Abstract
Schizophrenia (SZ) is a serious mental disorder that can mainly be distinguished by symptoms including delusions and hallucinations. This mental disorder makes difficult conditions for the person and her/his relatives. Electroencephalogram (EEG) signal is a sophisticated neuroimaging technique that helps neurologists to diagnose this mental disorder. Estimating and evaluating brain effective connectivity between electrode pairs is an appropriate way of diagnosing brain states in neuroscience studies. In this study, we construct a novel image from multi-channels of EEG based on the fusion of three effective connectivity, partial directed coherence (PDC), and direct directed transfer function (dDTF) and transfer entropy (TE) at three consecutive time windows. Then, this image was used as input of five well-known convolutional neural networks (CNNs) through transfer learning (TL) to learn patterns related to SZ patients to diagnose this disorder from normal participants from two public databases. Also, the majority voting method was used to improve these results based on ensemble results of the five CNNs, i.e., ResNet-50, Inception-v3, DenseNet-201, EfficientNetB0, and NasNet-Mobile. The highest average accuracy, specificity and sensitivity to diagnose SZ patients from healthy participants were obtained using EfficientNetB0 through the Leave-One-Subject-out (LOSO) Cross-Validation criterion equal to 96.67%, 96.23%, 96.82%, 95.15%, 94.42% and 96.28% for the first and second databases, respectively. Also, as we suggested, the ensemble approach of EfficientNetB0, ResNet-50 and NasNet-Mobile increased the accuracy by approximately 3%. Our results show the effectiveness of providing fused images from multichannel EEG signals to the ensemble of CNNs through TL to diagnose SZ than state-of-the-art studies.
Keywords: Electroencephalogram (EEG), Diagnose, Schizophrenia, Deep learning, Effective connectivity, Fusion
Introduction
Schizophrenia (SZ) is a chronic mental disorder that is mainly characterized by delusions and hallucinations (Krzystanek et al. 2019; Chatterjee et al. 2018). Due to these symptoms, these patients have difficult relations with family and surrounded people. Structure and function of the brain cortex of SZ patients are different from healthy participants. Therefore, interaction between cortical regions varies in these patients. Also, studies showed a decline in the gray matter volume and disruption in the integrity of white matter of these patients (Karlsgodt et al. 2010; Kubicki et al. 2005; DeLisi et al. 2022). Giraldo-Chica et al. (2018) declared a relation between deficiency of neural activity and cognition for these patients. Therefore, this disorder can be investigated and discriminated through brain mapping tools. Electroencephalography (EEG) is a brain mapping tool which widely used in neuroscience studies to evaluate mental states. Because this tool has good temporal resolution, affordable cost and available therefore, it is a suitable choice for this disease (Sabeti et al. 2011; Shalbaf et al. 2020; Khare and Bajaj 2022; Sairamya et al. 2022) and other mental disorders/states (Ullah et al. 2018; Shahabi et al. 2023; Bagherzadeh et al. 2022; Khan et al. 2022; de Paula et al. 2023; Zhou et al. 2023).
Extracting nonlinear features from EEG is a way to evaluate and detect SZ patients (Kutepov et al. 2020; Krishnan et al. 2020; Goshvarpour and Goshvarpour 2020; Akbari et al. 2021), for example, Kutepov et al. (2020) used the Lyapunov exponents. The largest Lyapunov exponent were calculated and compared from three measures of Rosenstein, Kantz and Wolf. The first method reveals the highest significant differences between SZ patients and healthy group using the student’s t-test method. Krishnan et al. (2020) extracted different types of entropy (approximate, sample, spectral, permutation and Singular Value Decomposition) from EEG signals that were decomposed using the multivariate empirical mode decomposition (MEMD) method. Then, appropriate features were reduced using the recursive feature elimination (RFE) method and classified via several machine learning methods such as the support vector machine (SVM) and random forest. The highest classification accuracy, sensitivity and specificity had been achieved 93%, 94% and 92%, respectively (SVM). Nonlinear features require a well knowledge about EEG signals characteristics.
The other way is extracting deep features using deep learning (DL) methods. DL is a challenging type of artificial intelligence (AI) that strongly solve computer vision problems. Recently, these methods are widely used in biomedical signal processing areas such as mental diagnosis. Long-short-term memory (LSTM) is a DL technique that is based on order dependency of time series. Chandran et al. (2021) extracted nonlinear features of approximate entropy and fractal dimension then applied them to an LSTM method and achieved the accuracy of 99%. Among DL techniques, Convolutional neural network (CNN) is the other effective technique that has the advantage of generalization and flexibility to process EEG signals (Craik et al. 2019). This technique was used in several neuroscience studies from EEG signals due to these advantages that causes high performance (Shahabi et al. 2023; Bagherzadeh et al. 2022; Korda et al. 2022). Also, CNNs were applied to detect SZ from EEG signals in (Phang et al. 2019; Shalbaf et al. 2020; Aslan and Akin 2022a, b; Korda et al. 2022; Sobahi et al. 2022). Generally, CNNs allow image inputs, therefore, one-dimensional EEG signals must be converted to two or three-dimensional image. The continuous wavelet transform (CWT) is a time-frequency method that was applied to build image from EEG signals of SZ patients (Shalbaf et al. 2020; Aslan and Akin 2022a; Korda et al. 2022; Sobahi et al. 2022). Also, Aslan and Akin (2022b) built images based on the Hilbert transform to detect schizophrenia from EEG signals. Moreover, Sun et al. (2021) decomposed EEG signals into three theta, alpha and beta frequency band then calculated the fuzzy entropy and fast Fourier transform (FFT) features from them. Then, constructed two-dimensional images based on them and fused CNN and LSTM models to detect SZ patients from healthy group and finally achieved the accuracy of 99.22%. These methods built two-dimensional images from each single EEG channels, therefore, inter-channel information is missing. In some studies, raw EEG channels are arranged to apply to one-dimensional CNN models (Singh et al. 2021).
One approach to transforming one-dimensional multichannel EEG signals into two-dimensional images suitable for deep neural network applications is through the concept of effective brain connectivity (Mullen 2010). Effective connectivity delves into the interdependence of different brain regions, describing the causal flow of information based on EEG channels. EEG proves to be a fitting tool for mapping effective brain connectivity. In other words, Effective connectivity characterizes the direction and spectral properties between localized EEG channels simultaneously. These techniques are used for visualization of time and frequency of multivariate dependent directed information flow and causality between any pair of channels in EEG signals. These brain connectivity measures are multichannel technique compared to complexity measures such as sample entropy, symbolic dynamic entropy and other entropies which are single channel assessment. These complexity measures extract information from single channel and inter-channel information is missing. These information from interaction of channels in effective brain connectivity measure help the recognition system to have higher accuracy and precision. Therefore, in this study we used effective connectivity technique. The resultant effective connectivity method is a four-dimensional location-location-time -frequency matrix that creates a two-dimensional color image. Transforming one-dimensional multichannel EEG signals into two-dimensional images was used to build images from Partial Directed Coherence (PDC) method, then, this image was applied to CNNs to recognize emotions (Bagherzadeh et al. 2022).
