Abstract
Asymptomatic carriers of Plasmodium falciparum represent important parasite reservoirs maintaining malaria transmission in the community. This study aimed on the one hand to screen the other household members living with children under seasonal malaria chemoprevention (SMC) coverage in order to determine the level of malaria infection in this population and on the other hand to determine the appropriate type of rapid diagnostic test (RDT) for this screening to detect these asymptomatic carriers in the community. During the 2022 SMC campaign (July to October), a cross-sectional survey was carried out in 745 participants who were screened by ultrasensitive rapid diagnostic test (usRDT), standard rapid diagnostic test (rRDT) and microscopy. Out of them, 395 had microscopy results available and were included in the data analysis. The prevalence of asymptomatic carriers of asexual forms of Plasmodium falciparum was 26.58% (105/395) while sexual forms were found in 5.32% (21/395) of the study population. Children from 5 to 15 years had the highest prevalence of P. falciparum asexual forms 35.76% (59/165) compared with older participants. Malaria positivity rate for rRDT and usRDT was 29.40% (219/745) and 40.49% (305/745) respectively. The usRDT had a higher sensitivity than the rRDT (72.38% (95% CI 62.8–80.66) vs. 60.95% (95% CI 50.94–70.33)). In terms of specificity, rRDT had a higher specificity 82.41% (95% CI 77.53–86.62) versus 69.66% (95% CI 64.01–74.89) for usRDT. This study reports a high prevalence of parasite carriers in household members of children under SMC coverage in Nanoro, Burkina Faso. In conclusion, usRDT seems more appropriate for strategies based on detection and treatment of parasite carriers within the community.
Keywords: Malaria, Ultrasensitive RDT, Standard RDT, Asymptomatic carriers, Nanoro
Background
In Burkina Faso as in most countries in sub-Saharan Africa, malaria is rampant despite the various preventive and curative interventions implemented in recent years. In 2021, in Burkina Faso, an estimated 12,231,036 malaria cases resulting in 4355 deaths were reported (Ministry of Health BF 2021). Children under 5 years represented the most vulnerable population in the country. To reduce malaria-related mortality and morbidity in children, the World Health Organization (WHO) recommended in 2012 the seasonal malaria chemoprevention (SMC) as an innovative preventive strategy in areas with seasonal malaria transmission (World Health Organization 2012). SMC consists of monthly and intermittent administration of treatment with amodiaquine and sulfadoxine-pyrimethamine (AQSP) to children (aged 3–59 months) during the period of high malaria transmission. SMC has shown high level of protection against clinical malaria (Wilson and on behalf of the IPTc Taskforce 2011; Meremikwu et al. 2012; Cairns et al. 2021). Since 2018, SMC was implemented at national level in Burkina Faso. However, a high number of malaria cases continue to occur in children aged less than 5 years suggesting that the expected impact from SMC intervention is not achieved yet (Ministry of Health BF 2021). This may be attributable to various reasons including operational challenges resulting in suboptimal intervention coverage (Cairns et al. 2020), lack of observance of the second and/or third doses of AQ by parents or caregivers (Somé et al. 2022), inadequate for certain groups such as malnourished children who are at risk of subprotective drug concentrations (Oshikoya et al. 2010; De Kock et al. 2018), and increased selection of parasites highly resistant to SP or AQ (Roh et al. 2023). In addition, the parasite reservoirs present around children under SMC coverage may continually maintain the infection cycle. Indeed, the transmission of Plasmodium to healthy individuals requires the presence of a parasite reservoir (symptomatic and asymptomatic carriers). In sub-Saharan Africa, it was reported that the majority of malaria infections are asymptomatic due to transient immunity acquired during different exposures (Lindblade et al. 2013). In this context, targeting the parasite reservoirs, especially asymptomatic carriers surrounding children under SMC coverage, would be necessary for optimizing the impact of SMC intervention. However, the precise identification of these reservoirs in the community for adequate management remains a major challenge given the low sensitivity of standard RDT (rRDT) for the detection of asymptomatic infections, which consists mostly of low parasite density infections. The limit of detection (LOD) of rRDT is between 100 and 200 parasites/microliter of blood in field studies (Bousema et al. 2014; Adams et al. 2015; Laban et al. 2015). Interestingly, ultrasensitive RDTs (usRDT) detecting the hrp2 antigen were developed recently with the aim to improving the rapid detection of asymptomatic carriers of Plasmodium falciparum (Cunningham et al. 2019). usRDT are known to be able to detect hrp2 antigen at concentrations as low as 10–40 pg/ml hrp2 as opposed to 800–1000 pg/ml hrp2 for rRDT (Kanwugu et al. 2019). Therefore, usRDT offer the possibility of detecting subpatent infections that will be missed by rRDT (Girma et al. 2019; Owalla et al. 2020). Nevertheless, there is limited data available on the effectiveness of usRDT in accurately identifying parasite reservoirs at the community level in endemic settings such as Burkina Faso. Therefore, this study aimed on the one hand to screen the other household members living with children under SMC coverage in order to determine the level of malaria infection in this population and on the other hand to determine the appropriate type of RDT for this screening to detect these asymptomatic carriers in the community.
