Abstract
Diabetic Peripheral Neuropathy (DPN) is a nerve damage that is treated with painkillers and steroids which have the drawback of interference with other medications and the dangers of side effects. Novelty of the proposed work is to develop a Near Infrared Region (NIR) based non-invasive therapy device called ‘DPNrelief-1.0V’developed with a 890 nm wavelength diodes. DPNrelief-1.0V delivers a total dosage of 6.174 J/cm2 with heat absorption by tissue of 61.74 Joules at 30 minutes. The device was tested by carrying out a pilot study with 8 patients where 4 were treatment group and control group. The DPNrelief-1.0V is validated by Nerve Conduction Study (NCS) test. The degenerated nerves pre-therapy showed less amplitude, Conduction Velocity (CV) and latency which was improved post-therapy by 100% in amplitude of nerve signal, 100% in CV and a decrease of 36.2% in latency. Independent t-test was conducted to find the difference between control and treatment, wherein a p value < 0.05 was obtained depicting significant difference between two groups. Furthermore, the performance of the device is validated by one-way test repeated measures Analysis of Variance (ANOVA), wherein a p value of < 0.05 was obtained depicting a difference in nerve condition pre-and post-therapy. The performance of DPNrelief-1.0V has outperformed Anodyne therapy device with lesser dosage, treatment time and portability and in curing the symptoms of DPN. DPNrelief-1.0V finds its potential in the field of medicine for treating DPN.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78144-5.
Keywords: Diabetic peripheral neuropathy, Near infrared spectroscopy therapy, Nerve conduction study, Hardware design, Therapy device
Subject terms: Biophysics, Neuroscience, Health care, Health occupations, Medical research, Neurology, Engineering, Optics and photonics
Introduction
DPN is an immedicable complication leading to pain, burning sensation, numbness, and irreversible nerve damage affecting hands, feet and legs1–4 which is treated by maintaining strict target blood glucose levels, medications, topical treatments, physical therapy, Transcutaneous Electrical Nerve Stimulation (TENS), and lifestyle changes, i.e., quitting smoking, alcohol, etc. but drawbacks of existing treatments are the side effects and intervention of the drugs with other diseases5,6.
Related literature
Photobiomodulation is implemented in research related to cancer7, Bowen’s disease8, knee osteoarthritis (OA)9, chronic neck pain10, post-surgical wounds11, and general-purpose applications which opens up the possibilities in DPN research12,13. The literature7–9 exceeds dosage recommendations proposed by International Commission on Non-Ionizing Radiation Protection (ICNIRP)14,15, which may lead to side effects. A dosage of 10 (J/cm2) is suggested per session, whereas harmful effects can be observed at higher doses8. Radiation and Limits of Exposure Recommendations are mentioned in Supplementary Material. Phototherapy finds its application in treating diabetic and related disorders in an optical window of 400-905 nm wavelength7–13. The wavelengths are chosen based on the target area of treatment and penetration depth as elaborated and depicted in Supplementary Fig. 1. So far, with the proper selection of wavelength and safe radiation, no research work is carried out in developing a phototherapy device for DPN.
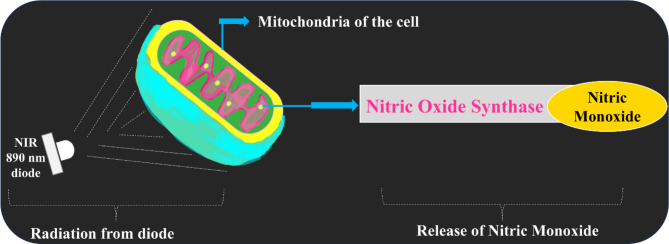
In a collective literature on diabetic disorders, 800–890 nm wavelength are chosen. The working principle in this therapeutic window is associated with release of Nitric Monoxide (NO) due to NIR irradiance as depicted in Fig. 1. NO is a vasodilator and neurotransmitter that prevents blood vessels and muscles from tightening16–22 thus improving blood circulation, rejuvenation, and vasodilation7,22. Irradiance of NIR on human skin is elaborated in Supplementary Material. In a study of avoiding falls due to DPN, Anodyne therapy system with sixty diodes of 890 nm treated 252 patients, showing a 78% of decrease in falls,79% decrease in fear of falling,72% increase in daily activities23. In a study of treating diabetic wounds, 808 nm laser is implemented on 120 rats, achieving reduced inflammation, enhanced epithelialization, and collagen fiber synthesis with energy densities of 5 J/cm2 and power densities of 0.1–0.3W/cm224. In a corresponding study of diabetic ulcers, thirty-two 890 nm and four 660 nm diodes achieved 90% healing with average power of 500 mW, yielding 100 mW/cm2 irradiance at day 90 with a fluence of 3.0 J/cm225. The research gaps identified from the existing literature are as follows:
Standard treatment time and fixed dosages are delivered, but personalized treatments based on the size of the affected area, pain intensity, treatment time, calculated dosages, and patients’ history with the condition are required for faster recovery.
The impact of pain relief medications is not considered, raising doubts if the improvement is with the device or medications.
None of the literature has focused on considering the gold standard diagnostic tests to validate the device’s effectiveness pre-and post-treatment23–25.
Fig. 1.
Effect of NIR on cellular chromophores localized in Mitochondria of the cell.
