Abstract
Freshwater mussel Parreysia corrugata is an ecologically important species endemic to south-east Asia, which has been suffering from a dramatic decline in recent years. The present study investigated the population dynamics and reproductive strategy of P. corrugata in a changing climate of Bangladesh waters. Mussels were sampled monthly from January to December 2021 from the Padma River near the Rajshahi city of Bangladesh. A total of 809 specimens were collected with a sizes ranging from 36.07 to 101.41 mm shell length and 7.92–87.54 g weight, respectively. The mussels displayed a growth pattern characterized by negative allometry in body weight-shell length relationship. The asymptotic length (L∞) was 106.05 mm, the growth coefficient (K) was 0.370 per year, and the growth performance index (Φ′) was 3.58. The recruitment of P. corrugata was occurred throughout the year, with a peak during the month of February. The annual total mortality (Z) was 1.15, and natural mortality (M) and fishing mortality (F) were 0.61 and 0.54 per year, respectively. The exploitation level was E = 0.47 which indicates a state of under exploitation. Sex was identifiable throughout the study period and the sex ratio of the mussels did not differ significantly from the 1:1 (M: F) ratio ((χ2 = 15.80, P > 0.05). In terms of gonadal development, maturation, and spawning activity, both sexes had similar trends. Histology identified five gonad development stages for both male and female mussels: early developing, late developing, ripe, spawning, and spent. Monthly GSI data showed significant seasonal fluctuations with the highest GSI in April and it was sharply declined in May indicating the peak spawning of the mussels, which was in consistent with condition index and gonadal histology. All the physicochemical parameters showed significant variation except for pH. The most important factors explaining mussel body condition (b value) and reproductive strategies (GSI and CI) were water temperature, dissolved oxygen and water level. Although no known major threats of this species have been documented from Bangladesh, conservation measures could be adopted to protect the natural habitats of P. corrugata from adverse physicochemical and hydrological factors.
Keywords: Gonad histology, Growth parameters, Multiple regression, Natural mortality, Parreysia corrugata, Padma river
1. Introduction
Freshwaters and their species are facing an increasing extinction threat due to environmental changes, which are frequently linked to climate catastrophes such as drought [[1], [2], [3]]. Freshwater mussels are economically important and play a vital role in benthic ecosystems functioning. They perform four critical ecosystem-level functions: trophic structure (modification of substrate and food webs), nutrient turnover, and alteration of habitat and indicator species to determine habitat conditions (invertebrate species composition and abundance). They influence the water column by filtering, grazing, and nutrient cycle alteration via organic deposits in sediments that are re-mineralized directly or by microbiological ways [4]. They have an effect on community structure and can affect community equilibrium, diversity, and interspecies connections. However, in a changing environment, environmental stressors associated with climate changes may cause alterations to bivalve resources, among which slow gamete development, atresia, and restoration are the most common phenomena [5]. As a result, assessing the exploitation level of stocks in a changing climate is critical for implementing appropriate management measures.
Morphometric traits and their dynamics in relation to temporal fluctuations and maturity stages are essential in understanding reproductive biology of aquatic animal [6]. Understanding the population dynamics and reproduction of mussels can help future conservation initiatives and pollution monitoring programs. Population dynamics research is an important decision-making method for sustainable stock management [7]. Fishery management is heavily reliant on calculating growth, mortality, and recruitment trends, as well as assessing the structure and sustainability of existing populations [8]. Suitable management techniques for resource planning and management can be implemented when determining the amount to which resources are used [9,10]. Further understanding of species demographic features and ecology will also aid in the identification of conservation units, resulting in successful integrated conservation initiatives. Furthermore, detailed field evaluations will aid in the identification of stressors and habitat limitations, which is a necessary step before applying recovery techniques including captive breeding.
Parreysia corrugata (Lamarck, 1807) is a bivalve mollusk species in the order Unionoida (freshwater mussels), superfamily Unionoidea, and family Unionidae. This species can be found all over Southeast Asia [11]. Though P. corrugata is not a commercially important food source, it does sustain small-scale fishing in Bangladesh and is a possible rival for freshwater pearl production [12]. These species are also eaten by local tribal people that live on the river's banks. Furthermore, Parreysia spp. is known to have therapeutic use and has been claimed to be utilized by indigenous people to lower blood pressure. It is also employed in the cement, lime, button, toy, and cosmetic production [13]. Despite its ecological and economic significance, there is a scarcity of biological information about this potential species that lives in Bangladesh's freshwater ecosystems.
Recent improvements in aquaculture development, environmental conservation, and surveillance of pollution research emphasize mollusks' significance [14,15]. Although no known major threats of this species have been documented in country's water bodies through overfishing and has been listed as a species of least concern by IUCN [16], alteration of their habitats by climate change, shocking drop in water depth, agricultural expansion and pollution making them vulnerable in natural water bodies [17].
Literature available on research of P. corrugata is mainly focused on the allometry and condition index [18,19], microbial analysis of tissue [13], population ecology [20], proximate and mineral compositions [20], genetic diversity [21] and bioactive compounds [22] in Indian waters. However, no studies were undertaken to ascertain the population dynamics and reproductive strategy of the species in Bangladesh waters. To ensure the success of P. corrugata conservation, gonado-somatic index, condition index, sex ratio, and histological examinations were performed on population traits such as age, growth, recruitment pattern, mortality, and reproductive strategy of the species resided in the Padma River. The purpose of presenting these data is to offer the essential baseline for assessing conservation status and enabling the establishment of successful conservation activities in response to changing climatic conditions.
2. Materials and methods
2.1. Description of the study area
The study was conducted in the Padma River near Rajshahi City (24°21′29.30”N, 88°37′30.55”E to 24°21′42.41”N, 88°34′31.18”E) located in the northwestern part of Bangladesh as shown in Fig. 1. The Padma River near Rajshahi City serves as a direct discharge site for vegetable markets, slaughterhouses, and household septic tanks. The surface water quality of the river is poor due to the dumping of untreated municipal discharges through various municipal drains [23,24]. Climate change is a significant concern that consistently contributes to the degradation of the environmental conditions within this river basin. The river basin has a unimodal rainfall pattern, with the monsoon season accounting for 63.11 % of total annual rainfall. Consequently, the dry season is characterized by hot and humid conditions and the elevated rate of evaporation decreasing the water depth of less than 2 m in some points during summer [25]. Farakka Barrage, located on the Ganges River in West Bengal, India, is also significantly decreasing the river's water flow. This reduction in water flow has led to stagnant water during dry seasons in some parts of the river, creating favorable conditions for pollutants to settle down easily into the water and sediments. Furthermore, when water availability is limited, agricultural operations in dry zones are intensified, resulting in the unrestricted discharge of fertilizers, pesticides, and other waste from agriculture into the river [23].
Fig. 1.
Location of the study area in the Padma River at Rajshahi district Bangladesh (Modified from google map).
2.2. Measurement of physicochemical and hydrological parameters
On-site measurements of physicochemical characteristics were made. A Celsius thermometer was used to measure the temperature of the water (WT), CO2, total alkalinity, and dissolved oxygen (DO) were measured using a mobile aquaculture kit (Model FF2, HACH, USA), and pH was measured using an electronic pH meter (Jenwary, 3020). Rainfall and water level information was obtained from the Bangladesh Water Development Board (BWDB), CL-205, Rajshahi Sadar, Bangladesh. A mechanical water flow meter (Model: 20307R6C, USA) was used to measure the flow of water.
2.3. Sampling and processing of mussels
A total of 50–110 individuals of P. corrugata were collected on each sampling month from January to December 2021. Therefore, a total of 809 individuals were collected during the study period by experienced personnel through hand picking from the randomly selected 3–5 sites. The number of mussels collected in each sampling date was used for further analysis. Immediately after collection, samples were transferred to the laboratory for analysis. In the laboratory, mussels were washed in running tap water and cleaned to remove debris. After that, the samples were wiped dry using absorbent paper before being measured for shell size and weight. A sub-sample of 30–40 mussels was used for the analysis of histology, gonado-somatic index (GSI) and condition index.
2.4. Biometric measurements
A total of 809 specimens were collected, with shell lengths ranged between 36.07 and 101.41 mm. Each specimen's shell length (maximum antero-posterior distance), shell height (maximum distance from hinge to ventral margin), and shell depth (maximum distance between outer edges of two valves) were measured with Vernier calipers to an accuracy of 0.01 cm. Using an electronic balance, the entire body weight of each mussel was measured to the nearest 0.01 g. After blotting, the shell was opened using a shell opener, and the tissue was taken and weighed individually. The tissue was dried for two days at 60 °C and carefully weighed to 0.01 g. Finally, the weights of the dry tissue and shell were estimated to the nearest 0.01 g.
2.5. Growth pattern and parameters
The mussels' length-weight relationship was established using the widely used equation W = aLb [26], where W is the total weight (g), L is the shell length (mm), 'a' is the intercept, and 'b' is the slope (growth coefficient). On log-log converted data, the parameters 'a' and 'b' were calculated using least square linear regression showed in equation (i).
| (i) |
The coefficient of determination (r2) was used to assess the quality of the linear regression [27], and the 95 % confidence limit of 'b' and statistical significance level of r2 were calculated. Growth is isometric if b = 3.0. Growth, on the other hand, is positive allometric if b > 3.0 and negative allometric if b < 3.0.
