comparison of HRV between patients (pre‐ and post‐ECT) and controls. (a) Low‐frequency (LF), (b) high‐frequency (HF), and (c) LF/HF ratio data are presented as box plots. *P < 0.05.
Pre‐ECT
One‐way ANOVA indicated significant differences in two of the three measured parameters. Both LF and HF values were significantly lower in the schizophrenia group compared with the healthy control group [LF: F(1, 49) = 4.593, p = 0.037, HF: F(1, 49) = 10.672, p = 0.002]. The LF/HF ratio showed a marginally significant trend towards being higher in the schizophrenia group compared with the healthy control group [F(1, 49) = 3.791, p = 0.057]. An independent samples t‐test for HR revealed significantly higher HR values in patients before ECT compared with those in healthy controls [t(49) = 3.943, p < 0.001].
Post‐ECT
One‐way ANOVA revealed no statistically significant differences in any of the measured parameters. LF values did not differ significantly between the post‐ECT group and the healthy control group [F(1, 49) = 1.514, p = 0.224]. Similarly, HF values showed no significant difference between the groups [F(1, 49) = 0.697, p = 0.408]. The LF/HF ratio also demonstrated no statistically significant difference between the post‐ECT patients and healthy controls [F(1, 49) = 0.093, p = 0.762]. An independent samples t‐test for HR between patients and controls was no longer statistically significant [t(49) = 0.654, p = 0.516].
Comparison of autonomic parameters between pre‐ECT and post‐ECT patients
Within‐subject changes in autonomic parameters
A repeated‐measures ANCOVA was conducted to examine the effect of ECT on three HRV indices in patients.
Main effects. There was no significant effect of time (pre‐ECT; post‐ECT) [F(1, 15) = 0.051, p = 0.825]. However, there was a significant main effect of frequency (LF; HF; LF/HF) [F(1.10, 16.63) = 17.632, p < 0.001].
Interaction effect. There was a significant interaction effect between time and frequency with HF power increasing and LF/HF ratio decreasing [F(1.44, 21.74) = 5.873, p = 0.015].
Pairwise comparisons with Bonferroni correction
LF did not change significantly between before ECT [mean (M) = 2.003, standard error (SE) = 0.138] and after ECT [(M) = 2.148, (SE) = 0.120] (mean difference = −0.145, SE = 0.163, p = 0.389) while HF increased significantly between before ECT [(M) = 1677, (SE) = 0.167] and after ECT [(M) = 2.089 (SE) = 0.136] (mean difference = −0.412, SE = 0.137, p = 0.009) and LF/HF decreased significantly between before ECT [(M) = 0.326, (SE) = 0.090] and after ECT [(M) = 0.059, (SE) = 0.113] (mean difference = 0.268, SE = 0.106, p = 0.023).
A paired‐samples t‐test showed a significant decrease in HR between pre‐ECT and post‐ECT patients [t(16) = 3.525, p = 0.003].
Between‐subjects effects: Age as a covariate
The analysis of between‐subjects effects revealed a significant effect of age as a covariate [F(1, 15) = 7.177, p = 0.017].
Correlations between HRV measures and PANSS scores
Comparison of PANSS scores before and after ECT demonstrated significant reductions in the total PANSS score and of each subscale, with the Negative Symptom subscale showing a significant decrease (p = 0.008) and all other subscales and the total score demonstrating even more pronounced reductions (p < 0.001).
Pre‐ECT
A significant negative correlation was observed between HF and PANSS scores (r = −0.686, p = 0.003), indicating that lower HF values were associated with overall symptom severity. Similarly, HF values had a significant negative correlation with the General Psychopathology subscale of PANSS (r = −0.623, p = 0.01), indicating that lower HF values were related to higher General Psychopathology scores. A significant positive correlation was found between the LF/HF ratio and positive scores (r = 0.709, p = 0.002), indicating that higher LF/HF ratios were associated with more severe positive symptoms. LF values also demonstrated significant negative correlations with both total PANSS scores (r = −0.512, p = 0.042) and the General Psychopathology subscale (r = −0.580, p = 0.018), indicating that higher LF values were associated with lower overall symptom severity and lower General Psychopathology scores.