The literature implies a possibility of designing a phototherapy device for DPN by addressing the above issues. Our motivation stems from the need for an efficient, reliable, long-term efficacy, and a safe therapy device for treating DPN. Expanding from the current literature, and overcoming the research gaps, our work proposes the DPNrelief-1.0V, a non-invasive, low-cost, lightweight, controllable, user-friendly, reliable, and portable therapy device. It is validated by performing a NCS test pre-and post-treatment where the precision of the device implies its application for healing and treating pain, burning sensation, and numbness, for better DPN management. The contribution of the proposed work is as follows:
The proposed novel therapy device, DPNrelief-1.0V is a non-invasive, low-cost, flexible, lightweight, operation-controllable, user-friendly, reliable, and portable.
Pain relief passes after an average of 3 sessions, burning sensation after 4, loosening and softening of the affected area after 5, and sensation reversal from numbness after 6; over 5 sessions are required on average for curing DPN.
With a total delivered dosage of 6.174 J/cm2 in 30 min, the device ensures safety while minimizing treatment duration.
Motor axonal neuropathy and sensory axonal neuropathy can be treated with DPNrelief-1.0V where the unstimulated nerves are stimulated post-therapy.
The proposed work is organized as follows: Sect. 2 presents the material and methods, including NIR for treating DPN, radiation and regulations, hardware design, DPN detection scale, healthcare data standards, and dosage calculations. Section 3 presents the results and discussion where simulation and working of DPNrelief-1.0V, clinical trials, validation and comparison with previous studies. The exposition on the conclusion is presented in Sect. 4.
Methods
The current section presents the Study Population and Healthcare Data Standards, Hardware Design of the Proposed Device, Operation and Experimental Procedure of DPNrelief-1.0V, Dosage Calculations of the Proposed Device.
Study population and healthcare data standards
Standard healthcare measures are thoroughly taken to minimize and prevent the risk of bacteria from known and unknown sources. Face masks and gloves are regularly changed with sanitizing of hands. The following are the steps taken for maintaining standard healthcare protocol and representation of data characteristics:
Ethical clearance is obtained from SRM Medical College Hospital & Research Centre with Ethical clearance number 8562/IEC/2023. All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent is taken from all patients before gathering any data and providing treatment.
Doctors from SRM Medical College Hospital & Research Centre have contributed to the current research.
Patient health records including name, age, sex, HbA1c, BMI, contact details, history of diabetes, history of DPN, days of stay in the hospital, medication history, medications on which the inpatients are during the hospital stay, and other health complications are recorded.
The affected area of the patient is cleaned with isopropyl alcohol before giving every session of the therapy and the therapy pad is cleaned with isopropyl alcohol after every session.
In this work, the patients are acquired based on inclusion criteria of at least 1 year of clinical diagnosis with diabetes mellitus and DPN. Furthermore, the patients are selected based on similar treatment protocol specially with history of no painkillers or steroids. The study population of 8 patients i.e., 4 for treatment group and 4 for control group are considered with age > 45 years.The study population was determined by doctors to study the effect of device on small population, patients with similar treatment regimen, demographics of patient population, based on frequency of participants,sources and logistics. Patient demographics are presented in Table 1. Patients with pregnancy, lactating women, hypoglycemic episodes, seizure disorder, and cancer are excluded from the study due to potential bias and interference of their medications with the treatment.
Table 1.
Patient demographics of pilot study from treatment and control group (N = 8).
| Mean age | 55 years |
| SD | 6.4 years |
| Age in Range | > 45 years |
| Gender | F = 6, M = 2 |
| Inclusion criteria | Male and female diabetic patients who are clinically diagnosed from diabetes mellitus and DPN for at least 1 year with similar treatment regimens, specifically with a history of no painkillers or steroids. |
| Exclusion criteria | Patients with pregnancy, lactating women, hypoglycemic episodes with unconsciousness, seizure disorder, and cancer are excluded from the study. |
SD, standard deviation; F, female; M, male.
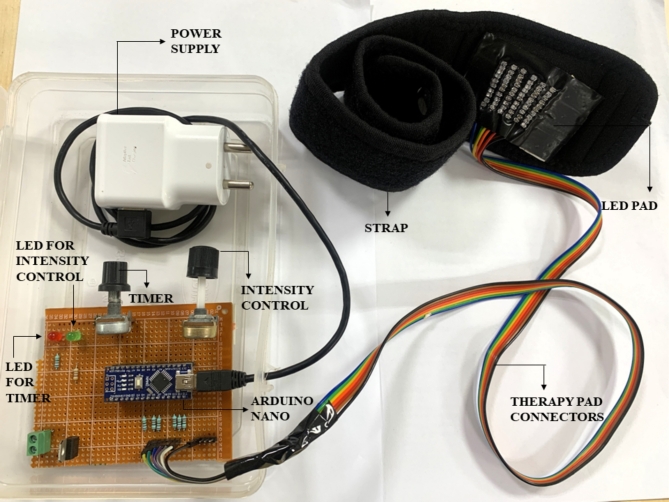
Hardware design of the proposed device
The hardware design of the proposed system is formulated on the optical therapeutic window of NIR spectrum. The block diagram of DPNrelief-1.0V is depicted in Supplementary Fig. 2. It consists of a power supply, Arduino nano as a microcontroller, timer, radiation intensity control, red, green, led, and a therapy pad with strap belt. In the proposed work, Surface mount (SMT) diodes of 890 nm are chosen for flexibility of therapeutic pad. The penetration depth of 890 nm is till 5 to 6 mm from surface of the skin where motor and sensory nerves are present19,21. The number of diodes is chosen based on the dosage calculated aligning to the radiation safety guidelines proposed by ICNIRP15,26. For effective penetration, the diodes with 17° angle of half intensity,100 mA of forward current, and radiant intensity of 25 mW/sr is chosen. The diodes were fabricated on a Polyvinyl chloride (PVC) material for flexibility in an array of 6 × 10 with Computer Numerical Control (CNC) machine as shown in the Supplementary Fig. 3. The area covered by array of diodes is 30 mm × 60 mm. Distance between each dioide is 5 mm. The fabricated PVC substrate was mounted on a multipurpose orthopedic brace strap with an adhesive. DPNrelief-1.0V operates with 5V power supply and 2A current. The detailed dosage calculations from Eq. (1) to Eq. (13) has been described in the Supplementary Material. The dosage calculated at 30 min is calculated as 6.174 (J/cm2) as mentioned in Eq. (8).The dermal heat observed by the tissue is 61.74 J as calculated in Eq. (9).Therapy pad connectors are slots designed for connecting the therapy pad to the Arduino’s pulse width modulator (PWM) pins for dosage intensity control. The PWM duty cycle is proportional to the output power of the diodes as calculated in Eq. (3) i.e., 1.8 w. The duty cycle is operated from 1 to 100%. The intensity is controlled at 25%, 50%,75%, and 100%. It can be observed from Eq. (10) to Eq. (13) that as the output power increases, the treatment time decreases. With each increase in the duty cycle, the output power increases, leading to increased dosage delivery. The weight of the whole hardware is 200 g with length of 4 inches and breadth of 3 inches making the system light and portable.