Monthly data were classified into 1-mm length classes, and length-frequency data were evaluated using the FAO-ICLARM Stock Assessment Tools (FiSAT) [28]. ELEFAN-1 was used to calculate the asymptotic length (L) and growth coefficient (K) of the von Bertalanffy Growth Equation (VBGF). The growth performance index was subsequently calculated using the equation (ii) utilizing the derived L and K values [29].
| (ii) |
The inverse von Bertalanffy growth equation [30] was used to compute the age of P. corrugata at varied shell lengths. The VBGF was then fitted to estimates of length-at-age curves [31] using non-linear squares estimation techniques, using the equation (iii).
| (iii) |
where, Lt denotes the mean length at age t and t0 denotes the hypothetical age at length zero [32]. By fitting the L and K into the inverse of the VBGF, the t0 value was calculated. The potential longevity (tmax) of P. corrugata was obtained using the equation (iv).
| (iv) |
2.6. Recruitment, mortality and exploitation rate
The annual recruiting pattern was measured using a monthly length-frequency distribution. The normal distribution was adjusted by NORMSEP in FiSAT [33]. The natural mortality (M) at the study site was calculated using Pauly's equation [34] showed in equation (v) and the annual mean water temperature (T) (24○C).
| (v) |
The length-converted catch curve approach [35] was used to calculate the overall mortality (Z) using the equation (vi).
| (vi) |
where, Ni is the number of individuals of relative age (i), and Δti is the time required for the mussel to develop over a length class i; a is the intercept, ti is the age or the relative age of individual mussel equivalent to the mid length of class i; and where b, with sign inverted, is an estimate of Z. The above-mentioned Z and M values were used to calculate fishing mortality (F) using the equation (vii).
| (vii) |
The exploitation level (E) was then calculated using Gulland's [36] equation (viii).
| (viii) |
2.7. Sex ratio and histology
Mussels were dissected in the lab with a fine needle to extract gonads and gills. To determine the sex of a gonad, it was microscopically checked for the presence of oocytes. Sex-ratio (♂/♀) was calculated using the equation: Sex-ratio = Number of males/Number of females [37]. Twenty specimens were histologically analyzed each month to identify the gametogenic stages. The visceral mass was preserved for 24 h in Davidson solution before being put into 70 % ethyl alcohol for processing. Dehydrated gonads were cleaned with xylene and embedded in paraffin wax. The embedded tissues were sliced into sections with a thickness of approximately 6 μm. These sections were then placed onto glass slides, where they underwent a process of dewaxing and rehydration using ethanol. Finally, the sections were stained using hematoxylin and eosin Y. The slides were observed microscopically at 40 × magnification using a Leica Dmi1 microscope. The histological sections were photographed using a Leica Mc 120 HD digital camera (Leica microsystems CMS GmbH, Wetzlar, Germany). According to Juhel et al. [38], the gonad development stages were identified via histological preparation as early developing, late developing, ripe, spawning, and spent.
2.8. Gonado-somatic and condition index
The gonado-somatic index was determined as the ratio of the mussel's body weight to its gonad weight using the equation (ix).
| (ix) |
The condition index (CI) indicates the physiological state of organisms and it was determined as the ratio of tissue dry weight and shell dry weight following the description of Uddin et al. [39] using the equation (x).
| (x) |
2.9. Statistical analysis
All data were expressed as the mean and standard deviation of mussel length, weight, and physicochemical characteristics of water. All data were analyzed to check normal distribution and variance before analysis [40]. Because the data were not normally distributed, the Mann-Whitney U test was used to detect the variations in the body measurement of the mussels. Spatial (sampling sites) and temporal (months) variation of the physicochemical parameters were evaluated using two-way analysis of variance. The differences in monthly mean values of GSI and CI were evaluated using one-way analysis of variance (ANOVA). In both cases, of ANOVA the significance was determined using Duncan multiple range test (DMRT) at P < 0.05. T-test was performed using the equation t = ((b-3)/standard error) to determine the significant difference of regression coefficient slope (b) from the isometric value [40]. Chi-square (χ2) analysis was employed to test for significant differences between the females and males ratio. Pearson correlation was run to determine the relationship between physicochemical, hydrological and biological parameters of P. corrugata (b value, GSI and CI). Finally, the relationship between environmental and biological parameters was determined using multiple regression analysis. Statistical analyses were accomplished with the SPSS version 25.0 program from IBM Corporation, New York, USA.
3. Results
3.1. Physicochemical and hydrological parameters
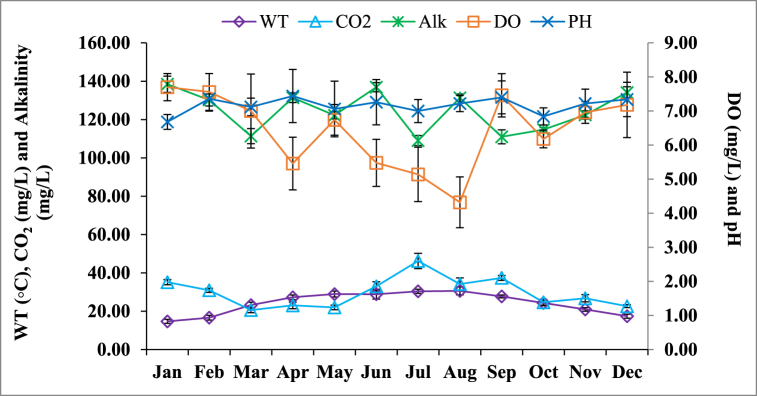
Physicochemical parameters showed significant monthly variations, while differences among the sampling sites were insignificant (Supplementary Table 1). Therefore, only the monthly changes in physicochemical parameters are described and shown in Fig. 2. The maximum water temperature was recorded in August (30.6 ○C) and the minimum in January (14.7 ○C). The annual mean WT exhibited significant differences (ANOVA, P < 0.05). CO2 displayed a considerable defined pattern and varied between 20.58 mg/L in March to 46.22 mg/L in July. Significant difference in mean values of alkalinity was observed with the maximum value recorded in January (138.42 mg/L) and the minimum in July (109.04 mg/L). The mean DO reached a high level of 7.70 mg/L in January and a lowest level of 4.32 mg/L in August. The mean annual difference of pH was not significant (ANOVA, P < 0.05) during the study period and varied within a narrow range of 6.68 in January to 7.44 in April.
Fig. 2.
Physicochemical parameters of the Padma River at Rajshahi district, Bangladesh. WT = Water temperature, CO2 = Carbon-di-oxide, Alk = Alkalinity, DO = Dissolved oxygen.
July recorded the highest amount of rainfall (332 mm) and February had the lowest (8 mm) during this study period. Rainfall had a similar pattern on the water level of the river (Supplementary Fig. 1). The maximum and minimum water levels were recorded in July and February, respectively, followed by the subsequent observation of rainfall patterns. The pattern of river flow rate and rainfall was similar, with July recording the greatest water flow (0.85 m/s) (Supplementary Fig. 2).
3.2. Biometric measurements
Descriptive statistics of body measurements of P. corrugata are shown in Table 1. Shell length, body weight, shell height, shell depth, tissue weight and shell weight of P. corrugata were ranged between 36.07 and 101.41 mm, 7.92–87.54 g, 22.05–47.90 mm, 13.39–33.09 mm, 1.21–20.89 g and 3.91–41.66 g with the mean value of 56.41 ± 8.02 mm, 28.93 ± 9.84 g, 36.40 ± 3.67 mm, 22.35 ± 2.49 mm, 6.19 ± 3.14 g and 16.53 ± 5.40 g, respectively. Mann-Whitney U test displayed no significant difference (P > 0.05) in all the biometric measurements between male and female P. corrugata.
Table 1.
Descriptive statistics of body measurements of P. corrugata (352 males, 457 females) collected from the Padma River.
| Measurements | Sex | Min | Max | Mean | SD | P-Value |
|---|---|---|---|---|---|---|
| Shell length (mm) | Male | 36.07 | 98.95 | 56.72 | 8.40 | 0.107 |
| Female | 36.85 | 101.41 | 56.17 | 7.72 | ||
| Combined | 36.07 | 101.41 | 56.41 | 8.02 | ||
| Shell height (mm) | Male | 22.05 | 47.24 | 36.46 | 3.70 | 0.293 |
| Female | 25.43 | 47.90 | 36.35 | 3.66 | ||
| Combined | 22.05 | 47.90 | 36.40 | 3.67 | ||
| Shell depth (mm) | Male | 13.39 | 30.70 | 22.41 | 2.49 | 0.260 |
| Female | 13.88 | 33.09 | 22.31 | 2.48 | ||
| Combined | 13.39 | 33.09 | 22.35 | 2.49 | ||
| Body weight (g) | Male | 7.92 | 87.54 | 29.30 | 9.90 | 0.052 |
| Female | 8.87 | 84.47 | 28.65 | 9.79 | ||
| Combined | 7.92 | 87.54 | 28.93 | 9.84 | ||
| Tissue weight (g) | Male | 1.42 | 20.45 | 5.98 | 3.01 | 0.100 |
| Female | 1.21 | 20.89 | 6.36 | 3.23 | ||
| Combined | 1.21 | 20.89 | 6.19 | 3.14 | ||
| Shell weight (g) | Male | 4.15 | 40.90 | 16.68 | 5.31 | 0.136 |
| Female | 3.91 | 41.66 | 16.41 | 5.47 | ||
| Combined | 3.91 | 41.66 | 16.53 | 5.40 |
Min.: minimum, Max.: Maximum, SD.: Standard Deviations, P-Value = Mann-Whitney.
