Abstract
Introduction
The study aimed to compare postoperative pain between alcohol-assisted and transepithelial photorefractive keratectomy in patients who received the novel analgesic preoperative nepafenac treatment 2 days preoperatively and 3 days postoperatively. Pain, stinging, tearing, light sensitivity, and stress levels were evaluated.
Methods
The study included a retrospective analysis of 55 patients divided into two groups: bilateral alcohol-assisted photorefractive keratectomy (aa-PRK) and transepithelial photorefractive keratectomy (transepithelial-PRK). Nepafenac was administered for pain control for all patients, with patients receiving four drops for 2 days before the surgery and 3 days postoperatively per clinical instructions. Patients completed questionnaires on the day of the surgery and for the first 5 days postoperatively. Statistical analysis was performed using XLSTAT (version 2023.1.2). t-Test was used to analyze and compare pain and symptom levels and Fisher’s exact test for categorical data. p-Values less than 0.05 were considered statistically significant.
Results
The study examined 55 patients (49% female) with a mean age of 25.11 ± 6.81 years who had undergone bilateral surface refractive surgery to correct myopic errors. The mean baseline standard error (SE) was −3.16 ± 2.20 D. Among these patients, 27 patients underwent aa-PRK and 28 patients underwent transepithelial-PRK. Higher levels of pain were significant in the aa-PRK group (p = 0.003). However, there was no significant difference between the groups in the average levels of stinging, tearing, or light sensation. Additionally, stress levels decreased over time in both groups, with levels becoming almost equal after 5 days, and there was no significant difference in the average stress levels between the two groups.
Conclusions
The study found that patients who underwent the transepithelial-PRK procedure had significantly lower pain levels compared with those who underwent aa-PRK after being treated with nepafenac per protocol. However, there was no significant difference between the two groups in terms of stinging, tearing, light sensation, and stress levels.
Keywords: Photorefractive keratectomy (PRK), Nepafenac, Nevanac, Nonsteroidal antiinflammatory drug (NSAID), Postoperative adverse events, Refractive surgery, Analgetic, Pain
Key Summary Points
| Why carry out this study? |
| Photorefractive keratectomy (PRK) is a very common laser refractive surgery in which patients usually experience postoperative pain. Easing the pain may benefit many patients worldwide. |
| Nepafenac, a topical nonsteroidal antiinflammatory drug (NSAID), is not usually prescribed for treatment of pain after refractive surgery. |
| In this study we aimed to compare postoperative pain between alcohol-assisted and transepithelial PRK in patients who received nepafenac treatment 2 days preoperatively and 3 days postoperatively to determine whether its use might reduce postoperative pain. |
| What was learned from the study? |
| After being treated with nepafenac, patients who underwent the alcohol-assisted PRK procedure had significantly higher pain levels compared with those who underwent transepithelial-PRK. |
| Stress levels decreased over time in both groups, with levels becoming almost equal after 5 days, and there was no significant difference in the average stress levels between the two groups. |
Introduction
Laser corneal refractive surgery is a commonly performed procedure that has high patient satisfaction rates, as supported by various studies [1–4]. Among refractive surgeries, there are two main common approaches: the first involves creating a corneal flap and then using an excimer laser for stromal ablation beneath it, known as laser-assisted in situ keratomileusis (LASIK). The second approach involves removing the epithelium and applying laser energy directly on the Bowman’s membrane, which can be done either manually with the assistance of alcohol (alcohol-assisted photorefractive keratectomy, aa-PRK) or with the help of an excimer laser system (transepithelial-PRK) [5].
Photorefractive keratectomy (PRK) has emerged as a widely practiced corneal refractive procedure since its inception in the late 1980s and has garnered global recognition for its efficacy and safety over the past decades [6]. However, it is imperative to note that despite its success, patients should be aware of the potential complications associated with the postoperative healing process, including significant discomfort and delayed visual recovery [7]. Due to major technological advancements in excimer laser systems and eye-tracking systems, the success rates of corneal reshaping procedures have markedly improved, leading to the performance of hundreds of millions of such procedures worldwide [2–4, 8].