Operation and experimental procedure of DPNrelief-1.0V
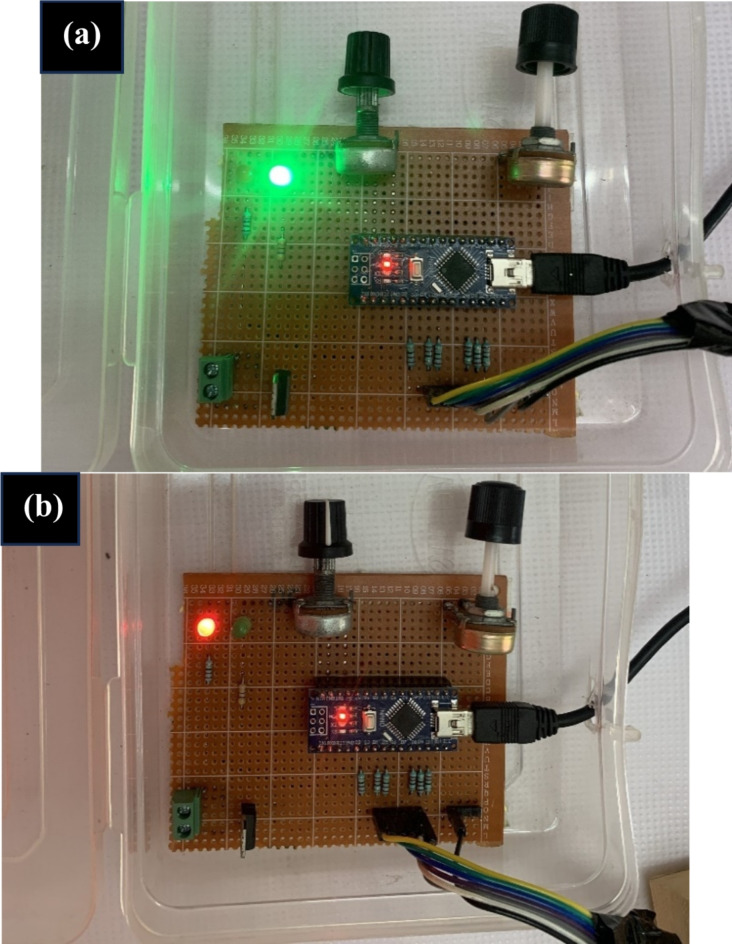
The working model of novel proposed hardware DPNrelief-1.0V is presented in Fig. 2. Two control signals are programmed in Arduino IDE to control the intensity, and timer in the device. The radiation intensity can be adjusted in 4 turns from potentiometer as shown in Fig. 2. Continuous dosage delivery of 30 min was programmed in the device. The operation of DPNrelief-1.0V is illustrated in Fig. 3 with dosage delivery ON and OFF condition in Fig. 3(a) and (b). The LED connected to intensity control turns ON (green) during dosage delivery and automatically switches off after 30 min, while the timer activates a red LED to signal the end of the operation. The experimental procedure is carried out by laying the patient in supine position and the device is switched ON. The desired dosage intensity between 0–25%-50%-75%-100% and desired time of delivery is also set. The device is turned OFF if the patient experiences any discomfort. The therapy is continued till the end of dosage if there are no signs of discomfort. Body temperature of the patient is noted at the start and end of the therapy to note if there is any temperature rise.
Fig. 2.
Proposed hardware DPNrelief-1.0V.
Fig. 3.
Operation of DPNrelief-1.0V (a) Dosage delivery with ON condition (b) End of dosage delivery.