Post‐ECT
The previously observed correlations for HF values and the LF/HF ratio were no longer significant post‐ECT treatment. LF values maintained significant negative correlations with PANSS total, Positive Symptoms, and General Psychopathology scores post‐ECT (PANSS total; r = −0.514, p = 0.042, Positive Symptom subscale; r = −0.660, p = 0.005, General Psychopathology subscale = −0.549, p = 0.027, respectively).
PANSS score reduction and changes in HRV indices
There was a significant positive correlation between changes in LF values and changes in PANSS total and General Psychopathology subscale scores (PANSS total; r = 0.500, p = 0.049, General Psychopathology subscale; r = 0.552, p = 0.027). This indicates that increases in LF values were associated with improvement in PANSS total scores and General Psychopathology scores.
DISCUSSION
This study examined changes in autonomic nervous system function in schizophrenia after ECT and also compared schizophrenia patients with age‐ and sex‐matched healthy controls. As we hypothesized, the results demonstrated that parasympathetic activity in patients was lower than in healthy controls. Following ECT treatment, patients showed a shift towards parasympathetic dominance, with their sympathetic–parasympathetic balance approaching that of healthy controls. In contrast to our prediction, we found no association between changes in symptoms and parasympathetic function.
Decreased parasympathetic activity in schizophrenia has been described. 15 Consistent with previous studies, our study also demonstrated a decrease in HF power, indicating reduced parasympathetic activity. Numerous studies provide a consistent view regarding parasympathetic activity. However, findings regarding the LF/HF ratio, an indicator of sympathetic activity, have been mixed; some studies report an increase, 16 while others found no change. 4 In our study, the LF/HF ratio in patients with schizophrenia tended to be higher than in healthy controls, although not with statistical significance. Overall, our findings regarding HF power and the LF/HF ratio did not deviate substantially from previously published reports.
In this study, we observed changes in autonomic nervous system activity related to the administration of ECT. There is a consensus that antipsychotic drugs affect HRV to varying degrees, with reports indicating that CPZ‐eq doses ≥500 mg strongly influence autonomic function. 5 We examined the impact of pre‐ECT CPZ‐eq on HRV but found no significant effect. Age is also considered to be a factor that affects HRV 12 and our study showed a significant influence, which we controlled for in our analysis. Parasympathetic and sympathetic activities showed remarkable changes with ECT. Parasympathetic activity increased while sympathetic activity decreased, resulting in parasympathetic dominance and a sympathetic–parasympathetic balance closer to that of healthy individuals. Additionally, the baseline HR in patients was significantly higher than that in healthy controls but decreased after ECT to a level comparable to that in healthy controls. HR is under dual regulation by efferent vagal nerve (parasympathetic) input to the sinoatrial and atrioventricular nodes, which originate from the medulla, and the sympathetic nervous system, which originates from the thoracic spinal cord. These systems interact reciprocally in response to acute or chronic physiological changes. This further corroborates our finding of parasympathetic dominance after ECT.
With respect to the relationship between autonomic nervous system function and psychiatric symptoms in schizophrenia, several studies have reported a negative correlation between parasympathetic function and symptom severity. 17 , 18 , 19 Our investigation also produced negative correlations between HF values and PANSS scores and general psychopathology before ECT. Additionally, we found a positive correlation between LF/HF and the Positive Symptom scores of PANSS. However, the existing literature shows mixed results. Some studies 20 , 21 have reported a positive correlation between certain PANSS scores and LF/HF, while Kim et al. found no significant association between various HRV measures, including LF/HF and PANSS scores. 22 This inconsistency may be caused by variations in methodology across studies, such as differences in ECG recording duration, patient populations, and the specific HRV indices analyzed. Our findings indicate that higher sympathetic activity may be associated with more severe positive symptoms. However, it is important to note that our study has limitations, including a small sample size and the inability to control for various factors, such as specific schizophrenia subtypes. Further research with larger sample sizes, more comprehensive control of confounding factors, and standardized methodologies is necessary to validate our findings.