Materials and methods
Study site
The present study was carried out in ten (10) villages of the rural commune of Soaw (Soaw, Kalwaka, Zoetgomde, Poesse, Rakalo, Kokolo, Kolokom, Mogdin, Bokin, Seguedin-Soaw) within the health district of Nanoro, Burkina Faso. The rural commune of Soaw is located in the center west of Burkina Faso, approximately 75 km from the capital Ouagadougou. According to data from the Nanoro health and demographic surveillance system (HDSS), the total population of the study area was estimated to be about 20,000 in 2018 (Derra et al. 2012). The climate there is of the Sudano-Sahelian type, with a rainy season from July to October followed by a dry season from November to June. Malaria is hyper-endemic with strong transmission during the rainy season, P. falciparum representing the dominant species (90%), followed by Plasmodium malariae (3–8%) and Plasmodium ovale (0.5–2%) (Sondo et al. 2015).
Study design
This is an ancillary study nested to a large SMC-RST project (Sondo et al. 2022). In the SMC-RST study, 526 isolate households with a least one child under SMC coverage were included and assigned to one of the two study arms (control arm receiving SMC alone, n = 263) or intervention arm (SMC + screening of household members and treatment if positive, n = 263). The unit of randomization was the household, and the eligibility of a household was defined by the presence of at least one child under SMC coverage. All roommates of children included in the intervention arm of the SMC-RST study were screened and treated. The inclusion and exclusion criteria for the SMC-RST study have been described elsewhere (Sondo et al. 2022).
This ancillary study was a cross-sectional study conducted from July to October 2022, i.e., during the 2022 SMC campaign, and focused on only households included in the intervention arm of the main SMC-RST study (n = 263). The population of the present study was other household members referred to as roommates living with children under SMC coverage, in the intervention arm of the SMC-RST study. Other household members (roommates) were defined as any individual (not in SMC target population) sharing a household with at least one child under SMC coverage (aged 3–59 months). At each monthly visit, while all the roommates were screened with standard RDT and treated if positive as per the main SMC-RST intervention process, only one (01) roommate among them (other than those included in the previous months) was selected and benefited with additional testing with ultrasensitive RDT as per this ancillary study. The choice of roommate to be included at each visit was chosen from among the asymptomatic carriers presents at the time of the visit in the household. If more than one roommate was available and eligible, we drew lots to select the roommate to be included. In addition, in order to cover all eligible housemates in the study households by the end of the SMC campaign, it was necessary, during some visits, to select two roommates from certain households. All villages were systematically targeted at each SMC round. In the present study, asymptomatic carrier was defined as a person with no symptoms, with a body temperature < 37.5 °C, and who reported no fever during the 2 days before blood collection. Participants were visited at home, and data on the sociodemographic characteristics were collected through a standardized numerical questionnaire. Capillary blood samples were taken from each participant for the detection of P. falciparum infection by rRDT, usRDT, and microscopy.
Malaria diagnosis by RDT
The AdvDx™ Malaria Pf test kit (004ADFEF025KI-1, Advy Chemical Pvt. Ltd, India) served as the standard RDT, and the NxTek™ Eliminate Malaria Pf test kit (05FK140, Abbot, Korea) was used as the ultrasensitive RDT. Both tests are based on in vitro diagnostic immuno-chromatographic assay for the qualitative detection of the hrp2 antigen specific of Plasmodium falciparum malaria in human whole blood. The tests were performed by trained field workers following the instructions of each manufacturer. Briefly, approximately 5 µl (μl) of capillary blood was collected using a sample dropper and then transferred to the sample port, followed by the addition of four drops of the buffer solution to the buffer port. The interpretation of the results was carried out after an incubation of 20 min. A sample was considered positive for P. falciparum malaria if the “Pf” test band and the “C” control band appeared in the result window. The presence of only the control band was considered a negative result. Results were declared invalid if the control band did not appear in the result window, thus warranting a new test.
Malaria diagnosis by microscopy
Thick and thin blood smear slides were taken in the field and sent to the parasitology laboratory/Soaw station of the Clinical Research Unit of Nanoro (CRUN) for microscopic diagnosis. This diagnosis consisted of the detection of asexual and sexual forms of the parasites and their enumeration in the thick smear, followed by an identification of Plasmodium species on the thin smear. To do this, the slides were dried and then stained with Giemsa (diluted at 3%) for 45 min according to the standard operating procedures of the CRUN. They were then examined on a light microscope Olympus CX23 (Olympus corporation, Japan) at × 100 oil immersion objective. Thick smears were declared negative when no asexual parasites were encountered after running at least 100 microscopic fields. When the slide was positive, the parasite density (DP) was determined by counting the number of asexual parasites per 200 white blood cells and calculated per microliter of blood by assuming the number of white blood cells to be at 8000/μl. Gametocytes were counted against 500 white blood cells, and densities were estimated using a factor of 8000 leukocytes/microliter. Each blood slide was independently blinded and read by two expert microscopists of CRUN qualified by the National Institute for Communicable Diseases (NICD, South Africa) (Tinto et al. 2014). In case of significant discordance (negative vs. positive; difference in Plasmodium species; difference between the two readers greater than twice the DP value), a third reading was performed by another expert microscopist who had no knowledge of the previous results. The two closest readings were selected as the matching result.
Sample size estimation
Details about the sample size calculation for the larger SMC-RST project were described previously (Sondo et al. 2022). Briefly, 526 isolate households with a least one child under SMC coverage were included, i.e., 263 households each of the two arms (control and intervention) in order to have 80% power to detect a 20% decrease in incidence of malaria after 1 year in comparison to a baseline incidence rate in the control arm (SMC) between 1.5 and 2.0 malaria cases per year, assuming a one-sided test with a significance level of 0.025 and large-sample z-test of the Poisson event rate difference (PASS software) (Sondo et al. 2022). The sample size of this study was calculated considering that the prevalence of asymptomatic malaria parasite carriage is estimated at 30% in the study area. As the study is ancillary to the SMC-RST study, the sample size was calculated assuming a finite population of 2630 participants and also so as to be 95% certain that the sample size estimate will be within 10% of the actual proportion of asymptomatic carriers of Plasmodium. Taking these assumptions into account, the required sample size was estimated at 745 participants.