The present study contributes to the field of neuroscience by proposing a novel approach to detect differences between paranoid SZ patients and healthy participants using EEG signals. The approach involves the fusion of three well-known brain effective connectivity techniques, namely transfer entropy (TE), PDC, and direct Directed Transfer Function (dDTF), to create a unique image from 1D EEG signals of multi channels from two public databases. This innovative approach provides high-level information about the direction of information flow between channels, which is crucial for detecting differences between paranoid SZ patients and healthy participants. Also, dDTF Moreover, this study highlights the importance of combining different brain effective connectivity techniques and using advanced machine learning algorithms to improve the accuracy of detection. The created fused connectivity images were used to fine-tune powerful pre-trained CNN models as TL models to detect paranoid SZ patients accurately. This approach has the potential to provide a new way of detecting paranoid SZ patients, which could lead to earlier diagnosis and better treatment outcomes. Moreover, an ensemble approach based on the majority voting method is applied to improve the prediction validity.
Materials and methods
EEG signals
We tested the proposed method on two public EEG databases. the first one was from the Institute of Psychiatry and Neurology in Warsaw, Poland (Olejarczyk and Jernajczyk 2017) and the second was from the Mental Health Research Center (MHRC) (Borisov et al. 2005).
First database
These EEG signals were recorded from 14 (7 women and 7 men) paranoid SZ patients with mean age of 28.1 ± 3.7 and 14 age and sex matched healthy participants during relaxation with closed eyes (Olejarczyk and Jernajczyk 2017). Nineteen EEG signals with the length and sampling frequency of 12 min and 250 Hz were recorded based on the 10–20 international electrode placement system (Fp2, Fp1, F3, F7, Fz, F8, F4, C4, Cz, C3, P4, Pz, P3, T4, T3, T5, T6, O1, O2). Paranoid SZ patients meet the International Classification of Diseases (ICD)–10 criteria with F20.0 (World Health Organization 1993). Patients that were in early stage of SZ, pregnant or having brain pathologic/ neurologic disorders (epilepsy, Alzheimer’s disease or Parkinson’s) were not included in recording. Also, these patients must not take medicine from seven days before the test day. Recording protocol of multichannel EEG signals was approved based on the Ethics Committee of the Institute of Psychiatry and Neurology in Warsaw, Poland. An example of 19 EEG channels from one paranoid SZ patient and one healthy participant is shown in Fig. 1.
Fig. 1.
An example from 10 s of 19 EEG channels for (a) a healthy participant and (b) a paranoid SZ patient
Second database
These EEG signals were recorded from 45 children with mean age of 12 ± 3 with SZ disorders included infant SZ and schizotypical and schizoaffective disorders based on ICD-10 (F20, F21 and F25) (Borisov et al. 2005). Also, 39 age matched healthy children were recruited in recording EEG signals as normal group (these group had not treated by the chemotherapy). EEG signals were recorded during awake and relaxed condition with closed eyes. Sixteen EEG signals with the length and sampling frequency of one minute and 128 Hz were recorded based on the 10–20 international electrode placement system (F3, F7, F8, F4, C4, Cz, C3, P4, Pz, P3, T4, T3, T5, T6, O1, O2). Recording protocol of multichannel EEG signals was approved based on the Ethics Committee of the Mental Health Research Center (MHRC). An example of 16 EEG channels from one SZ patient and one healthy participant is shown in Fig. 2.
Fig. 2.
An example from 10 s of 16 EEG channels for (a) a healthy child and (b) a SZ patient
Preprocessing step
Multichannel EEG signals were preprocessed using The EEGLAB toolbox, MATLAB software (2021b version). These signals were filtered using two low pass and high pass Finite Impulse Response (FIR) Butterworth filters at 0.5 and 45 cut off frequencies.
Effective connectivity methods
Effective connectivity investigates and calculates brain information flow between pairs of electrodes in rest state or task and therefore can be a useful tool in revealing mental states or disorders such as emotion (Gao et al. 2020; Bagherzadeh et al. 2022), drowsiness (Huang et al. 2015) and schizophrenia (Phang et al. 2019). In this research, we estimated three appropriate measures, from linear and nonlinear divisions, dDTF, PDC and TE to represent interactions of EEG signals between SZ patients and healthy participants. These measures had highly effective results in neuroscience studies (Korzeniewska et al. 2003; Astolfi et al. 2007; Vicente et al. 2011; Huang et al. 2015; Phang et al. 2019; Gao et al. 2020; Bagherzadeh et al. 2022).
Partial directed coherence
PDC is a parametric and linear effective connectivity method that estimates influences between two EEG signals ( ) causally at frequency domain. PDC of
) causally at frequency domain. PDC of  th EEG channel from
th EEG channel from  th channel at frequency component of
th channel at frequency component of 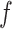 calculates by Eq. (1) (Astolfi et al. 2007; Bagherzadeh et al. 2022):
calculates by Eq. (1) (Astolfi et al. 2007; Bagherzadeh et al. 2022):
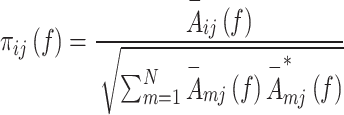 |
1 |
Where,  is a matrix whose belongs to autoregressive coefficients and
is a matrix whose belongs to autoregressive coefficients and 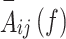 is its frequency component (
is its frequency component ( ) that is computed based on the model order of
) that is computed based on the model order of  via Eq. (2):
via Eq. (2):
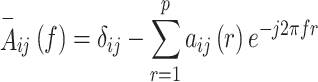 |
2 |
PDC is calculated in MATLAB by the Source Information Flow Toolbox (SIFT) version 0.1a (Mullen 2010).
Direct Directed transfer function
dDTF is another effective connectivity method that is linear and parametric and estimates frequency components between two pairs of EEG channels (Mullen 2010; Korzeniewska et al. 2003). This measure can be estimated for 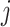 th and
th and 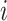 th channels from Eq. (3)
th channels from Eq. (3)
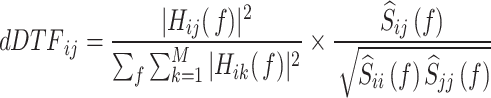 |
3 |
The transfer matrix of the system is indicated by 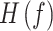 and the spectral density matrix of a Multi-Variable Auto-Regressive (MVAR) model indicates by
and the spectral density matrix of a Multi-Variable Auto-Regressive (MVAR) model indicates by 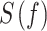
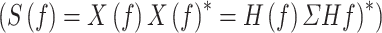 . dDTF was estimated in MATLAB via the Source Information Flow Toolbox (SIFT) version 0.1a (Mullen 2010).