However, these correlations disappeared after ECT. Changes in LF values before and after ECT correlated with changes in symptoms, but the physiological meaning of LF power is ambiguous, and it remains unclear whether sympathetic or parasympathetic activity contributes more to this measure. Additionally, while a negative correlation between clinical symptom improvement and parasympathetic activity during ECT has been reported, we did not find a correlation with changes in parasympathetic activity. This indicates that HRV is not a simple, direct indicator of psychiatric symptoms, 9 but is likely to be influenced by other complex and multifaceted factors. Nevertheless, it is noteworthy that one of the indicators of autonomic function was associated with symptom changes after ECT.
We observed changes in autonomic nervous system function following ECT and it is notable that the autonomic nervous system is controlled by a large network that includes the diencephalon, hypothalamus, brainstem, cortical areas, such as the prefrontal cortex, and limbic structures, like the hippocampus and amygdala. 23 The prefrontal cortex exerts inhibitory control over the amygdala, which in turn inhibits the lower hypothalamus. 24 This has led to the hypothesis that autonomic dysfunction in schizophrenia may be related to decreased activity in the prefrontal cortex, resulting in amygdala activation and subsequent suppression of parasympathetic function. Additionally, changes in the volume of brain regions associated with the autonomic nervous system, such as the hippocampus and amygdala, have been reported following ECT. 25 , 26
While hypothetical, it is possible that the hypothalamic–pituitary–adrenal (HPA) axis is related to post‐ECT changes in the autonomic nervous system. Both systems respond to stress with a degree of coordination and share anatomical connections through the hypothalamus. 27 , 28 HPA axis dysfunction has been reported in schizophrenia, and ECT influences the HPA axis. 29
Our present findings and these earlier studies indicate that ECT may directly or indirectly affect the brain regions belonging to or related to the autonomic nervous system network, leading to altered autonomic nervous system function in schizophrenia.
However, previous reports show no consistent imaging findings associated with ECT, and a coherent understanding of the relationship between HRV and clinical symptomatology remains elusive.
Our study has some limitations. First, our sample size was small and limited, which may affect the generalizability of our findings. While we did not observe significant differences in CPZ‐eq of antipsychotic medications before and after ECT, we cannot rule out the potential impact of medication dose changes on our results. Furthermore, we were unable to account for the impact of various medications, other than antipsychotics, that might influence HRV. Our analysis included age as a covariate; however, we did not investigate other potentially influential factors, such as body mass index, and cannot therefore exclude their possible effects on our results.
CONCLUSION
Our findings demonstrate that the autonomic balance in patients with schizophrenia treated with ECT shifted from sympathetic dominance to a state more closely resembling that of healthy controls, with increased parasympathetic activity.
AUTHOR CONTRIBUTIONS
Noriko Yoshida, Miho Miyajima, and Yoko Suzuki conceived and designed the study. Noriko Yoshida, Miho Miyajima, Yoko Suzuki, Takafumi Watanabe, Mayo Fujiwara, Rie Omoya, and Miho Miyajima managed the acquisition and collection of data. Noriko Yoshida performed statistical analyses and wrote the first draft of the manuscript. Yoko Suzuki was involved in the statistical analyses. Miho Miyajima supervised the research throughout the entire process as the corresponding author. Eisuke Matsushima, Hidehiko Takahashi, and Takashi Takeuchi critically revised the manuscript and supervised the study.
CONFLICT OF INTEREST STATEMENT
Takashi Takeuchi is an Editorial Board member of Psychiatry and Clinical Neurosciences Reports and a co‐author of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The study protocol was approved by the Ethics Committee of Tokyo Medical and Dental University Graduate School (No. 1104) and the Ethics Committee of Tokyo Metropolitan Health and Medical Corporation Toshima Hospital (No. 28‐10). All procedures were conducted in accordance with the Declaration of Helsinki.
