Abstract
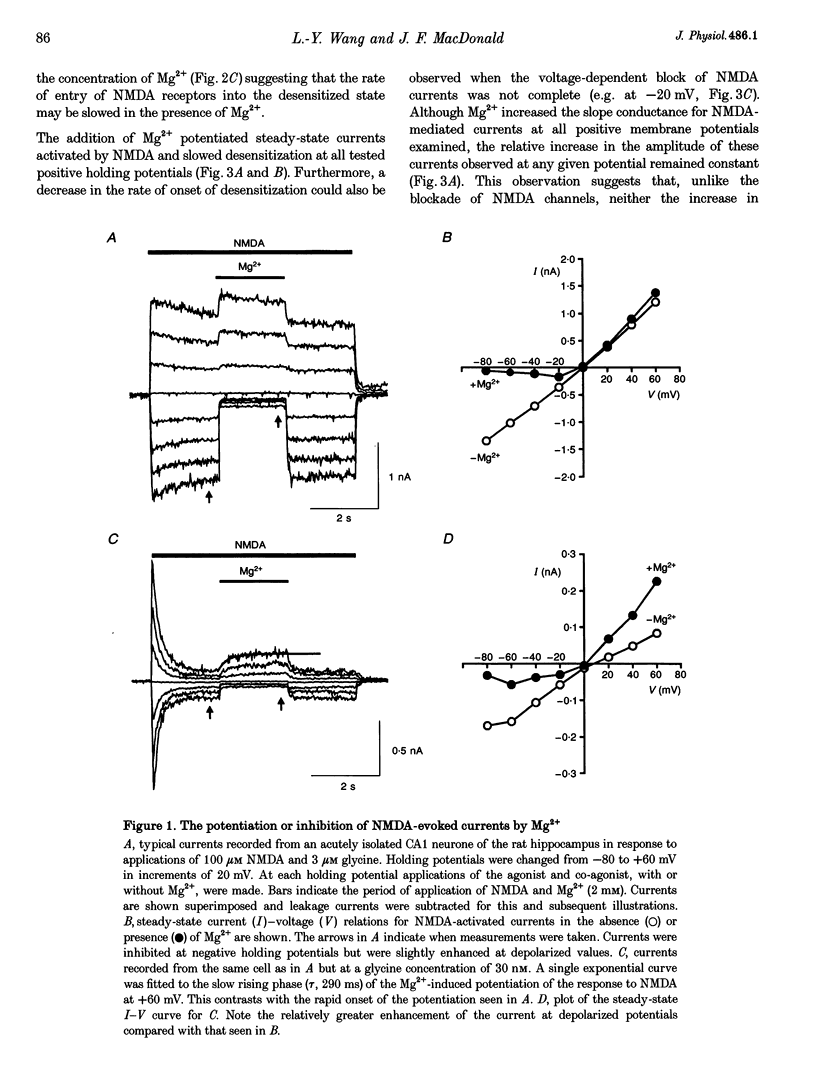