The use of the transepithelial-PRK method, which involves the vaporization of corneal tissue with an excimer laser, was first introduced in 1985. Subsequently, in 1995, the Food and Drug Administration (FDA) approved PRK for refractive surgery [9].
Pain following PRK surgery typically arises soon after the procedure and continues during the initial days of recovery as the corneal surface re-epithelializes, usually finalized approximately 3 days after the surgery [10, 11]. This discomfort is caused by the release of inflammatory agents resulting from the removal of the corneal epithelium, which enhances the sensitivity of nociceptors. Additionally, laser ablation damages stromal nerve fibers directly, and exacerbates the inflammatory reaction by harming stromal keratocytes [12].
The intensity of post-PRK pain can vary significantly and can be disabling since the cornea is the most richly innervated tissue in humans [13]. Pain management strategies following PRK comprise cooling techniques, applying a contact lens for covering the eye, oral analgesics [such as opioids, neuropathic medications, and nonsteroidal antiinflammatory drugs (NSAIDs)], and topical agents (including steroids, anesthetics, and NSAIDs). NSAIDs are suggested to operate by inhibiting the cyclooxygenase (COX) pathway, reducing nociceptor activity, and decreasing inflammatory mediator levels [12].
Nepafenac is a unique topical NSAID that differs from other drugs in this class as it is a prodrug with low intrinsic COX-inhibiting activity. Following intraocular enzymatic hydrolysis, it transforms into an active form called amfenac, and has the added advantage of excellent and rapid corneal penetration. In addition to its rapid analgesic effect, believed to be due to its inherent analgesic activity and quick saturation of the corneal epithelial layer, nepafenac is primarily converted to its active form, amfenac, in intraocular vascular tissues. This transformation occurs mainly in posterior structures, such as the retina and choroid, where higher hydrolase activity is present [14]. Therefore, administering nepafenac earlier during the preoperative period may enhance its analgesic effect later. Our group has modified the pain management protocol to involve administering topical nepafenac 2 days before and 3 days after surgery.
Several studies have examined the postoperative effects of aa-PRK and transepithelial-PRK, including adverse events and pain [15, 16]. To our knowledge, no study investigated the use of perioperative nepafenac, and there is no information in the literature regarding this certain pain management protocol, using nepafenac preoperatively and postoperatively in aa-PRK and transepithelial-PRK, and comparison of postoperative adverse events between the two procedures under this protocol. Thus, the aim of our study was to assess the impact of this protocol on patients who underwent aa-PRK and transepithelial-PRK.
Methods
All data for the study were collected and analyzed in accordance with the policies and tenets outlined in the Declaration of Helsinki. This study received approval by the Institutional Review Board of the TLV-0689-17 Medical Center. As the study data were deidentified and the study was retrospective, informed consent was not required.
Study Participants
This retrospective study included patients with myopia who underwent PRK by a single surgeon (E.L.) between October 2022 and June 2023 at Enaim Medical Center, Tel-Aviv, Israel. Patients were grouped into two groups according to whether they underwent aa-PRK or transepithelial-PRK. The routine follow-up process included physical examination after 1 day from the surgery and after 5 days to remove the therapeutic contact lens. In both examinations patients were asked about their pain, and in between the examinations, patients are being routinely asked via phone call, by a specific nurse, whether there are any pains or any other important issues.
Inclusion criteria were age over 18 years; stable refraction for at least 12 months; intraocular pressure (IOP) less than 21 mmHg; a period without wearing contact lenses (more than 2 weeks for rigid contact lenses and more than 5 days for soft contact lenses); and no history of autoimmune disease, diabetes, or previous ocular surgery.
