Abstract
Despite their efficacy, the currently available polio vaccines, oral polio vaccine (OPV) and inactivated polio vaccine (IPV), possess inherent flaws posing significant challenges in the global eradication of polio. OPV, which uses live Sabin attenuated strains, carries the risk of reversion to pathogenic forms and causing vaccine-associated paralytic poliomyelitis (VAPP) and vaccine-derived polio disease (VDPD) in incompletely vaccinated or immune-compromised individuals. Conventional IPVs, which are non-replicative, are more expensive to manufacture and introduce biohazard and biosecurity risks due to the use of neuropathogenic strains in production. These types of limitations have led to a call by the Global Polio Eradication Initiative and others for the development of updated polio vaccines. We are developing a novel Ultraviolet-C radiation (UVC) inactivation method that preserves immunogenicity and is compatible with attenuated strains of polio. The method incorporates an antioxidant complex, manganese-decapeptide-phosphate (MDP), derived from the radioresistant bacterium Deinococcus radiodurans. The inclusion of MDP protects the immunogenic neutralizing epitopes from damage during UVC inactivation. The novel vaccine candidate, ultraIPVTM, produced using these methods demonstrates three crucial attributes: complete inactivation, which precludes the risk of vaccine-associated disease; use of non-pathogenic strains to reduce production risks; and significantly enhanced yield of doses per milligram of input virus, which could increase vaccine supply while reducing costs. Additionally, ultraIPVTM retains antigenicity post-freeze–thaw cycles, a testament to its robustness.
Subject terms: Preclinical research, Inactivated vaccines, Inactivated vaccines
Introduction
The certification of global eradication of wild-type poliovirus types 2 and 3 (WPV2 and WPV3) by the World Health Organization (WHO) in 2015 and 2019, respectively, marked a significant victory in our collective fight against polio1–5. WPV1 infections have been limited in both number and geographical region with just two remaining countries, Pakistan and Afghanistan, reporting infections since the start of 20176–9. The workhorse of eradication efforts, the oral polio vaccine (OPV), is inexpensive and stimulates robust immunity in most vaccinated people. However, during replication in the gut, the disease attenuation phenotype can be reversed, and pathogenic virus can be shed. Infection from vaccine-derived poliovirus (VDPV) has become an increasingly serious and widespread problem10–12. In the years 2021, 2022, and 2023, the WHO reported 343, 1751, and 1 case of vaccine-derived paralytic polio from cVDP1, cVDP2, and cVDP3, respectively13. Because of the infections due to VDPV and eradication of wtPV2, trivalent OPV has been largely replaced by bivalent OPV containing PV1 and PV3 components or monovalent vaccines. A variety of replacement vaccines are in use or under consideration, including improved IPVs, novel oral polio vaccines (nOPVs), virus-like particles, and others14–16.
Inactivated polio vaccine (IPV) is a formalin-inactivated injectable product that stimulates robust systemic and partial gut immunity in vaccinated children17–20. Unlike OPV, IPV cannot cause VDPV, and it is used exclusively in numerous countries21. The majority of conventional IPVs (cIPV) are produced by treating purified, wild-type, neuropathogenic virus for 2–4 weeks with formalin, and some countries have approved a Sabin-based IPV (sIPV). As global eradication progresses, the use of pathogenic strains in the manufacturing process presents increasingly serious biohazard and biosecurity risks. Escape of the virus from manufacturing plants into the environment has been documented22–24. Such a breach of biosecurity could undo decades of eradication efforts if it led to infection in under-vaccinated populations in the future. Because of the risks associated with the virus remaining in the environment and a small, but concerning number of chronic shedders, the WHO predicts that continued vaccine pressure will be required for at least 2 or 3 decades after eradication25,26. These pressing challenges have inspired our quest for a safer, more cost-effective IPV alternative.
Multiple strategies for producing safer polio vaccines have been underway for several years. Early efforts to substitute attenuated Sabin for neuropathogenic strains discovered that formalin inactivation damages the primary neutralization epitope located on VP1 of PV1 Sabin27–29. Although some countries, such as Japan and China, have approved formalin-inactivated Sabin vaccines, others, such as the US, have not yet done so. More recently, alternative vaccines composed of either attenuated strains that are engineered to be more resistant to becoming pathogenic by mutation or recombination events or recombinant virus-like particles are in development to provide additional options15,21,30–42. nOPV, an engineered PV2 strain, is particularly interesting as it has been used since March 2021 and is the current PV2 strain used in OPVs. It is possible that the long-term safety and immunogenicity profiles of Sabin-based IPVs or new vaccines that use engineered attenuated strains will take many years to fully characterize and assess. Furthermore, recent reports indicate that the novel attenuated strains can evolve to cause poliomyelitis which calls into question the use of any attenuated polio vaccine. Therefore, it is prudent to continue with additional efforts that could result in improved inactivated polio vaccines.
In light of these challenges, we report here an alternative Sabin-based vaccine that is inactivated using a rapid irradiation process. Our collaborative team has developed a manganese-decapeptide-phosphate (MDP) antioxidant that protects surface amino acids from damage during exposure to various types of radiation, including gamma, x-rays, and UV light43–51. MDP was derived through analysis of the extreme radio-resistance of Deinococcus radiodurans. The bacteria accumulate manganese-peptide-phosphate complexes that protect DNA repair enzymes from radiation damage. This innovative approach allows the separation of desired nucleic acid damage from undesired surface epitope damage. While the use of radiation and UV light has been applied to sterilization of both medical equipment and vaccine development, MDP is the key factor that dramatically increases the immunogenicity of radiation-inactivated viruses and bacteria, setting a new paradigm in vaccine development.
Previous efforts to develop an IPV using gamma irradiation faltered when conditions could not be established for the production of immunogenic PV3 Sabin component50. The UVC-inactivated novel vaccine candidate described here, ultraIPVTM, sidesteps the use of neuropathogenic viruses in the manufacturing process, reduces the time required for inactivation, and produces more vaccine units per milligram of virus stock. Preclinical evaluations of our trivalent vaccine candidate in the Wistar rat, a recognized model for IPV immunogenicity studies, indicate that ultraIPVTM stimulates robust virus-neutralizing antibodies. In addition, the irradiation method is a platform technology that could facilitate a rapid response to emerging pathogens or improve the immunogenicity of other chemically-inactivated virus vaccines.
