Abstract
Although the uneven neuropsychological profile of William Syndrome (WS) is well established, less is known about social perception and how profile characteristics may affect the ability to predict other’s intentions, a main hallmark of social cognition. This study aimed at examining the neuropsychological profile, including social perception, of adolescents and adults with WS, and at verifying which neuropsychological outcome might account for their social prediction ability. Twenty-six individuals with WS were administered a comprehensive neuropsychological assessment, and a virtual reality scenario designed to assess social prediction in a dynamic, everyday life-like context. We found that social perception was a relative strength of the profile, although a dissociation emerged between impaired verbal ToM and relatively spared low-level components. Peaks and valleys were reported in other domains consistently with the expected profile. Both spatial and facial memory were significantly associated with the performance at the social prediction task. Results clarified that social perception per se should not be considered as typically impaired in WS. Weaknesses and strengths in specific abilities, particularly spatial and facial memory, might affect the ability to understand others’ intentions in WS beyond domain-specific mechanisms. These findings might inform future syndrome-specific rehabilitative interventions.
Keywords: Williams syndrome, Social perception, Social cognition, Theory of mind, Visual-spatial memory, Virtual reality
Subject terms: Social behaviour, Neurodevelopmental disorders
Introduction
Williams syndrome (WS) is a rare genetic disorder caused by microdeletion of 25–27 genes on chromosome 7q11.23, with an estimated prevalence of 1 in 7500 live births1. This condition is associated with characteristic physical features (e.g., distinctive facies, cardiovascular diseases and connective tissue abnormalities) and a specific pattern of cognitive impairments and social behaviour problems.
Most individuals with WS have a mild to moderate intellectual disability, even though a small percentage show a global intellectual functioning within the average2,3. Beyond intellectual disability, it is the uneven neuropsychological profile of WS that has long since gained attention from the medical-scientific community4–6. In details, a recent review7 reported, as characteristics of WS, deficits in visuospatial tests assessing memory, construction and discrimination, difficulties in executive functions, specifically inhibition and planning ability, and sensorimotor processing. Verbal working memory and language were pointed to as relative strengths of the WS profile, despite the acquisition of social-pragmatic language might be particularly impaired8,9. The uneven neuropsychological profile of WS is thought to be related to a specific dysfunction of the visual dorsal stream, which is the occipito-parietal network involved in spatial analysis and perceptual integration of visual stimuli10–12. The altered structural and functional connectivity of the visual dorsal stream in WS would affect the interlinked frontal and temporal networks, with effects on visuospatial processing and sensorimotor integration as well as higher-order cognitive functions13.
Hypersociability and overfriendliness are other peculiar features of WS; however, research documented specific challenges within the social behaviors of individuals with WS, such as poor social relationships, social disinhibition, and increased anxiety14–16. By comparing WS with Autism Spectrum Disorder, recent studies helped highlighting that, though social motivation and interest might be high17, individuals with WS may present specific impairments in social cognition18–20. In fact, while previous studies proposed that social cognition, and particularly Theory of Mind (ToM), could be spared in WS21,22, a growing body of evidence has shown deficits in different social perception skills, from facial affect recognition to understanding mental states23–26. Moreover, although facial recognition may be relatively preserved27, literature has consistently documented atypical tuning and attention to facial stimuli28–30. Still, social cognition of individuals with WS has not been fully considered in the broader context of their neuropsychological profile. This issue undermines claims that social perception skills may be relatively impaired or spared in this syndrome.
Importantly, most of the studies focused only on one or few specific domains, such as language, executive functions or visuospatial processing, and often adopted tests from different batteries; since cognitive functions are strongly interdependent, these issues limit the reliability of these results31–33. A comprehensive assessment of different cognitive areas including social perception with a co-normed test battery might provide new insights into the neuropsychological profile and social cognition of WS, considering differences between domains and within a single domain.
The first aim of this work was to provide a detailed neuropsychological profile of WS patients through the administration of the NEPSY-II neuropsychological battery34, which includes social perception subtests, along with “classical” neuropsychological domains. The choice of a multi-domain battery, which was validated in a single wide sample, allowed us to overcome methodological limitations of previous studies and to obtain reliable comparisons within the same individual and group, even without the recruitment of a new control group35. This way, it was possible to explore the neuropsychological profile at multiple levels, from between-domain comparisons to differences within the same cognitive domain, disentangling the contribution of specific subcomponents to the observed phenotype. For each participant, weaknesses in the profile were estimated on the basis of both chronological age and mental age, in order to highlight specific deficits at the individual level rather than focusing only on the group means36. Along with the previously described neuropsychological profile3, we anticipated that social perception would not be a weakness per se; however, we expected differences between its verbal and non-verbal components8,9.
Within a developmental framework, deficits in basic level processes would pose constraints to cognitive development, with cascading effects on later outcomes, particularly on more complex abilities such as social skills37,38. Accordingly, previous studies documented that, in congenital atypical development conditions, early impairments in basic sensorimotor and cognitive abilities can result into impaired social functioning39–41. In this vein, specific neuropsychological features in WS might account for social functioning in everyday life contexts, providing new insights into the developmental mechanisms of social cognition in this syndrome.
As a second aim, we investigated whether and which neuropsychological outcome might account for the ability to predict others’ intentions, assessed through a virtual reality (VR) scenario replicating an everyday life context. This ability is considered to be a fundamental hallmark of social cognition42 and was indicated as specifically impaired in WS43. Through VR, we could create immersive, ecologically sound scenarios, while maintaining a laboratory setting and control of the variables of interest44,45. The VR task exploited a design based on probabilistic learning of social events to assess social prediction. In a motivating and child-friendly virtual environment, individuals with WS were asked to anticipate the behaviour of competing avatars. This task was previously developed and adopted with patients with cerebellar malformations46, given the main functions of the cerebellum in prediction and error-signalling47. In this population, this task was documented to be associated with different levels of social processing, from action prediction to theory of mind48. Importantly, patients with WS present substantial alteration of cerebellar structure which are associated to their motor, cognitive and affective profile49,50. Accordingly, we expected that specific features of the neuropsychological profile of WS, particularly impairments in visuospatial processing, may be associated to social prediction41,51.
Results
For the Raven matrices, the mean Z-score of the whole sample was -3.22 ± 0.31, with scores ranging from -6.22 to 0.24 and 77% of participants scoring two SD below the normative mean. These results indicated a mild to moderate intellectual disability on average, although approximately one of four individuals fell within the normal range.
Scaled scores calculated for each domain, subtest, and subcomponent of the NEPSY-II, are reported in Table 1.
Table 1.