3.3. Growth pattern and growth parameters
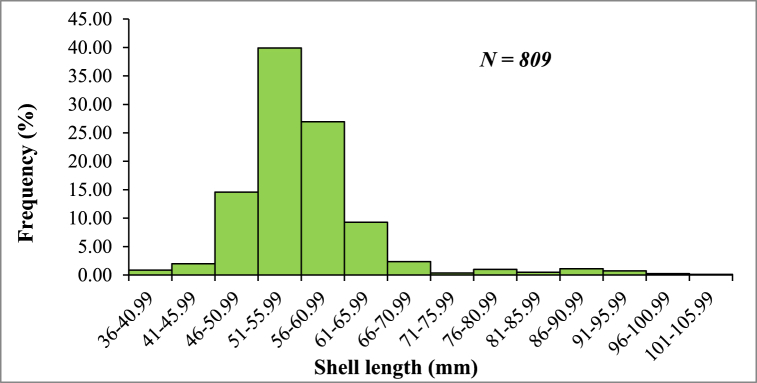
The size-frequency distribution of 809 specimens of P. corrugata analyzed is shown in Fig. 3. The shell length ranged between 36 and 101.41 mm. The overall size–frequency distribution showed the highest frequency (39.93 %) for the shell length group of 51.0–55.59 mm. On the contrary, the lowest frequency (0.12 %) was recorded for the shell length group of 101–105.99 mm.
Fig. 3.
Size frequency distribution of P. corrugata based on shell length (mm).
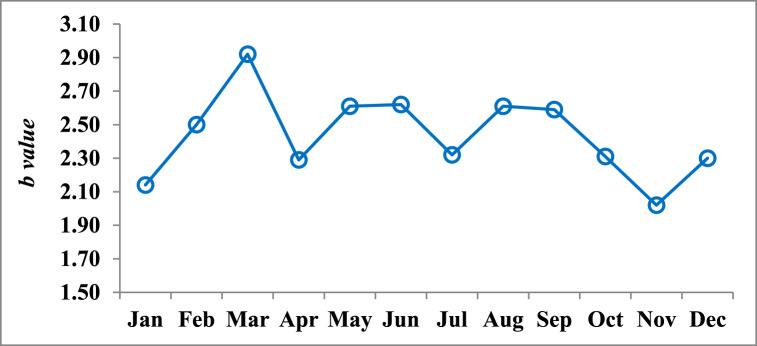
The relationship of SL with other body dimensions (BW, SH and SD) is shown in Table 2. All the relationships were significant (P < 0.001) with r2 values ranging from 0.436 to 0.658. Furthermore, the exponent b value was ranging from 0.57 to 1.98 suggesting a negative allometric growth pattern of P. corrugata for all the body measurements. The dataset containing monthly b values, which represent the equilibrium constant, is depicted in Fig. 4. The observed values for the shell length-total weight relationship varied between 2.02 in November and 2.92 in March.
Table 2.
Growth pattern of P. corrugata collected from the Padma River.
| Measurements | Equation | r2 | t-test (b) | P-value | Allometry |
|---|---|---|---|---|---|
| In(BW) – ln(SL) | ln(BW) = 1.98ln(SL) – 4.65 | 0.658 | −20.35 | 0.000 | – |
| In(SH) – ln(SL) | ln(SH) = 0.57ln(SL) + 1.29 | 0.493 | −119.26 | 0.000 | – |
| In(SD) – ln(SL) | ln(BW) = 0.58ln(SL) + 0.783 | 0.436 | −105.13 | 0.000 | – |
| In(SD) – ln(SH) | ln(BW) = 0.73ln(SL) – 0.475 | 0.465 | −82.12 | 0.000 | – |
–: indicates negative allometry.
Fig. 4.
Monthly fluctuations of b value of shell length-body weight relationship of P. corrugata.
Table 3 displays the conclusive evaluations of growth parameters for P. corrugata. The maximum observed length (Lmax) of P. corrugata was recorded as 101.41 mm, while the asymptotic length (L∞) was determined 106.05 mm. The estimated growth constant (K) of the studied mussel was 0.370 per year and the longevity (tmax) was approximately 8 years. The hypothetical age at zero length (to) was calculated as −2.00 years and the growth performance index (φ) was 3.58. The VBGF describing the growth of the P. corrugata population at the Padma River was as follows:
| Lt = 106.05 {1 – exp [– 0.37(t + 2.00)]} |
Table 3.
Estimates of growth parameters of P. corrugata from the Padma River.
| Growth parameters | Values |
|---|---|
| Asymptotic length (L∞) | 106.05 |
| Maximum observed length (Lmax) | 101.41 |
| Growth constant (K) | 0.370 |
| Longevity (tmax) | 8 |
| Theoretical age at zero length (to) | 2.00 |
| Growth performance index (Φ′) | 3.58 |
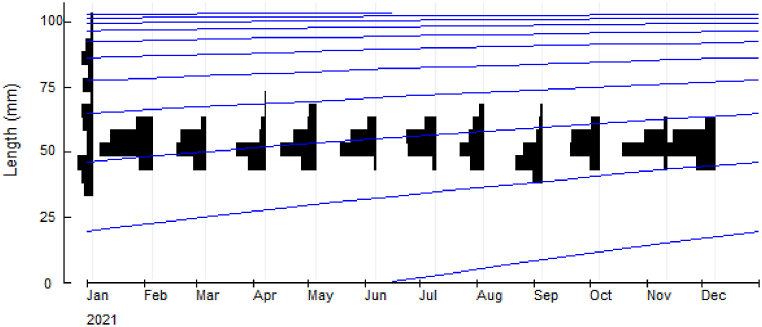
Restructured growth curve fitted with the monthly length-frequency distribution data obtained by ELEFAN I routine from January to December 2021 is shown in Fig. 5.
Fig. 5.
Length–frequency distributions with superimposed growth curves of P. corrugata fitted with a growth curve obtained by ELEFAN I routine from January to December 2021 (L∞ = 106.05 mm, K = 0.37 per year and N = sample size, 809.
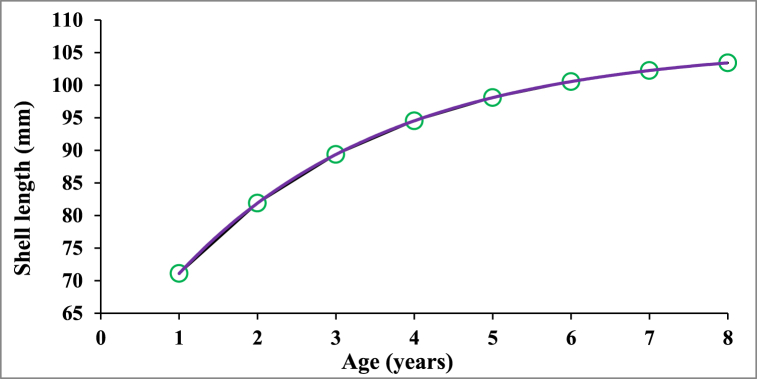
Fig. 6 represents the absolute increase in the growth of P. corrugata during the study period. The derived growth curve of P. corrugata in the Padma River showed that the sizes reached by the mussel population at year 1, 2, 3, 4, 5, 6, 7 and 8 were 71.10, 81.90, 89.37, 94.53, 98.09, 100.55, 102.25 and 103.42 mm, respectively.
Fig. 6.
Plot of age and growth of P. corrugata based on computed growth parameters (L∝ = 106.05 mm and K = 0.37 per year).
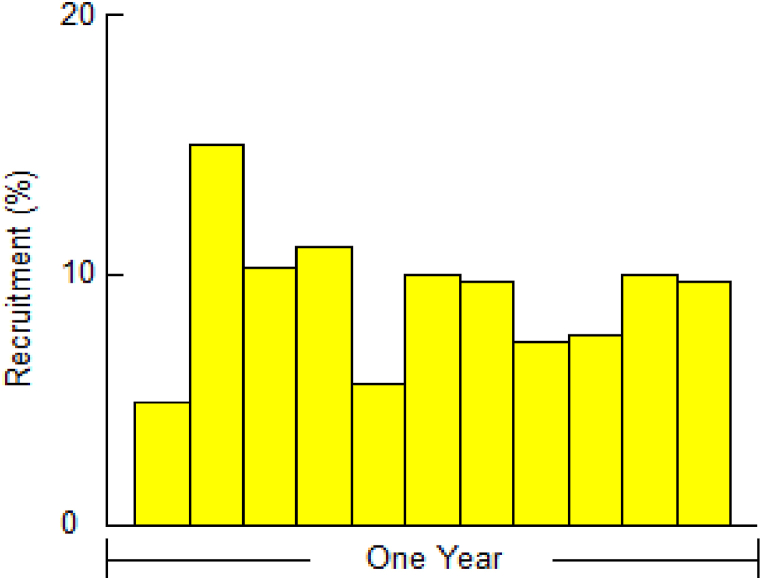
3.4. Recruitment pattern
Fig. 7 displays the annual recruitment pattern of P. corrugata as generated by the FiSAT-II software. The recruitment of P. corrugata in the Padma River was observed to occur continuously throughout the year. However, there was a distinct peak pulse in recruitment during the month of February which accounted for 14.81 % of the total recruitment observed during the entire study period.
Fig. 7.
Recruitment pattern of P. corrugata in the Padma River.