ETHICAL APPROVAL STATEMENT
Our study adhered to the principles of the Declaration of Helsinki and was implemented after being reviewed and approved by the Ethics Committee of Tokyo Medical and Dental University Graduate School (No. 1104) and the Ethics Committee of Tokyo Metropolitan Health and Medical Corporation Toshima Hospital (No. 28‐10).
PATIENT CONSENT STATEMENT
All participants provided written informed consent following a comprehensive explanation of the study procedures.
CLINICAL TRIAL REGISTRATION
N/A.
ACKNOWLEDGMENTS
This paper is dedicated to the memory of Dr. Katsuya Ohta, who served as the former Director of Onda‐daini Hospital and as Lecturer at the School of Medicine, Faculty of Medicine, Tokyo Medical and Dental University. Dr. Ohta's invaluable research and guidance in this field have significantly influenced our work. In addition, we would like to express our sincere gratitude to the patients, their families, and the staff of Toshima Hospital for their cooperation and support throughout this research. The authors have no funding to report.
Yoshida N, Miyajima M, Suzuki Y, Matsushima E, Watanabe T, Omoya R, et al Heart rate variability in schizophrenia: a comparative analysis before and after electroconvulsive therapy. Psychiatry Clin Neurosci Rep. 2024;3:e70030. 10.1002/pcn5.70030
DATA AVAILABILITY STATEMENT
The data are not publicly available.
REFERENCES
- 1. Rachow T, Berger S, Boettger MK, Schulz S, Guinjoan S, Yeragani VK, et al. Nonlinear relationship between electrodermal activity and heart rate variability in patients with acute schizophrenia. Psychophysiology. 2011. Oct; 48(10):1323–1332. [DOI] [PubMed] [Google Scholar]
- 2. Ieda M, Miyaoka T, Wake R, Liaury K, Tsuchie K, Fukushima M, et al. Evaluation of autonomic nervous system by salivary alpha‐amylase level and heart rate variability in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2014. Feb 1; 264(1):83–87. [DOI] [PubMed] [Google Scholar]
- 3. Stogios N, Gdanski A, Gerretsen P, Chintoh AF, Graff‐Guerrero A, Rajji TK, et al. Autonomic nervous system dysfunction in schizophrenia: impact on cognitive and metabolic health. NPJ Schizophr. 2021; 7(1):22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bar K, Letzsch A, Jochum T, Wagner G, Greiner W, Sauer H. Loss of efferent vagal activity in acute schizophrenia. J Psychiatr Res. 2005. Sep; 39(5):519–527. [DOI] [PubMed] [Google Scholar]
- 5. Iwamoto Y, Kawanishi C, Kishida I, Furuno T, Fujibayashi M, Ishii C, et al. Dose‐dependent effect of antipsychotic drugs on autonomic nervous system activity in schizophrenia. BMC Psychiatry. 2012. Nov; 12:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bär KJ Cardiac autonomic dysfunction in patients with schizophrenia and their healthy relatives—a small review. Fron Neurol; 2015;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta‐analysis. Biol Psychiatry. 2010. Jun 1; 67(11):1067–1074. [DOI] [PubMed] [Google Scholar]
- 8. Rahimian A, Rahmani B, Garshad J, Salarvand A, Mansourian M. Investigating the relationship between electroconvulsive therapy and heart rate variability: a systematic review. Adv Biomed Res. 2023;12:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ebert A, Jochum T, Ritter J, Boettger MK, Schulz S, Voss A, et al. Does parasympathetic modulation prior to ECT treatment influence therapeutic outcome? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1174–1180. [DOI] [PubMed] [Google Scholar]
- 10. Howes OD, McCutcheon R, Agid O, De Bartolomeis A, Van Beveren NJM, Birnbaum ML, et al. Treatment‐Resistant Schizophrenia: TreatmentResponse and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017. Mar 1; 174(3):216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. APA; 1994. [Google Scholar]