Performance at the NEPSY-II.
| Domain | Subtest | Subcomponent | Scaled scores | Individual weaknesses for chronological age (%) | Individual weaknesses for mental age (%) |
|---|---|---|---|---|---|
| Attention and executive functions | − 1.7 ± 0.8 | 85 | 65 | ||
| Visual attention | 2.4 ± 1.3 | 62 | 38 | ||
| Inhibition | − 4.5 ± 1.2 | 88 | 73 | ||
| Naming | − 0.4 ± 1.4 | 88 | 42 | ||
| Inhibition | − 8.3 ± 2.4 | 85 | 73 | ||
| Switching | − 4.7 ± 1.6 | 88 | 81 | ||
| Animal sorting | 2.4 ± 0.5 | 58 | 38 | ||
| Language | 0.8 ± 0.7 | 85 | 58 | ||
| Comprehension of instructions | 0.0 ± 1 | 81 | 69 | ||
| Speeded naming | 1.6 ± 1.2 | 53 | 42 | ||
| Memory and learning | − 1.7 ± 0.6 | 96 | 81 | ||
| Memory for faces | 7.1 ± 1 | 19 | 19 | ||
| Memory for designs | − 9.9 ± 1.1 | 96 | 96 | ||
| Content | − 10.4 ± 1.8 | 96 | 88 | ||
| Spatial | − 16.1 ± 2.1 | 96 | 92 | ||
| Word list interference | 2.2 ± 0.7 | 62 | 46 | ||
| Repetition | 3.0 ± 0.4 | 77 | 46 | ||
| Interference | 1.4 ± 1.3 | 58 | 46 | ||
| Sensorimotor functions | − 1.5 ± 1.2 | 92 | 69 | ||
| Fingertip tapping | − 3.8 ± 2.2 | 85 | 58 | ||
| Imitating hand positions | − 6.1 ± 1.5 | 96 | 88 | ||
| Manual motor sequences | 5.5 ± 0.7 | 35 | 27 | ||
| Social Perception | 2.5 ± 0.6 | 58 | 42 | ||
| Theory of mind | Verbal part | − 3 ± 1.2 | 88 | 69 | |
| Contextual part | 6.4 ± 0.9 | 27 | 27 | ||
| Affect recognition | 4.3 ± 0.8 | 35 | 31 | ||
| Visuospatial processing | − 0.8 ± 0.6 | 88 | 62 | ||
| Design copying | -5.7 ± 1 | 92 | 88 | ||
| Motor | − 10.3 ± 1.9 | 100 | 88 | ||
| Global | − 13.9 ± 2.3 | 88 | 88 | ||
| Local | − 4 ± 1.5 | 85 | 73 | ||
| Block construction | 1.1 ± 0.4 | 92 | 77 | ||
| Picture puzzles | − 1.2 ± 1.1 | 77 | 58 | ||
| Geometric puzzles | 2.8 ± 0.8 | 61 | 54 | ||
Scaled scores of the NEPSY-II battery obtained in each domain, subtest, and subcomponent, and percentage of participants with individual weaknesses with respect to both chronological and mental ages. Scaled scores are reported as mean ± SEM.
Neuropsychological domain comparisons
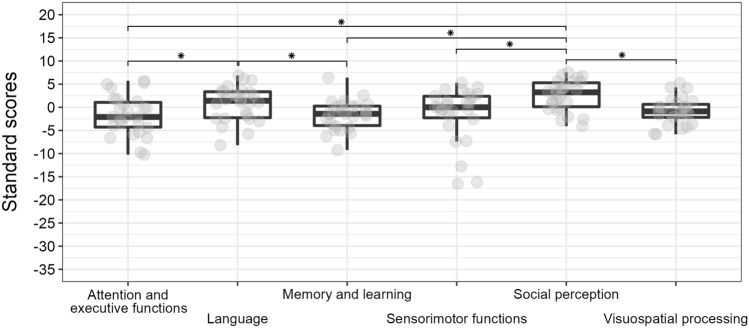
Comparisons between domains are reported in Fig. 1.
Fig.1.
Boxplot of domain scaled scores and their comparisons. Grey circles represent individual scores, asterisks represent significant comparisons.
The repeated measures analysis of variance (RM-ANOVA) comparing the different domains highlighted significant difference across domains (F5,125 = 9.87; p < 0.001; n2p = 0.28), with social perception less impaired than all other domains (all p < 0.001) but language (p = 0.390). Language was less impaired than attention and executive functions (p = 0.022) and memory and learning (p = 0.027). All other comparisons were non-significant (all p > 0.052). On individual level, social perception showed the lowest percentage of participants with weaknesses in that domain, while for all other domains more than half of the sample showed deficits even when considering their mental age.
Within domain comparisons
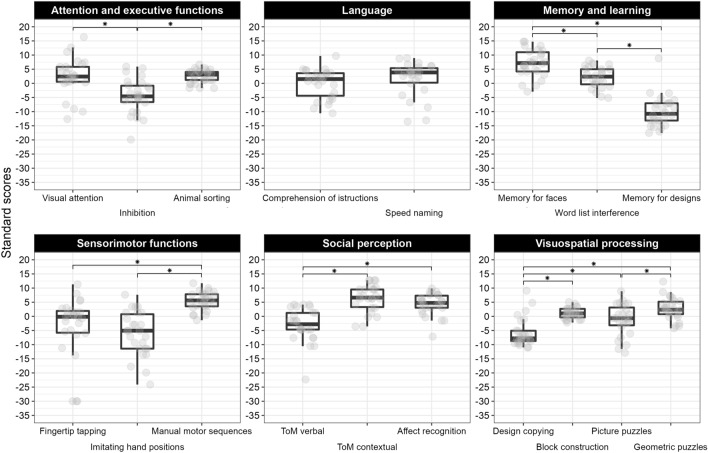
Comparisons between subtests of the same domain are reported in Fig. 2.
Fig. 2.
Boxplot of subtest scaled scores and their comparisons within each domain. Grey circles represent individual scores, asterisks represent significant comparisons.
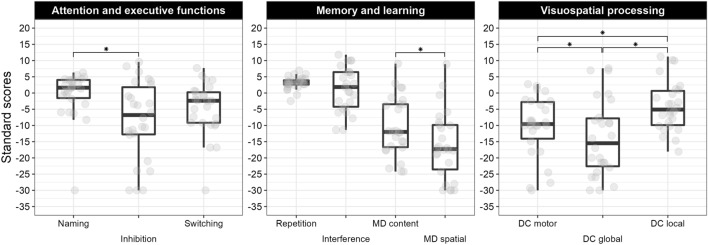
Comparisons between subcomponents of the same subtest are reported in Fig. 3.
Fig. 3.
Boxplot of subcomponent scaled scores and their comparisons within each domain. Grey circles represent individual scores, asterisks represent significant comparisons within each subtest subcomponents. MD means memory for designs, DC means design copying.
Attention and executive functions
A significant difference emerged between subtests (F2,50 = 17.17; p < 0.001; n2p = 0.41). Specifically, a worse performance was recorded on inhibition compared to both visual attention and animal sorting (p < 0.001), while no difference emerged between these two subtests (p > 0.99). Within the inhibition subtest, higher scores were obtained at the naming condition compared to the inhibition subcomponent (p = 0.006), while all other comparisons were non-significant (all p > 0.25).
Accordingly, the inhibition subtest, particularly the inhibition and switching subcomponents, emerged as a weakness of the individual profile in more than 70% of participants. Conversely, the percentage of participants showing individuals weaknesses lowered from over 50% to 38% for visual attention and animal sorting with respect to chronological and mental age, respectively.