The therapy is validated using NCS which is a gold standard electrodiagnostic test for diagnosing DPN. Conduction velocity (CV), amplitude, distal Latency (L) are parameters of Motor Nerve Conduction Velocity (MNCV), Sensory Nerve Conduction Velocity (SNCV), and F-wave from NCS report for identifying Motor and Sensory axonal neuropathy in DPN. CV is calculated to examine nerve’s strength and function whereas reduced or absent amplitude imply nerve dysfunction/damage. The L measures the time from stimulation of nerve to its activation27–29. Anatomy of the lower limbs effected by DPN, along with NCS and its parameters of measurement are presented in Supplementary Fig. 4, and Supplementary Fig. 5(a), 5(b),and Fig. 5(c). The dosage and treatment duration are based on the NCS report analysis. Visual to Analog Scale (VAS) is considered before initiating therapy session to access the presence or decrease in intensity of symptoms26,27,30. VAS is elaborated in Supplementary Fig. 6. The parameters of measurement from MNCV, F-Wave, and SNAP for diagnosing DPN are tabulated in Supplementary Table 1.
The radiant exposure is subjected to the primary principle of reciprocity. According to the Bunsen-Roscoe law of photobiology, the effect of treatment depends on the dosage given, which is also known as radiant exposure. The specifications of the device are tabulated in Table 2. The dosage is derived as the product of radiant exposure and treatment time in Eq. (1) as14,31,32,
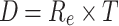 |
1 |
D = dosage, Re = radiant exposure, T = treatment time.
Table 2.
Specifications of DPNrelief-1.0V for therapy of 30 min.
| Metric | Value |
|---|---|
| P | 1.8 w |
| TPrad | 420 mw/sr |
| Radiant intensity | 1.68 w |
| Irradiance | 3.43 × 104 (w/m2) |
| Optical Efficiency | 23% |
| Dosage | 6.174 (J/cm2) |
| Dermal heat absorbed by tissue | 61.74 Joules |
P = power, TPrad = total radiant power.
Results
This section presents the results of the developed DPNrelief-1.0V, where Implementation of DPNrelief-1.0V in a Pilot Study where Experimental Validation of DPNrelief-1.0V, Statistical Validation of DPNrelief-1.0V is discussed.
Implementation of DPNrelief-1.0V in a pilot study
DPNrelief-1.0V is implemented in a pilot study where the device is tested on four patients (1 male and 3 female) from treatment group and compared the results with the 4 patients of control group who received no treatment. The proforma of patients in treatment group are tabulated in Table 3. It can be observed that the patients had been diagnosed with diabetes mellitus for a duration ranging from 7 to 17 years, and had been living with DPN for 1 to 2 years. It can be inferred from their medications and other complications that all patients followed a similar treatment regimen without any painkillers/steroids reinforcing that the outcomes are based solely as a result of the proposed therapy device.
Table 3.
Proforma of patients while treating from DPNrelief-1.0V.
| P | S | Age (years) |
HbA1C (%) |
BMI (kg/m2) |
Duration with DM (years) |
Duration with DPN (years) |
Type of DPN | No. of therapy sessions on each limb | Medications | Other complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 55 | 9.2 | 54 | 8 | 1 | Motor axonal neuropathy | 8 | Febuget 40, Shelcal 500 mg, Eltroxin 100mcg, Avas 40, Ecospirin75, Ceftriaxone 1gm |
Chromic kidney disease, Hypothyroid |
| 2 | M | 52 | 5.8 | 52 | 7 | 2 | Sensory axonal neuropathy | 14 | Udapa-M500, Rosuva gold, Prolomet XL, Telma 20 mg, Kerendia. |
Chromic kidney disease, Cardiovascular disease |
| 3 | F | 66 | 7.9 | 30 | 15 |
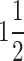
|
Sensory axonal neuropathy | 13 | Amlong 5 mg, Metosartan 50 mg, Verifica. M 50 mg, Rosuvastatin 20 mg, Saroglctazar 4 mg, Duloxetiene 20 mg, Ferisome | Hypothyroid, Lumbar radiculopathy |
| 4 | F | 49 | 9.8 | 18.44 | 17 | 1 | Motor axonal neuropathy | 11 |
Verifica M 500 mg/50 mg, Lantus 10U, Shelcal 500 mg |
Low appetite, Periarthritis shoulder, moderate non-proliferative diabetic neuropathy |
P = Patient numbers = Sex, HbA1C = Glycated Hemoglobin, DM = Diabetes Mellitus, DPN = Diabetic Peripheral Neuropathy, No = Number.
The results from NCS test are tabulated in Supplementary Table 2 for control group and Supplementary Table 3 for treatment group pre-and post-therapy. The highlighted section in indicates the improvement of CV, amplitude and L. From Supplementary Table 2, zero to low amplitude, low CV and increased latencies can be observed for Control group, which are similar to the treatment group pre-therapy, as shown in Supplementary Table 3. The hyphen presents parameter which is not measured for the specific nerve.