Data Collection and Symptom Survey
The medical files of all eligible patients were reviewed, and the following demographic and preoperative data were extracted: age, gender, analgesic regimen, date of surgery, preoperative manifest sphere, and preoperative manifest cylinder. The following intraoperative data were extracted: surgical technique (alcohol-assisted PRK or transepithelial PRK), optical zone diameter, ablation zone diameter. Data were obtained for both eyes of each patient.
Patients underwent a phone survey conducted twice daily (morning and evening) over the first five postoperative days. The survey evaluated:
Visual analogue scale (VAS) measuring pain intensity where pain is graded on a scale of 0–10, where zero indicates the absence of pain, and 10 indicates the worst possible pain.
The total number of analgesic tablets taken.
Presence of additional symptoms: photophobia, tearing, stinging sensation (recorded on a scale of 0–10 with 0 representing none and 10 representing maximal).
Stress levels at the day of surgery and once a day—indicating how stressed was the patient (on a scale of 1–10).
Surgical Technique
Prior to surgery, each patient received three drops of a topical anesthetic (benoxinate hydrochloride 0.4%) in the conjunctival fornix, and an eyelid speculum was inserted.
In the aa-PRK group, epithelial cells were removed using a dilute solution of 20% alcohol. The alcohol solution was instilled into an 8.5 mm alcohol well placed on the cornea. After 20–30 s, the alcohol was soaked with a sponge from within the well, and the well was removed. The cornea and ocular surface were irrigated with BSS to minimize toxicity to the limbal germinal epithelium. The epithelium was then easily removed using a hooky knife. This was followed by stromal excimer ablation using the Amaris 1050rs (Schwind, Kleinostheim, Germany) excimer laser platform. In the transepithelial-PRK group, epithelial removal was performed via excimer ablation using the same excimer laser platform, as a single step ablation combining both epithelial and stromal ablation. Epithelial thickness was set at 50–60 µm. The rest of the procedure was identical between the two groups.
A sponge soaked with 0.02% mitomycin C was placed on the stroma for 20 s immediately after ablation. Following thorough rinsing of mitomycin C, a contact lens was placed over the cornea. Following surgery, moxifloxacin 0.5% (four times a day), dexamethasone 0.1% (four times a day), and artificial tears (four times a day) were prescribed. Patients were examined at 1 day and 5 days.
Pain Management
All patients received the same preoperative and postoperative pain management protocol, including nepafenac administered for inflammation control and analgesic purposes. Patients received one drop four times daily, with similar interval between drops, for 2 days before the surgery and 3 days postoperatively in accordance with clinical instructions. All nepafenac drops were given using the standard bottles and patients were instructed to use the drops exactly four times a day, each time one drop only.
Statistical Analysis
Data were recorded in Microsoft Excel and analyzed using XLSTAT (version 2023.1.2). An estimation of the study’s power was made prior to initiation of the study by using the anticipated VAS score difference between the treatment group and the control group. We regarded a 1.5-point difference as clinically significant. Assuming a sample size of 48 patients, 24 in the group underwent aa-PRK and 24 in the group underwent transepithelial-PRK, with a one-sided p-value < 0.05 considered statistically significant and a 1.5-point VAS score difference between the groups with a standard deviation of 1.4 [17]—the study will have a power of > 99%.
For parameters recorded from both eyes, the maximal value from either the right or left eye was chosen for analysis since the primary outcome of the study is pain level. The normality of distribution was assessed using the Shapiro–Wilk test. Continuous variables were compared between the groups using the Mann–Whitney test. Analysis was performed for both mean and maximal pain levels recorded on each of the first five postoperative days. Repeated-measures t-test was used to analyze and compare pain and symptom levels throughout the 5-day study period, and Fisher’s exact test for categorical data. Multiple comparisons were not taken into account. A two-sided p-value < 0.05 was considered statistically significant in all analyses. All presented means are accompanied by their respective standard deviations.