Language
Performance at the comprehension of instructions and speeded naming subtests was comparable (t25 = -0.97; p = 0.341). Looking at the individual profiles, an important part of the sample (69%) still showed weaknesses in comprehension of instructions when considering their mental age.
Memory and learning
A significant difference between subtests (F2,50 = 99.42; p < 0.001; n2p = 0.41) indicated a relatively spared ability to memorize faces, with a mean score falling within the normative threshold, compared to verbal working memory (p = 0.001) and visual-spatial memory (p < 0.001). Moreover, performance was better at the word list interference subtest than at the memory for designs subtest (p < 0.001). Consistently for chronological and mental age, only the 19% of the sample presented weaknesses in memory for faces, while all participants but one (96%) showed specific deficits in memory for designs. In this subtest, individuals with WS were more impaired in the spatial compared to the content/visual component (t25 = 2.58; p = 0.016), even though almost all participants (≥ 88%) showed individual weaknesses in either subcomponent. Within the same domain, the two subcomponent scores of word list interference (i.e., repetition and interference) were comparable (t25 = 1.20; p = 0.242). Accordingly, the same percentage of participants (46%) showed deficits in these subcomponents when considering mental age.
Sensorimotor functions
Significant differences were detected between subtests (F2,50 = 21.87; p < 0.001; n2p = 0.47), with manual motor sequences significantly better than both fingertip tapping (p < 0.001) and imitating hand positions (p < 0.001). The difference between fingertip tapping and hand imitation was non-significant (p > 0.673). In keeping with the group mean analyses, a third of participants showed deficits in manual motor sequences, while more than half of the sample had difficulties with the fine-motor skills assessed by finger tapping and, particularly, imitating hand positions.
Social perception
A significant effect of the RM-ANOVA (F2,50 = 30.61; p < 0.001; n2p = 0.55) pointed to stronger difficulties in verbal ToM compared to both contextual ToM (p < 0.001) and affect recognition (p < 0.001). Conversely, on these two latter subtests, participants obtained comparable scores (p = 0.303) and approximately 30% of them were impaired. Most participants showed individual weaknesses in verbal ToM for either chronological (88%) and mental age (69%).
Visuospatial processing
The RM-ANOVA indicated a significant effect (F3,75 = 25.29; p < 0.001; n2p = 0.50), with the worst performance detected at design copying compared to all other subtests (all p < 0.001). Moreover, a significant advantage emerged for geometric compared to picture puzzles (p = 0.002), while all other comparisons were non-significant (all p > 0.190). On individual level, design copying was a weakness in almost all participants; however, also in the other subtests the percentage was above 50% even when considering their mental age. Significant differences between the subcomponents of design copying were detected (F2,50 = 35.47; p < 0.001; n2p = 0.59), with more impaired global perception compared to both graphomotor control and detail-based perception (all p < 0.001), and this latter ability being relatively more spared than motor control (p = 0.012). Almost the whole sample showing specific difficulties in the motor or the global component (88%).
Regression models on VR social prediction score
The model across domains was significant (F6,19 = 5.07; p = 0.003; AdjR2 = 0.49), with memory and learning as main predictor (p = 0.025; β = 0.48). All other predictors were non-significant (all p > 0.068). When testing the memory and learning subtests as regressors, the model was significant (F3,22 = 4.30; p = 0.016; AdjR2 = 0.28), with the score in memory for designs as the only significant predictor (p = 0.024; β = 0.42). The follow-up regression model with the memory for designs/content memory subcomponent was still significant (F3,22 = 3.23; p = 0.042; AdjR2 = 0.21) but did not highlight significant regressors (all p > 0.081). Conversely, the follow-up model including the spatial memory subcomponent indicated a significant contribution of spatial memory (p = 0.010; β = 0.47) and of memory for faces (p = 0.044; β = 0.35) (Table 2).
Table 2.
Results of Multiple Linear Regression Models with VR prediction score as dependent variable.
| Regressors | t | p | β |
|---|---|---|---|
| Model: all domains | |||
| F = 5.07; p = 0.003; AdjR2 = 0.49 | |||
| Attention and executive functions | 1.22 | 0.239 | 0.33 |
| Language | 1.28 | 0.216 | 0.36 |
| Memory and learning | 2.44 | 0.025 | 0.48 |
| Sensorimotor functions | -0.60 | 0.551 | -0.13 |
| Social perception | 1.05 | 0.305 | 0.20 |
| Visuospatial processing | -1.92 | 0.069 | -0.39 |
| Model: Memory and learning with content memory subcomponent | |||
| F = 3.23; p = 0.042; AdjR2 = 0.21 | |||
| Memory for faces | 1.73 | 0.096 | 0.32 |
| Word list interference | 0.97 | 0.344 | 0.17 |
| Memory for designs—Content memory | 1.82 | 0.082 | 0.33 |
| Model: Memory and learning with spatial memory subcomponent | |||
| F = 5.14; p = 0.008; AdjR2 = 0.33 | |||
| Memory for faces | 2.14 | 0.044 | 0.35 |
| Word list interference | 0.38 | 0.706 | 0.06 |
| Memory for designs—Spatial memory | 2.81 | 0.010 | 0.47 |
Significant results are reported in bold.
Lastly, significant correlations emerged between the learning coefficient, representing the trend of incremental number of correct choices for each participant, and both spatial memory (r = 0.52; p = 0.006) and memory for faces (r = 0.41; p = 0.038). These results indicated that spatial memory and memory for faces affected social prediction and probabilistic learning in our sample with WS.
Discussion
This study examined the neuropsychological profile, including social perception, of adolescents and adults with WS, and it tested whether and which neuropsychological outcome might better account for the performance in a VR task designed to assess social prediction in a dynamic, everyday life-like scenario. Our findings revealed that, even though individuals with WS displayed poor performance across all the examined cognitive domains, social perception was a relative strength of the profile, both at group and individual levels. Still, according to our hypothesis, an uneven profile was detected within this domain, with weaknesses at both group and individual levels in the verbal ToM subtest. Conversely, higher scores were recorded on affect recognition and contextual ToM, with only a third of our sample showing valleys in their individual profiles for one of these subtests. These results are in line with previous studies that suggested a differential impairment in high-level, explicit, socio-cognitive processes, partially overlapping with difficulties in narrative and pragmatic language8,9,52,53, compared to relatively spared low-level, implicit, mostly non-verbal social perception skills54–56. Interestingly, a recent study has confirmed that WS could be associated with an altered spontaneous judgment of face trustworthiness, but the ability to match emotional face expressions and social context descriptions appeared to be partially preserved57. Overall, our findings indicated that facial affect recognition and non-verbal ToM skills should not be considered as typical weaknesses in the neuropsychological profile of WS, at least in adolescent and adult individuals.