3.5. Mortality and exploitation rate
The annual mean water temperature during the sampling period was 24.41 °C. During the study period, estimated total mortality (Z) was 1.15 and 2.26 per year by length-converted catch curve, and Jones and van Zalinge method, respectively (Supplementary Fig. 3). However, natural mortality (M) and fishing mortality (F) of P. corrugata were estimated 0.61 and 0.54 per year, respectively. The estimated exploitation rate (E) was 0.47, which was below the optimal level of exploitation (E = 0.50). Therefore, the value of exploitation rate suggested that the population of P. corrugata of the Padma River is underexploited.
3.6. Sex ratio
A total of 809 P. corrugata individuals, including 352 males (43.51 %) and 457 females (56.49 %) were collected during the study period. The female to male sex ratio was 1:0.77 and did not deviate significantly from the equal representation of the female: male (1:1) ratio as expected (χ2 = 15.80, P > 0.05). However, monthly evaluation of sex ratio showed significant variation during the months of March, April, June, August and September (Supplementary Table 2).
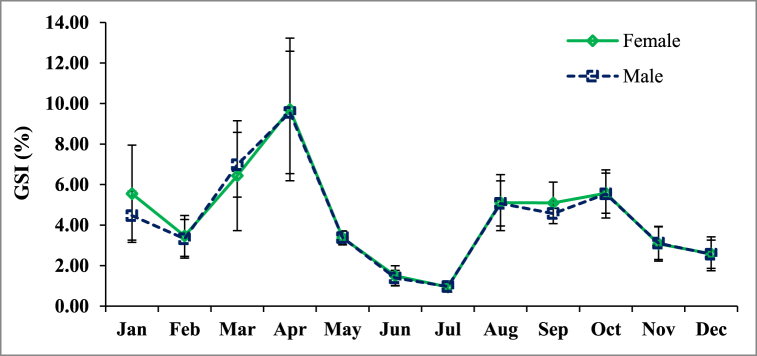
3.7. Gonadosomatic index (GSI)
Significant monthly variations were observed in the GSI values of female (ANOVA, F = 91.21, DF = 456, P < 0.0001) and male (ANOVA, F = 120.05, DF = 351, P < 0.0001) of P. corrugata (Fig. 8). The GSI of the mussels dramatically increased from February to April at reaching the highest value 9.71 for female and 9.56 for male, indicating most of the mussels were in the ripe stage during this time. A sharp decline of GSI was recorded both for male and female during April and May, which indicated the spawning peak period of the mussels in the habitat. For both sexes, the lowest GSI was recorded in July, after that it was again increased slowly with some fluctuations till October and then it dropped again till December. No considerable differences were noticed in the values of GSI between males and females.
Fig. 8.
Monthly variation of gonadosomatic index (GSI) of P. corrugata.
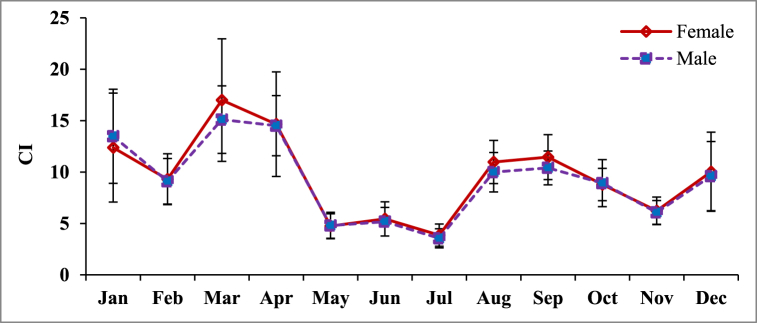
3.8. Condition index (CI)
Monthly variation in condition index of P. corrugata is shown in Fig. 9. The CIs of P. corrugata varied significantly during the study period both for females (ANOVA, F = 54.88, DF = 474, P < 0.0001) and males (ANOVA, F = 55.31, DF = 351, P < 0.0001). The mean maximum CI was recorded in March (Females, 17.00 ± 5.95; Males 15.10 ± 3.28). Condition index showed drastic decline during April and May and reached to lowest level in July. The sudden decline of CI during April and May also coincided with the GSI values, indicating the spawning loss of the mussel during the peak spawning period. Thereafter, a sudden increase of CI occurred during August and September. Again, after a slight decline, build up in condition was observed from December onward.
Fig. 9.
Monthly changes in condition index of P. corrugata.
3.9. Gametogenic development
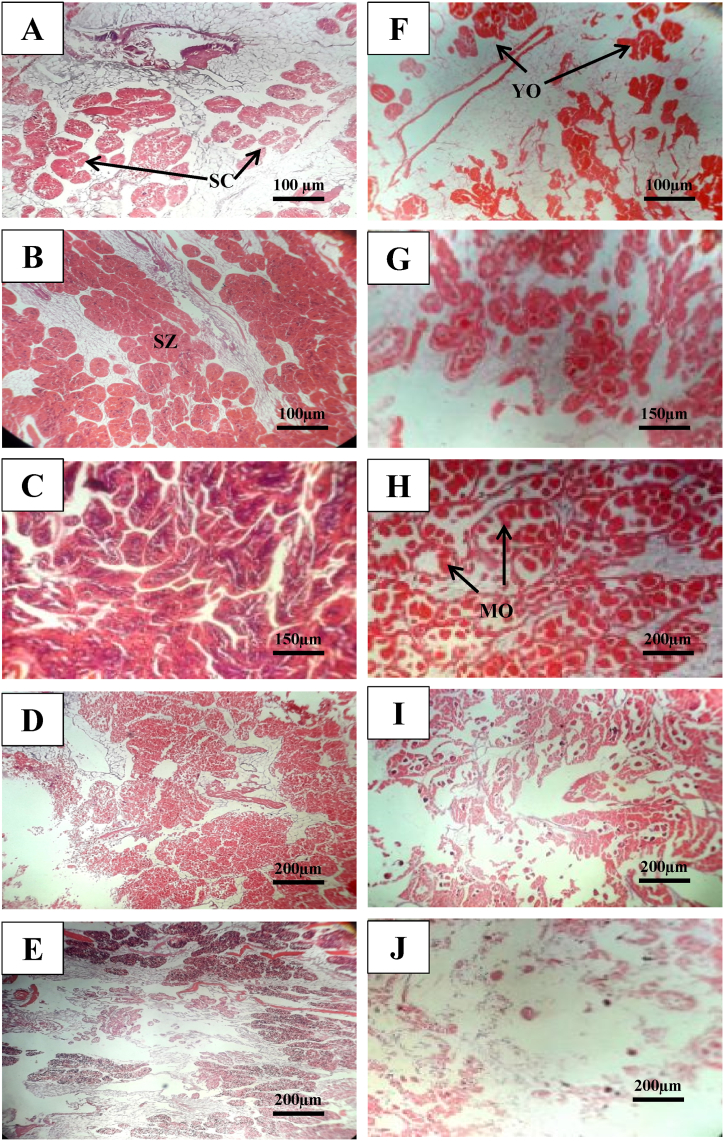
Histological observation revealed similar gametogenic development both for male and female. During the study five gonad developmental stages was observed both for males and females as early developing, late developing, ripe, spawning, and spent. Male and female gonad developing stages are shown in Fig. 10(A–E) and 10 (F-J), respectively. Occurrence of mature oocytes and spermatids in the histological sections indicated intense spawning events of P. corrugata during April and May.
Fig. 10.
Photomicrographs of histological section of male (A–E) and female (F–J) gonads of P. corrugata. A, F = Early developing stage, B, G = Late developing stage, C, H = Ripe stage, D, I = Spawning stage, E, J = Spent stage. SC = Spermatocyte, SZ = Spermatozoa, YO = Young Oocytes, MO = Mature Oocytes.
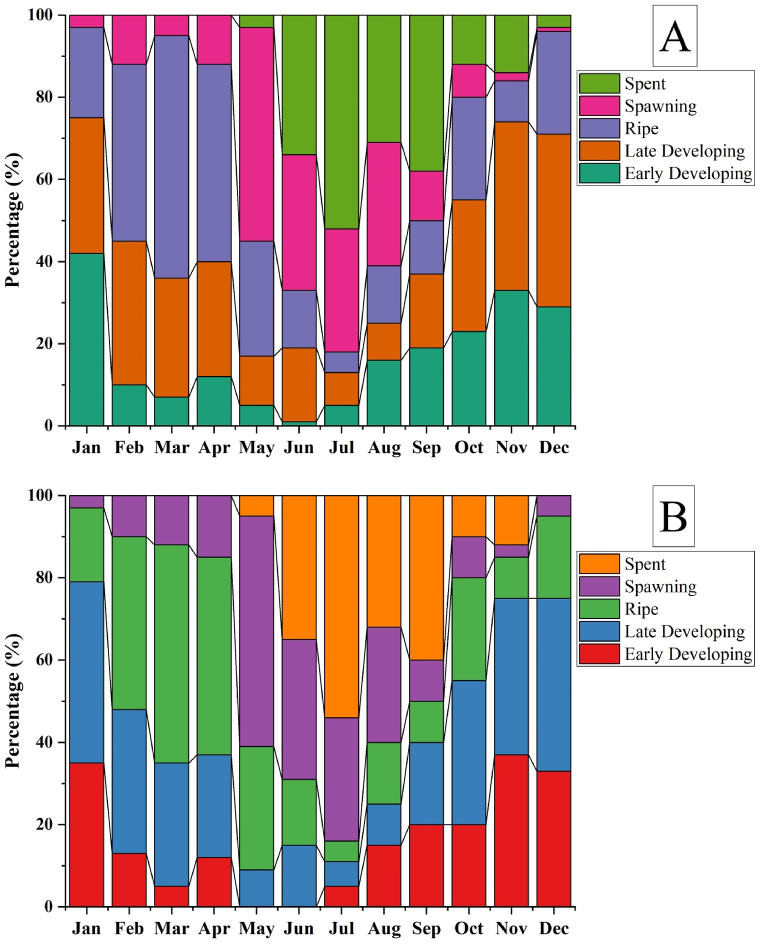
Fig. 11 (Male = A and Female = B) depicts the distribution of gonad development stages in P. corrugata. Late developing, ripe and spawning stages of P. corrugata were evident throughout the year indicating the continuous spawning nature of this species. However, >50 % of the mussels were at spawning stage in May, representing the peak spawning period of the mussels during this time. Between May to December, specimens with spent stage were observed and >50 % of the mussels were at spent stage in the month of July.