Experimental validation of DPNrelief-1.0V
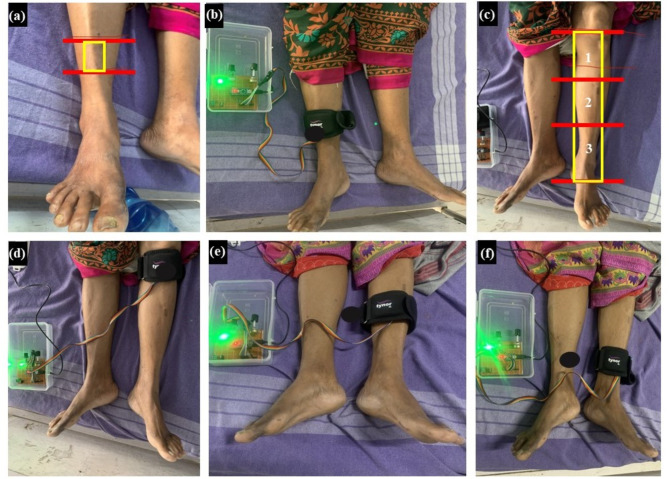
In the treatment group, two patients were diagnosed with motor axonal neuropathy and two with sensory axonal neuropathy. The affected areas from DPN are highlighted with red lines, with painful spots in yellow, as illustrated in Fig. 4. Other patients’ figures are presented in the Supplementary Fig. 7(a) to Fig. 7(n). NCS report outcome of a patient is shown in Table 4, highlighting nerve function improvements: nearing the threshold with CV > 40 m/s for MCNV and SNCV, amplitude > 10 mv for MNCV, amplitude > 10 µv for SNCV, L > 3.5 ms, L > 3 ms for MNCV and SNCV, Fmin < 40 ms and Fmax < 30 ms for F-wave. Noticeable subjective improvements in the treatment group are observed post-therapy by 100% in amplitude, 100% in CV, and 36.2% decrease in latency of Common Peroneal Nerve (CPN), Posterior Tibial Nerve (PTN), and Sural Peroneal Nerve (SPN), of the MNCV, and SNCV. For patients with partially stimulated/fully stimulated nerves, improvements in amplitude, CV, and latency were observed, indicating improvement in nerve function post-therapy, regardless of motor or sensory axonal neuropathy. Nerves effected due to demyelinating peripheral neuropathy (permanent nerve damage) remained unstimulated post-therapy. It was also observed that it took longer time to cure for the patients with longer duration of diabetes mellitus and DPN while gender distribution had no impact on treatment. 3 sessions are observed to reduce the pain, 4 sessions to reduce the burning sensation,5 sessions for loosening and softening of the affected area,6 sessions to feel the sensation after numbness. An average of 5 sessions are observed for curing DPN. Overall, symptomatic improvements in the patients were observed in the treatment group post-therapy demonstrating a positive effect of DPNrelief-1.0V.
Fig. 4.
DPNrelief-1.0V treatment applied to different areas for 30 min each (a) Affected area on right limb (b) Treatment on affected area at right limb (c) Affected area on left limb divided into sections for treatment (d) Treatment on affected area at right limb- first section (e) Treatment on affected area at right limb- second section (f) Treatment on affected area at right limb- third section.
Table 4.
MNCV, F-WAVE and SNCV Outcome of DPNrelief-1.0V in a patient of treatment group.
| P. no. | Test | CPN | PTN | SPN | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R.A | R.H | L.A | L.H | R.A | R.P | L.A | L.P | RM | L.M | R.S | L.S | ||
| Pre-therapy | ||||||||||||||
| MNCV | CV | 0 | 43.2 | 0 | 0 | 0 | 41.2 | 0 | 35.3 | – | – | – | – | |
| A | 3.09 | 2.40 | 4.76 | 3.58 | 11.2 | 7.92 | 12.7 | 10.5 | – | – | – | – | ||
| L | 4.31 | 13.0 | 5.12 | 15 | 4.81 | 14.5 | 4.62 | 13.9 | – | – | – | – | ||
| F-wave | Fmin | 55.7 | – | 55.5 | – | 57.3 | – | 57.3 | – | – | – | – | – | |
| Fmax | 58.6 | – | 59.2 | – | 63.5 | – | 59 | – | – | – | – | – | ||
| SNCV | CV | – | – | – | – | – | – | – | – | 30.5 | 40.0 | 44.8 | 33.5 | |
| A | – | – | – | – | – | – | – | – | 2.72 | 7.95 | 6.16 | 3.3 | ||
| L | – | – | – | – | – | – | – | – | 4.58 | 3.50 | 3.12 | 4.1 | ||
| Post-therapy | ||||||||||||||
| MNCV | CV | 0 | 44.7 | 0 | 39.5 | 0 | 45.4 | 0 | 38.8 | – | – | – | – | |
| A | 4.14 | 3.33 | 4.98 | 3.41 | 8.11 | 6.11 | 13.2 | 9.33 | – | – | – | – | ||
| L | 4.94 | 13.4 | 5 | 14.6 | 5.19 | 14 | 5.56 | 15.8 | – | – | – | – | ||
| F-wave | Fmin | 35.3 | – | 34.5 | – | 31.6 | – | 34.3 | – | – | – | – | – | |
| Fmax | 28.6 | – | 28 | – | 29.2 | – | 20.8 | – | – | – | – | – | ||
| SNCV | CV | – | – | – | – | – | – | – | – | 40.5 | 43.3 | 62.2 | 54.2 | |
| A | – | – | – | – | – | – | – | – | 10.1 | 10.9 | 10.7 | 10.9 | ||
| L | – | – | – | – | – | – | – | – | 3.55 | 3.23 | 2.55 | 2.55 | ||
CPN = common peroneal nerve, PTN = peroneal tibial nerve, SPN = sural peroneal nerve, RA = right ankle, R.H = right head of fibula, L. A = left ankle, L.H = left head of fibula, R.P = right pop fossa, L.P = left pop fossa, R.M = right mid leg, L.M = left mid leg, R.S = right sural nerve, L.S = left sural nerve, MCNV = motor nerve conduction velocity, SNCV = sensory nerve conduction velocity.