As expected from previous literature58,59, the language domain was found to be a relative strength. However, more than half of the sample showed weaknesses in comprehension of instructions. On one hand, in some trials this subtest requires comprehension of spatial relationships between stimuli (e.g., above, left), which was reported to be a specific difficulty in the receptive language abilities of patients with WS60. On the other, it has been argued that apparently good expressive language in WS might conceal comprehension difficulties, which may become more evident as task demands increase7. In this vein, given their inherent complexity, social interactions might represent one of the most likely contexts where receptive language problems may arise, with potential detrimental effects on verbal ToM and pragmatic skills.
Results on the other domains were overall consistent with the established profile of WS3,7,36. Within-domain analyses clarified that inhibition, visual-spatial memory, fine sensorimotor skills, and design copying were the most impaired abilities31,32,61. In this latter subtest, a dissociation was detected between global and detail-based visual-perceptual processes, in line with previous literature documenting a bias towards local processing in WS, which might interfere with the integration of parts into a complete stimulus62. Memory for faces was a strength at group level as well as in almost all individuals. This latter result confirms that the ability to memorise and recognise faces is mostly preserved, or at least consistent with the general cognitive functioning, in adolescents and adults with WS3,5,58. Interestingly, a recent study documented a dissociation in WS between early and late components of face processing, respectively associated with altered perceptual processing of low-level facial features and robust decoding of salient facial cues63. These findings may explain the seemingly contradictory evidence of atypical face tuning and processing64,65, yet preserved face recognition in WS.
Although the results reported here showed consistency on both group and individual levels, an important heterogeneity was observable among participants, posing constraints on the claim of a single cognitive profile in WS66,67. Moreover, the results at the between-domain analysis level were mostly driven by deficits in specific subtests or even subcomponents. In light of these aspects, individual assessment of specific abilities through multi-domain and co-normed batteries would be preferable in WS, rather than assessing and reporting aggregated scores and measures, as it is often the case with standard intelligence tests36.
Importantly, deficits in spatial memory appeared to impact on the memorisation and retrieval of avatars’ preferences in the social prediction VR task. This is in keeping with previous evidence of a close relationship between visuospatial skills and social cognition in atypical development conditions41. The score in memory for faces also predicted performance in the VR task, as the relatively spared facial recognition of WS patients might have helped them to recognise the avatars as a prerequisite for personal attribution of individual behavioural preference. According to these results, we can speculate that individuals with WS can rely on their facial recognition abilities in social interactions, whereas their spatial memory impairments might affect the processing and integration of spatial frames, such as other’s position and motion direction, which are critical for learning and navigating social contexts and for adaptive daily life behaviour68,69. The significant correlations with the learning coefficient further corroborated this hypothesis, highlighting how strengths and weaknesses in these abilities may interfere with probabilistic learning of social events in everyday life-like contexts70.
Taken together, these results are in keeping with a neuroconstructivist37,38,71 and evolutionary view72 of neurodevelopment, in which atypical development of basic skills could influence the emergence of later and more complex abilities well beyond the limits of a supposed modularity of brain and cognitive functions. Within this perspective, the proposed “dorsal stream vulnerability”12,27 would affect not only visuospatial processing, as confirmed by the spatial memory and global processing deficits reported here, but also other domains, particularly social cognition 73.
Literature about rehabilitation of ToM and socio-communication abilities in WS is scarce74,75. We speculate that interventions on specific cognitive skills, and in particular spatial memory, might be helpful to foster or compensate for abnormal social functioning. As an example, a navigational training in VR has already been developed for children with cerebral palsy, and might be adopted to enhance visuospatial skills in WS, with potential benefits on adaptive behaviour and social participation76,77. Moreover, a recent study documented improvements of social adaptive behaviour in two children with WS after a neuropsychological training targeting attention and visuospatial skills78. This encourages the development of syndrome-specific interventions that target social behaviour through the enhancement of basic cognitive functions, such as visuospatial and sensorimotor skills79. The use of the same VR scenario adopted here was effective for promoting social perception skills in patients with cerebellar malformations46,48,80, and might be proposed to individuals with WS.
Limitations must be acknowledged. First, the sample size was relatively small, despite it was in keeping with previous studies of this relatively rare disorder, and presented high age variability, asking for caution in generalizing the results. A recent study that adopted an even wider age range (6–39 years), however, did not find a significant relationship between age and cognitive abilities36. The small sample size also prevented us from detecting specific neuropsychological profiles and from investigating genotype–phenotype associations81. Nevertheless, the multi-level analyses, from domains to subcomponents to intra-individual weaknesses, indicated consistent results across patients. The adoption of a co-normed battery allowed us to overcome the methodological limitations of previous studies, obtaining reliable scaled scores for each subtest according to both chronological and mental ages. However, the comparison with multiple patient populations might provide a more complex picture of social perception impairments with respect to specific neuropsychological profiles82. The sample included adult individuals, who were compared to the Italian normative data for 16-year-olds. This issue may have blurred other potential differences between domains or subtests, although we avoided using approximation at the lowest extreme adopted in standard normative tables. Importantly, when considering individual mental age, the results consistently revealed the same neuropsychological profile, thereby affirming the reliability of our approach. Lastly, our findings support the role of spatial memory and facial recognition skills in the understanding of others’ intentions. Nevertheless, there are other aspects of the WS social phenotype that were not assessed in the tasks adopted here, such as hypersociability and anxiety, which could be better explained by other specific impairments like those in executive functions, particularly inhibition14,83.
In conclusion, this study shed new light on the neuropsychological profile and social cognition in WS. Social perception per se should not be considered as typically impaired in WS, with only high-level, verbal ToM representing a weakness of the profile. Moreover, the pattern of impairments and strengths in specific neuropsychological abilities, and particularly spatial and facial memory, might affect the ability to understand others’ intentions beyond domain-specific mechanisms.
Methods
Participants
Twenty-six individuals with a molecularly confirmed diagnosis of WS (15 males), aged 11–41 years (mean = 21.9; SD = 7.9), were recruited in collaboration with associations dedicated to WS (Associazione Famiglie Sindrome di Williams – AFSW; Associazione Persone Sindrome di Williams Italia – APW). Of them, 17 were adults (≥ 18 yo).
Ethics declarations
All participants and their parents/guardians were informed about aims of the study and were asked to sign a written informed consent. The procedures were approved by the local Ethics Committee of the Scientific Institute (IRCCS) E. Medea (Prot. N.34/18 – CE) and were in accordance with the Helsinki Declaration guidelines and later updates.
General procedure
All participants underwent a comprehensive neuropsychological assessment and participated to a VR assessment of social prediction abilities in an everyday life-like scenario.
Neuropsychological assessment
Participants were assessed individually by a trained psychologist (EF or NB) in four separate sessions, each lasting approximately 45 min and administered in two consecutive days.