. dDTF was estimated in MATLAB via the Source Information Flow Toolbox (SIFT) version 0.1a (Mullen 2010).
Transfer entropy
Transfer entropy is the nonlinear measure of effective connectivity that estimates the causal interactions between two signals or transient brain lobes based on conditional entropy. An advantage of this measure is that it does not require a priori assumption on connectivity patterns. TE measure was examined in the assessment of anesthesia (Ciprian et al. 2020), diagnosis of mental disorders such as Alzheimer’s and Mild Cognitive Impairment (McBride et al. 2015), detection of drowsiness (Huang et al. 2015), and recognition of emotion (Gao et al. 2020).
If we have two signals of 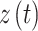 and
and 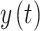 from a Markov process, the generalized Markov condition will be calculated from Eq. (4) (Niso et al. 2013):
from a Markov process, the generalized Markov condition will be calculated from Eq. (4) (Niso et al. 2013):
 |
4 |
Where
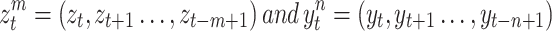 |
be 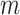 and
and  memory of Markov processes in
memory of Markov processes in  and
and 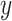 , respectively. Given to have the previous history
, respectively. Given to have the previous history  steps before, the probability of obtaining a value of
steps before, the probability of obtaining a value of 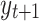 is on the right side of this equation and the probability given that we have both histories of
is on the right side of this equation and the probability given that we have both histories of 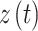 and
and 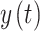 is calculated on the left side. If the dynamics or transition probabilities of
is calculated on the left side. If the dynamics or transition probabilities of  in the absence of causality from
in the absence of causality from 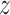 to
to 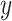 be independent of the past of
be independent of the past of 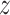 , then Eq. (4) is fully satisfied. Departure from this condition is calculated using divergence of the Kullback-Leibler between distributions of two probabilities to determine the TE from
, then Eq. (4) is fully satisfied. Departure from this condition is calculated using divergence of the Kullback-Leibler between distributions of two probabilities to determine the TE from 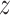 to
to  as Eq. (5):
as Eq. (5):
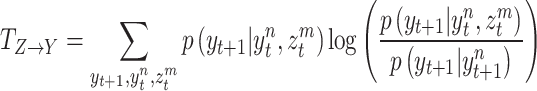 |
5 |
This equation computes flow of information from 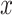 to
to  , directly. Therefore, the TE relation can be written based on Eq. (5) from the time series
, directly. Therefore, the TE relation can be written based on Eq. (5) from the time series  to
to 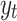 as Eq. (6):
as Eq. (6):
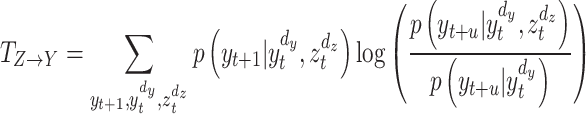 |
6 |
Where 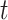 is the time-index (discrete value) and
is the time-index (discrete value) and 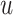 is the prediction time (discrete value).
is the prediction time (discrete value). 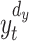 and
and  denote
denote 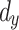 - and
- and 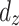 -dimensional delay vectors are defined as Eqs. (7) and (8), and
-dimensional delay vectors are defined as Eqs. (7) and (8), and  is the time delay.
is the time delay.
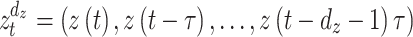 |
7 |
 |
8 |
TE is estimated in MATLAB via the HERMES connectivity toolbox (Niso et al. 2013).
Construct fused image
A novel image is constructed from multichannel EEG signals based on three linear and nonlinear effective connectivity methods, dDTF, PDC and TE. dDTF and PDC explicitly capture directional interactions between brain regions, distinguishing between driving and response regions. This directional information is valuable for understanding the flow of information within neural networks. dDTF and PDC allows for frequency-specific analysis of directed connectivity, providing insights into how different frequency bands contribute to information processing within the brain. This can be particularly useful in studying cognitive processes that are associated with specific frequency ranges. dDTF is also relatively robust to volume conduction and common sources of noise in EEG/MEG data. However, TE is a model-free measure of information flow, which means it can capture nonlinear and non-Gaussian dependencies between variables without assuming a specific model. In this method, the Wiener causality concept and the conditional mutual information in the context of information theory are combined. This makes these measures applicable to a wide range of systems, including those with complex and unknown dynamics. Therefore, each of these three methods has advantages and their combination can be very effective. After estimating three mentioned methos, three-time windows from each method are placed in a row consecutively and then, these are arranged in columns. This arrangement achieves the highest results in detection SZ patients from healthy participants. Figure 3 shows how the fused image is constructed.
Fig. 3.

Arrangement of a new fused connectivity image based on a fusion of three-time windows of EC measures (PDC, dDTF, and TE) with a size of 57 × 57 for the first database and 48 × 48 for the second. Each window is an EC measure with a size of 19 × 19 (for the first database) and 16 × 16 (for the second database). Three-time 5-second windows from each EC measure were arranged horizontally in an image, and PDC, dDTF, and TE were arranged vertically to construct an input fused EC image
CNN models along with transfer learning
CNN is the strongest division of deep learning methods with an integrated structure that extracts features, reduces features, and finally classifies with high performance among newly machine learning methods. A CNN is included three basic layers: the Convolutional layer (Conv) to extract low-level and high-level features, the pool layer to reduce the size of extracted feature maps in Conv and the Fully Connected (FC) layer to classify. There are some other layers such as the Rectified Linear Unit (ReLU) activation function that follows each Conv, the Softmax function that determines classes, and the cross-entropy that is the loss function. Also, there are two regularization techniques, the batch normalization and dropout, to prevent the overfitting problem. Pre-trained models are trained previously on huge databases like ImageNet (Krizhevsky et al. 2012) and places365 (Zhou et al. 2016). ImageNet has more than one million images from 1000 classes of animals and objects (Krizhevsky et al. 2012), and places365 includes images from 365 various places like parks, fields and subways (Zhou et al. 2016). Utilizing these networks with transfer learning causes fastness and easiness for training phase than from the scratch. Also, there are less requirement to record limited biomedical samples. Moreover, CNNs are more flexible and generalized than conventional machine learning methods for EEG signal processing tasks. Therefore, it is more reasonable to use these pre-trained networks as transfer learning models to detect SZ patients from normal class.