Results
A total of 55 patients were included, of which 27 were female (49%). Mean age was 25.11 ± 6.81 years. There were 27 patients in the group that underwent aa-PRK and 28 patients in the group that underwent transepithelial-PRK.
Table 1 provides an overview of the baseline and demographic features for both the entire cohort and each individual treatment group. Importantly, there were no significant differences between the groups.
Table 1.
Baseline characteristics of the entire cohort and of the treatment groups
| Entire cohort n = 55 |
aa-PRK group n = 27 |
Transepithelial-PRK group n = 28 |
p-Value | |
|---|---|---|---|---|
| Age (years) | 25.11 ± 6.89 | 25.07 ± 6.32 | 25.14 ± 7.37 | 0.971 |
| Gender (% female) | 49% | 37% | 61% | 0.363 |
| Sphere (D) | −2.77 ± 1.39 | −2.84 ± 1.80 | −2.70 ± 0.86 | 0.707 |
| Spherical equivalent (D) | −3.16 ± 1.43 | −3.32 ± 1.81 | −3.01 ± 0.95 | 0.438 |
| Optic zone (mm) | 7.18 ± 0.25 | 7.11 ± 0.27 | 7.24 ± 0.22 | 0.061 |
| Ablation zone (mm) | 8.42 ± 0.24 | 8.40 ± 0.25 | 8.44 ± 0.24 | 0.469 |
aa-PRK alcohol-assisted photorefractive keratectomy, Transepithelial-PRK transepithelial photorefractive keratectomy
Pain
Mean VAS pain scores over the first five postoperative days were 3.48 ± 2.25 in the group that underwent aa-PRK and 1.72 ± 1.88 in the group that underwent transepithelial-PRK (p = 0.003). Maximal VAS pain scores over the first five postoperative days were 7.15 ± 3.29 in the group that underwent aa-PRK and 4.25 ± 3.67 in the group that underwent transepithelial-PRK (p = 0.003).
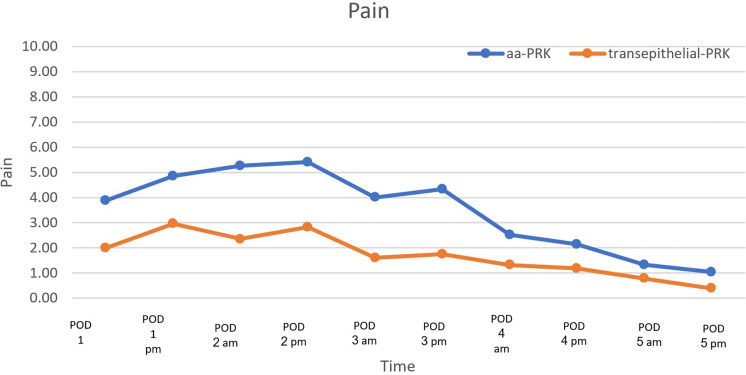
A repeated-measures analysis of variance (ANOVA) was performed to evaluate the effect of treatment type (aa-PRK vs transepithelial-PRK) on pain levels over time (days 1–5) and determined that treatment type was significantly associated with pain reduction over time, favoring the group that underwent transepithelial-PRK (p < 0.001, Fig. 1).
Fig. 1.
Visual analogue scale (VAS) scores of each treatment group throughout postoperative days 1–5. POD postoperative day, aa-PRK alcohol-assisted photorefractive keratectomy, Transepithelial-PRK transepithelial photorefractive keratectomy
The total number of analgesic tablets taken during postoperative days 1–5 was 4.07 ± 4.61 tablets in the group that underwent aa-PRK and 1.18 ± 1.52 tablets in the group underwent that transepithelial-PRK (p = 0.003).
Additional Factors Associated with Pain
The only factor significantly associated with postoperative pain was the type of PRK procedure performed (coefficient = 1.37, p = 0.032), indicating that trans-PRK is associated with a reduction of 1.37 in mean postoperative VAS pain scores compared with aa-PRK.