Data management and statistical analysis
Sociodemographic data and RDT results were entered in a REDCap database. Sociodemographic characterization and age stratification of asymptomatic malaria were performed by analyzing data from the subpopulation of participants with available microscopy results. Descriptive statistics was conducted for the sociodemographic characteristics and presented as frequencies and percentages. The performance of each RDT (standard RDT and ultrasensitive RDT) was assessed regarding expert microscopy results (gold standard) by calculating parameters such as sensitivity (Se), specificity (Sp), and the positive and negative predictive values (PPV and NPV). The concordance between rRDT and expert microscopy and between usRDT and expert microscopy was calculated using Cohen’s kappa coefficient of concordance (k). The k values < 0, 0.01–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1 represent mediocre, slight, fair, moderate, substantial, and near-perfect degrees of agreement, respectively (Landis and Koch 1977; McHugh 2012).
Results
Baseline characteristics of study population
A total of 745 household members of children under SMC coverage from the SMC-RST study were included in this study. RDT data from 745 included participants were used to assess the prevalence of asymptomatic malaria by rRDT and usRDT. Of the 745 participants included, 395 had microscopy data available. The age of the participants in the study subpopulation ranged from 5 to 83 years with a median age of 18 years, with a majority above 22 years of age (180/395). The female household members represented 55.70% (220/395) of the study population and males represented 44.30% (175/395). The sociodemographic characteristics of study subpopulation are shown in Table 1.
Table 1.
Sociodemographic characteristics of household members with microcopy results
| Characteristics | Frequency n (%) | |
|---|---|---|
| Age (in years) | 5–15 | 165 (41.77) |
| 16–26 | 74 (18.73) | |
| 27–37 | 58 (14.68) | |
| ≥ 38 | 98 (24.81) | |
| Sex | Female | 220 (55.70) |
| Male | 175 (44.30) |
Prevalence of asymptomatic carriers of malaria parasite
RDT positivity rate among household members of children under SMC coverage was 40.94% (305/745) determined by usRDT and 29.40% (219/745) using rRDT. The prevalence of asymptomatic carriers of asexual forms of P. falciparum detected by microscopy was 26.58% (105/395). Of the 105 asymptomatic carriers of asexual form of P falciparum, 103 (98.09%) were P. falciparum mono-infection and 2 (1.09%) P. falciparum mixed infections (1 P. falciparum + P. malariae and 1 P. falciparum + P. ovale). Also, 3 P. malariae mono-infection and 1 P. ovale mono-infection were detected by microscopy.
The parasite density of P. falciparum infections ranged from 24 to 42,645 parasites/microliter with a geometric mean of 551.42 parasites/microliter. The prevalence of sexual form of P. falciparum among household members was 5.32% (21/395), and the geometric mean of gametocyte estimated at 43.98 parasites/microliter (Table 2).
Table 2.
Prevalence of asymptomatic carriers of P. falciparum by microscopy, rRDT, and usRDT
| Method of diagnosis | Positive n (%) | Negative n (%) | Total |
|---|---|---|---|
| usRDT | 305 (40.94) | 440 (59.06) | 745 |
| 164 (41.52) | 231 (58.48) | 395 | |
| rRDT | 219 (29.40) | 526 (70.60) | 745 |
| 115 (29.11) | 280 (70.89) | 395 | |
| Microscopy | |||
| Asexual form | 105 (26.58) | 290 (73.42) | 395 |
Prevalence of asymptomatic carriage of asexual forms and gametocyte of P. falciparum by age group
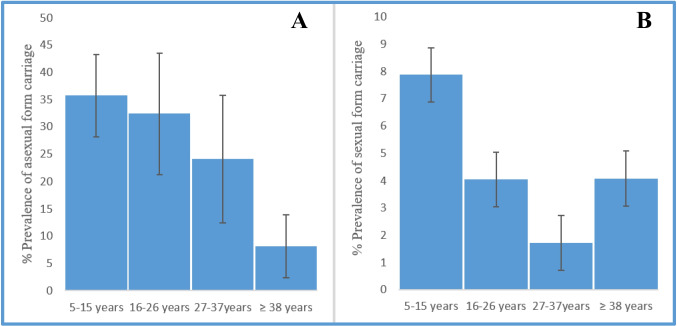
The prevalence of asymptomatic carriage of asexual form of P. falciparum was higher in children from 5 to 15 years old 35.76 (59/165). This was 32.43% (24/74) in the age group of 16 to 26 years old and 24.14% (14/58) and 8.16% (8/98) in the age groups 27 to 37 and ≥ 38 years old respectively (Fig. 1A). The prevalence of P. falciparum gametocytes in children between 5 and 15 years was 7.88% (13/165) (Fig. 1B).
Fig. 1.