Statistical validation of DPNrelief-1.0V
The results are validated with statistical analysis using independent t-test between control, treatment group and one-way repeated measures of Analysis of Variance (ANOVA) between pre-and post-therapy of treatment group. The statistical analysis is carried out using IBM SPSS Statistics 27 software.
Independent t-test between control and pre-therapy treatment group
The NCS outcome of control group and treatment group from Supplementary Table 2 and 3 were analyzed by employing independent t-test to access the difference between the groups. The test was performed on variables i.e., CV, A, L, Fmin and Fmax for MCNV, F-Wave and SNCV. The significance was set at p < 0.05 where p values greater than 0.05 implies no difference and less than 0.05 indicates significant differences33. All assumptions of independent t-test normality, independence, and equal variances were met. It can be observed from Table 5 that p < 0.05 is obtained for MCNV whereas p > 0.05 is obtained for F-Wave and SNCV. It can be inferred that there is no statistical difference between the control group and pre-therapy treatment group patients allowing to continue further analysis.
Table 5.
Independent T-test between control and treatment group.
| Test | Variables | p value | t value |
|---|---|---|---|
| MCNV | CV | 0.2 | 0.9 |
| A | 0.07 | 2.2 | |
| L | 0.09 | 0.7 | |
| F-wave | Fmin | 0.4 | 0.8 |
| Fmax | 0.6 | 0.4 | |
| SNCV | CV | 0.3 | 1.0 |
| A | 0.2 | 1.2 | |
| L | 0.1 | 11.1 |
CV = conduction velocity, A = amplitude, L = latency, df = degrees of freedom.
One-way repeated measures ANOVA between treatment group of pre-and post-therapy
The NCS outcome under treatment group consists of one independent factor with two levels i.e., pre-and post-therapy from Supplementary Table 3. The dependent variables are nerve measurements taken repeatedly under each level. One-way repeated measures ANOVA is chosen as the measurements are taken on the same patient. The significant assumption of repeated measures ANOVA is the test of sphericity which requires the variances of the differences between all pairs of conditions to be equal34,35. It was tested by Mauchly’s test where Greenhouse-Geiser corrections are carried out when assumption was violated. As depicted in Table 6, p < 0.05 is obtained indicating statistically significant differences between pre-and post-therapy. These results imply that the therapy had a significant effect on the nerve function in the treatment group.
Table 6.
One-way repeated measures ANOVA on pre-and post-treatment group.
| Test | Variables | p-value | df |
|---|---|---|---|
| MCNV | CV | .003 | 5 |
| A | .007 | 5 | |
| L | .001 | 5 | |
| F-wave | Fmin | .018 | 5 |
| Fmax | .033 | 5 | |
| SNCV | CV | .013 | 5 |
| A | .001 | 5 | |
| L | .001 | 5 |
CV = conduction velocity, A = amplitude, L = latency, df = degrees of freedom.
Discussion
The proposed work is the first among the few studies to design and develop a therapy device and test it in real-time on DPN patients. The photobiomodulation at 890 nm with NIR absorbance in the novel device has been shown to cure DPN.The device is non-invasive, low-cost, flexible, lightweight, operation-controllable, user-friendly, reliable, and portable. The proposed device offers significant benefits in treating DPN without the use of pain-killers or steroids, delivering a dosage of 6.174 J/cm2 in 30 min, which is lower than the dosage reported in literature works.
The key highlights of the current work are: i.) An 100% increase in amplitude,100% in CV and a reduction of 36.2% in latency can be observed. ii.) DPNrelief-1.0V proved to treat motor axonal neuropathy and sensory axonal neuropathy. iii.) Previously unstimulated nerves showed activation post-therapy iv.) Clear progression was followed in symptom relief: an average of 3 sessions reduced pain, 4 sessions reduced burning sensation, 5 sessions for loosening and softening of the affected area, 6 sessions to reverse numbness, and an average of 5 sessions for curing DPN. v.) DPNrelief-1.0V maintained stable body temperature without exceeding normal temperature of 98.6°F and no skin irritation during the therapy, implying the device’s safety. vi.) The device allows personalized treatment based on size of the affected area, pain intensity, treatment time and patients’ history. viii.) None of the patients required pain relief medications post-therapy.
After an observation period of one-three months, patients reported relief from pain, burning sensation, tingling sensation, and numbness. According to the neurologists and diabetologist, the DPNrelief-1.0V provided subjective relief from DPN symptoms which was validated by objective improvements in NCS reports. However, it was suggested by the neurologists that the remaining unstimulated nerves, even after the therapy, could be attributed due to long-term damage in the nerves caused by years of diabetes. They proposed that despite the absence of any DPN symptoms in these patients, continuous therapy sessions might reverse nerve damage overtime.
The novel DPNrelief-1.0V is compared to the previously developed non-invasive therapy devices. Unlike the previous studies, the proposed device is clinically tested with the gold standard of diagnosing DPN using the NCS test, and is user-controllable to avoid overdosage and underdosage which was not attempted in prior studies23–25. Patient safety is prioritized by monitoring body temperature changes and discomfort with a dosage of less than 6.174 J/cm2, factors missed in previous works23–25. The long-term effectiveness of the proposed device is validated through NCS test after treatment and monthly clinical follow-ups where the patient showed no signs of DPN symptoms, which is not attempted in previous studies23–25. The device’s efficacy is tested under normal operating conditions, including exposure to temperature variations, and interference of dust and sweat on skin, demonstrating its reliability in real-world conditions, a step not address in all of the previous works24,25.