Transfer learning utilized a pre-trained CNN that has previously learned to extract useful features from object and animal images and uses it as a starting point to learn a new task using a smaller number of training images. Using a pre-trained CNN network with transfer learning is typically much quicker and simpler than training a CNN model with random weights. To do this, the classification layers that had 1000 neurons to classify 1000 classes on the ImageNet database were changed to 2 neurons to classify two classes. Also, in the optimization phase, the cross-entropy was used as the loss function, and the adaptive moment estimation optimizer (ADAM) algorithm was used. Five robust pre-trained CNNs ResNet-50 (He et al. 2016), NasNet-Mobile (Zoph et al. 2018), DenseNet-201 (Huang et al. 2017), Inception-v3 (Szegedy et al. 2016) and EfficientNetB0 (Tan and Le 2019) are used to extract deep features and classify two groups of SZ patients and healthy participants from built images. Table 1 compares details of these networks. Finally, an ensemble model was developed based on the best pre-trained CNN models using a majority voting technique (Kuncheva et al. 2014) to improve detection rate.
Table 1.
Details of the pre-trained CNN models
| Net | Depth | Size (MB) | Parameters (millions) |
|---|---|---|---|
| ResNet-50 | 50 | 96 | 25.6 |
| Inception-v3 | 48 | 89 | 23.9 |
| DenseNet-201 | 201 | 77 | 20 |
| EfficientNetB0 | 82 | 20 | 5.3 |
| NasNet-Mobile | * | 20 | 5.3 |
all of these CNNs except Inception-v3 have 224 × 224 × 3 input size (Inception-v3 has 299 × 299 × 3 input size)
Statistical metrics
The evaluation phase was based on the Leave-One-Subject-Out (LOSO) cross-validation method to detect SZ class from normal. In this method, samples from all participants except one were used to fine-tune the network, and samples from that one were used to test performance of them. This procedure repeats while all participants were used as test set (28 times for and the first database and 84 times for the second one). The accuracy, sensitivity and specificity measures were calculated each time and finally average and standard deviation of them are reported (Sokolova and Lapalme 2009). These measures are computed by Eq. (9) to Eq. (11):
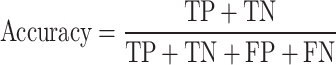 |
9 |
 |
10 |
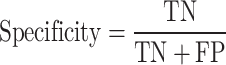 |
11 |
Where, true classification of SZ patients and healthy participants are counted in the true positive (TP) and true negative (TN) parameters, respectively. False classification of SZ patients as healthy participants is counted as false positive (FP) and vice versa as false negative (FN).
Results
Figure 4 shows the flowchart of the proposed SZ detection system from fused effective images and pre-trained CNN models. First, TE, PDC, and dDTF as the nonlinear and linear EC measures were calculated from five-second windows of EEG channels for each database, separately. Then, a new fused connectivity image is constructed. So that, three-time 5-second windows from each EC measure were arranged horizontally in an image, and PDC, dDTF, and TE were arranged vertically to construct an input fused EC image for the pre-trained CNN models. Therefore, a fused connectivity image with a size of 57 × 57 (48 × 48 for the second database) is created. The embedding dimension, number of neighbors and time delay to estimate TE were considered 8,4 and 10, respectively. Also, model order is selected 6 to estimate PDC and dDTF for both databases. Figure 5 represents an example of a fused EC image from one SZ patient (a) and healthy subject (b) for the first database.
Fig. 4.
Flowchart of the proposed system to detect SZ patients from healthy group from fused effective connectivity images and pre-trained CNNs
Fig. 5.
Fused connectivity images based on a fusion of three-time windows of EC measures (PDC, dDTF, and TE) with a size of 57 × 57 on the (a) SZ, and (b) normal (first database). Both dimensions are the location of EEG channels, and each element assigns the resultant EC value. Each window is an EC measure with a size of 19 × 19 (for the first database)
We trained and fine-tuned six pre-trained CNN models using fused connectivity images. To do this, the fully connected layer that had 1000 neurons to classify 1000 classes on the ImageNet database was changed to 2 neurons to classify SZ patients and healthy classes. Then, the classification layer was replaced by a new one to be matched for the new fully connected layer. Then, the Weight Learn Rate Factor and Bias Learn Rate Factor values were increased from 1 to 20 to make the fine-tune procedure faster in both mentioned layers than in the transferred (unchanged) layers. The initial learning rate was chosen at 0.0004 for all pre-trained CNN models. Also, the squared gradient decay factor, the mini-batch size, and max epochs were chosen 0.99, 64, and 30, respectively. Training curves of each pre-trained CNN model for both databases, which are average curves among LOSO cross-validation, are provided in Fig. 6 (a, b). Learning curves of all models show an exponential behavior before reaching a plateau and finally reaching to highest accuracy, which indicates the stability of all models for both databases. Vertical axes are accuracy values, and horizontal axes are epochs for each pre-trained CNN model during the training process.
Fig. 6.
Training progress of ResNet-50 (red color), NasNet-Mobile (black color), Inception-v3 (green color), DenseNet-201 (blue color) and EfficientNetB0 (magenta color) on fused images from (a) the first database and (b) the second database
Details of the performance of five mentioned pre-trained CNN models on TE, PDC, dDTF alone, and fused connectivity images to classify two SZ patients and healthy classes on new unseen data are represented by accuracy, sensitivity and specificity metrics in Tables 2 and 3 for the first and the second databases, respectively. Finally, an ensemble model was developed based on the best pre-trained CNN models using a majority voting technique to improve detection rate. The novel fused image and EfficientNetB0 achieved the highest accuracy among TE, PDC and dDTF images and pre-trained models (96.67% for the first database and 95.15% for the second database). Also, the novel fused connectivity images in the ensemble of EfficientNetB0, ResNet-50 and NasNet-Mobile achieved the highest accuracy of 99.61% and 96.43% for the first and the second databases, respectively. In comparison between three effective connectivity methods and five pre-trained CNN models separately for classifying two classes (SZ and healthy), the highest average accuracy was obtained using TE image and EfficientNetB0 by 94.31% and 92.52% for the first and the second databases, respectively. Also, ensemble of EfficientNetB0, ResNet-50 and NasNet-Mobile and this image achieves the average accuracy of 96.35% and 94.37% for the first and the second databases, respectively.
Table 2.