Prevalence of asymptomatic carriers of P. falciparum by age groups. The figure represents the prevalence of the carriers of parasite (detected by microscopy) in household members of children under SMC coverage. A The carriers of the asexual form of P. falciparum. B The carriers of gametocyte
Comparison of diagnostic performance of rRDT and usRDT versus microscopy
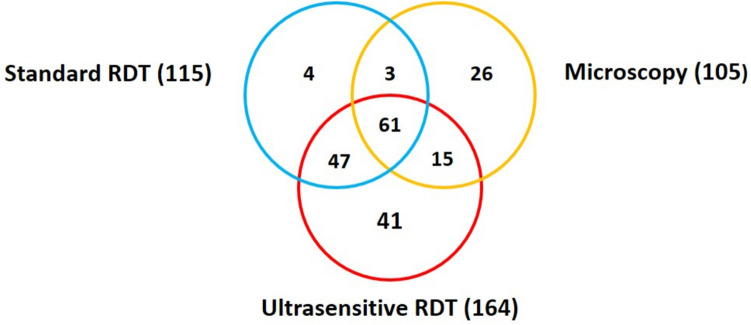
Of the 105 participants with a positive P. falciparum microscopy result considered true positives, the usRDT detected 76 (72.38%) while the rRDT was detected only 64 (60.95%). The false-positive rate was 53.66% (88/164) and 44.35% (51/115) with usRDT and rRDT respectively. A total of 56 participants that tested negative by rRDT tested positive by usRDT. Out of these, 26.79% (15/56) were confirmed to be P. falciparum positive by microscopy. In total, 25% (41/164) of the participants were diagnosed positive by usRDT and negative by microscopy and rRDT (Fig. 2).
Fig. 2.
Venn diagram showing the distribution of positive results according to the diagnostic test used with microscopy as reference test
The usRDT had a higher sensitivity than the rRDT (Se = 72.38% (95% confidence interval (CI) = 62.8 to 80.66) vs. 60.95% (95% CI = 50.94 to 70.33). In terms of specificity, rRDT had a higher specificity (82.41% 95% CI = 77.53 to 86.62) compared to 69.66% (95% CI = 64.01 to 74.89) for usRDT). Both RDTs had a comparable negative predictive value (87.45% (95% IC = 82.47 to 91.43) for usRDT and 85.36 (95% IC = 80.66 to 89.28) for rRDT. usRDT and rRDT showed substantial agreement (k = 0.70 for the usRDT and k = 0.76 for the rRDT) with the microscopy results (Table 3).
Table 3.
Diagnostic performance of rRDT and usRDT using microscopy as a reference
| Parameter | usRDT % [95% confidence interval] | rRDT (%) [95% confidence interval] |
|---|---|---|
| Sensitivity | 72.38 [62.8–80.66] | 60.95 [50.94–70.33] |
| Specificity | 69.66[64.01–74.89] | 82.41 [77.53–86.62] |
| Positive predictive value | 46.34 [38.53–54.28] | 55.65 [46.09–64.91] |
| Negative predictive value | 87.45 [82.47–91.43] | 85.36 [80.66–89.28] |
| Kappa coefficient (k) | 0.70 | 0.76 |
Diagnostic performance of rRDT and usRDT according parasite density
The sensitivity of the different RDT decreased with the parasite density; however, the usRDT was more sensitive than rRDT in both high (PD ≥ 200) and low ((PD < 200) parasite densities (Table 4).
Table 4.
Diagnostic performance of rRDT and usRDT according parasite density
| Diagnosis test | Parasite density | Sensitivity % [CI95%] | Specificity % [CI95%] |
|---|---|---|---|
| usRDT | < 200 | 51.52 [33.54–69.20] | 59.39 [54.14–64.49] |
| ≥ 200 | 40.61 [35.51–45.86] | 48.48 [30.80–66.46] | |
| rRDT | < 200 | 33.33 [17.96–51.83] | 71.27 [66.31–75.88] |
| ≥ 200 | 28.73 [24.12–33.69] | 66.67 [48.17–82.04] |
Discussion
This study reported on the prevalence of asymptomatic parasitaemia and on the performance of ultrasensitive hrp2 RDT and standard hrp2 RDT for detection of asymptomatic parasitaemia in household members of children under SMC coverage in Nanoro, Burkina Faso. High prevalence of asymptomatic carriers of asexual form of P. falciparum was observed in household members of children under SMC coverage. These asymptomatic carriers could compromise the effect of SMC intervention as these infections often go unnoticed, therefore untreated, and constitute major reservoirs of gametocytes for mosquito vectors, thus maintaining malaria transmission in the community (Alves et al. 2005; Lindblade et al. 2013). However, this finding is not surprising, especially in malaria-endemic areas where, despite the elimination of a large proportion of infected erythrocytes by immune-protective mechanisms, some may persist in the bloodstream and lead to asymptomatic parasitaemia (Dal-Bianco et al. 2007). Various studies reported higher prevalence of asymptomatic carriers in malaria endemic areas due to the transient immunity acquired over time (Laishram et al. 2012; Lindblade et al. 2013; Starzengruber et al. 2014). Malaria control strategies in endemic areas should therefore consider asymptomatic parasitaemia as a major obstacle to control efforts. Therefore, simultaneous screening and treatment of other asymptomatic household members with an antimalarial drug to eliminate the parasite reservoir could maximize the expected impact of the SMC intervention and global malaria control efforts.
Regarding age, adults and older children are more likely to be asymptomatic carriers because of the development of partial immunity due to repeated exposure to malaria parasites. The high prevalence of asymptomatic carriage of P. falciparum in children from 5 to 15 years old compared to other ages suggests a vulnerability of this age group, which represents an important reservoir for maintaining malaria transmission in the community. This was pointed out in various previous studies, highlighting school-aged children as greater contribution to the infectious reservoir and thereby undermining malaria elimination efforts (Ouédraogo et al. 2016; Coalson et al. 2016; Gonçalves et al. 2017). Therefore, the implementation of intermittent preventive treatment of malaria in school-aged (IPTsc) children as recommended by the WHO in 2022 (World Health Organization 2022) could reduce the parasite reservoir and prevent significant morbidity in this age group. There is some evidence that administration of IPTsc can confer a community-level benefit to those not receiving intermittent preventive treatment. Indeed, the administration of IPTc has been associated with a significant reduction in gametocyte carriage, which has a positive effect on malaria indicators at the community level (Clarke et al. 2017; Staedke et al. 2018; Rehman et al. 2019).