However, there are few limitations of the proposed work: i.) The LED pad of DPNrelief-1.0V is small which limits treatment area thus increasing therapy duration to cover larger areas. ii.) Therapy must be administered without any interruptions which requires patients to adhere to regular treatment schedule for optimal improvement. iii.) The device must be strictly used under medical supervision and is not designed for self-treatment at home to ensure safe dosage delivery and monitoring patient responses. iv.) Device is portable and not a wearable. As a future work, the LED pad will be designed to cover the larger treatment areas. The device may be designed as a wearable that may benefit patients who require hands-free therapy. Incorporating from the neurologists’ and diabetologists’ opinion, our future work is to provide continuous therapy to DPN patients to identify the possibility of reversing DPN in patients whose nerves are unstimulated post-therapy, with and without subjective symptoms. The implication from higher degree of improvement observed in NCS test results and the statistical validation highlight the importance of developing customized treatment plans, treatment titrations, a non-invasive alternative for optimal diagnosis. Therefore, with the precision of DPNrelief-1.0V, it can be employed in hospitals and clinics as a one-time purchase gadget offering a practical solution for diabetic management.
Conclusion
The novel NIR therapy device, DPNrelief-1.0V, has been clinically proven to potentially treat DPN symptoms and enhancing the nerve condition in both motor and sensory neuropathy. Our work addresses health disorder affecting millions worldwide, aligning with Sustainable Development Goals (SDG); SDG 3: Good Health and Well-being, SDG 9: Industry, innovation and infrastructure, SDG 10: Reduced inequalities. This new device is non-invasive, enduring potential benefits as it operates without interfering with other medications, or introducing any side effects related to radiation exposure. The timing of the session can be fixed at the patient’s convenience enhancing patients’ accessibility and compliance. The device is a one-time investment without the need for additional attachments, implying low cost and affordability in low-income populations. The potential of DPNrelief-1.0V provides a promising non-invasive alternative for treating DPN offering practical and approachable to improve patient outcomes and supports universal health coverage.
Supplementary Information
Acknowledgements
The authors would like to thank the patients who volunteered for the proposed work. The authors would like to thank K.Dilly Babu,Project Engineer, Department of ECE,SRM Institute of Science and Technology, Kattankulathur-Tamil Nadu, India, and A.Sethu Raman, Senior Technical Assistant, Department of ECE,SRM Institute of Science and Technology, Kattankulathur-Tamil Nadu, India for their support in designing the circuit for the proposed device. The authors would like to thank Dr.S.Robert Wilson, Professor, M.D (Paediatrics) ,D.M (Neurology), SRM Medical College Hospital and Research Center, Kattankulathur-Tamil Nadu, India for reviewing the reports and his guidance in contributing towards this research. The authors would also like to thank Mr.A.Thangamani, Neurotechnologist, Department of Neurology, SRM Medical College Hospital and Research Center, Kattankulathur-Tamil Nadu, India for his guidance on NCS test procedure and NCS reports.
Author contributions
S.V.K.R.R: Investigation, Methodology, Writing, Reviewing, Visualization. P : Investigation, Reviewing, Visualization, Supervision, Validation. N.Z: Investigation, Reviewing E.K: Investigation, Writing V.P: Reviewing S.V: Reviewing C.A: Writing M.D: Writing NR: Validation KJ: Investigation, Reviewing, Validation MG: Investigation, Reviewing, Validation.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78144-5.
References
- 1.Pop-Busui, R. et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care40(1), 136–154. 10.2337/dc16-2042 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llewelyn, J. G. The diabetic neuropathies: types, diagnosis and management. J. Neurol. Neurosurg. Psychiatry74(90002), 1519. 10.1136/jnnp.74.suppl_2.ii15 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang, H. et al. New perspective in diabetic neuropathy: from the periphery to the brain, a call for early detection, and precision medicine. Front. Endocrinol.10, 10. 10.3389/fendo.2019.00929 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diabetic neuropathy: Types, symptoms, and causes. www.medicalnewstoday.com. https://www.medicalnewstoday.com/articles/245310#types (2019, March 19).
- 5.Ali, R. A. Management of diabetic neuropathy. Malays. J. Med. Sci.10(2), 27–30 (2003). [PMC free article] [PubMed] [Google Scholar]
- 6.Ang, L., Cowdin, N., Mizokami-Stout, K. & Pop-Busui, R. Update on the management of diabetic neuropathy. Diabetes Spectrum31(3), 224–233. 10.2337/ds18-0036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gálvez, E. N. et al. Analysis and evaluation of the operational characteristics of a new photodynamic therapy device. Photodiagn. Photodyn. Ther.37, 102719. 10.1016/j.pdpdt.2022.102719 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Moseley, H. et al. Ambulatory photodynamic therapy: a new concept in delivering photodynamic therapy. Br. J. Dermatol.154(4), 747–750. 10.1111/j.1365-2133.2006.07145.x (2006). [DOI] [PubMed] [Google Scholar]
- 9.Liu, K., Chen, H., Wang, Y., Wang, M. & Tang, J. Wearable flexible phototherapy device for knee osteoarthritis. Electronics10(16), 1891. 10.3390/electronics10161891 (2021). [Google Scholar]
- 10.Gur, A. et al. Efficacy of 904 Nm gallium arsenide low level laser therapy in the management of chronic myofascial pain in the neck: a double-blind and randomize-controlled trial. Lasers Surg. Med.35(3), 229–235. 10.1002/lsm.20082 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Lagan, K., Alyson Clements, B., McDonough, S., & Baxter, G. Low Intensity laser therapy (830nm) in the management of minor postsurgical wounds: A controlled clinical study. Lasers Surg. Med.28(1), 27–32. 10.1002/1096-9101(2001)28:1%3C27::aid-lsm1013%3E3.0.co;2-4 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Phan, D. T. et al. Development of a LED light therapy device with power density control using a Fuzzy logic controller. Med. Eng. Phys.86, 71–77. 10.1016/j.medengphy.2020.09.008 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Kohli, H., Srivastava, S., Sharma, S. K., Chouhan, S. & Oza, M. Design of programmable LED based phototherapy system. Int. J. Opt. 1–8. 10.1155/2019/6023646 (2019).