Detection results of five pre-trained CNN models alone and the best ensemble of these models (EfficientNetB0, ResNet-50 and NasNet-Mobile) on TE, PDC, dDTF alone and fused connectivity images for the classification of paranoid SZ patients from normal class (the first database) using LOSO Cross-Validation. The values are presented using: Mean (± SD).
| Image | CNN | Accuracy | Specificity | Sensitivity |
|---|---|---|---|---|
| Fused | EfficientNetB0 | 96.67 ± 0.44 | 96.23 ± 0.39 | 96.82 ± 0.58 |
| (PDC, TE, dDTF) | NasNet-Mobile | 95.02 ± 0.66 | 94.62 ± 0.54 | 95.42 ± 0.63 |
| ResNet-50 | 94.22 ± 0.71 | 93.56 ± 0.65 | 94.81 ± 0.65 | |
| DenseNet-201 | 93.90 ± 0.66 | 93.25 ± 0.45 | 94.27 ± 0.43 | |
| Inception-v3 | 93.69 ± 0.62 | 93.25 ± 0.52 | 94.13 ± 0.60 | |
| Ensemble | 99.51 ± 0.55 | 99.23 ± 0.55 | 99.74 ± 0.53 | |
| TE | EfficientNetB0 | 94.31 ± 0.52 | 93.39 ± 0.48 | 94.74 ± 0.50 |
| NasNet-Mobile | 93.72 ± 0.65 | 92.71 ± 0.65 | 94.22 ± 0.54 | |
| ResNet-50 | 93.48 ± 0.60 | 92.85 ± 0.69 | 93.75 ± 0.45 | |
| DenseNet-201 | 92.78 ± 0.64 | 92.30 ± 0.50 | 93.20 ± 0.60 | |
| Inception-v3 | 92.50 ± 0.63 | 92.15 ± 38 | 92.72 ± 0.62 | |
| Ensemble | 96.35 ± 0.52 | 95.60 ± 0.53 | 96.61 ± 0.54 | |
| dDTF | EfficientNetB0 | 92.17 ± 0.46 | 91.54 ± 0.39 | 92.30 ± 0.59 |
| NasNet-Mobile | 91.46 ± 0.52 | 91.06 ± 0.52 | 92.57 ± 0.60 | |
| ResNet-50 | 91.23 ± 0.55 | 90.53 ± 0.60 | 91.54 ± 0.57 | |
| DenseNet-201 | 90.34 ± 0.62 | 90.14 ± 0.55 | 90.75 ± 0.62 | |
| Inception-v3 | 90.02 ± 0.59 | 89.62 ± 0.46 | 90.43 ± 0.66 | |
| Ensemble | 94.33 ± 0.55 | 93.72 ± 0.42 | 95.11 ± 0.53 | |
| PDC | EfficientNetB0 | 92.84 ± 0.43 | 92.33 ± 0.45 | 92.53 ± 0.59 |
| NasNet-Mobile | 92.16 ± 0.67 | 91.50 ± 0.42 | 92.54 ± 0.62 | |
| ResNet-50 | 91.35 ± 0.65 | 90.64 ± 0.40 | 91.53 ± 0.64 | |
| DenseNet-201 | 91.39 ± 0.54 | 90.42 ± 0.35 | 91.64 ± 0.55 | |
| Inception-v3 | 91.14 ± 0.50 | 90.28 ± 0.35 | 91.50 ± 0.62 | |
| Ensemble | 95.25 ± 0.43 | 94.50 ± 0.40 | 95.56 ± 0.50 |
Table 3.
Detection results of five pre-trained CNN models alone and the best ensemble of these models (EfficientNetB0, ResNet-50 and NasNet-Mobile) on TE, PDC, dDTF alone and fused connectivity images for the classification of SZ patients from normal class (the second database) using LOSO Cross-Validation. The values are presented using: Mean (± SD).
| Image | CNN | Accuracy | Specificity | Sensitivity |
|---|---|---|---|---|
| Fused | EfficientNetB0 | 95.15 ± 0.53 | 94.42 ± 0.47 | 96.28 ± 0.49 |
| (PDC, TE, dDTF) | NasNet-Mobile | 94.73 ± 0.56 | 95.71 ± 0.54 | 94.26 ± 0.55 |
| ResNet-50 | 94.24 ± 0.64 | 93.41 ± 0.55 | 95.23 ± 0.55 | |
| DenseNet-201 | 94.13 ± 0.65 | 94.59 ± 0.54 | 93.45 ± 0.61 | |
| Inception-v3 | 92.21 ± 0.65 | 91.64 ± 0.66 | 93.58 ± 0.64 | |
| Ensemble | 96.43 ± 0.52 | 97.72 ± 0.54 | 96.19 ± 0.58 | |
| TE | EfficientNetB0 | 92.52 ± 0.63 | 91.37 ± 0.60 | 92.80 ± 0.57 |
| NasNet-Mobile | 91.64 ± 0.58 | 92.34 ± 0.62 | 90.84 ± 0.63 | |
| ResNet-50 | 91.29 ± 0.58 | 90.70 ± 0.57 | 91.66 ± 0.54 | |
| DenseNet-201 | 91.24 ± 0.71 | 91.69 ± 0.50 | 90.47 ± 0.64 | |
| Inception-v3 | 90.61 ± 0.76 | 91.39 ± 38 | 90.25 ± 0.69 | |
| Ensemble | 94.37 ± 0.62 | 94.12 ± 0.59 | 95.21 ± 0.56 | |
| dDTF | EfficientNetB0 | 90.42 ± 0.75 | 90.12 ± 0.67 | 91.21 ± 0.72 |
| NasNet-Mobile | 90.03 ± 0.64 | 90.22 ± 0.57 | 89.49 ± 0.63 | |
| ResNet-50 | 89.88 ± 0.62 | 90.16 ± 0.64 | 89.53 ± 0.59 | |
| DenseNet-201 | 88.55 ± 0.66 | 89.04 ± 0.69 | 88.14 ± 0.60 | |
| Inception-v3 | 89.46 ± 0.65 | 89.74 ± 0.58 | 89.19 ± 0.65 | |
| Ensemble | 91.73 ± 0.62 | 91.39 ± 0.61 | 92.82 ± 0.60 | |
| PDC | EfficientNetB0 | 91.88 ± 0.61 | 91.50 ± 0.60 | 92.25 ± 0.66 |
| NasNet-Mobile | 91.32 ± 0.65 | 89.24 ± 0.62 | 91.56 ± 0.65 | |
| ResNet-50 | 90.07 ± 0.56 | 90.35 ± 0.64 | 88.59 ± 0.57 | |
| DenseNet-201 | 89.22 ± 0.56 | 89.56 ± 0.69 | 90.39 ± 0.63 | |
| Inception-v3 | 88.02 ± 0.56 | 88.36 ± 0.69 | 87.45 ± 0.66 | |
| Ensemble | 92.55 ± 0.53 | 92.05 ± 0.59 | 93.29 ± 0.61 |
Discussion
In this study, a novel image was constructed based on fusion of a nonlinear effective connectivity measure (TE) and two linear effective connectivity measures (PDC and dDTF) from multichannel EEG channels from two databases. These images were constructed to automatically detect SZ patients and healthy participants using five powerful pre-trained CNN models (ResNet-50, Inception-v3, NasNet-Mobile, DenseNet-201 and EfficientNetB0) through transfer learning. In the other words, these particular models trained previously on the huge ImageNet database were transferred to extract deep features from newly built fused connectivity images and classify the two desired classes. To develop a more accurate and stable model, we combined the capabilities of all of these pre-trained CNN models by majority voting among their predictions and made an ensemble model which performs better than any of the base models. The best performance among three effective connectivity methods and mentioned pre-trained models separately belongs to TE images and EfficientNetB0 from 19 (16, second database) EEG signals (96.67% (first database) and 95.15%). Finally, the ensemble of EfficientNetB0, ResNet-50 and NasNet-Mobile, on new fused connectivity images gained a superior performance with an average accuracy of 99.51% and 98.46 for first and second databases, respectively.
