Abstract
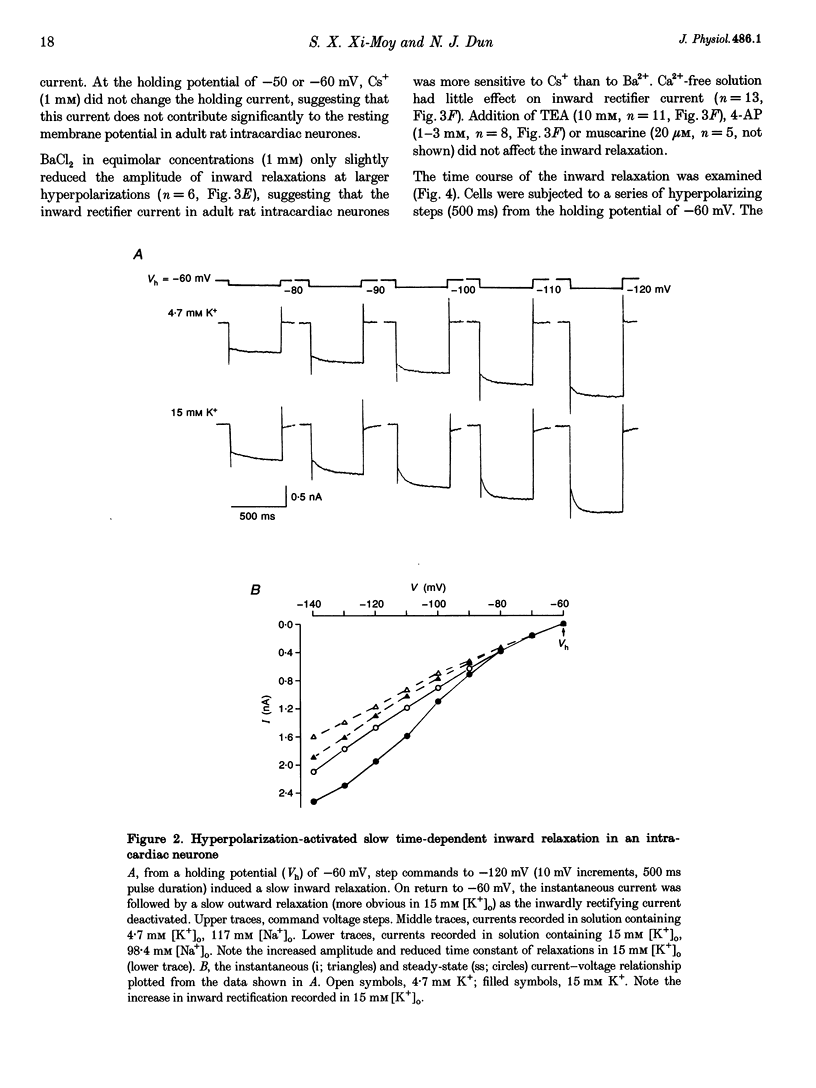
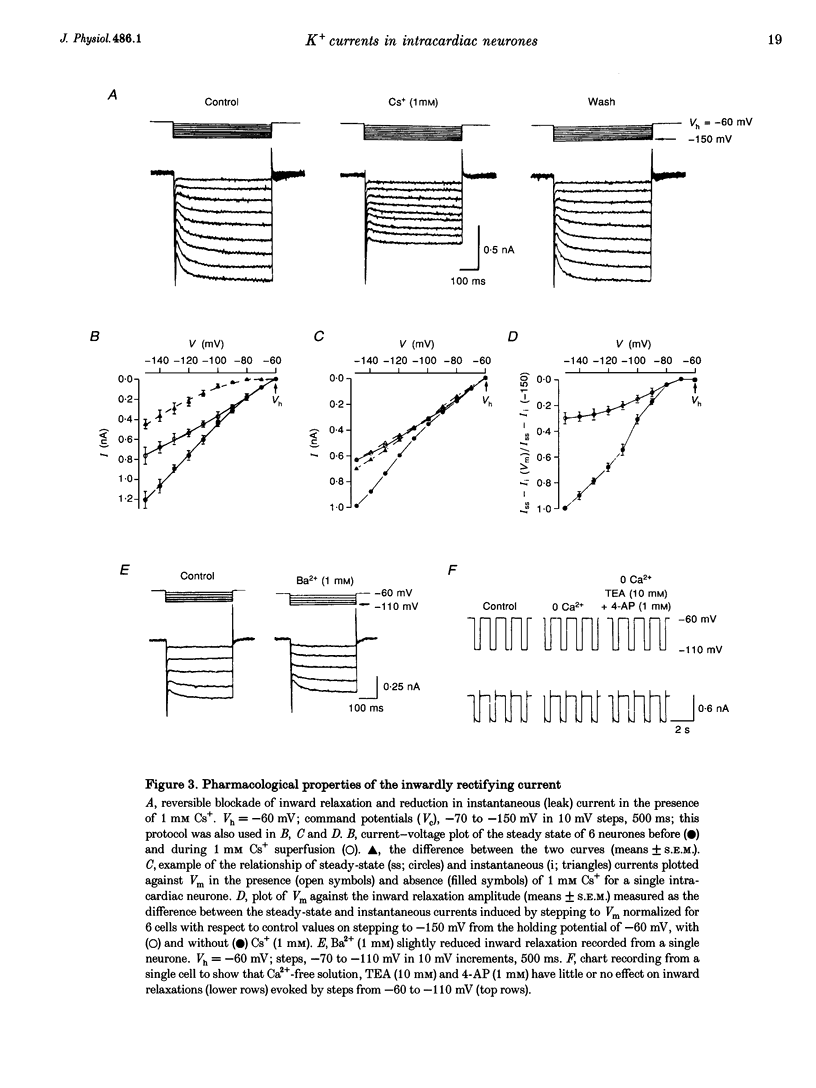
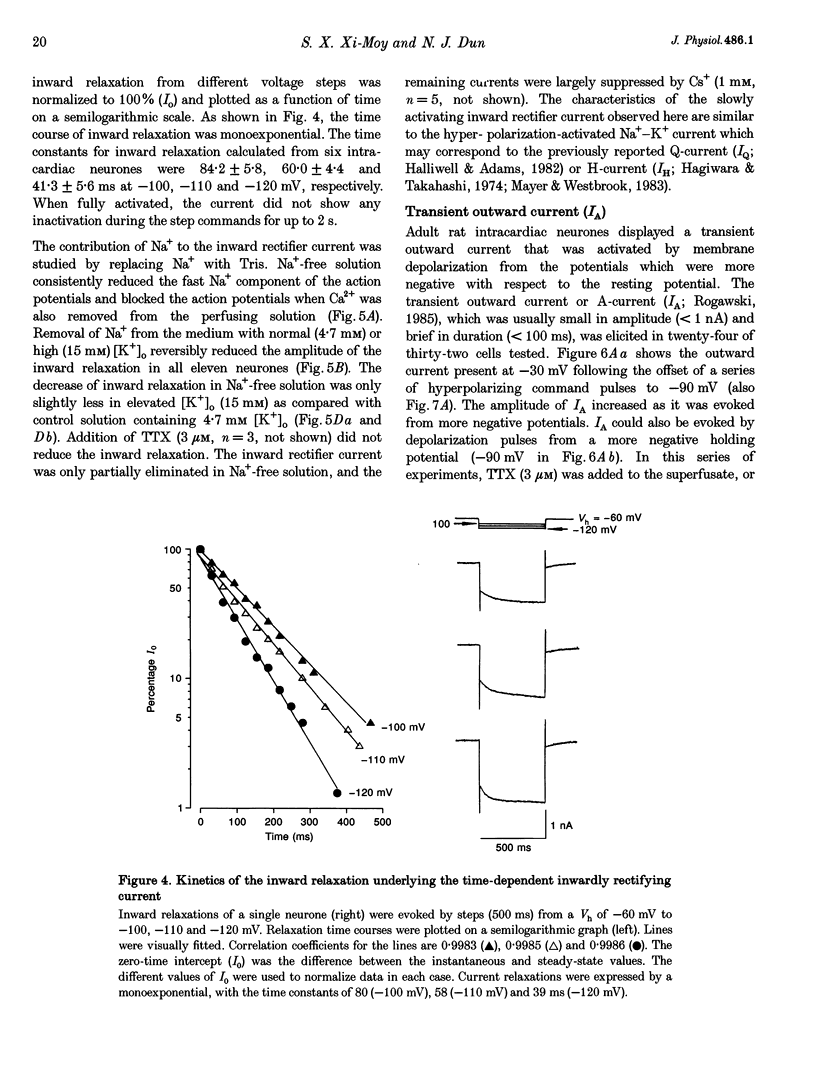
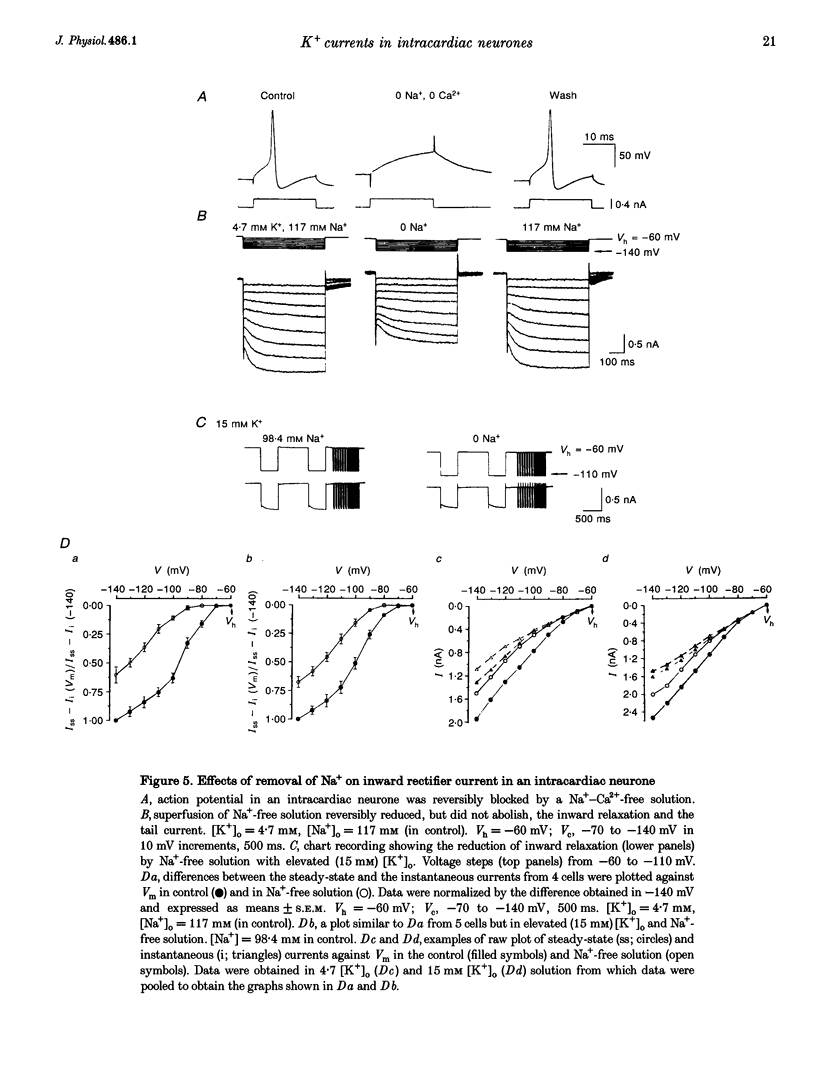
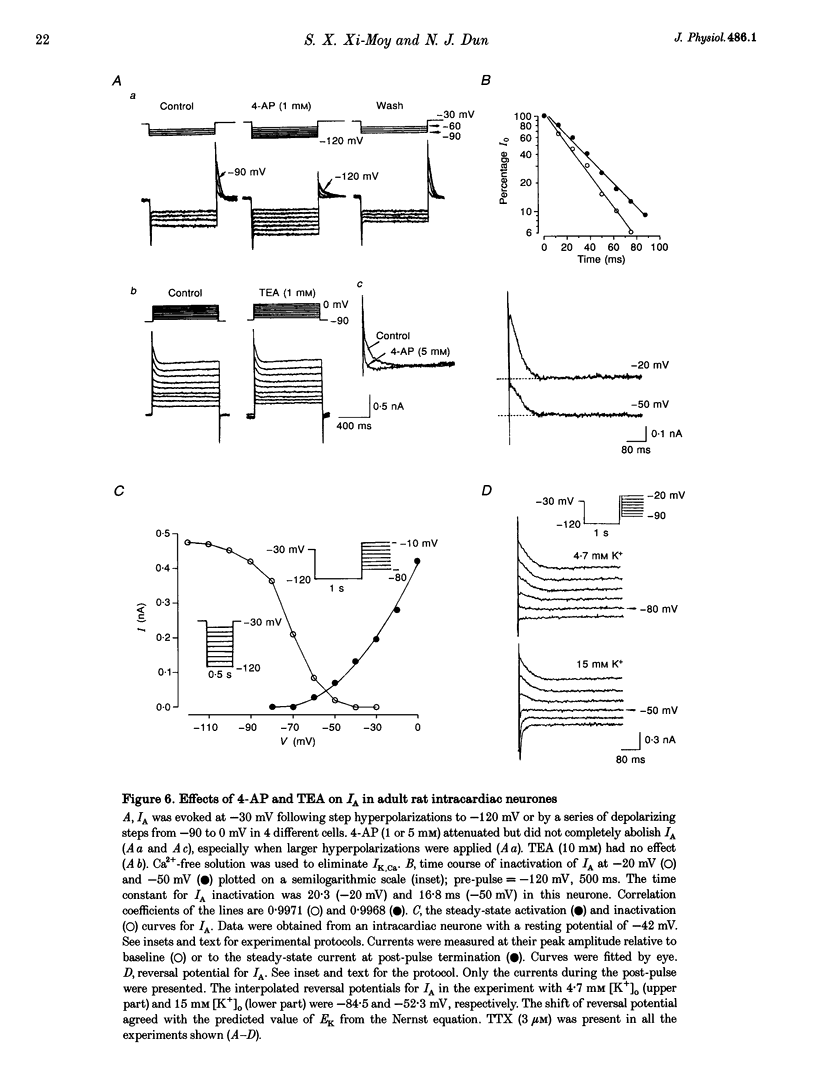
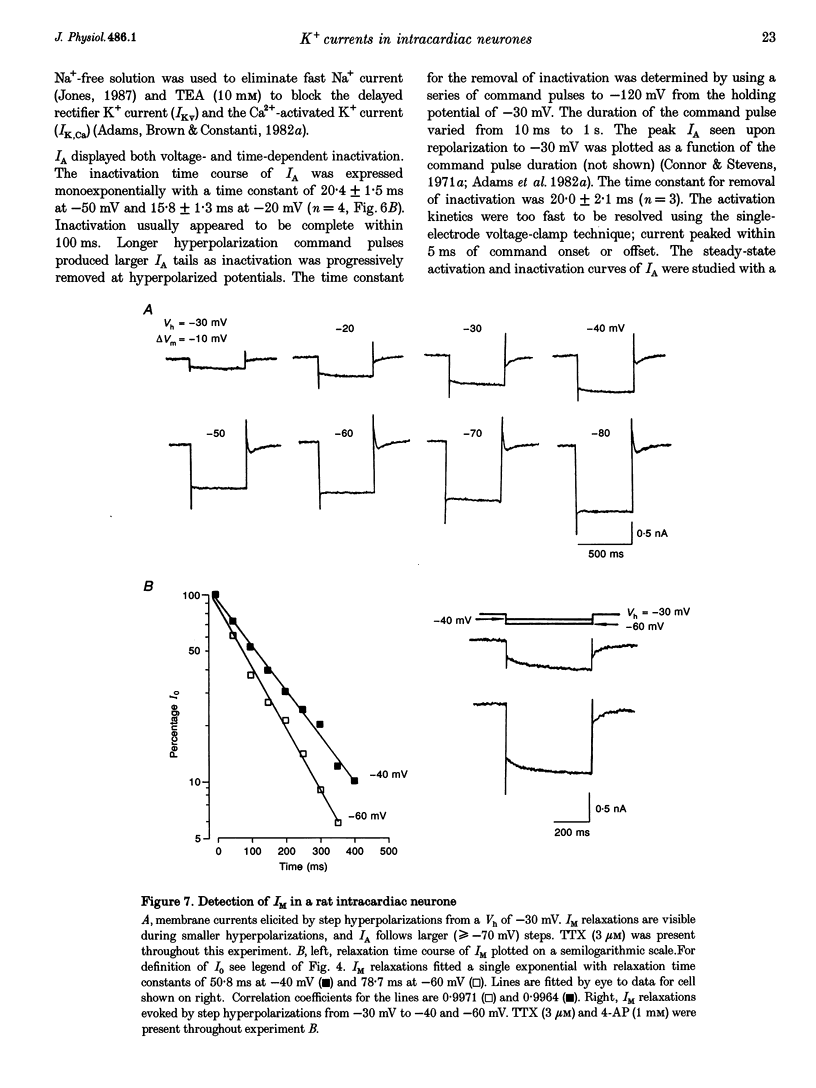
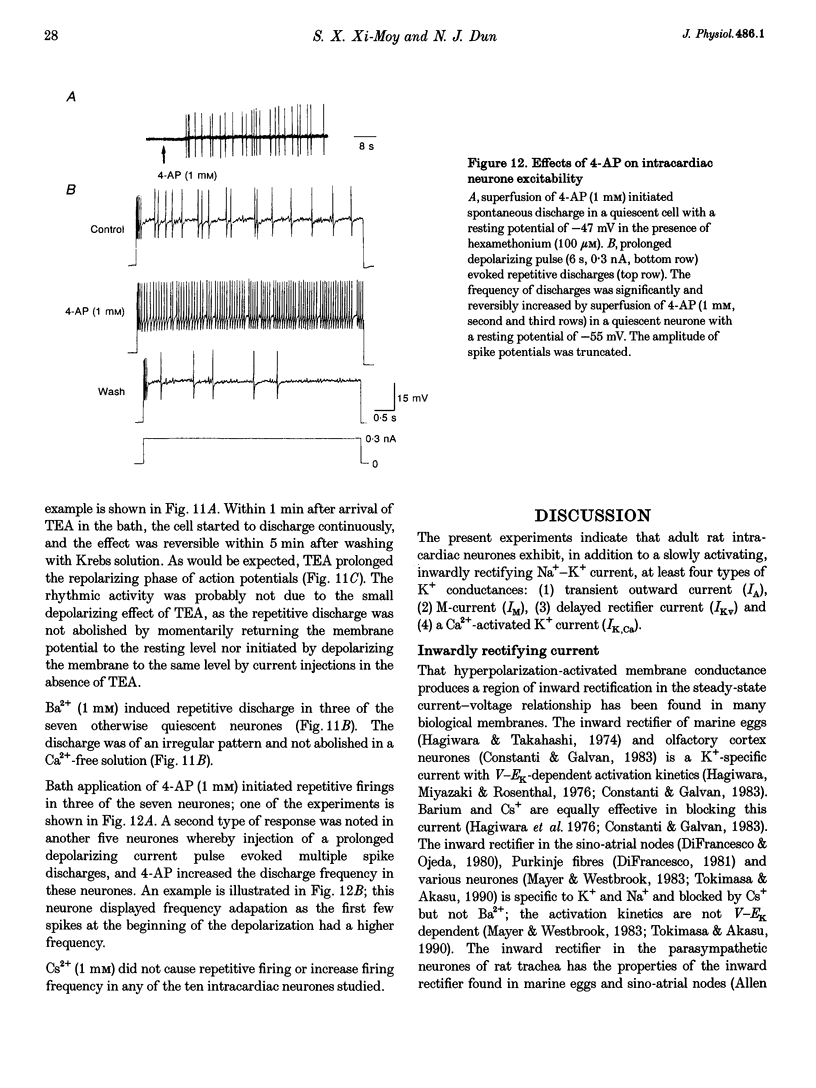
1. Properties of K+ currents were studied in isolated adult rat parasympathetic intracardiac neurones with the use of single-electrode voltage-clamp techniques. 2. A hyperpolarization-activated inward rectifier current was revealed when the membrane was clamped close to the resting level (-60 mV). The slowly developing inward relaxation had a mean amplitude of 450 pA at -150 mV, an activation threshold of -60 to -70 mV and a relaxation time constant of 41 ms at -120 mV. The current was reversibly blocked by Cs+ (1 mM) and became smaller with reduced [K+]o and [Na+]o, indicating that this inward rectifier current probably is a time- and voltage-dependent Na(+)-K+ current. 3. Step depolarizations from the holding potential of -80 mV evoked a transient (< 100 ms at -40 mV) outward K+ current (IA) which was blocked by 4-aminopyridine (4-AP, 1 mM). The time constants for IA