Results
Attenuated polio viruses are rapidly inactivated using UVC light
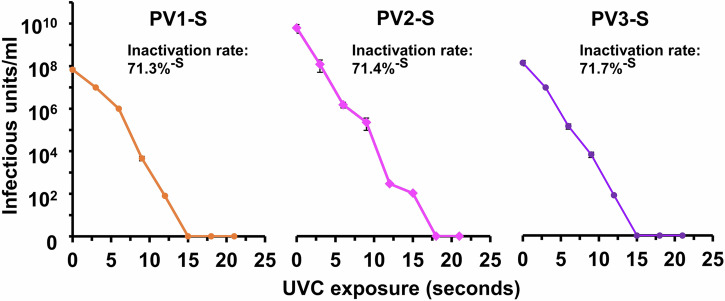
The formalin-inactivation process used for standard IPV products requires careful maintenance of temperature, pH, and other conditions over many days or weeks. The UVC-inactivation process is rapid, straightforward, and requires no elaborate equipment. Figure 1 shows typical UVC inactivation curves for the three Sabin viruses compounded with the MDP protectant using an inexpensive light wand emitting 5 mW/cm2 for up to 21 s of exposure. Each of the three viruses demonstrated similar inactivation kinetics with inactivation rates of approximately 71% per second at 5 mW/cm2. No infectivity could be detected after 15 or 18 s of exposure (75–90 mJ) when inactivated at concentrations of ~100 μg/mL. Following UVC-inactivation, the samples can be chromatographed in a size-exclusion column or washed in filtration cartridges (100,000 MWCO) to remove MDP components and increase the purity, if needed.
Fig. 1. Decrease in infectivity of PV treated with UVC irradiation.
TCID50 virus titers are graphed vs. seconds of exposure to a UVC lamp outputting 4.8 mW/cm2. Error bars are one standard deviation above and below the means. The Y-axis divisions are in Log-10 infectivity units. The zero point reflects no detectable infectivity and is not to be confused with 0log10.
MDP protects the immunogenicity during UVC inactivation
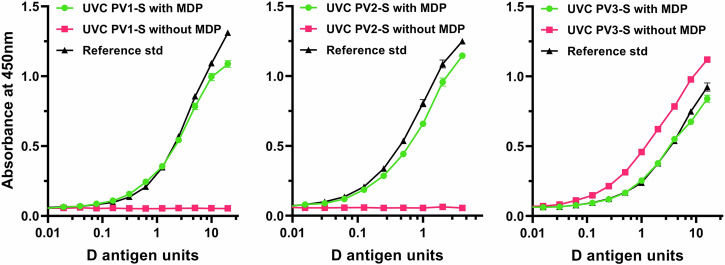
Polio D antigen content provides a prediction of vaccine potency52. Standard IPV products are produced from wild-type strains and are formulated to contain 40 D antigen units of PV1, 8 of PV2, and 32 of PV3. Prior to the analysis of immunogenicity in an animal model, the D antigen content of the UVC-inactivated virus preparations was measured using a standard ELISA. Figure 2 shows D antigen ELISA titration curves of the three Sabin viruses after exposure to 120 mJoules UVC light (approximately 24 s at 5 mW/cm2). As expected, irradiation of PV1 and PV2 Sabin viruses without the MDP protectant resulted in loss of D antigen concentration. Unexpectedly, PV3 Sabin appeared to retain D antigen concentrations whether or not MDP was used during UVC inactivation of the virus. The D antigen ELISA data were used to determine the antigen concentration of each inactivated virus for equilibration to conventional IPVs prior to immunization studies.
Fig. 2. D antigen determination of PV1-S, PV2-S and PV3-S preparations UVC inactivated with and without the MPD complex present.
In this experiment, MDP-containing samples were normalized to the D antigen content of a reference standard (IPOL) and samples irradiated without MDP are shown for comparison.
Extended irradiation
As reported by Plotkin and Orenstein, a small number of early batches of IPV were incompletely inactivated at Cutter Laboratories and tragically led to infection, paralysis, and death in a small number of vaccinees, now referred to as “The Cutter Incident”53. For this reason, the WHO has published guidelines for the analyses of inactivated polio vaccine to reduce the risk of releasing a vaccine lot that contains residual infectivity54–56. Briefly, test viruses are applied to susceptible cell monolayers, which are examined for CPE after 3–5 days. If no CPE is observed, the cells are freeze-thawed to release potential low-level viruses, and the lysed samples are used to inoculate a second round of indicator cells. A lack of CPE after five sequential passages indicates that no replication-competent virus was present in the initial inocula. Figure 3 shows such an analysis of UVC-inactivated PV1, PV2, and PV3 Sabin viruses that were exposed to 5 mW/cm2 for 30 s (150 mJ).
Fig. 3. Analysis of potential low-level residual infectivity.
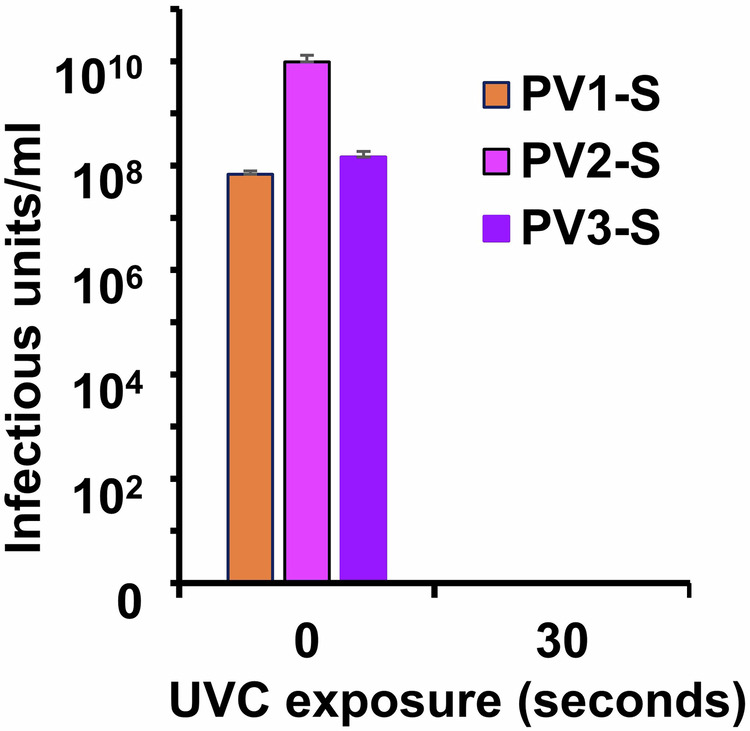
PV1-, PV2-, and PV3-Sabin strains were complexed with the MDP protectant and exposed to 120 mJ UVC irradiation. The irradiated preparations were placed on MRC-5 indicator cells which were passaged five times to detect potential infectivity by CPE. No infectivity could be detected.