- 12. Valentini M, Parati G. Variables influencing heart rate. Prog Cardiovasc Dis. 2009. Jul; 52(1):11–19. [DOI] [PubMed] [Google Scholar]
- 13. Kay SR, Flszbeln A, Qpjer LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia [Internet]. 1967;13:261–276. Available from https://academic.oup.com/schizophreniabulletin/article/13/2/261/1919795 [DOI] [PubMed] [Google Scholar]
- 14. Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015. Aug 1; 69(8):440–447. [DOI] [PubMed] [Google Scholar]
- 15. Montaquila JM, Trachik BJ, Bedwell JS Heart rate variability and vagal tone in schizophrenia: a review. J Psychiatr Res. 2015;69:57–66. [DOI] [PubMed] [Google Scholar]
- 16. Chang JS, Yoo CS, Yi SH, Hong KH, Oh HS, Hwang JY, et al. Differential pattern of heart rate variability in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009. Aug 31; 33(6):991–995. [DOI] [PubMed] [Google Scholar]
- 17. Toichi M, Kubota Y, Murai T, Kamio Y, Sakihama M, Toriuchi T, et al. The influence of psychotic states on the autonomic nervous system in schizophrenia. Int J Psychophysiol. 1999;31(2):147–152. [DOI] [PubMed] [Google Scholar]
- 18. Huang WC, Liu WS, Chen T, Chen WH, Huang WL. Parasympathetic activity as a potential biomarker of negative symptoms in patients with schizophrenia. Asia Pac Psychiatry. 2020. Sep 1; 12(3):e12392. [DOI] [PubMed] [Google Scholar]
- 19. Fujibayashi M, Matsumoto T, Kishida I, Kimura T, Ishii C, Ishii N, et al. Autonomic nervous system activity and psychiatric severity in schizophrenia. Psychiatry Clin Neurosci. 2009. Aug; 63(4):538–545. [DOI] [PubMed] [Google Scholar]
- 20. Sandsten KE, Jensen MT, Saebye D, Null K, Northoff G, Parnas J. Altered cardiac autonomic functioning associates with self‐disorders in schizophrenia. Schizophr Res. 2024. Aug 1; 270:57–62. [DOI] [PubMed] [Google Scholar]
- 21. Chung MS, Yang AC, Lin YC, Lin CN, Chang FR, Shen S, et al. Association of altered cardiac autonomic function with psychopathology and metabolic profiles in schizophrenia. Psychiatry Res. 2013. Dec 30; 210(3):710–715. [DOI] [PubMed] [Google Scholar]
- 22. Kim JH, Yi SH, Yoo CS, Yang SA, Yoon SC, Lee KY, et al. Heart rate dynamics and their relationship to psychotic symptom severity in clozapine‐treated schizophrenic subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):371–378. [DOI] [PubMed] [Google Scholar]
- 23. Schulz S, Bolz M, Bär KJ, Voss A. Central‐and autonomic nervous system coupling in schizophrenia. Philos Trans A Math Phys Eng Sci. 2016. May 13; 374(2067):20150178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thayer JF, Sollers JJ, Labiner DM, Weinand M, Herring AM, Lane RD, et al. Age‐related differences in prefrontal control of heart rate in humans: a pharmacological blockade study. Int J Psychophysiol. 2009. Apr; 72(1):81–88. [DOI] [PubMed] [Google Scholar]
- 25. Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry. 2016. Feb 15; 79(4):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomann PA, Wolf RC, Nolte HM, Hirjak D, Hofer S, Seidl U, et al. Neuromodulation in response to electroconvulsive therapy in schizophrenia and major depression. Brain Stimulation. 2017. May 1; 10(3):637–644. [DOI] [PubMed] [Google Scholar]
- 27. Mueller B, Figueroa A, Robinson‐Papp J Structural and functional connections between the autonomic nervous system, hypothalamic–pituitary–adrenal axis, and the immune system: a context and time dependent stress response network. Neurol Sci. 2022;43: 951–960. [DOI] [PubMed] [Google Scholar]
- 28. Rotenberg S, McGrath JJ. Inter‐relation between autonomic and HPA axis activity in children and adolescents. Biol Psychol. 2016. May 1; 117:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bolwig TG. How does electroconvulsive therapy work? theories on its mechanism. Can J Psychiatry. 2011;Vol. 56:13–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available.