inactivation were 20 ms at -50 mV and 16 ms at -20 mV. The steady-state activation and (removal of) inactivation curve showed a small overlap between -70 and -40 mV; the reversal potential of IA was close to EK. 4. Step hyperpolarizations from the depolarized potentials, i.e. -30 mV, revealed a slow inward relaxation associated with the deactivation of a time- and voltage-dependent current. The inward relaxation became faster at more hyperpolarized potentials and reversed at -85 and -53 mV in 4.7 and 15 mM [K+]o. This current was blocked by muscarine (20 microM) and Ba2+ (1 mM) but not affected by Cs+ (1 mM); this current may correspond to the M-current (IM). 5. Depolarization-activated outward K+ currents were evoked by holding the membrane close to the resting potential in the presence of tetrodotoxin (TTX, 3 microM), 4-AP (1 mM) and Ba2+ (1 mM). The amplitude of the outward relaxation and the tail current became smaller as the [K+]o was elevated. The outward tail current was reduced in a Ca(2+)-free solution and the residual current was eliminated by the addition of tetraethylammonium (TEA, 10 mM); the reversal potential was shifted in a direction predicted by the Nernst equation. These findings suggest the presence of delayed rectifier K+ current and Ca(2+)-activated K+ current. 6. Superfusion of TEA, Ba2+ and 4-AP, but not Cs+, induced rhythmic discharges in some of the otherwise quiescent intracardiac neurones.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

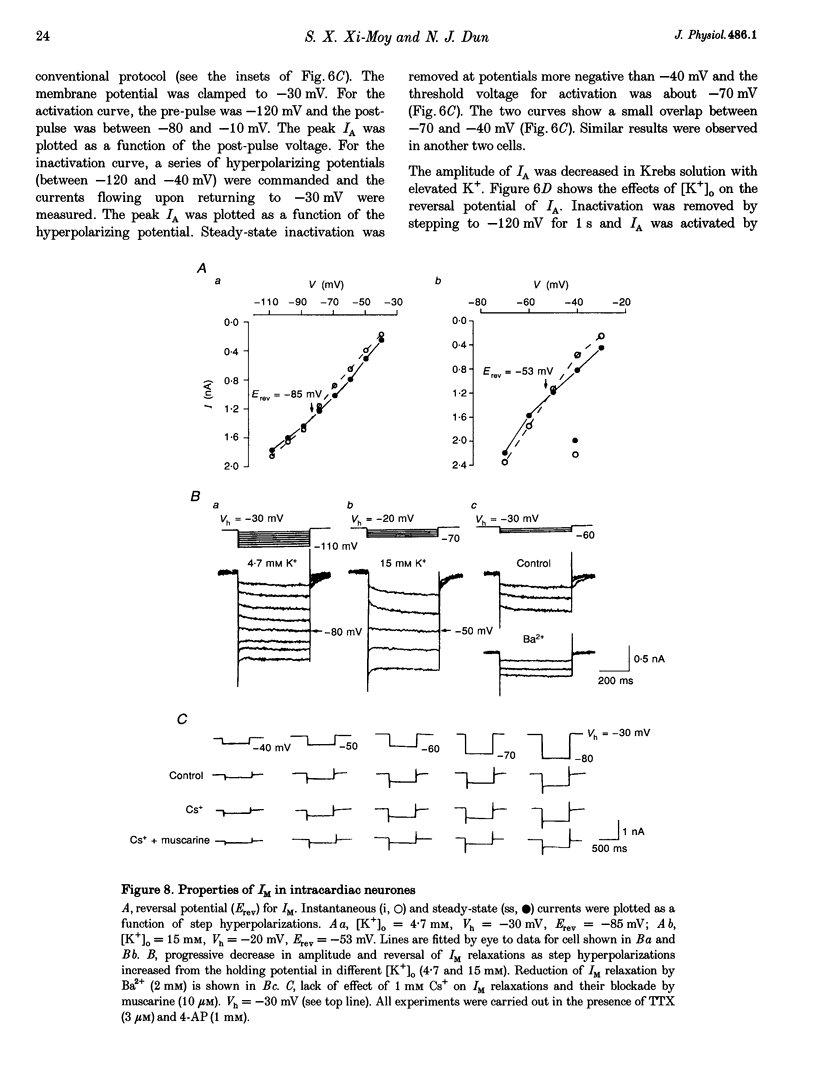
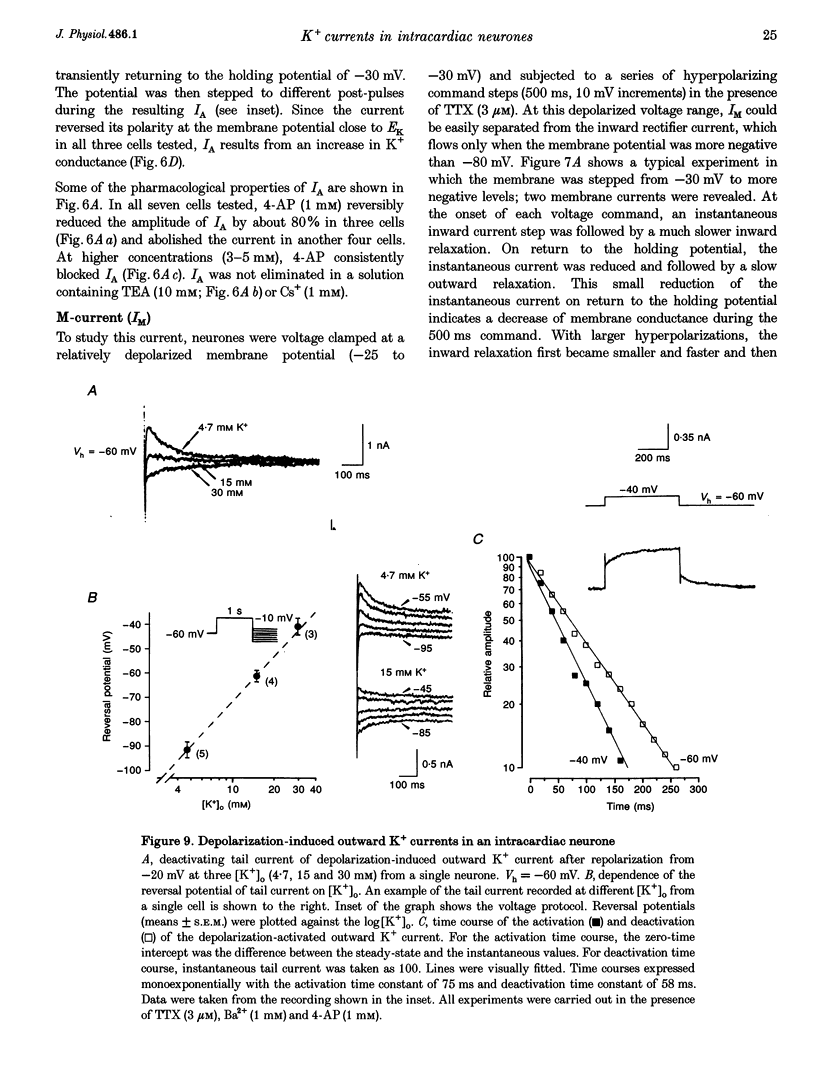
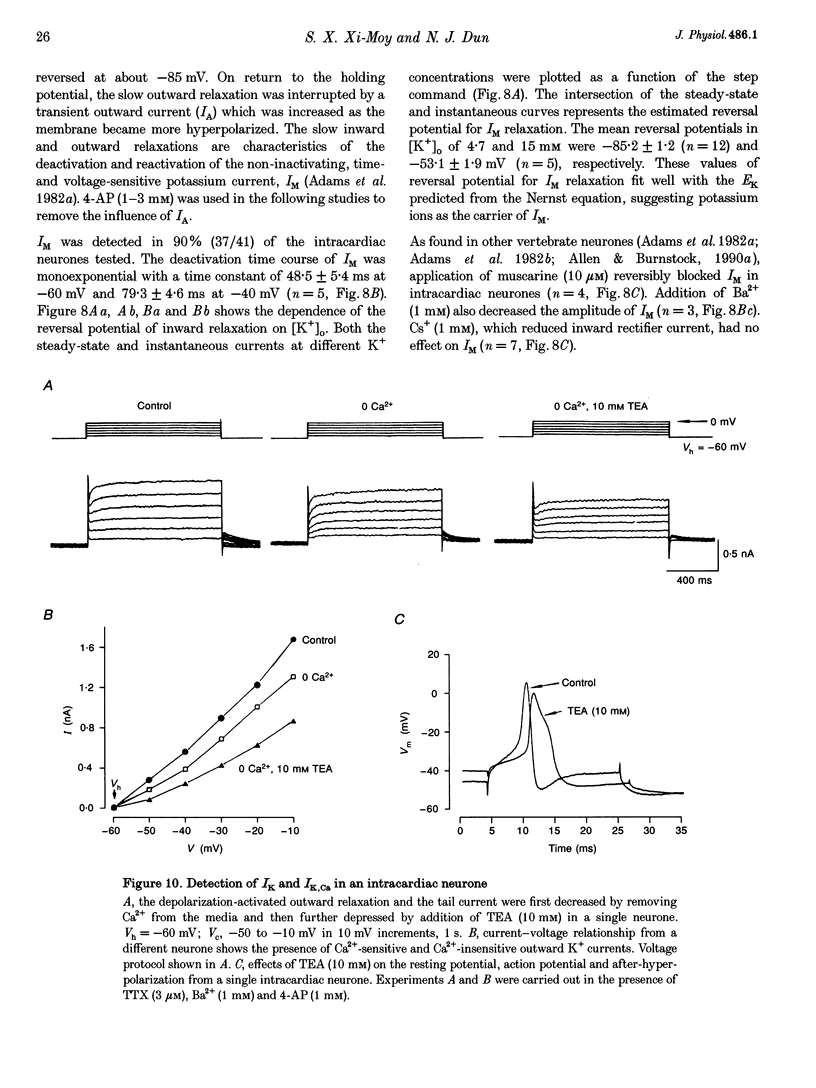
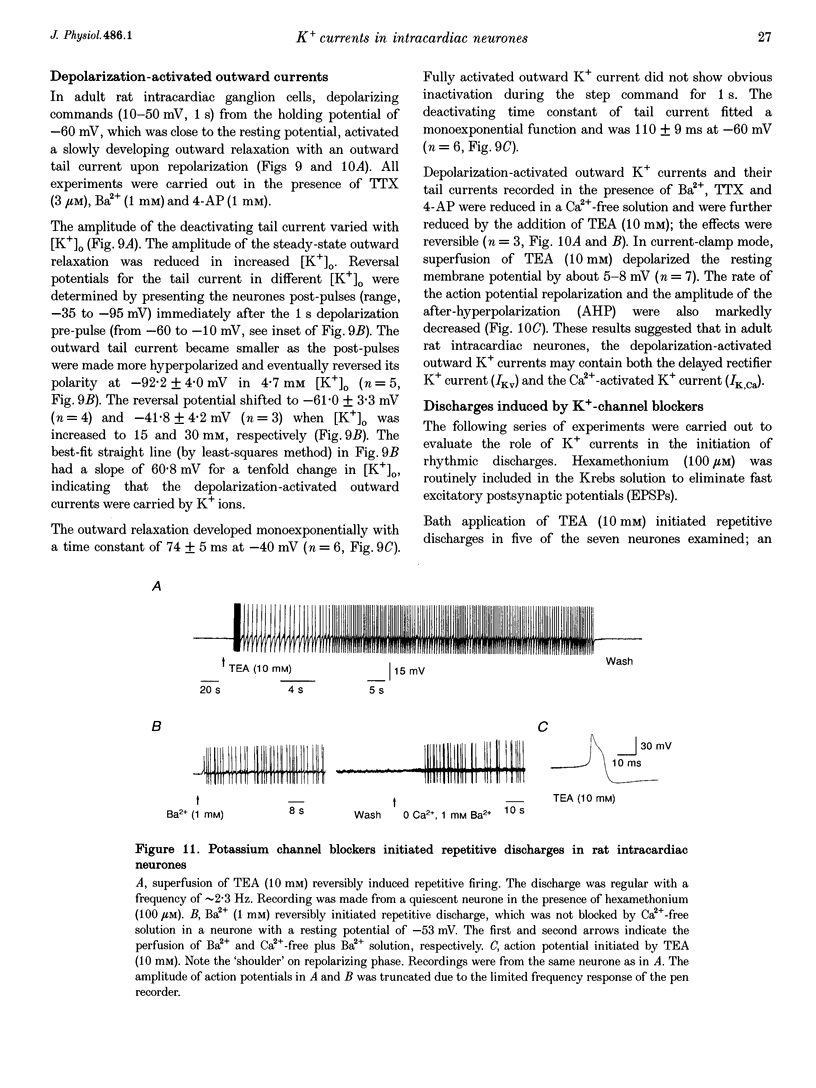



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Tokimasa T. Potassium currents in submucous neurones of guinea-pig caecum and their synaptic modification. J Physiol. 1989 Sep;416:571–588. doi: 10.1113/jphysiol.1989.sp017778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. A voltage-clamp study of the electrophysiological characteristics of the intramural neurones of the rat trachea. J Physiol. 1990 Apr;423:593–614. doi: 10.1113/jphysiol.1990.sp018042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. M1 and M2 muscarinic receptors mediate excitation and inhibition of guinea-pig intracardiac neurones in culture. J Physiol. 