Cissé et al. in a stepped-wedge trial in Senegal reported that the expansion of SMC to children under 10 was associated with a 27% reduction in malaria in persons who had not received SMC, an effect that was not seen when SMC was limited to children 3–59 months (Cissé et al. 2016).
In this study, the prevalence of sexual forms of P. falciparum in household members was low compared with findings from a previous study that showed a high prevalence of gametocyte carriage in settings with a high load of asymptomatic infections (Vantaux et al. 2018). Difference between the two studies results may be attributable to many factors. The higher sensitivity of the RT-PCR used in the previous study compared with the microscopy used in this study could be the most important factor, as gametocytes tend to occur at low densities (Churcher et al. 2013; Sturrock et al. 2013). Moreover, polyclonality, asexual parasite density, multiplicity of infections, asexual genotype, acquired host immunity, immune responses, and seasonality of parasite transmission may also influence gametocytogenesis and therefore the prevalence of gametocyte (Nassir et al. 2005; Ouédraogo et al. 2008; Bousema and Drakeley 2011; Lamptey et al. 2018; Touray et al. 2021). Since gametocytemia is one of the indicators of intensity of transmission within an area, distinguishing gametocyte carriers in population would enable better characterization of the parasite reservoir (Lindblade et al. 2013). The use of more sensitive diagnostic tools for the detection of submicroscopic gametocytes and search for factors linked to gametocytogenesis would therefore be necessary to provide appropriate guidance for the future implementation of strategies aimed at reducing and interrupting transmission.
The findings showed that usRDT detected more asymptomatic P. falciparum carriers than the rRDT and microscopy confirming its superiority in detecting asymptomatic P. falciparum infections over the rRDT and microscopy. Similarly, Landier et al. are observed in a large field survey of asymptomatic carriers in Myanmar that usRDT were more sensitive the rRDT and microscopy for the detection of asymptomatic parasitaemia (Landier et al. 2018). The superiority of the usRDT results indeed from its LOD which is relatively low compared to the LOD of the rRDT and the expert microscopy, thus offering it the capacity to detect very low parasite densities (Jimenez et al. 2017; Das et al. 2018; Mpina et al. 2022). Parasite density being controlled by acquired immunity in infected hosts (Bousema et al. 2014), populations in high-transmission areas are more likely to have submicroscopic infections (Okell et al. 2012; Mosha et al. 2013) which are not detectable by microscopy and rRDT. Low parasite density could therefore have affected the proportion of asymptomatic infections detected by microscopy and rRDT (Okell et al. 2009). Other studies have also documented similar findings (Das et al. 2017, 2018).
The high sensitivity of the usRDT compared to the rRDT in the detection of asymptomatic carriers as observed in this study corroborates with its ability to identify parasites below the detection threshold of the rRDT (Das et al. 2018). Similar results had been reported in different studies on different asymptomatic populations (Girma et al. 2019; Briand et al. 2020; Acquah et al. 2021; Manjurano et al. 2021; Yimam et al. 2022). However, the usRDT did not show increased sensitivity compared to rRDT as was the case in studies conducted in Indonesia (62% for rRDT vs. 84% for usRDT) (Unwin et al. 2020), Colombia (64.3% for rRDT vs. 71.4% usRDT) (Vásquez et al. 2018), and Myanmar (44% for usRDT vs. 0% for rRDT) (Das et al. 2017). In terms of usRDT, it had lower specificity than rRDT. This could be due to the influence of several parameters such as the persistence of the hrp2 antigen in the blood (Mouatcho and Goldring 2013), the genetic variability of hrp2 and its homology with hrp3 (Baker et al. 2005), and mutations or deletions in the Pfhrp2 gene (Houzé et al. 2011; Koita et al. 2012; Maltha et al. 2012) to high malaria transmission in the study site (Hopkins et al. 2008; Baiden et al. 2012). Another source of variation in sensitivity could be the reference test used and its associated LOD (Ding et al. 2023). Factors such as transport and storage conditions and the inability of the parasite to express the hrp2 target antigen can affect TDR performance. All this merits further studies. However, the high sensitivity of the usRDT compared to the rRDT makes it potentially more useful for community diagnosis of asymptomatic carriers especially in a perspective of malaria elimination.
Limitations of the study
The lack of microscopy results in number of participants is the major limitation of this study. Indeed, at first, participants were including without slides (the two types of RDT only). The inclusion of microscopy resulted from a recommendation by steering committee, and this was caught up from month 2 leaving the firstly included participants without microscopy results. Another limitation was the use of microscopy as gold standard rather than molecular tools such as PCR that could have detected more asymptomatic infections.
Conclusion
The present study reports higher prevalence of parasite carriers in other household members of children under SMC coverage in the health district of Nanoro, Burkina Faso. More specifically, school-aged children were mostly affected. This could lead to frequent reinfection of children under SMC coverage, compromising the effect of SMC intervention. Also, the usRDT seems more appropriate for strategies based on detection and treatment of parasite carriers within the community. Finally, this study supports the implementation of intermittent preventive treatment of malaria in school-aged (ITPsc) children in Burkina Faso.