Freeze-thaw stability
Both IPOLR and VeroPOLR require storage at 4 °C with shelf-lives of 3 and 2 years, respectively. The vaccine circulars that accompany the products warn against freezing the vials. Because it is possible that some agencies and organizations may want to store stockpiles of ultraIPVTM for many years, we examined antigenic stability after freeze–thaw cycles. Using −80 °C as an overnight storage temperature, lots of ultraIPVTM were subjected to either one or three freeze-thaw cycles and then tested for D antigen activity. Similar to those reported with formalin-inactivated sIPV57, no appreciable loss of D antigen concentrations were observed after one freeze–thaw cycle and only minor losses were observed after three cycles (Fig. 4).
Fig. 4. Stability of immunogenicity after freeze-thaw.
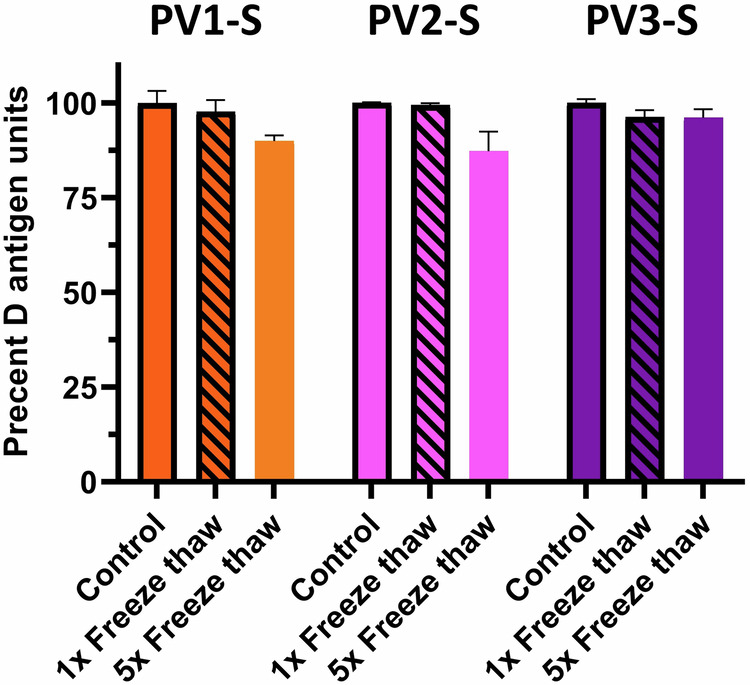
D antigen concentrations were determined after 0, 1, and 5 rounds of freeze–thaw cycles.
D antigen content per milligram of virus
The D-antigen assays used to formulate IPV vaccines utilize antibodies to neutralizing epitopes to measure antigenic content. The ratio of D-antigen units (DU) to virus mass (micrograms) may vary between products and vaccine lots. We determined the D antigen content of ultraIPVTM, IPOLR, and VeroPolR using wild-type reference standards. One dose was defined as containing 40, 8, and 32 D antigen units of PV1, PV2, and PV3 components, respectively, for each of the three preparations. We then used mass spectroscopy (LC–MS/MS) to determine the viral protein content in micrograms per dose of PV1, PV2, and PV3 in ultraIPVTM and the two conventional vaccines, IPOLR and VeroPolR. Total masses of VP1, VP2, VP3, and VP4 viral proteins were computed from the sum of the masses of the tryptic/ chymotryptic peptides. Table 1 shows the micrograms per dose based on the MS data. For example, each dose of ultraIPVTM (40:8:32 DU formulation) contained 4, 0.04, and 2.2 μg of the Sabin strains of PV1, PV2, and PV3, respectively. These data were used to calculate the number of doses that can be produced per milligram.
Table 1.
MS-based analysis of doses per milligram of ultraIPVRTM
| Vaccine | Virus component | Micrograms per dosea | Doses per milligram |
|---|---|---|---|
| UltraIPVTM (Biological Mimetics, Inc.) | |||
| PV1 | 4 | 250 | |
| PV2 | 0.04 | 25,000 | |
| PV3 | 2.2 | 454 | |
| VeroPolR (Statens Serum Institut) | |||
| PV1 | 67.5 | 14.8 | |
| PV2 | 18 | 55.6 | |
| PV3 | 54 | 18.5 | |
| IPOLR (Sanofi Pasteur) | |||
| PV1 | 99.5 | 10.1 | |
| PV2 | 55.2 | 18.1 | |
| PV3 | 67.3 | 14.9 | |
aMicrograms per dose determined by mass spectroscopy.