1990 Mar;422:463–480. doi: 10.1113/jphysiol.1990.sp017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. M-currents: an update. Trends Neurosci. 1988 Jul;11(7):294–299. doi: 10.1016/0166-2236(88)90089-6. [DOI] [PubMed] [Google Scholar]
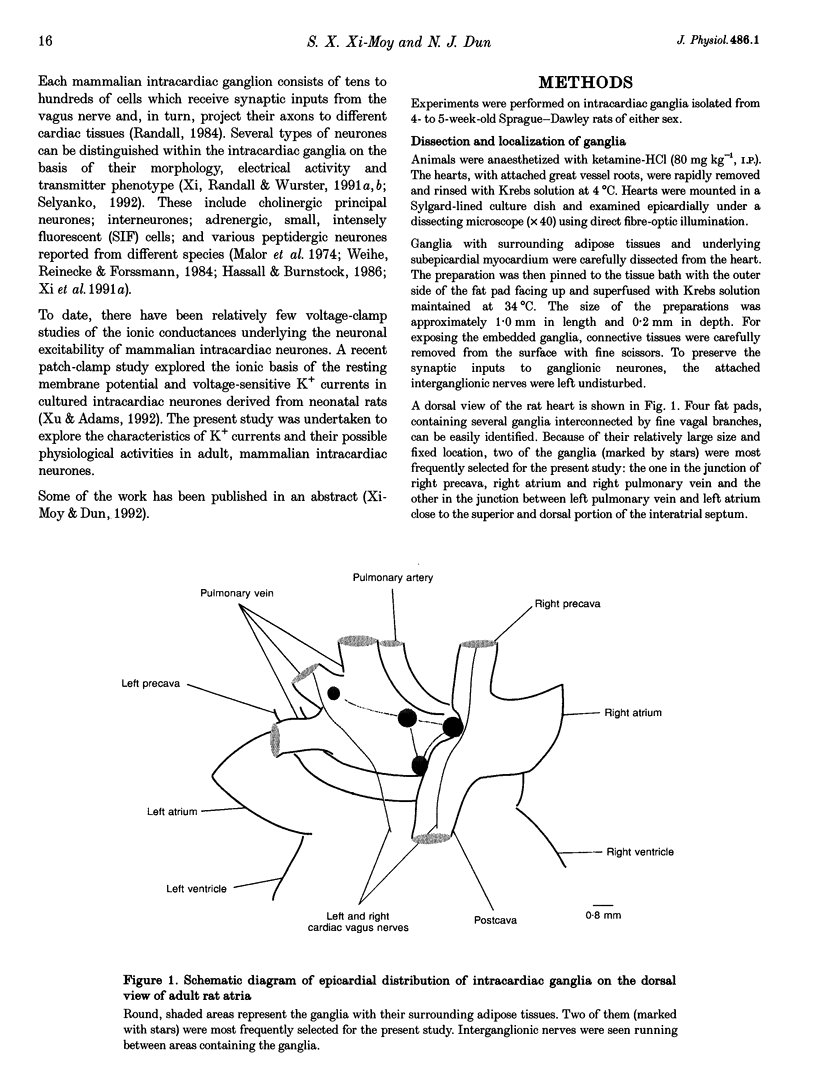
- Burkholder T., Chambers M., Hotmire K., Wurster R. D., Moody S., Randall W. C. Gross and microscopic anatomy of the vagal innervation of the rat heart. Anat Rec. 1992 Mar;232(3):444–452. doi: 10.1002/ar.1092320313. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK, in Purkinje fibres. J Physiol. 1980 Nov;308:353–367. doi: 10.1113/jphysiol.1980.sp013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Membrane conductances of photoreceptors. Prog Biophys Mol Biol. 1981;37(2):91–147. doi: 10.1016/0079-6107(82)90021-9. [DOI] [PubMed] [Google Scholar]
- Flamm R. E., Harris-Warrick R. M. Aminergic modulation in lobster stomatogastric ganglion. I. Effects on motor pattern and activity of neurons within the pyloric circuit. J Neurophysiol. 1986 May;55(5):847–865. doi: 10.1152/jn.1986.55.5.847. [DOI] [PubMed] [Google Scholar]