- 14.International commission on non-ionizing radiation protection icnirp guidelines on limits of exposure to incoherent visible and infrared radiation. [DOI] [PubMed]
- 15.Akhalaya, M. Y., Maksimov, G. V., Rubin, A. B., Lademann, J. & Darvin, M. E. Molecular action mechanisms of solar infrared radiation and heat on human skin. Ageing Res. Rev.16, 1–11. 10.1016/j.arr.2014.03.006 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Walton, D. M., Minton, S. D. & Cook, A. D. The potential of transdermal nitric oxide treatment for diabetic peripheral neuropathy and diabetic foot ulcers. Diabetes Metab. Syndr. Clin. Res. Rev.13(5), 3053–3056. 10.1016/j.dsx.2018.07.003 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Finlayson, L. et al. Depth penetration of light into skin as a function of wavelength from 200 to 1000 nm. Photochem. Photobiol.98(4), 974–981. 10.1111/php.13550 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Van Tran, V., Chae, M., Moon, J.-Y. & Lee, Y.-C. Light emitting diodes technology-based photobiomodulation therapy (PBMT) for dermatology and aesthetics: Recent applications, challenges, and perspectives. Opt. Laser Technol.135, 106698. 10.1016/j.optlastec.2020.106698 (2021). [Google Scholar]
- 19.Ash, C., Dubec, M., Donne, K. & Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci.32(8), 1909–1918. 10.1007/s10103-017-2317-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson, T. A., & Morries, L. Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiat. Dis. Treat. 2191. 10.2147/ndt.s78182 (2015). [DOI] [PMC free article] [PubMed]
- 21.Huang, Y.-Y., Sharma, S. K., Carroll, J., & Hamblin, M. R. Biphasic dose response in low level light therapy – an update. Dose-Response9(4), dose-response.1. 10.2203/dose-response.11-009.hamblin (2011). [DOI] [PMC free article] [PubMed]
- 22.Chung, H. et al. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng.40(2), 516–533. 10.1007/s10439-011-0454-7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell, M. W., Carnegie, D. H. & Burke, T. J. Reversal of diabetic peripheral neuropathy with phototherapy (MIRETM) decreases falls and the fear of falling and improves activities of daily living in seniors. Age Ageing35(1), 11–16. 10.1093/ageing/afi215 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Lau, P. et al. Photobiostimulation effect on diabetic wound at different power density of near infrared laser. J. Photochem. Photobiol. B: Biol.151, 201–207. 10.1016/j.jphotobiol.2015.08.009 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Minatel, D. G., Frade, M. A. C., França, S. C. & Enwemeka, C. S. Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers Surg. Med.41(6), 433–441. 10.1002/lsm.20789 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Klimek, L. et al. Visual analogue scales (VAS): Measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care. Allergo J. Int.26(1), 16–24. 10.1007/s40629-016-0006-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavee, J. Nerve conduction studies: Basic concepts. Handbook of Clinical Neurology160, 217–224. 10.1016/B978-0-444-64032-1.00014-X (2019). [DOI] [PubMed] [Google Scholar]
- 28.Britannica. Year in Review | Timeline of Historical Events | Britannica. www.britannica.com. https://www.britannica.com/topic/2023-The-Year-in-Review (2023)
- 29.Lakra, B., Mohapatra, S., & Satapathy, U. Study on electrophysiological changes of peripheral nerves in type 2 diabetes mellitus. Natl. J. Physiol. Pharm. Pharmacol.0, 1. 10.5455/njppp.2022.12.08273202153082021 (2021).
- 30.Bouhassira, D. & Attal, N. Diagnosis and assessment of neuropathic pain: The saga of clinical tools. Pain152(Supplement), S74–S83. 10.1016/j.pain.2010.11.027 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Joe. Complete guide to light therapy dosing. Red Light Man. https://redlightman.com/blog/complete-guide-light-therapy-dosing/ (2016).
- 32.Maung, M. M. et al. DC Motor angular position control using PID controller with friction compensation. Int. J. Sci. Res. Publ.8(11), 8321. 10.29322/ijsrp.8.11.2018.p8321 (2018). [Google Scholar]
- 33.Jasrai, L. One-Way ANOVA Repeated Measures 19.1 Introduction, Feb. 2021.
- 34.Kim, H.-Y. Statistical notes for clinical researchers: A one-way repeated measures ANOVA for data with repeated observations. Restor. Dent. Endod.40(1), 91. 10.5395/rde.2015.40.1.91 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schober, P. & Vetter, T. R. Repeated measures designs and analysis of longitudinal data. Anesthesia Analgesia127(2), 569–575. 10.1213/ane.0000000000003511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.