The UVC-inactivated poliovirus vaccine candidate is highly immunogenic in Wistar rats
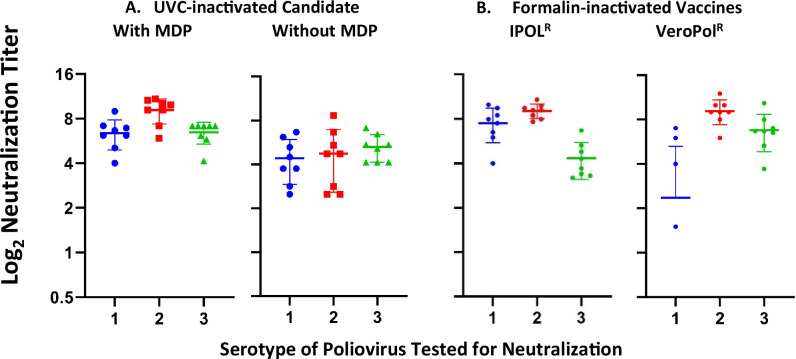
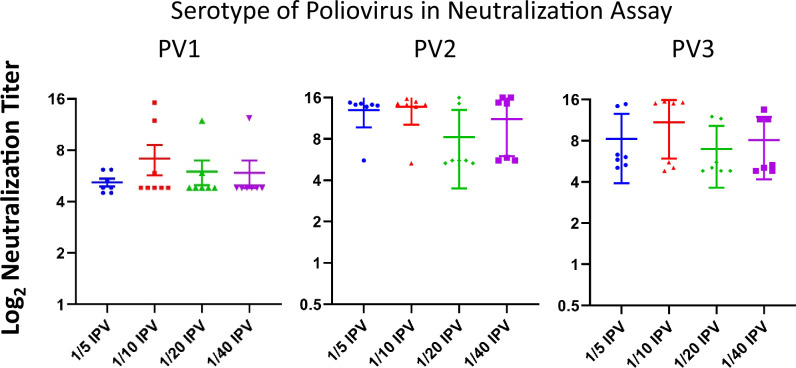
The accepted human poliovirus correlate of immune protection is the presence of neutralizing antibodies with titers of 1:8 (23) or higher35,36. For assessing the immunogenicity of IPV products, the Wistar rat has been an accepted model because its vaccine-elicited neutralizing titers closely predict human titers34,35,37. For the studies reported herein, the immunogen content of ultraIPVTM was adjusted to 40 D antigen units of PV1, 8 of PV2, and 32 of PV3 per dose13,15,35,36. Figure 5 presents the log-2 neutralization titers from Wistar rats immunized with two doses of either ultraIPVTM, IPOLR, or VeroPolR delivered IM on Days 1 and 21, with the titers determined on Day 49. Each point represents the titer from a single animal. Horizontal lines show mean immunization group titers. The neutralizing titers of ultraIPV are above the protective correlate and compare favorably with the commercial vaccines. Figure 6 shows the neutralization titers of a second experiment in which rats were immunized twice with 1/5, 1/10, 1/20, and 1/40 of a standard human dose (40:8:32 D antigen units). The data from sera sampled 3 weeks after the boost show titers above the level of 1:8 (log2 = 3), which is an accepted correlate of protection in humans29,35,36.
Fig. 5. Log2 virus neutralization titers of sera from rats immunized with one human dose of IPV defined as 40 D units PV1, 8 DU PV2, and 32 DU PV3.
PV1, PV2, and PV3 viruses used in the neutralization assay are shown as 1, 2, and 3 on the X-axis. Panel A Neutralization titers from rats immunized with ultraIPVTM. Panel B Titers from rats immunized with IPOLR (Left) and VeroPolR (Right).
Fig. 6. Neutralization titers of rat sera from fractional immunizations of ultraIPVTM.
Groups of 8 Wistar rats were immunized with fractional doses of ultraIPVTM in which one human dose is defined as 40:8:32 D antigen units of PV1, PV2, and PV3, respectively. Sera from individual rats were assayed for neutralization of the three serotypes using a standard TCID50 assay. Mean neutralization titers are reported as horizontal lines with error bars showing one standard deviation.
Discussion
The development of an effective vaccine necessitates a delicate balance between mitigating adverse reactions, managing the pathogenicity of the agent during manufacturing, and ensuring vaccine efficacy. Polio was responsible for disabling 15,000–20,000 individuals annually in the US during the late 1940s. Post introduction of IPV and OPV, these figures drastically decreased to approximately 100 per year in the 1960s and to around 10 annually in the 1970s53,58. Since the start of the Global Polio Eradication Initiative, an estimated 2.2 million instances of deaths and 20 million cases of paralytic polio were prevented worldwide from 1988 to 201859.
OPV has been the workhorse throughout much of the vaccination campaigns. The use of OPV is accompanied by the risk of reversion to neuropathic forms during replication in the gut. The reversion rates have been estimated to be on the order of 1 in 125,000 birth cohorts in a Norway study, with about half in vaccinees and half in by-standers60, 1 in 143,000 in India61, and approximately 1 in 750,000 from a review of documented cases worldwide62,63. Throughout most of the seven decades of OPV use, the risk of paralytic disease from natural infection with wild-type viruses far out-weighed the risks from attenuated vaccine viruses that have evolved into pathogenic strains. However, as the global burden of wild-type infection has declined, the risks of evolved viruses have eclipsed those of wild-type infections, and OPV has been replaced with IPV in most countries.
Historically, IPV has been produced by formalin inactivation of wild-type strains of PV1, PV2, and PV3 viruses. As global eradication efforts continue, the use of neuropathogenic viruses in manufacturing has become an increasingly serious biohazard and biosecurity risk. Despite manufacturers’ rigorous safety measures, at least two accidental leaks into the surrounding environment have been documented23,64,65.
In light of the risks posed by OPV reversions and IPV’s wild-type strains, alternative vaccine strategies are required. New OPV products that incorporate novel OPV (nOPV) vaccine strains have been engineered to be more reversion resistant. A 2023 statement from the Global Polio Eradication Initiative reported that nOPV2, which has evolved into pathogenic strains, had been recovered from the stools of seven children with paralytic polio who were immunized with nOPV266. Although this number is lower than what would have been anticipated with OPV2 (Sabin strain), any such evolved viruses are concerning.
Sabin-based IPV vaccines have been developed and approved for use in some countries, with attenuated strains used in manufacturing to reduce biohazard and biosecurity risks. However, the inactivation of PV1 Sabin with formalin has been linked to damage to a neutralizing epitope67,68. In addition, a study of 300 infants vaccinated with three doses of Sabin-IPV showed reduced immunity against wt-PV1 compared to infants vaccinated with conventional IPV69. Based on our findings of reduced antigen damage caused by UVC-inactivation compared to formalin-inactivation, the reduced immunogenicity against the wild-type PV1 component may have been caused by formalin cross-linking of amino acids within neutralization epitopes. If so, the reduction in efficacy against wt-PV1 may be avoided with the use of ultraIPVTM.