The main contribution and advantages of this study is a way to represent a new image from 1D multi-channel EEGs based on the fusion of three well-known brain effective connectivity methods of TE, PDC and dDTF as input to the pre-trained CNN models. The Continuous Wavelet Transform (CWT) is the classical time-frequency method used to represent the 1D signals to the 2D image to feed pre-trained CNN models (Shalbaf et al. 2020; Korda et al. 2022; Aslan and Akin 2022a). These scalogram methods provide time and frequency information, but, effective connectivity methods used in this study provide nonlinear high-level contents related to SZ patients and healthy group obtained from the brain information flow between channels at separate regions. Between these two classes, the flow of information from different regions changes transiently, and this new fused effective connectivity image successfully estimates and represents them. As said earlier, the effective connectivity measure successfully was applied to process EEG signals for various applications (mental states), and our results demonstrate its effectiveness in detection of SZ patients.
In the comparison of three effective connectivity methods for classifying the two mentioned classes, the highest average accuracy was obtained using TE images. TE images in ensemble models had achieved higher accuracies compared to PDC and dDTF images for both databases (96.35% vs. 95.25% and 94.33% (for the first database) in Tables 2 and 94.37% vs. 92.55% and 91.73% (for the second database) in Table 3). This is because TE is based on the information theory and estimates the nonlinear relation of EEG channels; the other two measures are linear. Finally, the fusion of connectivity images provides more valuable information to detect the two mentioned classes. Observing Tables 2 and 3, we found that fusion of these connectivity methods in ensemble models had achieved higher accuracies than only images by TE, PDC, and dDTF for both databases (99.51% vs. 96.35%, 95.25%, and 94.33% for the first database and 96.43% vs. 94.37%, 92.55% and 91.73% for the second database, respectively).
The best performance to recognize two classes of SZ patients and healthy group among single pre-trained CNNs belongs to EfficientNetB0 with an accuracy of 96.67% and 95.15% on fused connectivity images from the first and second databases, respectively (Tables 2 and 3). This CNN designed from stem block (rescaling, normalization, zero padding, 2D convolution, batch normalization and activation layers) and five specific blocks that construct sub blocks and caused different structure from others. Despite its deeper architecture than ResNet-50 and Inception-v3, the other advantage of this network is being light. This CNN is designed for mobile phones and has 5.3 million parameters from ImageNet and occupies 20 Megabit; therefore, it could be an amazing choice to detect SZ patients and healthy participants quickly and precisely on a mobile phone or similar devices. Finally, a fusion of the best pre-trained CNN models of EfficientNetB0, ResNet-50 and NasNet-Mobile increased the accuracy by about 2∼3% for the first database (96.67% vs. 99.51% (novel fused image), 96.35% vs. 94.31% (TE), 95.25% vs. 92.84% (PDC) and 92.17% vs.94.33% (dDTF)) and about 1∼2% for the second database (95.15% vs. 96.43% (novel fused image), 92.52% vs. 94.37% (TE), 91.88% vs. 92.55% (PDC) and 90.42% vs.91.73% (dDTF) for the second database) and achieved the highest accuracy.
A comparison of obtaining results and recent related studies on the detection of two mentioned classes from EEG signals of the same database are mentioned in Table 4. The accuracy of this study for paranoid (the first database) or other types of SZ (the second database) detection system is the greatest, the newly proposed method has the advantage of fusion of effective connectivity methods and an ensemble of the most powerful pre-trained CNNs through the transfer learning approach that caused higher accuracy. In other words, we have presented fusion in input connectivity images and predictive output levels, i.e., a fusion of effective connectivity methods to create the most discriminative image from brain connectivity of sample population (SZ patients and healthy participants) and fusion in the output level of pre-trained CNNs.
Table 4.
Comparison of obtaining results and recent deep learning studies on detection of SZ patients and healthy group from both EEG databases
| Ref | Processing Method | Accuracy (%) | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|
| Phang et al. 2019 | multi-domain connectome CNN | 91.69 | 92.50 | 91.11 |
| Shalbaf et al. 2020 | CWT, CNN-SVM | 98.60 | 96.92 | 99.65 |
| Singh et al. 2021 | spectral features, Hjorth parameters, CNN, LSTM | 98.96 | 98.88 | 99.05 |
| Aslan and Akin 2022a | CWT, CNN |
99.5, 98 (second database) |
- |
99, 98 (second database) |
| Aslan and Akin 2022b | Hilbert spectrum image, pre-trained CNNs (VGG-16, DenseNet121, ResNet152, Inception V3) |
98.2, 96.02 (second database) |
- | - |
| Proposed method |
Fused effective connectivity image, ensemble of pre-trained CNNs (ResNet-50, NasNet-Mobile, EfficientNetB0) |
99.51, 96.43 (second database) |
99.23, 97.72 (second database) |
99.74, 96.19 (second database) |
However, limited EEG samples is an important limitation of deep learning models and can cause models vulnerable to bias and lack of generalizability. In this research, we tried to overcome this issue using transfer learning models. Another limitation of this study was high computational cost to do the fine-tuning and evaluation steps through the LOSO. This study also had high computational cost to fine-tune and evaluate through the LOSO.
The test results show that the accuracy, specificity, and sensitivity values produced in the first database are higher than in the second database. The reason for this can be that the first database has longer duration (12 min vs. 1 min) and higher number of EEG channels (19 vs. 16) which caused several images than the second database and therefore higher accuracy based on fine-tuning pre-trained CNNs on several images from the test set. Also, as the first database has lower subjects (28 vs. 84) than the second, it has lower variations among subjects and this caused higher performance.