Additional Symptoms
Repeated-measures ANOVA was performed to evaluate the effect of treatment type (aa-PRK versus transepithelial-PRK) on additional symptoms over time (days 1–5). The models determined that the treatment type did not significantly affect levels of photophobia (p = 0.411), tearing (p = 0.306), or stinging sensation (p = 0.118) over the postoperative period.
Discussion
Previous studies have investigated the comparison of postoperative adverse events between aa-PRK and transepithelial-PRK, with a focus on pain, and our study was the first to investigate the use of nepafenac for analgesic purposes. The study conducted by Zarei-Ghanavati et al. reported a significant difference in pain levels only on the first day after surgery, with patients who underwent transepithelial-PRK reporting greater pain than those who underwent aa-PRK [18]. Hashemi et al. found that patients who underwent conventional PRK have experienced less pain and discomfort in the first postoperative day compared with patients who underwent transepithelial-PRK and mechanical epithelial debridement PRK [19]. Our study revealed a different pattern of postoperative pain levels compared with prior studies that compared aa-PRK and transepithelial-PRK. We observed a trend toward greater pain levels in patients who underwent aa-PRK, which was statistically significant on postoperative days 2 and 3. This discrepancy may be explained by the improvement in surgical technology, but this will require further investigation in further studies. Furthermore, the average levels of pain during the first five postoperative days were higher in patients who underwent aa-PRK than those who underwent transepithelial-PRK, highlighting the differential effect of the two procedures on postoperative pain.
Gharieb et al. proposed that increased postoperative pain in the transepithelial-PRK group could be linked to the extended ablation process involving greater laser energy, resulting in higher cytokine release [20]. Contrary to this hypothesis, our investigation revealed no association between a larger optical zone and postoperative pain. Despite the group that underwent transepithelial-PRK having a larger optical zone, there was a notable reduction in postoperative pain within this cohort.
In previous research, nepafenac has been suggested as a means of alleviating postoperative stinging following PRK surgery. Nonetheless, Donnenfeld et al. have reported that this treatment is less effective than other NSAIDs [21]. Despite these findings, no study has yet examined the disparities in postoperative stinging between aa-PRK and transepithelial-PRK procedures. Our study found a non-significant trend in most days toward higher tearing levels in patients underwent aa-PRK.
Moreover, the gradual reduction in pain levels beyond the third day, despite the discontinuation of nepafenac, can be attributed to the time required for epithelial healing, which is typically completed around 3 days postoperatively [11]. The lower pain levels observed in patients who underwent transepithelial-PRK compared with those who underwent aa-PRK highlight the potential benefits of technological advancements, such as the use of an excimer laser for corneal tissue removal during refractive procedures.
Recent research indicates that there are differences in tear levels following aa-PRK and transepithelial-PRK procedures. Zarei-Ghanavati et al. found that patients who underwent transepithelial-PRK had significantly higher tear levels on postoperative days 1 and 3 compared with those who underwent aa-PRK. Additionally, there was a tendency toward higher tearing levels in the group that underwent transepithelial-PRK even 7 days after the surgery [18]. However, our study did not reveal a significant difference in postoperative stinging between the two groups, and the average tearing level was actually higher in the group underwent aa-PRK than in the group underwent transepithelial-PRK.
The identification of a biphasic pattern of stinging, tearing, and light sensitivity in postoperative patients who have undergone aa-PRK or transepithelial-PRK and received nepafenac is a novel finding. This discovery could have implications for surgeons when providing postoperative care instructions and selecting postoperative pharmacological treatments. Additionally, the pattern observed, with the lowest point occurring immediately after the end of nepafenac therapy, may suggest an association between nepafenac and stinging, tearing, and light sensitivity. Furthermore, the presence of this pattern indicates that nepafenac has an immediate impact on these adverse events while also exerting a long-lasting effect on pain.