Conflicting thoughts on the ability of IPV to lead to and maintain eradication have been published70. It is well-accepted that OPV stimulates high levels of mucosal immunity after replicating in the intestines. The ability of IPV to lead to and maintain eradication is not as clear, possibly due to difficulties in quantitating the level of polio-specific IgA in stool samples. However, Norway phased out OPV in favor of IPV in 1979, and since then, all reported cases of poliomyelitis have been imported53,71. In studies where vaccinated children were challenged with OPV, those immunized with IPV shed less fecal and nasopharyngeal virus than naïve, yet more than those initially vaccinated with OPV53. In addition, children immunized with three doses of cIPV had similar levels of nasopharyngeal sIgA antibodies as seen in those immunized with three doses of OPV53,72. Thus, it appears clear that IPVs can stimulate some level of mucosal immunity which could assist in eradication and maintaining the state of eradication.
In this report, we present data showing similar inactivation kinetics of the three polio Sabin serotypes (Fig. 1) and confirmation data showing a lack of residual infectivity after 30 s UVC treatments. We unexpectedly found that when calibrated to formalin-inactivated viruses using standard D-antigen ELISA, the UVC-inactivated viruses contained far less mass of virus protein, suggesting that the UVC-MDP inactivation method is gentler to the antigens by preserving epitopes. In addition, we found a disconnect between the D-antigen ELISA data derived from the three viruses inactivated with and without the MDP complex (Fig. 2), and the neutralization data (Fig. 5). UVC inactivation of PV1 and PV2 without MDP caused an almost complete reduction of D antigen while the reduction in neutralization stimulated by the immunogens was more modest. In contrast, the D antigen content of PV3 inactivated with or without MDP was relatively constant. The PV3 result is reproducible and not yet understood. The PV3 data may reflect the complexity of characterizing polio immunogens based on the concentration of a single epitope such as reported by the D antigen ELISA. Finally, we observed fairly consistent magnitudes of neutralization titers when assessing partial vaccine doses (Fig. 6). These results may reflect the timing of the experimental samples where immunizations occurred on Days 1 and 21 and the serum for neutralization was collected on Day 49. We hypothesize that lower doses may result in reduced neutralization titers when the sera is collected several months or years after the final immunization.
The vaccine candidate described in this report, ultraIPVTM, incorporates at least three enhancements over previous vaccines. The inactivated candidate is produced using attenuated Sabin strains, which reduce manufacturing risks. The inactivation process takes less than a minute compared to 2–4 weeks for formalin inactivation. In addition, the increased number of doses per milligram of input virus could lead to reduced costs and increased supplies, an important feature when phasing out less expensive OPV vaccines. Moreover, the use of UVC instead of formalin inactivation may avoid damage to neutralizing epitopes, which could increase immunity to wt-PV1. In ongoing studies, we plan to develop ultraIPVTM through IND-enabling studies and then clinical trials. We believe that the regulatory development pathway will benefit from the long safety and efficacy record of IPV products and that we will need to demonstrate that the immunogenicity profiles (e.g., stimulated neutralizing titers) are not significantly lower and that the toxicity profiles are not significantly higher than approved conventional IPVs. We recognize that the novel inactivation process may require additional analysis to satisfy safety concerns, and the use of attenuated strains may require additional immunogenicity analyses.
Methods
Virus production
Viruses were propagated in shaking suspension culture of H1-HeLa using standard technologies50. Crude intracellular viruses were purified by centrifugation through a 30% w/v sucrose cushion at 120,000×g for 6 h, purification on Toyopearl Sulfate-650F cation exchange resin (Tosoh Biosciences) using 100 mM sodium citrate buffer at pH 6.0 with NaCl elution, and Toyopearl HW-65F size-exclusion resin (Tosoh Biosciences).
Virus infectivity titer assays
10-fold dilutions of virus samples were titrated for infectivity in MRC5 cells using standard techniques50. Titers were determined using the Spearman–Kärber formula (log10 50% endpoint dilution = −(x0−d/2 + d∑ri/ni), where x0 = log10 of the reciprocal of the final dilution at which all wells are positive; d = log10 of the dilution factor; ni = number of replicate wells used; ri = number of positive wells)73–75.
UVC inactivation of viruses
0.1–0.3 mg/ml of PV1-S, PV2-S, and PV3-S were formulated with the MDP complex consisting of 25 mM potassium phosphate buffer (pH 7.4), 2.5 mM MnCl2 and 3 mM decapeptide (DP1: DEHGTAVMLK) as previously described50. MDP-virus samples were placed into thin-wall 0.2 ml PCR tubes, ambient air was purged with argon, and the tubes were placed onto a Model UVP UVG-54 UVC wand (Analytik Jenna US, Upland, CA) outputting 5 mW per square centimeter. UVC output was measured using a UV512C digital light meter (General). Samples exposed for 30 s received ~120 mJ of light energy. Irradiation times are adjusted to compensate for reduced light output as the lamp ages.
Analysis of UVC-treated viruses
To determine the inactivation kinetics, samples of PV1-S, -2S, and -3S were exposed to increasing doses of UVC and then titered in 96-well plates. The wells were scored as infected or not infected based on microscopic examination of cytopathic effects (CPE). Titers were determined using the Spearman–Kärber formula as above. To determine whether inactivated viruses contained traces of residual infectivity, six-well plates of MRC-5 cells were inoculated with UVC-treated viruses and incubated for 4–5 days. The plates were examined microscopically for CPE and subjected to three freeze–thaw cycles if none was observed. A portion (~25%) of the material from the final freeze–thaw was placed on fresh MRC-5 indicator plates and incubated for another 4–5 days. The process was carried out through five passages to determine that no residual infectivity remained as per WHO guidelines34.