- Flamm R. E., Harris-Warrick R. M. Aminergic modulation in lobster stomatogastric ganglion. II. Target neurons of dopamine, octopamine, and serotonin within the pyloric circuit. J Neurophysiol. 1986 May;55(5):866–881. doi: 10.1152/jn.1986.55.5.866. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., North R. A., Tokimasa T. Muscarinic agonists and potassium currents in guinea-pig myenteric neurones. Br J Pharmacol. 1989 Jan;96(1):193–203. doi: 10.1111/j.1476-5381.1989.tb11800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J. W., Kelly M. E., Pennefather P. S. Electrophysiological function of the delayed rectifier (IK) in bullfrog sympathetic ganglion neurones. Pflugers Arch. 1989 Mar;413(5):482–486. doi: 10.1007/BF00594177. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984 Nov;4(11):2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984 Nov;4(11):2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith W. H. Membrane properties of cell types within guinea pig basal forebrain nuclei in vitro. J Neurophysiol. 1988 May;59(5):1590–1612. doi: 10.1152/jn.1988.59.5.1590. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Johnson B. R. Potassium channel blockade induces rhythmic activity in a conditional burster neuron. Brain Res. 1987 Jul 28;416(2):381–386. doi: 10.1016/0006-8993(87)90923-1. [DOI] [PubMed] [Google Scholar]