Fig. 11.
Monthly frequencies of gonad development stages of P. corrugata. A = Male and B = Female.
3.10. Correlation between GSI, CI and physicochemical parameters
Among the physicochemical and hydrological parameters, only WT, DO and water level have strong correlation with b value, CI and GSI (Table 4). WT has strong positive correlation with b value and moderate negative correlations with CI and GSI. DO have moderate negative correlation with b value and moderate positive correlation with CI. Furthermore, water level has strong positive correlation with GSI. Although hydrological parameters have no significant influence on b value, CI and GSI, they have positive influence on these biological parameters.
Table 4.
Correlation matrix of physicochemical and hydrological parameters with CI and GSI of P. corrugata.
| Parameters | b value | CI | GSI |
|---|---|---|---|
| Temperature | 0.557∗ | 0.415∗∗ | 0.316∗ |
| DO | 0.368 | 0.399∗∗ | 0.245 |
| CO2 | 0.152 | 0.202 | 0.007 |
| pH | 0.113 | 0.032 | 0.108 |
| Alkalinity | 0.212 | 0.053 | 0.094 |
| Rainfall | 0.144 | 0.470 | 0.038 |
| Water level | 0.033 | 0.154 | 0.801∗∗ |
| Water flow | 0.143 | 0.362 | 0.271 |
∗Correlation is significant at the 0.05 level (2-tailed).∗∗ Correlation is significant at the 0.01 level (2-tailed).
3.11. Regression between physicochemical and hydrological parameters with CI and GSI
The multiple regression analysis generated different R2 values for b value, CI and GSI as indicated in Table 5. In the regression analysis, 52.8, 37.2 and 13.7 % of the variation in b value, CI and GSI was explained by the five physicochemical and three hydrological parameters.
Table 5.
Coefficient of determination showing amount of variation in biological parameters of P. corrugata explained by the physicochemical and hydrological parameters.
| Dependent variables | R | R2 | Adjusted R2 | Std. Error of the Estimate | Change Statistics |
||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | F Change | df1 | df2 | Sig. F Change | |||||
| b value | 0.452a | 0.528 | 0.312 | 6.785 | 0.528 | 3.785 | 8 | 3 | 0.005 |
| CI | 0.572a | 0.372 | 0.265 | 4.710 | 0.372 | 5.244 | 8 | 3 | 0.001 |
| GSI | 0.333a | 0.111 | 0.028 | 16.585 | 0.111 | 1.346 | 8 | 3 | 0.259 |
Predictors: (Constant), Temperature, DO, CO2, pH, alkalinity, rainfall, water level, water flow.
The amplitude and direction of the slope of a regression line are determined by the partial regression coefficient, b value (Supplementary Table 3). The b values associated with WT and hydrological parameters showed positive sign indicating positive influence on b values of P. corrugata. b values for condition index showed negative sign for WT (−4.54) and DO (−2.66), suggesting that for every increase in one unit of WT and DO, the regression equation anticipated a decrease of 4.54 and 2.66 condition indexes, respectively. At the same time, CO2 (3.74), pH (0.51), alkalinity (0.17) and other hydrological parameters showed positive signs, indicating corresponding increase of condition index with the increase of one unit of these parameters. In case of GSI, the b values showed negative sign for WT (−1.58) and pH (−1.02), implying that in one unit of increase in WT and pH predicting 1.58 % and 1.02 % decrease of GSI, respectively. However, b values associated with DO, CO2, alkalinity, and other hydrological parameters portrait positive influence on GSI.
4. Discussion
This work contributes to the primary literature by reporting the population characteristics and reproductive strategy of P. corrugata over a one-year period. Monthly measurements of physicochemical and hydrological parameters were also taken to determine their impact on growth (b-value) and reproductive attempts (CI and GSI).
The utilization of body morphometrics is widely employed as a valuable tool in the fields of conservation, biological assessment, and resource management for aquatic organisms [41,42]. The length of the mussels exhibited variability, ranging from 36.07 to 101.41 mm, with an average length of 56.41 ± 8.02 mm. The overall size–frequency distribution showed the highest frequency of young mussels of 51–55.59 mm (39.93 %) size class. Study conducted by Malathi and Thippeswamy [19] reported the total length ranged between 17 and 56.6 mm and the average length was 41.45 mm in P. corrugata obtained from the river Malthi, Western Ghat, India which is corroborated by the present findings. They also reported higher abundance of younger mussels in their samples. The observed decrease in the occurrence of larger mussels in this study may be attributed to the cessation of shell growth and subsequent mortality in older individuals. This could be since habitats do not consistently provide optimal conditions for mussel growth.
During the study period, the growth coefficient for shell length-total body weight (b = 1.98) of P. corrugata demonstrated negative allometric growth pattern, which showed that the relative growth of length was greater than that of the body weight. The data on monthly b values also indicate negative allometric growth pattern of P. corrugata in the Padma River. The presence of a negative growth allometric pattern in molluscan organisms appears to be widespread, and similar growth models have been documented in L. marginalis in Bangladesh [19,43]. The ‘b’ values computed in the present investigation are lower compared to the studies conducted by Ramesha and Thippeswamy [18] and Malathi and Thippeswamy [19] on the same species from the rivers Kempuhole and Malthi, India, respectively, who reported the monthly ‘b’ values ranging between 2.61 to 2.95 and 2.27 to 2.86, respectively. In most bivalves, the growth coefficient ‘b’ typically falls within the range of 2.4–4.5 [44]. The relationship is considered isometric when the growth coefficient is equal to 3. Therefore, the P. corrugata of the Padma River are generally long and slender compared to the similar population of Indian waters.
Despite the insignificant differences of the sex ratio from 1:1, P. corrugata in the Padma River showed the dominance of females. The observed deviations in the mussel sex ratio, with a female-to-male ratio of 1:0.77, could potentially be ascribed to fluctuations in environmental factors, food availability, the age and size of mollusks and various epigenetic and genetic mechanisms which can have an influence on the sex determination of bivalves [[45], [46], [47]]. Earlier [23,25,48,49] it was noted that the Padma River is contaminated with heavy metals and are affected by other anthropogenic load factors. The presence of these pollutants may have an adverse effect on the native mussel population of P. corrugata in the Padma River. Sex change mechanisms in bivalves can be either female to male (protogyny) or male to female (protandry), whereas protandry is more common than protogyny [50,51]. The size advantage hypothesis of Ghiselin [52] can make assumptions about why female bivalves dominate in the present study. This model favors protandry in species where female size promotes fecundity but male size does not. However, further research is needed to clarify this issue.
Growth factors can be used to assess population growth in various environmental situations [53]. L∞ is a metric used to show the average length that a certain stock would attain if allowed to develop indefinitely, whereas the K value determines how quickly the species reaches its L∞ [53]. Furthermore, φ is a length-based growth index that reflects the interaction of L∞ and K and has been utilized to offer a population's prospective growth rate [53]. During the study period, L∞ (101.41), Lmax (106.05), t0 (-2.00) and φ (3.58) of P. corrugata were higher compared to the study conducted by Malathi and Thippeswamy [20] who reported L∞, Lmax, t0, φ to be 60.76, 56.06, −0.04 and 3.239, respectively. On the contrary, growth coefficient (k) recorded (0.370) in the present study was lower compared to Malathi and Thippeswamy [20]. Furthermore, the studied growth parameters were also lower compared to the findings of Ramesha and Sophia [54] measured for the same species. P. corrugata individuals in the present study were larger and long-lived when compared with the findings of Ramesha and Sophia [54] and Malathi and Thippeswamy [20] who reported the lifespan 4.83 and 6 years, respectively. Strayer et al. [55] found that freshwater mussels often survive for a long time and grow slowly, which fact corroborated the results of the current study. Jones and Neves [56] have reported that negative correlation between growth constant (k) and lifespan and showed that an increase in ‘k’ was correlated with a reduction in lifespan and maximum size. Therefore, it can be assumed that lower ‘k’ value was responsible for higher lifespan and maximum size of P. corrugata in the Padma River. In addition, sex, water depth, population size, and geographical distribution [57] are hypothesized to affect growth parameters of bivalves, which may explain the current variation. Generally, the value of parameter t0 close to zero indicates the higher abundance of young individuals. However, the estimate of t0 in the present study was far from 0 and it has no real biological meaning [58].
P. corrugata appears to have a single, large recruitment peak in February, consistent with an explanation in which recruitment is continuous. The peak month of spawning (May) is hypothesized to coincide with the recruitment peak seen in this study. This situation may be explained by the increased flood condition of the river which was accompanied with higher water depth and flow. Jones and Neves [56] reported that flood conditions may inhibit recruitment of juveniles and induce mortality in adult mussel. The creation of a complex connection between the female mussel and fish host, the facilitation of byssal-thread attachment, feeding, and growth in juveniles, and the resulting increase in survival, are all benefits of moderate and reduced water flow. Juvenile Margaritifera falcata were more successfully recruited during low discharge years in a California stream [59]. However, further research is needed to fully understand how environmental factors affect recruitment.