First, Colored or Raven Standard Progressive Matrices were administered as a measure of general cognitive functioning84. Then, participants were assessed with selected subtests (Table 1) of the Italian NEPSY–II version34,85. Although the NEPSY-II is standardised for children and adolescents aged 3–16 years, this battery was previously used to assess the neuropsychological profile of adult clinical populations presenting cognitive impairments86. This way, it was possible to have a uniform assessment across the whole sample. The subtests were selected to assess various neuropsychological domains and to be administered to individuals of different ages and cognitive levels. Specifically, all subtests with normative data from 7 to 16 years of age were administered to assess attention and executive functions, sensorimotor functions and social perception. In the language domain, we selected comprehension of instructions and speeded naming to evaluate receptive and expressive language, respectively. For memory and learning, we chose subtests presenting verbal (word list interference), social (memory for faces) and visual-spatial stimuli (memory for designs). For visuospatial processing, we included all subtests except for arrows and route finding, as these two tasks were not normally distributed in the Italian NEPSY-II standardization data. Some subtests included subcomponents assessing specific abilities within the same construct (Table 3).
Table 3.
Description of the selected NEPSY-II subtests.
| Domain | Subtest | Subcomponent | Description | Main assessed ability |
|---|---|---|---|---|
| Attention and executive functions | Visual attention | Paper–pencil cancellation test | Visual, selective attention | |
| Inhibition | Naming | Automatic naming of visual stimuli | Control of verbal response | |
| Inhibition | Learned naming of visual stimuli | Inhibitory control of verbal response | ||
| Switching | Switch between response types | Flexibility in control of verbal response | ||
| Animal Sorting | Card sorting task | Categorisation | ||
| Language | Comprehension of Instructions | Pointing appropriate stimuli in response to oral instruction | Receptive language | |
| Speeded naming | Rapid naming of visual stimuli | Rapid semantic access and production | ||
| Memory and learning | Memory for faces | Immediate and delayed facial recognition | Encoding and retrieval of facial stimuli | |
| Memory for designs | Content | Immediate and delayed recalling of visual stimuli and their positions on a grid | Visual memory | |
| Spatial | Spatial memory | |||
| Word list interference | Repetition | Recalling words of a list | Verbal working memory span | |
| Interference | Recalling words following interference | Verbal working memory following interference | ||
| Sensorimotor functions | Fingertip tapping | Rapid imitation of basic finger motions | Rapid motor programming | |
| Imitating hand positions | Imitation of hand postures | Imitation | ||
| Manual motor sequences | Imitation of hand movement sequences | Encoding and retrieval of rhythmic motor programmes | ||
| Social Perception | Theory of Mind | Verbal part | Verbal inference of others’ point of view from texts or pictures | Understanding mental functions (e.g., belief, pretending etc.) |
| Contextual part | Selecting appropriate affect for displayed scenarios | Understanding others’ emotional states related to social context | ||
| Affect recognition | Recognizing faces depicting same affects | Facial affect recognition | ||
| Visuospatial processing | Design copying | Motor | Copying two-dimensional geometric figures | Graphomotor control |
| Global | Global visual-perceptual analysis | |||
| Local | Detail-based visual-perceptual analysis | |||
| Block construction | Reproducing three-dimensional constructions from models | Visuomotor skills | ||
| Picture Puzzles | Identifying locations of smaller pictures within a larger picture | Recognizing part-whole relationships | ||
| Geometric Puzzles | Matching of geometrical shapes in different orientations | Mental rotation |
Assessment of social prediction in a daily life-like VR context
Social prediction was assessed through a VR paradigm, embedded in a Gait Real-time Analysis Interactive Lab (GRAIL, Motek, Amsterdam, NL), originally developed for evaluation and rehabilitation of patients with cerebellar malformations46,48,80. Participants were immersed in a sweet-stand scenario, and they were asked to compete, in each trial, with one of four avatars for reaching one of three sweet stands. The GRAIL therapist instructed participants to move towards the sweet stand chosen by the avatar and activate it before the avatar could. The four avatars (two males and two females) were clearly distinguishable by their clothing, hair, and facial features. At the start of each trial, one avatar appeared next to the participant and then moved along a 9-m straight path, turning onto one of the three 3-m long branches towards one of the three sweet stands (Fig. 4).
Fig. 4.
Virtual environment used to assess social prediction abilities. The VR scenario presents a path branching into three streets, where three sweet stands are located (A). Gait Real-time Analysis Interactive Lab (GRAIL) with the social scenario projected on the 180° cylindrical screen (B). In each trial, the participant competes with one of four avatars (C).
Avatars’ preferences were biased towards pre-established probabilities. Three avatars exhibited a preference for one sweet stand in 80% of the trials, while the fourth avatar chose stands pseudo-randomly, with an equal 33% preference for each stand. Participants could only overtake the avatar before the crossroad, requiring them to predict the avatar’s choice without perceptual clues, thus testing social prediction in an ecological context. When participants reached the avatar’s chosen sweet stand before the avatar, they earned a point and received both auditory (clapping sound) and visual (activation of the object) reinforcement. Participants always saw which sweet stand the avatar reached, even in unsuccessful trials, allowing them to use this information in subsequent trials.
Given a maximum time of 15 s per trial, with short interval between trials, and a total of 80 trials, the assessment lasted ~ 30 min. During the session, events occurred in a pseudorandom order according to the pre-established probabilities. The avatar-object probabilistic associations were counterbalanced between subjects. For further details please see46,48,80.
Data handling and statistical analysis
For the Raven matrices, standardized Z-scores (mean = 0; SD = 1) were estimated according to normative tables. Raw scores at the NEPSY-II subtests were transformed into scaled scores (mean = 10, SD = 3) according to the Italian normative sample85. It is important to note that the Italian normative data extend only to 16 years of age. Since we expected defective performances due to intellectual disability, adult individuals were compared to the normative data for 16-year-olds, in line with previous studies80,86. However, scaled scores were calculated for both chronological age and mental age (mean = 10.9, SD = 5.3), derived from the performance at the Raven matrices. Scaled scores > 7 represented preserved abilities, while scaled scores falling below 2 SD (< 4) indicated impaired performance. We purposefully refrained from approximating scores at the lowest extreme to mitigate the anticipated floor effect arising from the intellectual disabilities observed in many participants. Consequently, the scaled scores for each subtest exhibited a broader range (from -30 to 19), encompassing negative values that denoted varying degrees of impaired performance, in contrast to the narrower range typically found in standard normative tables (from 1 to 19). Scaled scores were calculated also for the subcomponents, to consider possible dissociations within the same subtest (e.g., spatial vs. visual memory in memory for designs). Immediate and delayed recall conditions of the memory for designs subtest were collapsed into a single scaled score since they were highly correlated (r = 0.74; see supplementary table 1). Moreover, domain scores were computed as the mean scaled score on the subtests within the same domain. For each domain, subtest and subcomponent, we estimated the percentage of participants with a performance lower than 2 SD (standard score < 4). These percentages were calculated considering each participant’s chronological age and mental age.
For the VR social-prediction task, we computed a prediction score by assigning one point every time the participant moved according to the avatars’ preferred choice (i.e., the 80% avatar-object association). This allowed us not to consider as correct predictions those trials in which the participant anticipated the avatar by randomly choosing one of the objects48. Furthermore, to take into account the learning component, we computed the incremental number of correct choices considering the three avatars with a preference for one sweet stand for each participant and we fitted these data with a straight line (according to visual inspection). The fitting line was computed for each participant and the alpha value, namely the slope of the fitting straight line, was calculated as a measure of probabilistic learning and defined as learning coefficient (see Supplementary Fig. 1).