- Hassall C. J., Burnstock G. Intrinsic neurones and associated cells of the guinea-pig heart in culture. Brain Res. 1986 Jan 29;364(1):102–113. doi: 10.1016/0006-8993(86)90991-1. [DOI] [PubMed] [Google Scholar]
- Jones S. W. On the resting potential of isolated frog sympathetic neurons. Neuron. 1989 Aug;3(2):153–161. doi: 10.1016/0896-6273(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Jones S. W. Sodium currents in dissociated bull-frog sympathetic neurones. J Physiol. 1987 Aug;389:605–627. doi: 10.1113/jphysiol.1987.sp016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malor R., Taylor S., Chesher G. B., Griffin C. J. The intramural ganglia and chromaffin cells in guinea pig atria: an ultrastructural study. Cardiovasc Res. 1974 Nov;8(6):731–744. doi: 10.1093/cvr/8.6.731. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravec M., Moravec J. Intrinsic innervation of the atrioventricular junction of the rat heart. Am J Anat. 1984 Nov;171(3):307–319. doi: 10.1002/aja.1001710307. [DOI] [PubMed] [Google Scholar]
- Selyanko A. A. Membrane properties and firing characteristics of rat cardiac neurones in vitro. J Auton Nerv Syst. 1992 Jul;39(3):181–189. doi: 10.1016/0165-1838(92)90011-5. [DOI] [PubMed] [Google Scholar]
- Selyanko A. A., Zidichouski J. A., Smith P. A. The effects of muscarine and adrenaline on patch-clamped frog cardiac parasympathetic neurones. J Physiol. 1991 Nov;443:355–370. doi: 10.1113/jphysiol.1991.sp018837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard P. D., Bunney B. S. Repetitive firing properties of putative dopamine-containing neurons in vitro: regulation by an apamin-sensitive Ca(2+)-activated K+ conductance. Exp Brain Res. 1991;86(1):141–150. doi: 10.1007/BF00231048. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Kuba K. The Ca2+-sensitive K+-currents underlying the slow afterhyperpolarization of bullfrog sympathetic neurones. Pflugers Arch. 1987 Oct;410(3):234–242. doi: 10.1007/BF00580271. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. Cyclic AMP regulates an inward rectifying sodium-potassium current in dissociated bull-frog sympathetic neurones. J Physiol. 