Natural mortality for P. corrugata was found to be much higher than fishing mortality in this research, indicating a stock imbalance which contradicts the findings of Malathi and Thippeswamy [20], and Ramesha and Sophia [54] who reported higher fishing mortality of the same species in Indian waters. However, the present study agrees with the findings of the researchers in Bangladesh and Malaysia [60,61] in observing a greater rate of natural mortality. Natural mortality is the result of various natural factors such as eutrophication, predation, disease, parasitism, and other similar causes. Moreover, in particular ecological environments with a rapid decline in water levels, a portion of the population died due to the mussels' inability to undertake migration towards the available water sources [20]. Therefore, environmental factors might be the main reason of higher natural mortality of P. corrugata in the Padma River. The computed exploitation level of P. corrugata in the Padma River was in under-exploitation level (0.47). The greater value of E (>0.50) indicates the pressure on the species, and when F = M, the yield is maximized.
The GSI and CI have been utilized in bivalves to examine growth and maturity in a specific stock [62,63]. The condition index provides a detailed picture of the physiological status of individuals in each group [64] and allows us to estimate the gonadal tissues sacrificed during the reproductive cycle [65]. In many bivalves, gonad growth before spawning increases total size since gonads make up the majority of the visceral mass. Variation in the condition index in such animals indicates the reproductive state. Increased condition is caused by the buildup of gametes in follicles and resulting bulkiness of the gonad, whereas decreased condition is caused by the release of gametes from follicles and the associated shrinkage of gonadal mass. During the study period, March had the highest GSI and CI (Female CI = 17.00 5.95, Male CI = 15.10 3.28), and July had the lowest (Female CI = 3.87 1.08, Male CI = 3.56 0.93). The variations in condition index and spawning time suggest that mussel harvesting during March–April and January may be biologically and economically feasible. On the other hand, as the peak spawning of P. corrugata in the Padma River occurred during May to July, declaration of bans during these months could be beneficial for the conservation of this species in their habitat.
During the study period, both male and female individuals displayed a similar gametogenic trend. Siddique et al. [66] found that patterns of gonadal development, maturity, and spawning in L. marginalis were similar in males and females, with the sexes displaying similar monthly patterns. During the study period, P. corrugata were found to spawn throughout the year indicating their continuous or prolific spawning nature. Prolific spawning was also evident by Siddique et al. [66] in L. marginalis. The extensive production of mature oocytes and spermatids in histology shows that this stock has improved spawning events during the early rainy season (April to May). The observed phenomenon can potentially be attributed to several factors, including enhanced food accessibility, appropriate hydrological conditions, and an increased number of potential mating partners [67]. Furthermore, higher temperature during April and May in the present study might be the triggering factor for spawning of P. corrugata. Gaikward and Kamble [68] made a parallel observation, reporting that the high rate of spawning was found at temperatures over 20 °C and indicating that the ideal spawning temperature of L. marginalis from the Panchganga River in Maharashtra, India, was between 20 and 25 °C.
Freshwater mussel distribution, abundance, and behavior can be impacted by a variety of ecological parameters such as water temperature, pH levels, food availability, and sediment composition [69]. The shell formation and tissue maturation of mollusks exhibit a strong correlation with the ambient water temperature [70,71]. Elevated CO2 and alkalinity levels have a deleterious impact on the growth of bivalves at both the juvenile and larval stages [72]. The optimal pH range for promoting the growth of aquatic animals varies between 5 and 10 [73]. Dissolved oxygen (DO) levels exceeding 5 mg/L are deemed sufficient to promote the proliferation of most aquatic organisms [74]. This study suggests that the DO level remains favorable throughout the year, except for August. The water characteristics described in recent studies, on the other hand, remained appropriate for supporting aquatic growth. Distribution, density, and growth of mussel are also influenced by hydrological factors. Studies showed that habitat characteristics, such as water depth and current affect the survival of freshwater mussels [75]. The Padma River is distinguished by irregular precipitation, which has a direct impact on the water flow pattern as well as the water level. During periods of reduced flow, aquatic species may encounter diminished levels of dissolved oxygen, elevated water temperatures, and desiccation. Conversely, heightened flow rates and hydraulic conditions can have equally deleterious consequences during periods of increasing flow. Low flow can expose aquatic species to reduced dissolved oxygen concentrations, higher water temperatures, and arid conditions, but strong flow and hydraulics can be as damaging [76].
Our results showed that two physicochemical variables (WT and DO) and one hydrological factor (water level) of river were key factors for predicting growth and reproductive physiology of P. corrugata in the Padma River. In our study these two biological parameters, CI and GSI, were negatively influenced by temperature and positively by DO and water level. Temperature and DO are the most important factors influencing aquatic organisms’ reproduction. Based on the findings of Britton et al. [77], it has been observed that environmental factors, such as temperature, dissolved oxygen (DO), and rainfall, have a persistent impact on the growth and reproductive processes of aquatic organisms. Several studies also reported direct relation between environmental parameters and biological parameters of bivalves [78,79].
4.1. Conservation implications
This research work presents a quantitative analysis of population factors and reproductive strategy of P. corrugata in the Padma River. The data gathered in this study has the potential to assess its future viability in this River, as well as in other ecologically comparable rivers that are being considered for population re-introduction and augmentation to achieve the objectives outlined in the recovery plan. Further investigation is necessary to comprehend the mechanisms by which this species thrives in the river. However, it is evident that P. corrugata exhibits distinct demographic characteristics that are influenced by both population parameters and environmental conditions. Therefore, it is likely that a range of ecological mechanisms contribute to the regulation of mussel populations, encompassing such factors as drought, flood events, seasonal variations in temperatures, predation, availability of host fish, and the quality of the environment needs to be considered. Further research is required to investigate the extent to which extrinsic ecological factors contribute to the regulation of bivalve populations.
5. Conclusion
Current investigation showed that the P. corrugata population in the Padma River is underexploited. Furthermore, higher natural mortality over fishing mortality indicated the major role of physicochemical and hydrological parameters in structuring the population of P. corrugata in the Padma River. Hence, considering the swift urbanization and climate change phenomena, comprehending the fundamental ecological mechanisms will aid us in developing more effective strategies for the implementation of protection and conservation initiatives. The data gathered in this study will offer a current evaluation of the conservation status of P. corrugata in the waters of Bangladesh. It can serve as a point of reference and a guiding tool for future research and management endeavors aimed at enhancing the conservation of this species.
CRediT authorship contribution statement
Md Ayenuddin Haque: Writing – original draft, Visualization, Software, Methodology, Formal analysis. Rayhana Akhtar Raka: Investigation, Data curation. Md Raihan Ali: Investigation, Data curation. Md Jasim Uddin: Writing – review & editing. Md Mostafizur Rahman Mondol: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization.