The scaled scores calculated for chronological age were inserted in the analyses for describing the neuropsychological profile. A hierarchical analysis approach was used, starting from comparisons across NEPSY-II domains to comparisons across different subtests within each domain and across different subcomponents within each single subtest. First, an RM-ANOVA was conducted inserting the six domain scaled scores as dependent variables. Then, a series of RM-ANOVAs or paired-sample t-test (for language) were run within each domain, inserting scaled scores of each subtest as within-subject variable. Please note that the different ToM subcomponents were entered into this level of analysis as separate measures to compare directly different levels of social processing. In a similar vein, differences across subcomponents of the same subtest were investigated through RM-ANOVAs for inhibition and design copying, and by means of paired-sample t-tests for memory for designs and word list interference.
A similar hierarchical approach was adopted for the VR social-prediction task. First, a multiple linear regression was conducted inserting the prediction score as dependent variable and the NEPSY-II domain scores as regressors. Then, only for a significant domain, a multiple linear regression model was tested with the subtest scores of this domain as independent variables. Lastly, follow-up multiple linear regressions were executed inserting subcomponents of the significant subtests separately to avoid collinearity issues. To consider the effects of neuropsychological outcomes on probabilistic learning, subtests and subcomponents emerged as significant in the regression models were correlated with the learning coefficient.
All analyses were performed with Statistica software version 8.0 (Statsoft, Tulsa, OK). Effect sizes for ANOVAs were reported as partial eta squared (n2p), adopting conventional cut-off of 0.01, 0.06, and 0.14 for small, medium, and large effect sizes, respectively87,88. Bonferroni post-hoc tests were used to analyse significant effects in the RM-ANOVA. Significance threshold was set at p = 0.05 for all statistical tests.
An a-priori power analysis using the G*Power software89 with the “as in GPower 3.0” option, setting the alpha-level at 0.05, desired power at 90% and correlation between repeated measures at 0.5, showed that a sample size of N ≥ 24 allowed us to detect differences across the six domains, in an RM one-way ANOVA design, with a medium effects size of f = 0.25, corresponding to n 2p = 0.06, which was considered to highlight effects that may have clinical relevance90.
Supplementary Information
Acknowledgments
The authors are grateful to Associazione Famiglie Sindrome di Williams – AFSW and Associazione Persone Sindrome di Williams Italia – APW. Special thanks to the president of AFSW Donato Pignataro and to Mrs Daniela Baseggio of APW for their enthusiastic support of the project. We would also thank all the people and families who took part in the study.
Author contributions
Niccolò Butti: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – Original draft, Visualization; Elisabetta Ferrari: Investigation, Data curation, Formal Analysis, Writing – Original draft, Visualization; Viola Oldrati: Investigation, Data curation, Formal Analysis, Writing – Original draft, Visualization; Emilia Biffi: Methodology, Software, Formal analysis, Resources, Writing – Review and Editing, Funding acquisition; Chiara Gagliardi: Conceptualization, Methodology, Resources, Writing – Review and Editing; Romina Romaniello: Conceptualization, Methodology, Resources, Writing – Review and Editing; Sandra Strazzer: Resources, Writing – Review and Editing, Supervision; Renato Borgatti: Conceptualization, Methodology, Writing – Review and Editing, Resources, Supervision; Cosimo Urgesi: Conceptualization, Methodology, Formal analysis, Resources, Writing – Review and Editing, Supervision, Project administration, Funding acquisition.
Funding
This work was supported by grants from the Italian Ministry of Health (Ricerca Corrente 2022–2023-2024, Scientific Institute, IRCCS E. Medea; Bando Ricerca Finalizzata 2016, Prot. GR-2016–02363640; to C.U.; Ricerca Corrente UTOPIA 2022–2023 and DESTINATION 2024, Scientific Institute, IRCCS E. Medea; to E.B.).
Data availability
The anonymized datasets generated for this study are publicly available at this link: https://zenodo.org/records/7852311
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79289-z.
References
- 1.Strømme, P., Bjømstad, P. G. & Ramstad, K. Prevalence estimation of Williams syndrome. J. Child Neurol.17, 269–271 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Morris, C. A. et al. Health care supervision for children with Williams syndrome. Pediatrics10.1542/peds.2019-3761 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Atkinson, J. Williams Syndrome. In Oxford Research Encyclopedia of Psychology (ed. Atkinson, J.) (Oxford University Press, 2022). [Google Scholar]
- 4.MacDonald, G. W. & Roy, D. L. Williams syndrome: a neuropsychological profile. J. Clin. Exp. Neuropsychol. Off. J. Int. Neuropsychol. Soc.10, 125–131 (1988). [DOI] [PubMed] [Google Scholar]
- 5.Udewin, O. & Yule, W. A cognitive and behavioural phenotype in Williams syndrome. J. Clin. Exp. Neuropsychol.13, 232–244 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Mervis, C. B. et al. The Williams syndrome cognitive profile. Brain Cogn.44, 604–628 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Royston, R., Waite, J. & Howlin, P. Williams syndrome: Recent advances in our understanding of cognitive, social and psychological functioning. Curr. Opin. Psychiatry32, 60–66 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Alfieri, P. et al. A comparison between linguistic skills and socio-communicative abilities in Williams syndrome. J. Intellect. Disabil. Res.61, 866–876 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Van Den Heuvel, E., Manders, E., Swillen, A. & Zink, I. Developmental trajectories of structural and pragmatic language skills in school-aged children with Williams syndrome. J. Intellect. Disabil. Res.60, 903–919 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Atkinson, J. et al. Neurobiological models of visuospatial cognition in children with Williams Syndrome: measures of dorsal-stream and frontal function. Dev. Neuropsychol.23, 139–172 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Atkinson, J. et al. A specific deficit of dorsal stream function in Williams’ syndrome. Neuroreport10.1097/00001756-199705260-00025 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Atkinson, J. et al. Visual and visuospatial development in young children with Williams syndrome. Dev. Med. Child Neurol.10.1111/j.1469-8749.2001.tb00213.x (2007). [DOI] [PubMed] [Google Scholar]