1990 Jan;420:409–429. doi: 10.1113/jphysiol.1990.sp017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe E., Reinecke M., Forssmann W. G. Distribution of vasoactive intestinal polypeptide-like immunoreactivity in the mammalian heart. Interrelation with neurotensin- and substance P-like immunoreactive nerves. Cell Tissue Res. 1984;236(3):527–540. doi: 10.1007/BF00217219. [DOI] [PubMed] [Google Scholar]
- Xi-Moy S. X., Randall W. C., Wurster R. D. Nicotinic and muscarinic synaptic transmission in canine intracardiac ganglion cells innervating the sinoatrial node. J Auton Nerv Syst. 1993 Mar;42(3):201–213. doi: 10.1016/0165-1838(93)90365-2. [DOI] [PubMed] [Google Scholar]
- Xi X. H., Thomas J. X., Jr, Randall W. C., Wurster R. D. Intracellular recordings from canine intracardiac ganglion cells. J Auton Nerv Syst. 1991 Feb;32(2):177–182. doi: 10.1016/0165-1838(91)90068-e. [DOI] [PubMed] [Google Scholar]
- Xi X., Randall W. C., Wurster R. D. Morphology of intracellularly labeled canine intracardiac ganglion cells. J Comp Neurol. 1991 Dec 8;314(2):396–402. doi: 10.1002/cne.903140213. [DOI] [PubMed] [Google Scholar]
- Xu Z. J., Adams D. J. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J Physiol. 1992 Oct;456:405–424. doi: 10.1113/jphysiol.1992.sp019343. [DOI] [PMC free article] [PubMed] [Google Scholar]