Acknowledgements
The authors would like to thank Expertise France L’Initiative for the financial supports of the project. Special thanks to Veronica Noseda and Cyrielle Thomas for their kind support. We are grateful to the study participants and all the community of the department of Soaw for their acceptance in participating to the study. We thank the study staff i.e. field workers, fields supervisors, laboratory technicians, data managers and the personnel of the Clinical Research Unit of Nanoro (CRUN) in Burkina Faso who played an important role for the successful completion of the present study.
Author contribution
SP, KB, RT, DK, MT, IH, RE, TG, SO, MD, GP, TH designed the study, KE, MS, SP, KB, KC, BI, RT, DK, MT, IH, RE, SB, TH implemented the study and supervised field work, KE, SP, IB, RT contributed in data management and statistical analysis, KE, SP, RT,DK, SO,SB, TH contributed in drafting the manuscript and all authors read and approved the manuscript.
Funding
This study is funded by Expertise France, L’Initiative, through the SMC-RST project (20SANIN204).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was an ancillary to a larger study entitled “Boosting the impact of seasonal malaria chemoprevention (SMC) through simultaneous screening and treatment of household members of children receiving SMC in Burkina Faso” acronym SMC-RST which has been approved by the Ethics Committee for Health Research of Burkina Faso (Deliberation No.: 2021–03-059 of 10 March 2021). A signed informed consent was obtained from participants or their parents/guardians (if they are minors) before enrollment. An impartial, literate witness (not a member of the study staff) was presented in case the parents/guardians were illiterate. The parent(s)/guardian(s) and, if applicable, the witness signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acquah FK, Donu D, Obboh EK et al (2021) Diagnostic performance of an ultrasensitive HRP2-based malaria rapid diagnostic test kit used in surveys of afebrile people living in Southern Ghana. Malar J 20:125. 10.1186/s12936-021-03665-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M, Joshi SN, Mbambo G et al (2015) An ultrasensitive reverse transcription polymerase chain reaction assay to detect asymptomatic low-density Plasmodium falciparum and Plasmodium vivax infections in small volume blood samples. Malar J 14:520. 10.1186/s12936-015-1038-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves FP, Gil LHS, Marrelli MT et al (2005) Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol 42:777–779. 10.1093/jmedent/42.5.777 [DOI] [PubMed] [Google Scholar]
- Baiden F, Webster J, Tivura M et al (2012) Accuracy of rapid tests for malaria and treatment outcomes for malaria and non-malaria cases among under-five children in rural Ghana. PLoS ONE 7:e34073. 10.1371/journal.pone.0034073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, McCarthy J, Gatton M et al (2005) Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J INFECT DIS 192:870–877. 10.1086/432010 [DOI] [PubMed] [Google Scholar]
- Bousema T, Drakeley C (2011) Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24:377–410. 10.1128/CMR.00051-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Okell L, Felger I, Drakeley C (2014) Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 12:833–840. 10.1038/nrmicro3364 [DOI] [PubMed] [Google Scholar]
- Briand V, Cottrell G, Tuike Ndam N et al (2020) Prevalence and clinical impact of malaria infections detected with a highly sensitive HRP2 rapid diagnostic test in Beninese pregnant women. Malar J 19:188. 10.1186/s12936-020-03261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns M, Ceesay SJ, Sagara I et al (2021) Effectiveness of seasonal malaria chemoprevention (SMC) treatments when SMC is implemented at scale: case–control studies in 5 countries. PLoS Med 18:e1003727. 10.1371/journal.pmed.1003727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns ME, Sagara I, Zongo I et al (2020) Evaluation of seasonal malaria chemoprevention in two areas of intense seasonal malaria transmission: secondary analysis of a household-randomised, placebo-controlled trial in Houndé District, Burkina Faso and Bougouni District. Mali Plos Med 17:e1003214. 10.1371/journal.pmed.1003214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churcher TS, Bousema T, Walker M et al (2013) Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. eLife 2:e00626. 10.7554/eLife.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cissé B, Ba EH, Sokhna C et al (2016) Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: a stepped-wedge cluster-randomised trial. PLoS Med 13:e1002175. 10.1371/journal.pmed.1002175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SE, Rouhani S, Diarra S et al (2017) Impact of a malaria intervention package in schools on Plasmodium infection, anaemia and cognitive function in schoolchildren in Mali: a pragmatic cluster-randomised trial. BMJ Glob Health 2:e000182. 10.1136/bmjgh-2016-000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coalson JE, Walldorf JA, Cohee LM et al (2016) High prevalence of Plasmodium falciparum gametocyte infections in school-age children using molecular detection: patterns and predictors of risk from a cross-sectional study in southern Malawi. Malar J 15:527. 10.1186/s12936-016-1587-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J, Jones S, Gatton ML et al (2019) A review of the WHO malaria rapid diagnostic test product testing programme (2008–2018): performance, procurement and policy. Malar J 18:387. 10.1186/s12936-019-3028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Bianco MP, Köster KB, Kombila UD et al (2007) High prevalence of asymptomatic Plasmodium falciparum infection in Gabonese adults. Am J Trop Med Hyg 77:939–942 [PubMed] [Google Scholar]