D antigen ELISA
Sabin virus-specific D antigen ELISAs were used to quantify the concentration of inactivated virus and calibrate the amount of material per dose with commercially prepared IPV products52. The antibodies and reference standards were generously provided by Drs. Konstantin Chumakov and Diana Kouiavskaia at the FDA Office of Vaccines Research and Review. Briefly, plates were coated with polyclonal antibodies raised against PV1, 2, or 3 overnight at 4 °C. After washing and blocking, test samples and standards were incubated in the wells overnight at 4 °C. The following day, plates were washed and probed with biotinylated anti-poliovirus 1, 2, or 3 polyclonal antibodies and detected with Extravidin-HRP and 3,3’,5,5’-tetramethylbenzidine substrate. Four-parameter logistic regression of the values was used to calculate unknown sample concentrations over a range of dilutions using GraphPad Prism 8.2.1. The D antigen concentrations were used to formulate standard immunization doses, each containing 40, 8, and 32 D antigen units of serotypes 1–3, respectively, in 0.5 mL volumes to correspond to commercial IPV products.
Rat immunization
Animal studies were performed under humane conditions using protocols reviewed and approved by the Institutional Animal Care and Welfare Committee at Cocalico Biologicals, Inc. (Denver, PA). All studies were performed in accordance with the guidelines of the American Veterinary Medical Association (AVMA) under the registrations of Animal Welfare Assurance number D16-00398 (A3669-01) and USDA Research License 23-B-0028. Wistar rats, a widely accepted animal model for polio vaccine analysis, between 6 and 8 weeks of age and of mixed sex were used for immunization studies. Animals were housed in a pathogen-free facility, provided food and water without restriction, and observed twice daily to assess potential health problems. Rats were immunized by intramuscular injection into the quadriceps without adjuvant on Days 1 and 21. On Day 49, the rats were anesthetized with isoflurane, bled by cardiac puncture, and then humanely euthanized by CO2 inhalation following AMVA guildelines. Serum samples were prepared from coagulated blood to assess antiviral immune responses. No differences in health observations, weight, or behavior were detected between immunization groups before or after immunization. No adverse events were observed from the immunizations.
Virus neutralization assay
Neutralization assays were performed using standard procedures50. Briefly, serial two-fold dilutions of serum were incubated 1 h with 100 CCID50 of each poliovirus in separate assays. The virus-serum mixtures were applied to six replicate wells in 96-well plates of MRC5 monolayers at room temperature. After a 1 h incubation, the plates were washed to remove unbound virus, media was added to the wells, and the plates were incubated for 4–6 days at 37 °C. Wells were scored as infected or uninfected by microscopic visualization of CPE. Neutralization titers were derived using the Spearman–Kärber method73–76. The titer represents the reciprocal of the highest dilution of serum that causes a 50% reduction in the number of infected wells. The neutralization titers were graphed as Log2 values.
Statistical analysis
In order to evaluate the immunogenicity of irradiated vaccines and commercially prepared IPV vaccines, unpaired, parametric, one-tailed Student’s t-tests were performed. Neutralizing titers stimulated by irradiated vaccine samples were compared to neutralizing titers stimulated by either IPOLR or VeroPolR independently to test the hypothesis that the irradiated vaccines are more immunogenic than either of the commercially prepared vaccines used in comparison. Analyses were performed using GraphPad, Prism version 8.2.1. P values are reported within the figures
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Acknowledgements
The authors are indebted to Drs. Konstantin Chumakov and Dianna Kouiavskaia (FDA, Bethesda, MD, USA) for scientific discussions and for providing D antigen ELISA reagents and methods. We thank Dr. Jing-Jiang Hao (Poochon Scientific, Inc., Frederick, MD, USA) for the expert mass spectroscopic analysis of the vaccine candidates. Funding: This research was funded, in part, by a grant from the National Institutes of Health (2R44AI120260-02) and a research contract from the Defense Threat Reduction Agency (HDTRA1-17-C-0030).
Author contributions
G.J.T. designed the study. R.V.B., J.K.T., and G.J.T. propagated, purified, inactivated and titered the viruses. J.K.T. and A.V.K. performed virus neutralization analyses. S.J.D. performed D antigen quantitation studies and performed freeze–thaw experiments. J.K.T., S.J.D., D.A.M., and G.J.T. performed statistical analyses of the data. G.J.T. wrote the first draft of the manuscript, and all co-authors, including H.N.M., M.J.D., M.F.M., and T.J.W. reviewed and edited the paper. All authors have approved the submission of this manuscript.
Data availability
The data presented in this report can be extracted from the figures for additional analyses. The authors welcome requests from qualified laboratories for additional information related to UVC-inactivation of vaccines with the MDP complex.
Competing interests
G.J.T., J.K.T., R.V.B., T.J.W., S.J.D., D.A.M., and A.V.K. are employees of Biological Mimetics, Inc., which is the recipient of the NIH grant and DTRA contract that provided the majority of the funding for the project. All other authors have no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gregory J. Tobin, Email: tobin@bmi-md.com
Stephen J. Dollery, Email: dollery@bmi-md.com
References
- 1.Batson, A. et al. Polio eradication vaccine investment: how do we ensure polio vaccines are available to keep the world polio-free after transmission of wild poliovirus (wPV) has been interrupted?. BMJ Glob. Health6, e006447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenner, L. et al. Global progress toward poliovirus containment, 2019–2020. Morbid. Mortal. Wkly. Rep.70, 359 (2021). [Google Scholar]
- 3.GPEI. Current Research Areas. https://polioeradication.org/tools-and-library/current-research-areas/affordable-ipv/ (2021).
- 4.Langar, H. et al. The quest for a new polio vaccine. Vaccines8, 538 (2020).32957489 [Google Scholar]
- 5.WHO. Two out of three wild poliovirus strains eradicated. https://www.who.int/news-room/feature-stories/detail/two-out-of-three-wild-poliovirus-strains-eradicated (2019).