- 13.Gagliardi, C. et al. A different brain: Anomalies of functional and structural connections in Williams syndrome. Front. Neurol.10.3389/fneur.2018.00721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng-Cordell, E., Hanley, M., Kelly, A. & Riby, D. M. Anxiety in Williams syndrome: the role of social behaviour, executive functions and change over time. J. Autism Dev. Disord.48, 796–808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng, R., Järvinen, A. & Bellugi, U. Characterizing associations and dissociations between anxiety, social, and cognitive phenotypes of Williams syndrome. Res. Dev. Disabil.35, 2403–2415 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng, R., Bellugi, U. & Järvinen, A. Anxiety and autonomic response to social-affective stimuli in individuals with Williams syndrome. Res. Dev. Disabil.10.1016/j.ridd.2016.08.017 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Frigerio, E. et al. Is everybody always my friend? Perception of approachability in Williams syndrome. Neuropsychologia10.1016/j.neuropsychologia.2005.05.008 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Vivanti, G., Hamner, T. & Lee, N. R. Neurodevelopmental disorders affecting sociability: recent research advances and future directions in autism spectrum disorder and Williams syndrome. Curr. Neurol. Neurosci. Rep.18, 94 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Asada, K. & Itakura, S. Social phenotypes of autism spectrum disorders and williams syndrome: similarities and differences. Front. Psychol.3, 247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niego, A. & Benítez-Burraco, A. 2022 Autism and Williams syndrome: truly mirror conditions in the socio-cognitive domain?. Int. J. Dev. Disabil.10.1080/20473869.2020.1817717 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmiloff-Smith, A., Klima, E., Bellugi, U., Grant, J. & Baron-Cohen, S. Is there a social module? Language, face processing, and theory of mind in individuals with Williams syndrome. J. Cogn. Neurosci.7, 196–208 (1995). [DOI] [PubMed] [Google Scholar]
- 22.Tager-Flusberg, H., Boshart, J. & Baron-Cohen, S. Reading the windows to the soul: evidence of domain-specific sparing in Williams syndrome. J. Cogn. Neurosci.10, 631–639 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Hanley, M., Riby, D. M., Caswell, S., Rooney, S. & Back, E. Looking and thinking: how individuals with Williams syndrome make judgements about mental states. Res. Dev. Disabil.34, 4466–4476 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Godbee, K. & Porter, M. A. Attribution of negative intention in Williams syndrome. Res. Dev. Disabil.34, 1602–1612 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Lacroix, A., Guidetti, M., Rogé, B. & Reilly, J. Recognition of emotional and nonemotional facial expressions: A comparison between Williams syndrome and autism. Res. Dev. Disabil.30, 976–985 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Plesa Skwerer, D., Verbalis, A., Schofield, C., Faja, S. & Tager-Flusberg, H. Social-perceptual abilities in adolescents and adults with Williams syndrome. Cogn. Neuropsychol.10.1080/02643290542000076 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Atkinson, J. & Braddick, O. From genes to brain development to phenotypic behavior ‘Dorsal-stream vulnerability’ in relation to spatial cognition, attention, and planning of actions in Williams syndrome (WS) and other developmental disorders. In Progress in Brain Research (eds Atkinson, J. & Braddick, O.) (Elsevier B, 2011). [DOI] [PubMed] [Google Scholar]
- 28.Doherty-Sneddon, G., Riby, D. M., Calderwood, L. & Ainsworth, L. Stuck on you: Face-to-face arousal and gaze aversion in Williams syndrome. Cogn. Neuropsychiatry14, 510–523 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Vivanti, G., Fanning, P. A. J., Hocking, D. R., Sievers, S. & Dissanayake, C. Social attention, joint attention and sustained attention in autism spectrum disorder and williams syndrome: convergences and divergences. J. Autism Dev. Disord.47, 1866–1877 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Kleberg, J. L. et al. Williams syndrome: reduced orienting to other’s eyes in a hypersocial phenotype. J. Autism Dev. Disord.53, 2786–2797 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes, S. M., Riby, D. M., Park, J., Fraser, E. & Campbell, L. E. Executive neuropsychological functioning in individuals with Williams syndrome. Neuropsychologia48, 1216–1226 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Menghini, D., Addona, F., Costanzo, F. & Vicari, S. Executive functions in individuals with Williams syndrome. J. Intellect. Disabil. Res.54, 418–432 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Mervis, C. B. & John, A. E. Cognitive and behavioral characteristics of children with Williams syndrome: Implications for intervention approaches.. Am. J Med. Genet. Part C Semin. Med. Genet.154C, 229–248 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korkman, M., Kirk, U. & Kemp, S. NEPSY—Second Edition (NEPSY-II). (Harcourt Assessment, 2007).
- 35.Russell, E. W., Russell, S. L. K. & Hill, B. D. The fundamental psychometric status of neuropsychological batteries. Arch. Clin. Neuropsychol.20, 785–794 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Miezah, D., Porter, M., Batchelor, J., Boulton, K. & Campos Veloso, G. Cognitive abilities in Williams syndrome. Res. Dev. Disabil.104, 103701 (2020). [DOI] [PubMed] [Google Scholar]
- 37.D’Souza, H. & Karmiloff-Smith, A. Neurodevelopmental disorders. Wiley Interdiscip. Rev. Cogn. Sci.8, e1398 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Westermann, G. et al. Neuroconstructivism. Dev. Sci.10, 75–83 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Houwen, S., Visser, L., van der Putten, A. & Vlaskamp, C. The interrelationships between motor, cognitive, and language development in children with and without intellectual and developmental disabilities. Res. Dev. Disabil.10.1016/j.ridd.2016.01.012 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Leonard, H. C. & Hill, E. L. Review: The impact of motor development on typical and atypical social cognition and language: A systematic review. Child Adolesc. Ment. Health10.1111/camh.12055 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Ferrari, E. et al. Cognitive predictors of social processing in congenital atypical development. J. Autism Dev. Disord.53, 3343–3355 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Frith, C. D. & Frith, U. Mechanisms of social cognition. Annu. Rev. Psychol.10.1146/annurev-psych-120710-100449 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Sparaci, L., Stefanini, S., D’Elia, L., Vicari, S. & Rizzolatti, G. What and Why understanding in autism spectrum disorders and williams syndrome: Similarities and differences. Autism Res.7, 421–432 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Riva, G., Wiederhold, B. K. & Mantovani, F. Neuroscience of virtual reality: from virtual exposure to embodied medicine. Cyberpsycholo. Behav. Soc. Netw.10.1089/cyber.2017.29099.gri (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maggio, M. G. et al. The growing use of virtual reality in cognitive rehabilitation: fact, fake or vision? a scoping review. J. Natl. Med. Assoc.10.1016/j.jnma.2019.01.003 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Butti, N. et al. Virtual reality social prediction improvement and rehabilitation intensive training (VR-SPIRIT) for paediatric patients with congenital cerebellar diseases: study protocol of a randomised controlled trial. Trials21, 82 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Overwalle, F. et al. Consensus Paper: Cerebellum and Social Cognition. The Cerebellum19, 833–868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urgesi, C. et al. Social prediction in pediatric patients with congenital, non-progressive malformations of the cerebellum: From deficits in predicting movements to rehabilitation in virtual reality. Cortex144, 82–98 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Osório, A. et al. Cerebral and cerebellar MRI volumes in Williams syndrome. Res. Dev. Disabil.35, 922–928 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Menghini, D. et al. Cerebellar vermis abnormalities and cognitive functions in individuals with Williams syndrome. Res. Dev. Disabil.34, 2118–2126 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Hamilton, A. F. & d. C., Brindley, R. & Frith, U.,. Visual perspective taking impairment in children with autistic spectrum disorder. Cognition10.1016/j.cognition.2009.07.007 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Marini, A., Martelli, S., Gagliardi, C., Fabbro, F. & Borgatti, R. Narrative language in Williams syndrome and its neuropsychological correlates. J. Neurolinguistics23, 97–111 (2010). [Google Scholar]