- Das S, Jang IK, Barney B et al (2017) Performance of a high-sensitivity rapid diagnostic test for Plasmodium falciparum malaria in asymptomatic individuals from Uganda and Myanmar and naive human challenge infections. Am J Trop Med Hyg 97:1540–1550. 10.4269/ajtmh.17-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Peck RB, Barney R et al (2018) Performance of an ultra-sensitive Plasmodium falciparum HRP2-based rapid diagnostic test with recombinant HRP2, culture parasites, and archived whole blood samples. Malar J 17:118. 10.1186/s12936-018-2268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kock M, Tarning J, Workman L et al (2018) Population pharmacokinetic properties of sulfadoxine and pyrimethamine: a pooled analysis to inform optimal dosing in African children with uncomplicated malaria. Antimicrob Agents Chemother 62:e01370-e1417. 10.1128/AAC.01370-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derra K, Rouamba E, Kazienga A et al (2012) Profile: Nanoro health and demographic surveillance system. Int J Epidemiol 41:1293–1301. 10.1093/ije/dys159 [DOI] [PubMed] [Google Scholar]
- Ding XC, Incardona S, Serra-Casas E et al (2023) Malaria in pregnancy (MiP) studies assessing the clinical performance of highly sensitive rapid diagnostic tests (HS-RDT) for Plasmodium falciparum detection. Malar J 22:60. 10.1186/s12936-023-04445-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girma S, Cheaveau J, Mohon AN et al (2019) Prevalence and epidemiological characteristics of asymptomatic malaria based on ultrasensitive diagnostics: a cross-sectional study. Clin Infect Dis 69:1003–1010. 10.1093/cid/ciy1005 [DOI] [PubMed] [Google Scholar]
- Gonçalves BP, Kapulu MC, Sawa P et al (2017) Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 8:1133. 10.1038/s41467-017-01270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins H, Bebell L, Kambale W et al (2008) Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J INFECT DIS 197:510–518. 10.1086/526502 [DOI] [PubMed] [Google Scholar]
- Houzé S, Hubert V, Le Pessec G et al (2011) Combined deletions of pfhrp2 and pfhrp3 genes result in Plasmodium falciparum malaria false-negative rapid diagnostic test. J Clin Microbiol 49:2694–2696. 10.1128/JCM.00281-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A, Rees-Channer RR, Perera R et al (2017) Analytical sensitivity of current best-in-class malaria rapid diagnostic tests. Malar J 16:128. 10.1186/s12936-017-1780-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwugu ON, Helegbe GK, Aryee PA et al (2019) Prevalence of asymptomatic malaria among children in the Tamale Metropolis: how does the PfHRP2 CareStart™ RDT perform against microscopy? J Trop Med 2019:1–7. 10.1155/2019/6457628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koita OA, Doumbo OK, Ouattara A et al (2012) False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg 86:194–198. 10.4269/ajtmh.2012.10-0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laban NM, Kobayashi T, Hamapumbu H et al (2015) Comparison of a PfHRP2-based rapid diagnostic test and PCR for malaria in a low prevalence setting in rural southern Zambia: implications for elimination. Malar J 14:25. 10.1186/s12936-015-0544-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laishram DD, Sutton PL, Nanda N et al (2012) The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malar J 11:29. 10.1186/1475-2875-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamptey H, Ofori MF, Kusi KA et al (2018) The prevalence of submicroscopic Plasmodium falciparum gametocyte carriage and multiplicity of infection in children, pregnant women and adults in a low malaria transmission area in Southern Ghana. Malar J 17:331. 10.1186/s12936-018-2479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landier J, Haohankhunnatham W, Das S et al (2018) Operational performance of a Plasmodium falciparum ultrasensitive rapid diagnostic test for detection of asymptomatic infections in Eastern Myanmar. J Clin Microbiol 56:e00565-e618. 10.1128/JCM.00565-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159. 10.2307/2529310 [PubMed] [Google Scholar]
- Lindblade KA, Steinhardt L, Samuels A et al (2013) The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11:623–639. 10.1586/eri.13.45 [DOI] [PubMed] [Google Scholar]
- Maltha J, Gamboa D, Bendezu J et al (2012) Rapid diagnostic tests for malaria diagnosis in the Peruvian Amazon: impact of pfhrp2 gene deletions and cross-reactions. PLoS ONE 7:e43094. 10.1371/journal.pone.0043094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjurano A, Omolo JJ, Lyimo E et al (2021) Performance evaluation of the highly sensitive histidine-rich protein 2 rapid test for Plasmodium falciparum malaria in North-West Tanzania. Malar J 20:58. 10.1186/s12936-020-03568-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22:276–282 [PMC free article] [PubMed] [Google Scholar]
- Meremikwu MM, Donegan S, Sinclair D et al (2012) Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database Syst Rev. 10.1002/14651858.CD003756.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry Of Health BF (2021) Statistial yearbook 2021. Burkina Faso. https://www.sante.gov.bf/fileadmin/annuaire_2021_mshp.pdf
- Mosha JF, Sturrock HJ, Greenhouse B et al (2013) Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malar J 12:221. 10.1186/1475-2875-12-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouatcho JC, Goldring JPD (2013) Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol 62:1491–1505. 10.1099/jmm.0.052506-0 [DOI] [PubMed] [Google Scholar]
- Mpina M, Stabler TC, Schindler T et al (2022) Diagnostic performance and comparison of ultrasensitive and conventional rapid diagnostic test, thick blood smear and quantitative PCR for detection of low-density Plasmodium falciparum infections during a controlled human malaria infection study in Equatorial Guinea. Malar J 21:99. 10.1186/s12936-022-04103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassir E, Abdel-Muhsin A-MA, Suliaman S et al (2005) Impact of genetic complexity on longevity and gametocytogenesis of Plasmodium falciparum during the dry and transmission-free season of eastern Sudan. Int J Parasitol 35:49–55. 10.1016/j.ijpara.2004.10.014 [DOI] [PubMed] [Google Scholar]