Our study revealed continuous decrease in stress levels for both aa-PRK and patients who underwent transepithelial-PRK during the postoperative period. Additionally, our findings indicated no significant differences in stress level changes between the two groups, suggesting that patients perceive similar levels of risk associated with each procedure.
Our study findings should be interpreted in light of several limitations. First, the retrospective design of the study and the fact that all patients underwent a comprehensive medical screening, may introduce selection bias. The absence of a control group further limits the conclusions that can be drawn from our findings. Second, there were notable differences in demographic criteria between the two groups, particularly in preoperative SE, which may have affected our results. Third, the study group was relatively small, so confounding variables were not taken into account and gender allocation to aa-PRK and transepithelial-PRK was not equal. Finally, longer-term follow-up is necessary to fully assess the outcomes of both procedures, and the potential loss of follow-up may limit the validity of our study results. Future studies will include a larger cohort, will have a prospective design, and will include a control group, enabling them to explore potential contributing factors to pain to reduce bias and to provide meaningful insights.
Conclusions
Our study highlights the importance of preoperative and postoperative management in addressing adverse events that can significantly improve patients’ well-being. By focusing on pain levels, stinging and tearing sensations, light sensitivity, and stress, surgeons can ensure optimal patient outcomes and provide effective care during this critical period. We believe that our findings will deepen surgeons’ understanding of perioperative patient management and ultimately enhance their ability to provide high-quality care to their patients.
Acknowledgements
We thank the participants of the study.
Author Contributions
Nir Gomel—design, writing, and drafting; Nadav Shemesh—writing and drafting; Nir Sorkin—design, statistical analysis, and drafting; Nadav Levinger—writing and drafting; Shmuel Levinger—design, writing, and drafting; Ami Hirsch—design and drafting; Asaf Achiron—design, writing, and drafting; and Eliya Levinger—design, writing, and drafting.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Nir Gomel, Nadav Shemesh, Nir Sorkin, Nadav Levinger, Shmuel Levinger, Ami Hirsch, Asaf Achiron, and Eliya Levinger have nothing to disclose.
Ethical Approval
All data for the study were collected and analyzed in accordance with the policies and tenets outlined in the Declaration of Helsinki. This study received approval by the Institutional Review Board of the TLV-0689-17 Medical Center. As the study data was deidentified and the study was retrospective, informed consent was not required.
Footnotes
Nir Gomel and Nadav Shemesh contributed equally to this work.
References
- 1.Achiron A, Gur Z, Aviv U, Hilely A, Mimouni M, Karmona L, et al. Predicting refractive surgery outcome: machine learning approach with big data. J Refract Surg. 2017;33:592–7. 10.3928/1081597X-20170616-03. [DOI] [PubMed] [Google Scholar]
- 2.Hamam KM, Gbreel MI, Elsheikh R, Benmelouka AY, Ouerdane Y, Hassan AK, et al. Outcome comparison between wavefront-guided and wavefront-optimized photorefractive keratectomy: a systematic review and meta-analysis. Indian J Ophthalmol. 2020;68:2691–8. 10.4103/ijo.IJO_2921_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Li M, Cen Z. Excimer laser corneal refractive surgery in the clinic: a systematic review and meta-analysis. Comput Math Methods Med. 2022;2022:7130422. 10.1155/2022/7130422. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Ang M, Gatinel D, Reinstein DZ, Mertens E, Alió del Barrio JL, Alió JL. Refractive surgery beyond 2020. Eye. 2021;35:362–82. 10.1038/s41433-020-1096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez AH, Galvis V, Tello A, Parra MM, Rojas MÁ, Arba MS, et al. Fellow eye comparison between alcohol-assisted and single-step transepithelial photorefractive keratectomy: late mid-term outcomes. Rom J Ophthalmol. 2020;64:176–83. [PMC free article] [PubMed] [Google Scholar]