- 6.Chard, A. N. et al. Progress toward polio eradication—worldwide, January 2018–March 2020. Morb. Mortal. Wkly. Rep.69, 784–789 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Famulare, M. et al. Assessing the stability of polio eradication after the withdrawal of oral polio vaccine. PLoS Biol16, e1009690 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan, W. K. et al. Oral polio vaccine response in the MAL-ED birth cohort study: considerations for polio eradication strategies. Vaccine37, 352–365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel, M. & Cochi, S. Addressing the challenges and opportunities of the polio endgame: lessons for the future. J. Infect. Dis.216, S1–S8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherkasova, E. A. et al. Spread of vaccine-derived poliovirus from a paralytic case in an immunodeficient child: an insight into the natural evolution of oral polio vaccine. J. Virol.79, 1062–1070 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopalco, P. L. Wild and vaccine-derived poliovirus circulation, and implications for polio eradication. Epidemiol. Infect.145, 413–419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minor, P. D. Attenuation and reversion of the Sabin vaccine strains of poliovirus. Dev. Biol. Stand.78, 17–26 (1993). [PubMed] [Google Scholar]
- 13.WHO Global Circulating Vaccine-derived Poliovirus Report July 30, 2024https://polioeradication.org/this-week/variant-polio-cvdpv-cases/.
- 14.Bandyopadhyay, A. S. et al. A randomized phase 4 study of immunogenicity and safety following monovalent oral type 2 Sabin polio vaccine challenge in IPV-vaccinated children in Lithuania. J. Infect. Dis.223, 119–127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bockstal, V. et al. An inactivated poliovirus vaccine using Sabin strains produced on the serum-free PER.C6® cell culture platform is immunogenic and safe in a non-human primate model. Vaccine36, 6979–6987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybicki, E. P. Plant molecular farming of virus-like nanoparticles as vaccines and reagents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.12, 1587 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Brickley, E. B. et al. Intestinal Immune Responses to Type 2 Oral Polio Vaccine (OPV) challenge in infants previously immunized with bivalent OPV and either high-dose or standard inactivated polio vaccine. J. Infect. Dis.217, 371–380 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Medina, E. et al. Inactivated polio vaccines from three different manufacturers have equivalent safety and immunogenicity when given as 1 or 2 additional doses after bivalent OPV: results from a randomized controlled trial in Latin America. Vaccine35, 3591–3597 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilton, T. Methods for the quality control of inactivated poliovirus vaccines. Methods Mol. Biol.1387, 279–297 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Wood, D. J., Heath, A. B. & Sawyer, L. A. A WHO Collaborative study on assays of the antigenic content of inactivated poliovirus vaccines. Biologicals23, 83–94 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Thompson, K. M. et al. Trade-offs of different poliovirus vaccine options for outbreak response in the United States and other countries that only use inactivated poliovirus vaccine (IPV) in routine immunization. Vaccine42, 819–827 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandyopadhyay, A. S. et al. Facility-associated release of polioviruses into communities—risks for the posteradication era. Emerg. Infect. Dis.25, 1363–1369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duizer, E., Rutjes, S., de Roda Husman, A. M. & Schijven, J. Risk assessment, risk management and risk-based monitoring following a reported accidental release of poliovirus in Belgium, September to November 2014. Eur. Surveill.21, 30169 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Kew, O. M. et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. World Health Organ.83, 16–23 (2005). [PMC free article] [PubMed] [Google Scholar]
- 25.Minor, P. D., Lane, B., Mimms, S. & Bar, P. Scientific consultation on the safety and containment of new poliovirus strains for vaccine production, clinical/regulatory testing and research. Report of a meeting held at NIBSC, Potters Bar, Hertfordshire, UK, 6/7th July 2016. Biologicals48, 92–100 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Polio Eradication Strategy 2022–2026: Delivering on a Promise (World Health Organization, Geneva, 2021).
- 27.Chumakov, K. et al. Inactivated vaccines based on alternatives to wild-type seed virus. Dev. Biol. (Basel)105, 171–177 (2001). [PubMed] [Google Scholar]
- 28.Cramer, J. P. et al. Safety and immunogenicity of experimental stand-alone trivalent, inactivated Sabin-strain polio vaccine formulations in healthy infants: a randomized, observer-blind, controlled phase 1/2 trial. Vaccine38, 5313–5323 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tano, Y. et al. Antigenic characterization of a formalin-inactivated poliovirus vaccine derived from live-attenuated Sabin strains. Vaccine25, 7041–7046 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Bandyopadhyay, A. S. & Macklin, G. R. Final frontiers of the polio eradication endgame. Curr. Opin. Infect. Dis.33, 404–410 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Chumakov, K. & Ehrenfeld, E. New generation of inactivated poliovirus vaccines for universal immunization after eradication of poliomyelitis. Clin. Infect. Dis.47, 1587–1592 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawt, L. et al. Differences in antigenic structure of inactivated polio vaccines made from Sabin live-attenuated and wild-type poliovirus strains: impact on vaccine potency assays. J. Infect. Dis.221, 544–552 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Hu, Y. et al. Immunogenicity and safety of a sabin strain-based inactivated polio vaccine: a Phase 3 Clinical Trial. J. Infect. Dis.220, 1551–1557 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Konopka-Anstadt, J. L. et al. Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. NPJ Vaccines5, 26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okayasu, H. et al. Development of inactivated poliovirus vaccine from Sabin strains: a progress report. Biologicals44, 581–587 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Sanders, B. P. et al. Production of high titer attenuated poliovirus strains on the serum-free PER.C6(®) cell culture platform for the generation of safe and affordable next generation IPV. Vaccine33, 6611–6616 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Sanders, B. P. et al. Cold-adapted viral attenuation (cava): highly temperature sensitive polioviruses as novel vaccine strains for a next generation inactivated poliovirus vaccine. PLoS Pathog.12, e1005483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu, H. Development and introduction of inactivated poliovirus vaccines derived from Sabin strains in Japan. Vaccine34, 1975–1985 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Shin, W. J. et al. Development of thermostable lyophilized Sabin inactivated poliovirus vaccine. mBio9, e02287–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Damme, P. et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet394, 148–158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan, S. et al. Immunogenicity and safety of different sequential schedules of Sabin strain-based inactivated poliovirus vaccination: a randomized, controlled, open-label, phase IV clinical trial in China. VaccineS0264-410X, 30971–30973 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Yeh, M. T. et al. Engineering the live-attenuated polio vaccine to prevent reversion to virulence. Cell Host Microbe27, 736–751 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daly, M. J. et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science306, 1025–1028 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Daly, M. J. et al. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol.5, e92 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daly, M. J. et al. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS ONE5, e12570 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dollery, S. J. et al. Radiation-inactivated Acinetobacter baumannii vaccine candidates. Vaccines (Basel)9, 96 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dollery, S. J. et al. Select whole-cell biofilm-based immunogens protect against a virulent Staphylococcus isolate in a stringent implant model of infection. Vaccines10, 833 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dollery, S. J. et al. Whole-cell vaccine candidates induce a protective response against virulent Acinetobacter baumannii. Front. Immunol.13, 941010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaidamakova, E. K. et al. Preserving immunogenicity of lethally irradiated viral and bacterial vaccine epitopes using a radio-protective Mn2+–peptide complex from Deinococcus. Cell Host Microbe12, 117–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobin, G. J. et al. A novel gamma radiation-inactivated sabin-based polio vaccine. PLoS ONE15, e0228006 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broder, K. C. et al. Irradiated whole cell Chlamydia vaccine confers significant protection in a murine genital tract challenge model. npj Vaccines10.1038/s41541-024-00968-z (2004). [DOI] [PMC free article] [PubMed]
- 52.Kouiavskaia, D., Puligedda, R. D., Dessain, S. K. & Chumakov, K. Universal ELISA for quantification of D-antigen in inactivated poliovirus vaccines. J. Virol. Methods276, 113785 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Vidor, E. J. & Modlin, J. F. Poliovirus vaccine—inactivated. In Plotkin’s Vaccines (eds Orenstein, W. A., Offit, P. A., Edwards, K. M. & Plotkin, S. A.) Ch. 49 (Elsevier Inc., 2022).