- Okell LC, Bousema T, Griffin JT et al (2012) Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 3:1237. 10.1038/ncomms2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okell LC, Ghani AC, Lyons E, Drakeley CJ (2009) Submicroscopic infection in Plasmodium falciparum –endemic populations: a systematic review and meta-analysis. J INFECT DIS 200:1509–1517. 10.1086/644781 [DOI] [PubMed] [Google Scholar]
- Oshikoya KA, Sammons HM, Choonara I (2010) A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Clin Pharmacol 66:1025–1035. 10.1007/s00228-010-0851-0 [DOI] [PubMed] [Google Scholar]
- Ouédraogo AL, De Vlas SJ, Nébié I et al (2008) Seasonal patterns of Plasmodium falciparum gametocyte prevalence and density in a rural population of Burkina Faso. Acta Trop 105:28–34. 10.1016/j.actatropica.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Ouédraogo AL, Gonçalves BP, Gnémé A et al (2016) Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 213:90–99. 10.1093/infdis/jiv370 [DOI] [PubMed] [Google Scholar]
- Owalla TJ, Okurut E, Apungia G et al (2020) Using the ultrasensitive Alere Plasmodium falciparum malaria Ag HRP-2™ rapid diagnostic test in the field and clinic in Northeastern Uganda. Am J Trop Med Hyg 103:778–784. 10.4269/ajtmh.19-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman AM, Maiteki-Sebuguzi C, Gonahasa S et al (2019) Intermittent preventive treatment of malaria delivered to primary schoolchildren provided effective individual protection in Jinja, Uganda: secondary outcomes of a cluster-randomized trial (START-IPT). Malar J 18:318. 10.1186/s12936-019-2954-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh ME, Zongo I, Haro A et al (2023) Seasonal malaria chemoprevention drug levels and drug resistance markers in children with or without malaria in Burkina Faso: a case-control study. J Infect Dis 228:926–935. 10.1093/infdis/jiad172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somé AF, Zongo I, Sagara I et al (2022) Factors influencing second and third dose observance during seasonal malaria chemoprevention (SMC): a quantitative study in Burkina Faso. Mali and Niger Tropicalmed 7:214. 10.3390/tropicalmed7090214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondo P, Bihoun B, Valea I, et al (2015) La part du paludisme dans les maladies fébriles en saison sèche dans la région de Nanoro, Burkina Faso. West African Journal of Research for Health. https://www.researchgate.net/publication/321058112
- Sondo P, Tahita MC, Ilboudo H et al (2022) Boosting the impact of seasonal malaria chemoprevention (SMC) through simultaneous screening and treatment of household members of children receiving SMC in Burkina Faso: a protocol for a randomized open label trial. Arch Public Health 80:41. 10.1186/s13690-022-00800-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedke SG, Maiteki-Sebuguzi C, Rehman AM et al (2018) Assessment of community-level effects of intermittent preventive treatment for malaria in schoolchildren in Jinja, Uganda (START-IPT trial): a cluster-randomised trial. Lancet Glob Health 6:e668–e679. 10.1016/S2214-109X(18)30126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzengruber P, Fuehrer H-P, Ley B et al (2014) High prevalence of asymptomatic malaria in south-eastern Bangladesh. Malar J 13:16. 10.1186/1475-2875-13-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock HJW, Hsiang MS, Cohen JM et al (2013) Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS Med 10:e1001467. 10.1371/journal.pmed.1001467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinto H, Valea I, Sorgho H et al (2014) The impact of clinical research activities on communities in rural Africa: the development of the Clinical Research Unit of Nanoro (CRUN) in Burkina Faso. Malar J 13:113. 10.1186/1475-2875-13-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touray AO, Mobegi VA, Wamunyokoli F et al (2021) Prevalence of asymptomatic P falciparum gametocyte carriage among school children in Mbita, Western Kenya and assessment of the association between gametocyte density, multiplicity of infection and mosquito infection prevalence. Wellcome Open Res 5:259. 10.12688/wellcomeopenres.16299.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin VT, Ahmed R, Noviyanti R et al (2020) Use of a highly-sensitive rapid diagnostic test to screen for malaria in pregnancy in Indonesia. Malar J 19:28. 10.1186/s12936-020-3110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantaux A, Samreth R, Piv E et al (2018) Contribution to malaria transmission of symptomatic and asymptomatic parasite carriers in Cambodia. J Infect Dis 217:1561–1568. 10.1093/infdis/jiy060 [DOI] [PubMed] [Google Scholar]
- Vásquez AM, Medina AC, Tobón-Castaño A et al (2018) Performance of a highly sensitive rapid diagnostic test (HS-RDT) for detecting malaria in peripheral and placental blood samples from pregnant women in Colombia. PLoS ONE 13:e0201769. 10.1371/journal.pone.0201769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AL, on behalf of the IPTc Taskforce (2011) A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc). PLoS ONE 6:e16976. 10.1371/journal.pone.0016976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2012) WHO policy recommendation: seasonal malaria chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. World Health Organization. https://iris.who.int/handle/10665/337978
- Yimam Y, Mohebali M, Abbaszadeh Afshar MJ (2022) Comparison of diagnostic performance between conventional and ultrasensitive rapid diagnostic tests for diagnosis of malaria: a systematic review and meta-analysis. PLoS ONE 17:e0263770. 10.1371/journal.pone.0263770 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.