- 6.McDonnell PJ, Moreira H, Clapham TN. Photorefractive keratectomy for astigmatism. Initial clinical results. Arch Ophthalmol. 1991;109:1370–3. [DOI] [PubMed] [Google Scholar]
- 7.Sorkin N, Perez MA, Rootman DS. Excimer laser surface treatment: photorefractive keratectomy. In: Mannis MJ, Holland EJ, editors. Cornea: fundamentals, diagnosis and management. 5th ed. Amsterdam: Elsevier; 2022. p. 1682–94. [Google Scholar]
- 8.Duvdevan N, Mimouni M, Dominitz Y, Sela T, Munzer G, Achiron A, et al. Flap protection during laser-assisted in situ keratomileusis improves refractive outcomes in high myopic astigmatism. Clin Exp Vis Eye Res. 2019;2:11–7. 10.15713/ins.clever.22. [Google Scholar]
- 9.Stern C. New refractive surgery procedures in ophthalmology and the influence on Pilot’s fitness for flying. Eur J Med Res. 1999;4:382–4. [PubMed] [Google Scholar]
- 10.Skevas C, Katz T, Wagenfeld L, Richard G, Linke S. Subjective pain, visual recovery and visual quality after LASIK, EpiLASIK (flap off) and APRK—a consecutive, non-randomized study. Graefe’s Arch Clin Exp Ophthalmol. 2013;251:1175–83. 10.1007/s00417-012-2181-7. [DOI] [PubMed] [Google Scholar]
- 11.Tomás-Juan J, Murueta-Goyena Larrañaga A, Hanneken L. Corneal regeneration after photorefractive keratectomy: a review. J Optom. 2015;8:149–69. 10.1016/j.optom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golan O, Randleman JB. Pain management after photorefractive keratectomy. Curr Opin Ophthalmol. 2018;29:306–12. 10.1097/ICU.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 13.Müller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–42. 10.1016/S0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues EB, Farah ME, Bottós JM, Aggio FB. Nonsteroidal anti-inflammatory drugs (NSAIDs) in the treatment of retinal diseases. Retin Pharmacother. 2010. 10.1016/B978-1-4377-0603-1.00034-X. [Google Scholar]
- 15.Gaeckle HC. Early clinical outcomes and comparison between trans-PRK and PRK, regarding refractive outcome, wound healing, pain intensity and visual recovery time in a real-world setup. BMC Ophthalmol. 2021;21:181. 10.1186/s12886-021-01941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashemi H, Alvani A, Aghamirsalim M, Miraftab M, Asgari S. Comparison of transepithelial and conventional photorefractive keratectomy in myopic and myopic astigmatism patients: a randomized contralateral trial. BMC Ophthalmol. 2022;22:68. 10.1186/s12886-022-02293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 18.Zarei-Ghanavati S, Shandiz JH, Abrishami M, Karimpour M. Comparison of mechanical debridement and trans-epithelial myopic photorefractive keratectomy: a contralateral eye study. J Curr Ophthalmol. 2019;31:135–41. 10.1016/j.joco.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashemi H, Alvani A, Aghamirsalim M, Miraftab M, Asgari S. Comparison of transepithelial and conventional photorefractive keratectomy in myopic and myopic astigmatism patients: a randomized contralateral trial. BMC Ophthalmol. 2022;22(1):68. 10.1186/s12886-022-02293-2. (published 2022 Feb 11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gharieb HM, Awad-Allah MAA, Ahmed AA, Othman IS. Transepithelial laser versus alcohol assisted photorefractive keratectomy safety and efficacy: 1-year follow-up of a contralateral eye study. Korean J Ophthalmol. 2021;35:142–52. 10.3341/kjo.2020.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnenfeld ED, Holland EJ, Durrie DS, Raizman MB. Double-masked study of the effects of nepafenac 0.1% and ketorolac 0.4% on corneal epithelial wound healing and pain after photorefractive keratectomy. Adv Ther. 2007;24:852–62. 10.1007/BF02849978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.