- 54.WHO. Requirements for poliomyelitis vaccine (inactivated) WHO Annex 2. World Health Organ Tech. Rep. Ser.910, 32e65 (2002). [Google Scholar]
- 55.WHO. Guidelines for the Safe Production and Quality Control of Inactivated Poliomyelitis Vaccine Manufactured from Wild Polioviruses. WHO Technical Report Series. 926 (WHO, 2004).
- 56.WHO. Guidelines on the Quality, Safety, and Efficacy of Inactivated Poliomyelitis Vaccines (World Health Organization, 2018).
- 57.Cai, W. et al. Potency of the Sabin inactivated poliovirus vaccine (sIPV) after exposure to freezing temperatures in cold chains. Hum. Vaccin. Immunother.16, 1866–1874 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization. History of Polio Vaccination—A Crippling and Llife-threatening Diseasehttps://www.who.int/news-room/spotlight/history-of-vaccination/history-of-polio.vaccination (World Health Organization, 2024).
- 59.World Health Organization. https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (2023).
- 60.Minor, P. D., Ferguson, M., Evans, D. M. & Almond, J. W. The Sabin attenuated poliovirus vaccine: a critical review of recent studies. J. Virol.68, 5585–5592 (1994). [Google Scholar]
- 61.John, T. J. & Dharmapalan, D. Relevance of medical ethics in public health: case study of polio eradication. Qeioshttps://www.qeios.com/read/I8W77Q (2023).
- 62.Bull, J. J. Evolutionary reversion of live viral vaccines: can genetic engineering subdue it? Virus Evol. 1, vev005 (2015). [DOI] [PMC free article] [PubMed]
- 63.Burns, C. C., Diop, O. M., Sutter, R. W. & Kew, O. M. Vaccine-derived polioviruses. J. Infect. Dis.210, S283–S293 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Aylward, R. B. et al. Global poliovirus vaccine supply: trends and implications for eradication. Vaccine37, 5764–5770 (2019). [Google Scholar]
- 65.Dowdle, W. R. & Wolff, C. Post-eradication poliovirus facility-associated community risks. Biologicals34, 127–132 (2006). [DOI] [PubMed] [Google Scholar]
- 66.GPEI 2023 Interview with Simona Zipursky (World Health Organization) and Ananda S. Bandyopadhyay (Bill and Melinda Gates Foundation)https://polioeradication.org/news-post/two-years-since-rollout-of-novel-oral-polio-vaccine-type-2-nopv2-hows-it-all-working-out/ (2023).
- 67.Chumakov, K. M. et al. Development and evaluation of a safe and immunogenic inactivated poliovirus vaccine. J. Infect. Dis.162, 1275–1282 (1990). [Google Scholar]
- 68.Minor, P. D. & Dunn, G. Virus neutralisation assays for detection and quantitation of neutralising antibody to polioviruses. In SARS- and Other Coronaviruses 1st edn (ed. Lal, S. K.) 189–200 (Elsevier, 2010).
- 69.Ong-Lim, A. L. et al. Safety and immunogenicity of 3 formulations of a Sabin inactivated poliovirus vaccine produced on the PER.C6® cell line: a phase 2, double-blind, randomized, controlled study in infants vaccinated at 6, 10 and 14 weeks of age. Hum. Vaccin. Immunother.18, 2044255 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Connor, R. I. et al. Mucosal immunity to poliovirus. Mucosal Immunol.15, 1–9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bottinger, M. The elimination of polio in the Scandinavian countries. Public Health Rev.21, 27–33 (1993). [PubMed] [Google Scholar]
- 72.Faden, H. et al. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J. Infect. Dis.162, 1291–1297 (1990). [DOI] [PubMed] [Google Scholar]
- 73.Karber, G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuce. Arch. Exp. Pathol. Pharmakol.162, 480–483 (1931). [Google Scholar]
- 74.Spearman, C. The method of right and wrong cases (constant stimuli) without Gauss’s formula. Br. J. Psychol.2, 227–242 (1908). [Google Scholar]
- 75.Mahy, B. W. J. & Kangro, H. O. Virology Methods Manual (Academic Press, Cambridge, 1996).
- 76.Ramakrishnan, M. A. Determination of 50% endpoint titer using a simple formula. World J. Virol.5, 85–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this report can be extracted from the figures for additional analyses. The authors welcome requests from qualified laboratories for additional information related to UVC-inactivation of vaccines with the MDP complex.