Conclusion
This study proposes a novel approach to detect differences between paranoid SZ patients and healthy participants by creating a unique image from 1D EEG signals of multi channels. The approach involves the fusion of three well-known linear and nonlinear brain effective connectivity techniques, namely transfer entropy (TE), PDC, and direct Directed Transfer Function (dDTF), obtained from two public databases. These techniques provide high-level information about the direction of information flow between channels, which is essential for detecting differences between paranoid SZ patients and healthy participants. Then, these new images were used to fine-tune five particular pre-trained CNN models through the transfer learning approach to classify two classes of SZ patients and healthy participants. Among these models, the EfficientNetB0 outperformed and achieved the highest accuracy of 96.67% on new fused images. Finally, an ensemble model based on the best pre-trained CNN models of EfficientNetB0, ResNet-50, and NasNet-Mobile on these new images combined all capabilities fully and improves the performance with an average accuracy of 99.51%. Therefore, the outstanding performance achieved in this study shows the usefulness of the proposed method compared to deep learning and machine learning studies. This study highlights the importance of combining different brain effective connectivity techniques and using advanced machine learning algorithms to improve the accuracy of detection.
Overall, this approach has the potential to provide a new way of detecting paranoid SZ patients from fMRI data. In fMRI, image of each brain slice segmented into several regions and connectivity could be estimated from each region and use as unput of CNNs. In the future study, we will try to apply these novel images from multichannel EEG and utilize particular development of deep structures such as Long short-term memory (LSTM) neural network that is a recurrent cell with learning capability.
Author contributions
Sara Bagherzadeh: Conceptualization, Investigation, Methodology, Formal analysis, Coding, Writing – original draft. Ahmad Shalbaf: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The present article is financially supported by “Research Department of School of Medicine Shahid Beheshti University of Medical Sciences” (Grant No 29635).
Data availability
The first and second datasets can be accessed through https://repod.pon.edu.pl/dataset/eeg-in-schizophrenia and http://brain.bio.msu.ru/eeg_schizophrenia.htm, respectively.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akbari H, Ghofrani S, Zakalvand P, Sadiq MT (2021) Schizophrenia recognition based on the phase space dynamic of EEG signals and graphical features. Biomed Signal Process Control 69:102917 [Google Scholar]
- Aslan Z, Akin M (2022a) A deep learning approach in automated detection of schizophrenia using scalogram images of EEG signals. Phys Eng Sci Med 45:83–96 [DOI] [PubMed] [Google Scholar]
- Aslan Z, Mehmet A (2022b) Empirical mode decomposition and convolutional neural network-based approach for diagnosing psychotic disorders from EEG signals. Applied Intelligence: 1–13
- Astolfi L, Cincotti F, Mattia D, Marciani MG, Baccala LA, de Vico Fallani F, Babiloni F (2007) Comparison of different cortical connectivity estimators for high-resolution EEG recordings. Hum Brain Mapp 28(2):143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherzadeh S, Maghooli K, Shalbaf A, Maghsoudi A (2022) Recognition of emotional states using frequency effective connectivity maps through transfer learning approach from electroencephalogram signals. Biomed Signal Process Control 75:103544 [Google Scholar]
- Borisov SV, Kaplan AY, Gorbachevskaya NL, Kozlova IA (2005) Analysis of EEG structural synchrony in adolescents with schizophrenic disorders. Hum Physiol 31:255–261 [PubMed] [Google Scholar]
- Chatterjee I, Agarwal M, Rana B, Lakhyani N, Kumar N (2018) Bi-objective approach for computer-aided diagnosis of schizophrenia patients using fMRI data. Multimedia Tools Appl 77:26991–27015 [Google Scholar]
- Ciprian C, Masychev K, Ravan M, Reilly JP, Maccrimmon D (2020) A machine learning approach using effective connectivity to predict response to clozapine treatment. IEEE Trans Neural Syst Rehabil Eng 28(12):2598–2607 [DOI] [PubMed] [Google Scholar]
- Craik A, He Y, Contreras-Vidal JL (2019) Deep learning for electroencephalogram (EEG) classification tasks: a review. J Neural Eng 16:031001 [DOI] [PubMed] [Google Scholar]
- De Paula PO, da Silva Costa TB, de Faissol Attux RR, Fantinato DG (2023) Classification of image encoded SSVEP-based EEG signals using Convolutional neural networks. Expert Syst Appl 214:119096 [Google Scholar]
- DeLisi LE, Szulc KU, Bertisch HC, Majcher M, Brown K (2022) Understanding structural brain changes in schizophrenia. Dialogues in clinical neuroscience [DOI] [PMC free article] [PubMed]
- Gao Y, Wang X, Potter T, Zhang J, Zhang Y (2020) Single-trial EEG emotion recognition using Granger Causality/Transfer Entropy analysis. J Neurosci Methods 346:108904 [DOI] [PubMed] [Google Scholar]
- Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND (2018) Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol Psychiatry 83(6):509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshvarpour A, Goshvarpour A (2020) Schizophrenia diagnosis using innovative EEG feature-level fusion schemes. Phys Eng Sci Med 43:227–238 [DOI] [PubMed] [Google Scholar]
- He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. In Proceedings of the IEEE conference on computer vision and pattern recognition: 770–778
- Huang CS, Pal NR, Chuang CH, Lin CT (2015) Identifying changes in EEG information transfer during drowsy driving by transfer entropy. Front Hum Neurosci 9:570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Liu Z, Maaten LVD, Weinberger KQ (2017) Densely connected convolutional networks. CVPR IEEE Comput Soc : 2261–2269
- Karlsgodt KH, Sun D, Cannon TD (2010) Structural and functional brain abnormalities in schizophrenia. Curr Dir Psychol Sci 19(4):226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MS, Salsabil N, Alam MG, Dewan MA, Uddin MZ (2022) CNN-XGBoost fusion-based affective state recognition using EEG spectrogram image analysis. Sci Rep 12(1):14122 [DOI] [PMC free article] [PubMed]
- Khare SK, Bajaj V (2022) A hybrid decision support system for automatic detection of Schizophrenia using EEG signals. Comput Biol Med 141:105028 [DOI] [PubMed] [Google Scholar]