Ethical statement
Sampling and analysis of population parameters of P. corrugata was approved by the guidelines and regulations of the Department of Fisheries, Faculty of Agriculture, University of Rajshahi, Bangladesh (Memo no. 907/23 Fish.).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39916.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Fey S.B., Siepielski A.M., Nussle S., Cervantes-Yoshida K., Hwan J.L., Huber E.R., et al. Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proc. Natl. Acad. Sci. U.S.A. 2015;112:1083–1088. doi: 10.1073/pnas.1414894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughn C.C., Atkinson C.L., Julian J.P. Drought induced changes in flow regimes lead to long-term losses in mussel provided ecosystem services. Ecol. Evol. 2015;5:1291–1305. doi: 10.1002/ece3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenger S.J., Subalusky A.L., Freeman M.C. The missing dead: the lost role of animal remains in nutrient cycling in North American Rivers. Food Webs. 2018;18 doi: 10.1016/j.fooweb.2018.e00106. [DOI] [Google Scholar]
- 4.McKindsey C.W., Archambault P., Callier M.D., Olivier F. Influence of suspended and off-bottom mussel culture on the sea bottom and benthic habitats: a review. Can. J. Zool. 2011;89(7):622–646. [Google Scholar]
- 5.Rambaldi E., Lanni L., Pelusi P., Binda F., Cererasi S., Poggi A., Ukmar E., Avetrani P. Evaluation of the natural beds of bivalve molluscs (Telline, Donax trunculus and razor clams, Siliqua patula) along the coastal strip of the province of Latina and management indications for sustainable fishing/evaluation of natural bivalve stocks. Biol. Mar. Mediterr. 2010;17:328. [Google Scholar]
- 6.Kashefi P., Bani A., Ebrahimi E. Morphometric and meristic variations between non-reproductive and reproductive kutum females (Rutilus frisiikutum, Kamensky, 1901), in the southwest Caspian Sea. Ital. J. Zool. 2012;79:337–343. doi: 10.1080/11250003.2011.642414. [DOI] [Google Scholar]
- 7.Kumari S., Sarkak U.K., Mandhir S.K., Lianthuamluaia L., Panda D., Chakraborty S.K., Karnatak G., Kumar V., Puthiyottil M. Studies on the growth and mortality of Indian river shad, Gudusia chapra (Hamilton, 1822) from panchet reservoir, India. Environ. Sci. Pollut. Res. 2018;25:33768–33772. doi: 10.1007/s11356-018-3232-3. [DOI] [PubMed] [Google Scholar]
- 8.Vivekanandan E. Stock assessment of tropical marine fishes. Indian Council of Agricultural Research. 2005:115. New Delhi. [Google Scholar]
- 9.Amin S.M.N., Zafar M., Halim A. Age, growth, mortality and population structure of the oyster, Crassostrea madrasensis, in the Moheskhali Channel (southeastern coast of Bangladesh) J. Appl. Ichthyol. 2008;24:18–25. [Google Scholar]
- 10.Yapi J.N., Blé M.C., Etchian A.O., Kadjo V., Yao K. Population dynamics of mangrove oyster, Crassostrea gasar of the lagoons Ebrié and Aby (Côte d'Ivoire) Int. J. Sci. Basic Appl. Res. 2017;36(8):122–137. [Google Scholar]
- 11.Ramakrishna A. Dey. Zoological Survey of India; Kolkata: 2007. Handbook of Indian Freshwater Molluscs. [Google Scholar]
- 12.Tanu M.B., Islam M.S., Barman A.C., Sku S., Hossen M.N., Dey S.K. Pearl producing mussel diversity and distribution in Meghna river of Bangladesh. Int. J. Nat. Soci. Sci. 2019;6(1):54–59. [Google Scholar]
- 13.Upadhye M.V., Patil R.C., Jadhav U. Microbiological analysis of tissues of Parreysia corrugata. Curr. World Environ. 2010;5(1):209–211. http://www.cwejournal.org/?p=1158 Available from: [Google Scholar]
- 14.Chowdhury G.W., Zieritz A., Aldridge D.C. Ecosystem engineering by mussels supports biodiversity and water clarity in a heavily polluted lake in Dhaka, Bangladesh. Freshw. Sci. 2016;35:188–199. doi: 10.1086/684169. [DOI] [Google Scholar]
- 15.Niogee S.R., Tonni K.F., Barman A.C., Tanu M.B., Sku S., Uddin M.J. Ovarian cycle of freshwater pearl mussel, Lamellidens marginalis (Lamarck, 1819) collected from a culture pond in Bangladesh. Asian Fish Sci. 2019;32:117–123. doi: 10.33997/j.afs.2019.32.3.004. [DOI] [Google Scholar]
- 16.Budha P.B., Daniel B.A. Parreysia favidens IUCN 2012. IUCN Red List of Threatened Species. 2010 [Google Scholar]
- 17.Nahar D., Islam M.R., Islam M.S., Jasmine S., Mondol M. Growth pattern of freshwater bivalve mollusk Lamellidens marginalis Lamarck, 1819 from the northwest Bangladesh. J. Biosci. 2019;27:121–132. doi: 10.3329/jbs.v27i0.44677. [DOI] [Google Scholar]
- 18.Ramesha M.M., Thippeswamy S. Allometry and condition index in the freshwater bivalve Parreysia corrugata (Muller) from river Kempuhole, India. Asian Fish Sci. 2009;22:203–214. [Google Scholar]
- 19.Malathi S., Thippeswamy S. Morphometry, length-weight, and condition in Perreysia corrugata (muller 1774) (Bivalvia: unionidae) from river Malthi in the western ghats, India. Int. J. Biol. Sci. 2011;2(1):43–52. [Google Scholar]
- 20.Malathi S., Thippeswamy S. Population ecology of freshwater mussel Parreysia corrugata (mullar 1774) from river Malthi, tributary of river tunga in the western ghats, India. Recent Res. Sci. Technol. 2013;5(4):20–26. [Google Scholar]
- 21.Upadhye M.V., Jadhav U. Evaluation of genetic diversity of Pearly mussel, Parreysia corrugata by randomly amplified polymorphic DNA (RAPD) Biomedical Pharmacol. J. 2010;3(1):135–139. [Google Scholar]
- 22.Santhiya N., Ramasamy M. GC -MS analysis of bioactive compounds from freshwater mussels of Parreysia corrugata (Muller 1774) and their pharmacological activities. J. Drug Deliv. Therapeut. 2019;9(4-A):155–158. [Google Scholar]
- 23.Haque M.A., Jewel M.A.S., Hasan J., Islam M.M., Ahmed S., Alam L. Seasonal variation and ecological risk assessment of heavy metal contamination in surface waters of the Ganges river (Northwestern Bangladesh) Malaysian J. Anal. Sci. 2019;23(2):300–311. doi: 10.17576/mjas-2019-2302-14. [DOI] [Google Scholar]
- 24.Haque M.A., Jewel M.A.S., Atique U., Paul A.K., Naher N., Iqbal S. Seasonal and spatial variation of flagellate communities in a tropical river. Limnol. 2020;85 [Google Scholar]
- 25.Haque M.A., Jewel M.A.S., Zinat A., Jasmine S., Khatun M.S., Hasan J. Phytoplankton community structure and environmental variables as indicators of organic pollution in Padma River, Bangladesh. Int. J. Ecol. Environ. Sci. 2019;45(1):19–29. [Google Scholar]
- 26.Ricker W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board of Canada. 1975;191:382. [Google Scholar]
- 27.Scherrer B., Biostatistique Morin. SPSS Inc; USA: 1984. Montreal, Paris. SPSS Inc., 1999. Systat Version 9. [Google Scholar]
- 28.Gayanilo F.C., Sparre P., Pauly D. FAO Computerized Information Series, Fisheries. FAO; Rome: 1996. The FAO-ICLARM stock assessment tools (FiSAT) users guide; p. 126. spp. [Google Scholar]
- 29.Pauly D., Munro J.L. Once more on the comparison of growth in fish and invertebrate. Fishbyte. 1984;2:21. [Google Scholar]
- 30.Sparre P., Venema S.C. Introduction to the tropical fish stock assessment. Rome, Italy: food and agriculture organization of the united nations. Manual. Rev. 1998;2 FAO fisheries technical paper, No. 306. [Google Scholar]
- 31.Pauly D., Soriano-Bartz M., Moreau J., Jarre A. A new model accounting for seasonal cessation of growth in fishes. Aus. J. Mar. Fresh. Res. 1992;43:1151–1156. [Google Scholar]
- 32.Newman S.J. Growth, age estimation and preliminary estimates of longevity and mortality in the Moses perch, Lutjanus russelli (Indian Ocean form), from Continental Shelf Waters off North-Western Australia. Asian Fish Sci. 2002;15:283–294. [Google Scholar]
- 33.Pauly D., Caddy J.F. A modification of Bhattacharya‘s method for the analysis of mixtures of normal distributions. FAO. FAO Fish. Cir. 1985;781:16. Rome. [Google Scholar]
- 34.Pauly D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980;39(2):175–192. [Google Scholar]
- 35.Pauly D. Fish population dynamics in tropical waters: a manual for use with programmable calculators. ICLARM Studies and Reviews. 1984;8:325. [Google Scholar]
- 36.Gulland J.A. International Commission of Northwest Atlantic Fisheries Organization Special Publication; 1965. Survival of the Youngest Stages of Fish and its Relation to Year-Class Strength; pp. 365–371. 6. [Google Scholar]
- 37.Dao M., Diallo B., Kabore-Zoungrana C. Sex ratio and sexual dimorphism in a dioecious species from dry tropical areas: Piliostigmare ticulatum (leguminosae-caesalpinioideae) Ann. Des. Sci. Agron. 2011;15:107–122. [Google Scholar]
- 38.Juhel G., Culloty S.C., O'Riordan R.M., O'Connor J., De Faoite L., McNamara R. A histological study of the game-togenic cycle of the freshwater mussel Dreissena polymorpha (Pallas, 1771) in Lough Derg, Ireland. J. Molluscan Stud. 2003;69:365–373. [Google Scholar]
- 39.Uddin M., Yang H.-S., Choi K.-S., Kim H.-J., Hong J.-S., Cho M. Seasonal changes in Perkinsus olseni infection and gametogenesis in manila clam, Ruditapes philippinarum, from seonjaedo island in incheon, off the west coast of korea. J. World Aquacult. Soci. 2010;41:93–101. doi: 10.1111/j.1749-7345.2009.00337.x. [DOI] [Google Scholar]
- 40.Zar J.H. fifth ed. Prentice-Hall/Pearson; Upper Saddle River, NJ, USA: 2010. Biostatistical Analysis; p. 944. xii. [Google Scholar]