- 53.Lorusso, M. L. et al. Indicators of theory of mind in narrative production: A comparison between individuals with genetic syndromes and typically developing children. Clin. Linguist. Phonetics21, 37–53 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Tager-Flusberg, H. A componential view of theory of mind: evidence from Williams syndrome. Cognition76, 59–90 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Weisman, O. et al. Comparing the broad socio-cognitive profile of youth with Williams syndrome and 22q11.2 deletion syndrome. J. Intellect. Disabil. Res.61, 1083–1093 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campos, R., Martínez-Castilla, P. & Sotillo, M. False belief attribution in children with Williams syndrome: the answer is in the emotion. J. Intellect. Disabil. Res.61, 1003–1010 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Gomez, A., Costa, M., Lio, G., Sirigu, A. & Demily, C. Face first impression of trustworthiness in Williams syndrome: dissociating automatic vs decision based perception. Cortex132, 99–112 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Martens, M. A., Wilson, S. J. & Reutens, D. C. Research Review: Williams syndrome: a critical review of the cognitive, behavioral, and neuroanatomical phenotype. J. Child Psychol. Psychiatry49, 576–608 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Mervis, C. B. & Becerra, A. M. Language and communicative development in Williams syndrome. Ment. Retard. Dev. Disabil. Res. Rev.13, 3–15 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Landau, B. & Zukowski, A. Objects, motions, and paths: spatial language in children with Williams syndrome. Dev. Neuropsychol.23, 105–137 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Vicari, S., Brizzolara, D., Carlesimo, G. A., Pezzini, G. & Volterra, V. Memory abilities in children with Williams syndrome. Cortex32, 503–514 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Porter, M. A. & Coltheart, M. Global and local processing in Williams Syndrome, autism, and Down Syndrome: Perception, attention, and construction. Dev. Neuropsychol.30, 771–789 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Gomez, A., Lio, G., Costa, M., Sirigu, A. & Demily, C. Dissociation of early and late face-related processes in autism spectrum disorder and Williams syndrome. Orphanet J. Rare Dis.17, 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlova, M. A., Heiz, J., Sokolov, A. N. & Barisnikov, K. Social cognition in Williams syndrome: Face tuning. Front. Psychol.7, 1131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D’Souza, D. et al. Face processing in Williams syndrome is already atypical in infancy. Front. Psychol.6, 760 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porter, M. A. & Coltheart, M. Cognitive heterogeneity in Williams syndrome. Dev. Neuropsychol.27, 275–306 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Pezzini, G., Vicari, S., Volterra, V., Milani, L. & Ossella, M. T. Children with Williams syndrome: Is there a single neuropsychological profile. Dev. Neuropsychol.15, 141–155 (1999). [Google Scholar]
- 68.Broadbent, H. J., Farran, E. K. & Tolmie, A. Egocentric and allocentric navigation strategies in Williams syndrome and typical development. Dev. Sci.17, 920–934 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Pavlova, M., Sokolov, A. & Krägeloh-Mann, I. Visual navigation in adolescents with early periventricular lesions: Knowing where, but not getting there. Cereb. Cortex17, 363–369 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Kleberg, J. L. et al. 2023 Social feedback enhances learning in Williams syndrome. Sci. Rep.131(13), 1–11 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karmiloff-Smith, A., Brown, J. H., Grice, S. & Paterson, S. Dethroning the Myth: Cognitive Dissociations and Innate Modularity in Williams Syndrome. In Williams Syndrome (eds Karmiloff-Smith, A. et al.) (Routledge, 2019). [DOI] [PubMed] [Google Scholar]
- 72.Bruner, E. & Iriki, A. Extending mind, visuospatial integration, and the evolution of the parietal lobes in the human genus. Quat. Int.10.1016/j.quaint.2015.05.019 (2016). [Google Scholar]
- 73.Gagliardi, C. et al. A different brain: anomalies of functional and structural connections in Williams syndrome. Front. Neurol.9, 721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diez-Itza, E., Martínez, V., Pérez, V. & Fernández-Urquiza, M. Explicit oral narrative intervention for students with Williams syndrome. Front. Psychol.8, 2337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisher, M. H. & Morin, L. Addressing social skills deficits in adults with Williams syndrome. Res. Dev. Disabil.71, 77–87 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Biffi, E. et al. Learning my way: a pilot study of navigation skills in cerebral palsy in immersive virtual reality. Front. Psychol.11, 591296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nossa, R. et al. Could an immersive virtual reality training improve navigation skills in children with cerebral palsy? a pilot controlled study. J. Clin. Med.11, 6146 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Domínguez-García, C. M. et al. Neuropsychological intervention in attention and visuospatial skills in two patients with Williams syndrome with different types of genetic deletion. Appl. Neuropsychol. Child10.1080/21622965.2022.2063723 (2022). [DOI] [PubMed] [Google Scholar]
- 79.Wuang, Y. P. & Tsai, H. Y. Sensorimotor and visual perceptual functioning in school-aged children with Williams syndrome. J. Intellect. Disabil. Res.61, 348–362 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Butti, N. et al. Feasibility and efficacy of a virtual reality social prediction training in children and young adults with congenital cerebellar malformations. J. Autism Dev. Disord.2024, 1–17. 10.1007/S10803-024-06349-8 (2024). [DOI] [PubMed] [Google Scholar]
- 81.Serrano-Juárez, C. A. et al. Neuropsychological genotype-phenotype in patients with Williams syndrome with atypical deletions: a systematic review. Neuropsychol. Rev.33, 891–911 (2023). [DOI] [PubMed] [Google Scholar]
- 82.Morel, A. et al. Overview of social cognitive dysfunctions in rare developmental syndromes with psychiatric phenotype. Front. Pediatr.6, 102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Porter, M. A., Coltheart, M. & Langdon, R. The neuropsychological basis of hypersociability in Williams and down syndrome. Neuropsychologia10.1016/j.neuropsychologia.2007.05.006 (2007). [DOI] [PubMed] [Google Scholar]
- 84.Raven, J. C. Revised manual for raven’s progressive matrices and vocabulary scale revised manual for raven’s progressive matrices and vocabulary scale (NFER Nelson, 1982). [Google Scholar]
- 85.Urgesi, C., Campanella, F. & Fabbro, F. NEPSY-II, Contributo alla Taratura Italiana (Giunti OS, 2011). [Google Scholar]
- 86.Butti, N. et al. New insights into the neuropsychological profile and intellectual quotient variability in joubert syndrome compared to other congenital cerebellar malformations. The Cerebellum10.1007/s12311-023-01580-y (2023). [DOI] [PubMed] [Google Scholar]
- 87.Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol.10.3389/fpsyg.2013.00863 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen, J. Statistical Power Analysis for the Behavioral Sciences Statistical Power Analysis for the Behavioral Sciences (Routledge, 2013). [Google Scholar]
- 89.Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods39, 175–191 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Cohen, J. A power primer. Psychol. Bull.112, 155–159 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The anonymized datasets generated for this study are publicly available at this link: https://zenodo.org/records/7852311