- Korda AI, Ventouras E, Asvestas P, Toumaian M, Matsopoulos GK, Smyrnis N (2022) Convolutional neural network propagation on electroencephalographic scalograms for detection of schizophrenia. Clin Neurophysiol 139:90–105 [DOI] [PubMed] [Google Scholar]
- Korzeniewska A, Mańczak M, Kamiński M, Blinowska KJ, Kasicki S (2003) Determination of information flow direction among brain structures by a modified directed transfer function (dDTF) method. J Neurosci Methods 125(1–2):195–207 [DOI] [PubMed] [Google Scholar]
- Krishnan PT, Raj ANJ, Balasubramanian P, Chen Y (2020) Schizophrenia detection using multivariate empirical Mode decomposition and entropy measures from multichannel EEG signal. Biocybernetics Biomedical Eng 40:1124–1139 [Google Scholar]
- Krizhevsky A, Sutskever I, Hinton GE (2012) Imagenet classification with deep convolutional neural networks. Adv Neural Inf Process Syst : 25
- Krzystanek M, Borkowski M, Skałacka K, Krysta K (2019) A telemedicine platform to improve clinical parameters in paranoid schizophrenia patients: results of a one-year randomized study. Schizophr Res 204:389–396 [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley RW, Shenton ME (2005) Evidence for white matter abnormalities in schizophrenia. Curr Opin Psychiatry 18(2):121–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncheva LI (2014) Combining pattern classifiers: methods and algorithms. Wiley
- Kutepov IE, Dobriyan VV, Zhigalov MV, Stepanov MF, Krysko AV, Yakovleva TV, Krysko VA (2020) EEG analysis in patients with schizophrenia based on Lyapunov exponents. Inf Med Unlocked 18:100289 [Google Scholar]
- McBride J, Zhao X, Munro N, Jicha G, Smith C, Jiang Y (2015) Discrimination of mild cognitive impairment and Alzheimer’s disease using transfer entropy measures of scalp EEG. J Healthc Eng 6(1):55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen T (2010) Source information Flow Toolbox (SIFT). An Electrophysiological Information Flow Toolbox for EEGLAB theoretical handbook and user Manual. Swartz Center for Computational Neuroscience and Institute for Neural Computation and Department of Cognitive Science, University of California, San Diego [Google Scholar]
- Nikhil Chandran A, Sreekumar K, Subha DP (2021) EEG-based automated detection of schizophrenia using long short-term memory (LSTM) network advances in machine learning and Computational Intelligence. Springer, Singapore, pp 229–236 [Google Scholar]
- Niso G, Bruña R, Pereda E, Gutiérrez R, Bajo R, Maestú F, Del-Pozo F (2013) HERMES: towards an integrated toolbox to characterize functional and effective brain connectivity. Neuroinformatics 11:405–434 [DOI] [PubMed] [Google Scholar]
- Olejarczyk E, Jernajczyk W (2017) Graph-based analysis of brain connectivity in schizophrenia. PLoS ONE 12(11):e0188629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang CR, Noman F, Hussain H, Ting CM, Ombao H (2019) A multi-domain connectome convolutional neural network for identifying schizophrenia from EEG connectivity patterns. IEEE J Biomedical Health Inf 24:1333–1343 [DOI] [PubMed] [Google Scholar]
- Sabeti M, Katebi SD, Boostani R, Price GW (2011) A new approach for EEG signal classification of schizophrenic and control participants. Expert Syst Appl 38(3):2063–2071 [DOI] [PubMed] [Google Scholar]
- Sairamya NJ, Subathra MSP, George ST (2022) Automatic identification of schizophrenia using EEG signals based on discrete wavelet transform and RLNDiP technique with ANN. Expert Syst Appl 192:116230 [Google Scholar]
- Shahabi MS, Shalbaf A, Nobakhsh B, Rostami R, Kazemi R (2023) Attention-based convolutional recurrent deep neural networks for the prediction of response to repetitive transcranial magnetic stimulation for major depressive disorder. Int J Neural Syst 33(02):2350007 [DOI] [PubMed] [Google Scholar]
- Shalbaf A, Bagherzadeh S, Maghsoudi A (2020) Transfer learning with deep convolutional neural network for automated detection of schizophrenia from EEG signals. Phys Eng Sci Med 43:1229–1239 [DOI] [PubMed] [Google Scholar]
- Singh K, Singh S, Malhotra J (2021) Spectral features based convolutional neural network for accurate and prompt identification of schizophrenic patients. Proc Institution Mech Eng Part H: J Eng Med 235(2):167–184 [DOI] [PubMed] [Google Scholar]
- Sobahi N, Ari B, Cakar H, Alcin OF, Sengur A (2022) A New Signal to Image Mapping Procedure and Convolutional neural networks for efficient Schizophrenia detection in EEG recordings. IEEE Sens J 22:7913–7919 [Google Scholar]
- Sokolova M, Lapalme G (2009) A systematic analysis of performance measures for classification tasks. Inf Process Manag 45:427–437 [Google Scholar]
- Sun J, Cao R, Zhou M, Hussain W, Wang B, Xue J, Xiang J (2021) A hybrid deep neural network for classification of schizophrenia using EEG Data. Sci Rep 11(1):1–1633414495 [Google Scholar]
- Szegedy C, Vanhoucke V, Ioffe S, Shlens J, Wojna Z (2016) Rethinking the inception architecture for computer vision Proceedings of the IEEE conference on computer vision and pattern recognition: 2818–2826
- Tan M, Le Q (2019) Efficientnet: Rethinking model scaling for convolutional neural networks. International conference on machine learning: 6105–6114
- Ullah I, Hussain M, Aboalsamh H (2018) An automated system for epilepsy detection using EEG brain signals based on deep learning approach. Expert Syst Appl 107:61–71 [Google Scholar]
- Vicente R, Wibral M, Lindner M, Pipa G (2011) Transfer entropy—a model-free measure of effective connectivity for the neurosciences. J Comput Neurosci 30:45–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, G (1993) The ICD-10 classification of Mental and behavioural disorders. Diagnostic Criteria for Research
- Zhou B, Khosla A, Lapedriza A, Torralba A, Oliva A (2016) Places: An image database for deep scene understanding arXiv preprint arXiv:1610.02055
- Zhou X, Ling BWK, Zhou Y, Law NF (2023) Phase space reconstruction, geometric filtering based Fisher discriminant analysis and minimum distance to the Riemannian means algorithm for epileptic seizure classification. Expert Syst Appl 219:119613 [Google Scholar]
- Zoph B, Vasudevan V, Shlens J, Le QV (2018) Learning transferable architectures for scalable image recognition. Proceedings of the IEEE conference on computer vision and pattern recognition: 8697–8710
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The first and second datasets can be accessed through https://repod.pon.edu.pl/dataset/eeg-in-schizophrenia and http://brain.bio.msu.ru/eeg_schizophrenia.htm, respectively.