- 41.Mozsár A., Boros G., Sály P., Antal L., Nagy S.A. Relationship between Fulton's condition factor and proximate body composition in three freshwater fish species. J. Appl. Ichthyol. 2015;31:315–320. doi: 10.1111/jai.12658. [DOI] [Google Scholar]
- 42.Okuthe G.E., Bhomela B. Morphology, histology and histochemistry of the digestive tract of the banded tilapia, Tilapia sparrmanii (Perciformes: cichlidae) Zoologia. 2020;37:1–14. [Google Scholar]
- 43.Hossain M.A., Hussain M., Sarker T.R., Saha S., Iqbal M.M. Reproductive and morphometric traits of freshwater mussel Lamellidens marginalis and associated hydrology in the Ratargul freshwater Swamp Forest, Bangladesh. Egyptian J. Aqua. Res. 2023;49:161–170. [Google Scholar]
- 44.Wilbur K.M., Owen G. In: Wilbur K.M., Yonge C.M., editors. vol. 1. Academic Press; New York, USA: 1964. Growth; pp. 211–242. (Physiology of Mollusca). [Google Scholar]
- 45.Chelyadina N.S., Popov M.A., Kapranov S.V. Morphometric characteristics, sex structure and gonadal ripening of Mytilus galloprovincialis Lam. Cultivated in lake donuzlav (northwestern Crimea, Black sea) Aquacult. Int. 2022;31:103–116. [Google Scholar]
- 46.Gosling E. second ed. Wiley Blackwell; 2015. Marine Bivalve Molluscs; p. 524. Sussux, UK. [Google Scholar]
- 47.Breton S., Capt C., Guerra D., Stewart D. In: Transitions between Sexual Systems: Understanding the Mechanisms of, and Pathways between, Dioecy, Hermaphroditism and Other Sexual Systems. Leonard J.L., editor. Springer International Publishing; Cham, Switzerland: 2018. Sex-determining mechanisms in bivalves; pp. 165–192. [Google Scholar]
- 48.Jewel M.A.S., Haque M.A., Amin R., Hasan J., Alam L., Mondal S., Ahmed S. Heavy metal contamination and human health risk associated with sediment of Ganges River (northwestern Bangladesh) Nat. Environ. Pollut. Technol. 2020;19(2):783–790. [Google Scholar]
- 49.Haque M.A., Jewel M.A.S., Masud A.A., Rahman M.S., Hasan J. Assessment of bacterial pollution in sediment of Padma River, Rajshahi, Bangladesh. Curr. World Environ. 2018;13(1):66–74. doi: 10.12944/CWE.13.1.07. [DOI] [Google Scholar]
- 50.Hoagland K.E. Use of the terms protandry, protogyny, and hermaphroditism in malacology. Am. Malacol. Bull. 1984;3:85–88. [Google Scholar]
- 51.Mackie G.L. In: The Mollusca. Tompa A.S., Verdonk N.H., Van Den Biggelaar J.A.M., editors. vol. 7. Academic Press; New York: 1984. Bivalves; pp. 351–418. (Reproduction). [Google Scholar]
- 52.Ghiselin M.T. The evolution of hermaphroditism among animals. Q. Rev. Biol. 1969;44:189–208. doi: 10.1086/406066. [DOI] [PubMed] [Google Scholar]
- 53.Mirzaei M.R., Yasin Z., Tan S.H.A. Length-weight relationship, growth and mortality of Anadara granosa in Penang Island, Malaysia: an approach using length-frequency data sets. J. Mar. Biol. Assoc. U. K. 2014:1–10. doi: 10.1017/S0025315414001337. [DOI] [Google Scholar]
- 54.Ramesha M.M., Sophia S. Morphometry, length-weight relationships and condition index of Parreysia favidens (benson, 1862) (Bivalvia: unionidae) from river seeta in the western ghats, India. Indian J. Fish. 2015;62(1):18–24. [Google Scholar]
- 55.Strayer D.L., Downing J.A., Haag W.R., King T.L., Layzer J.B., Newton T.J., Nichols S.J. Changing perspectives on pearly mussels, America's most imperiled animals. J. Biosci. 2004;54:429–439. [Google Scholar]
- 56.Jones J.W., Neves R.J. Influence of life-history variation on demographic responses of three freshwater mussel species (Bivalvia: unionidae) in the Clinch River, USA. Aquatic Conserv: Mar. Freshw. Ecosyst. 2011;21:57–73. [Google Scholar]
- 57.Krampah E.A., Yankson K., Blay J. Aspects of reproduction of the brown mussel Perna perna at the Iture rocky beach near Cape Coast, Ghana. African J. Mar. Sci. 2016;38(4):503–512. [Google Scholar]
- 58.Allen M.S., Hightower J.E. In: Inland Fisheries Management in North America. Hurbert W.A., Quist M.C., editors. Berthes-da, Maryland: American fisheries Society; 2010. Fish Population dynamics: mortality, growth and recruitment; pp. 43–79. [Google Scholar]
- 59.Howard J.K., Cuffey K.M. Factors controlling the age structure of Margaritifera falcata in 2northern California streams. J. North American Benthol. Soci. 2006;25(3):677–690. [Google Scholar]
- 60.Amin S.M.N., Halim M.A., Bara M., Zafar M., Arshad A. Population dynamics and exploitation level of green-lipped mussel (Perna viridis) using FiSAT from the offshore island of the Cox's Bazar Coast of Bangladesh. Pertanika J Tropi. Agri. Sci. 2005;28(2):103–109. [Google Scholar]
- 61.Khan M.A.A., Assim Z.B., Ismail N. Population dynamics of the greenlipped mussel, Perna viridis from the offshore waters of Naf river coast, Bangladesh. Chiang Mai J. Sci. 2010;37:344–354. [Google Scholar]
- 62.Uddin M.J., Jeung H.D., Yang H.S., Kim B.K., Ju S.J., Choi K.S. Quantitative assessment of reproductive effort of the Manila clam Ruditapes philippinarum in a lagoon on Jeju Island (Korea) using enzyme-linked immunosorbent assay. Invertebrate Repro. Develop. 2013;57:316–324. doi: 10.1080/07924259.2013.793219. [DOI] [Google Scholar]
- 63.Galvao P., Longo R., Torres J.P.M., Malm O. Estimating the potential production of the brown mussel Perna perna (Linnaeus, 1758) reared in three tropical bays by different methods of condition indices. J. Mar. Biol. 2015 doi: 10.1155/2015/948053948053. [DOI] [Google Scholar]
- 64.Kefi F.J., Boubaker S., El Menif N.T. Relative growth and reproductive cycle of the date mussel Lithophaga lithophaga (Linnaeus, 1758) sampled from the Bizerte Bay (Northern Tunisia) Helgoland Mar. Res. 2014;68:439–450. [Google Scholar]
- 65.Chelyadina N.S., Pospelova N.V., Popov M.A. Comparative characteristics of indices to assess the quality of mussel production by an example of cultivated Mytilus galloprovincialis (Сrimea, theВlack Sea) Turk. J. Fish. Aqua. Sci. 2018;19(9):719–726. [Google Scholar]
- 66.Siddique M.A., Khatun M.A., Rahman M.M., Ahmed G.U., Moniruzzaman M., Uddin M.J. Annual gametogenic cycle of the freshwater pearl mussel, Lamellidens marginalis (Lamarck, 1819) collected from a perennial lentic habitat of Bangladesh. Molluscan Res. 2020;40:36–43. [Google Scholar]
- 67.Silva-Cavalcanti J.S., Costa M.F., Alves L.H.B. Seasonal variation in the abundance and distribution of Anomalocardia flexuosa (Mollusca, Bivalvia, Veneridae) in an estuarine intertidal plain. PeerJ. 2018;6:e4332. doi: 10.7717/peerj.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaikwad S.S., Kamble N.A. Gametogenic phenology in freshwater molluskan species; Lamellidens marginalis and Parreysia corrugata. Asian J. Biol. Life Sci. 2013;2:1–5. [Google Scholar]
- 69.Zieritz A., Bogan A.E., Rahim K.A.A., Sousa R., Jainih L., Harun S., Razak N.F.A., Gallardo B., McGowan S., Hassan R., Lopes-Lima M. Changes and drivers of freshwater mussel diversity and distribution in northern Borneo. Biol. Conserv. 2018;219:126–137. [Google Scholar]
- 70.Fitzer S.C., Torres Gabarda S., Daly L., Hughes B., Dove M., O'Connor W., Byrne M. Coastal acidification impacts on shell mineral structure of bivalve mollusks. Ecol. Evolut. 2018;8:8973–8984. doi: 10.1002/ece3.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chatzinikolaou E., Keklikoglou K., Grigoriou P. Morphological properties of gastropod shells in a warmer and more acidic future ocean using 3D microcomputed tomography. Front. Mar. Sci. 2021;8:427. doi: 10.3389/fmars.2021.645660. [DOI] [Google Scholar]
- 72.Talmage S.C., Gobler C.J. Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of northwest Atlantic bivalves. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyd C.E., Lichtkoppler F. Auburn University; Alabama: 1979. Water Quality Management in Fish Ponds Research and Development Series No. 22, International Centre for Aquaculture (J.C.A.A) Experimental Station; pp. 45–47. [Google Scholar]
- 74.Bhatnagar A., Singh G. Culture fisheries in village ponds: a multi-location study in Haryana, India. Agri. Biol. J. North America. 2010;1:961–968. doi: 10.5251/abjna.2010.1.5.961.968. [DOI] [Google Scholar]
- 75.Silva A.M.F., Yalin M.S. Taylor & Francis Group; 2017. Fluvial Processes. [Google Scholar]
- 76.Stoeck K., Geist J. Hydrological and substrate requirements of the thick‐shelled river mussel Unio crassus (Philipsson 1788) Aqua. Conserv. Mar. Fresh. Eco. 2016;26:456–469. doi: 10.1002/aqc.2598. [DOI] [Google Scholar]
- 77.Britton J.R., Davies G.D., Pegg J. Spatial variation in the somatic growth rates of European barbel Barbus barbus: a UK perspective. Ecol. Freshw. Fish. 2013;22:21–29. [Google Scholar]
- 78.Biandolino F., Parlapiano I., Grattagliano A., Fanelli G., Prato E. Comparative characteristics of percentage edibility, condition index, biochemical constituents and lipids nutritional quality indices of wild and farmed scallops (Flexopecten glaber) Water. 2020;12:1777. doi: 10.3390/w12061777. [DOI] [Google Scholar]
- 79.Yıldız H., Vural P., Acarlı S. Condition index, meat yield and biochemical composition of Mediterranean mussel (Mytilus galloprovincialis Lamarck, 1819) from Canakkale Strait, Turkey. Alınteri Zirai Bilimler Dergisi. 2021;36(1):308–314. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.