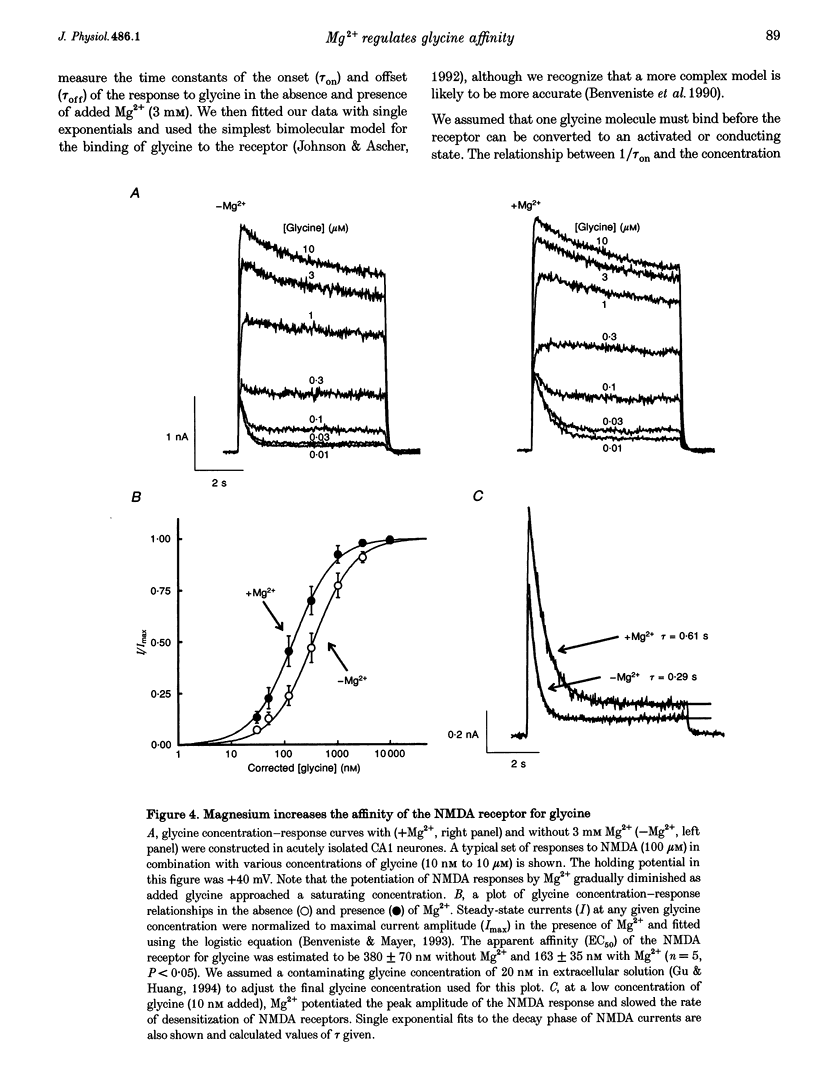
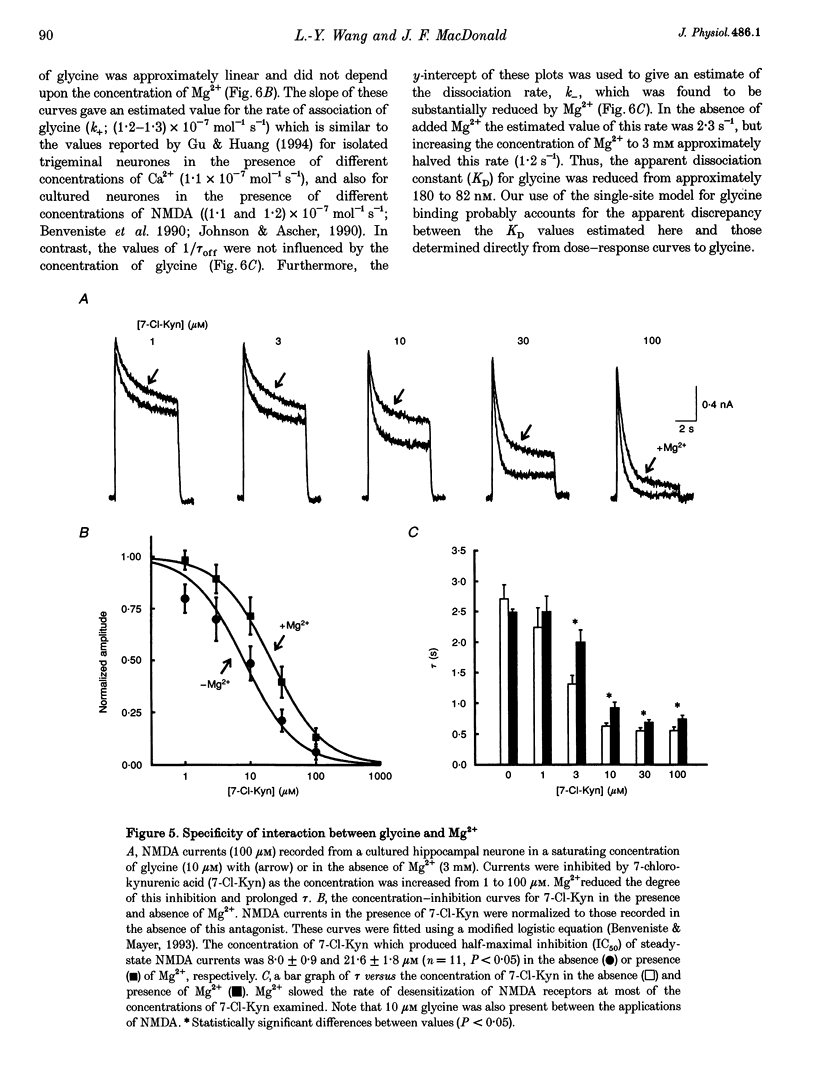
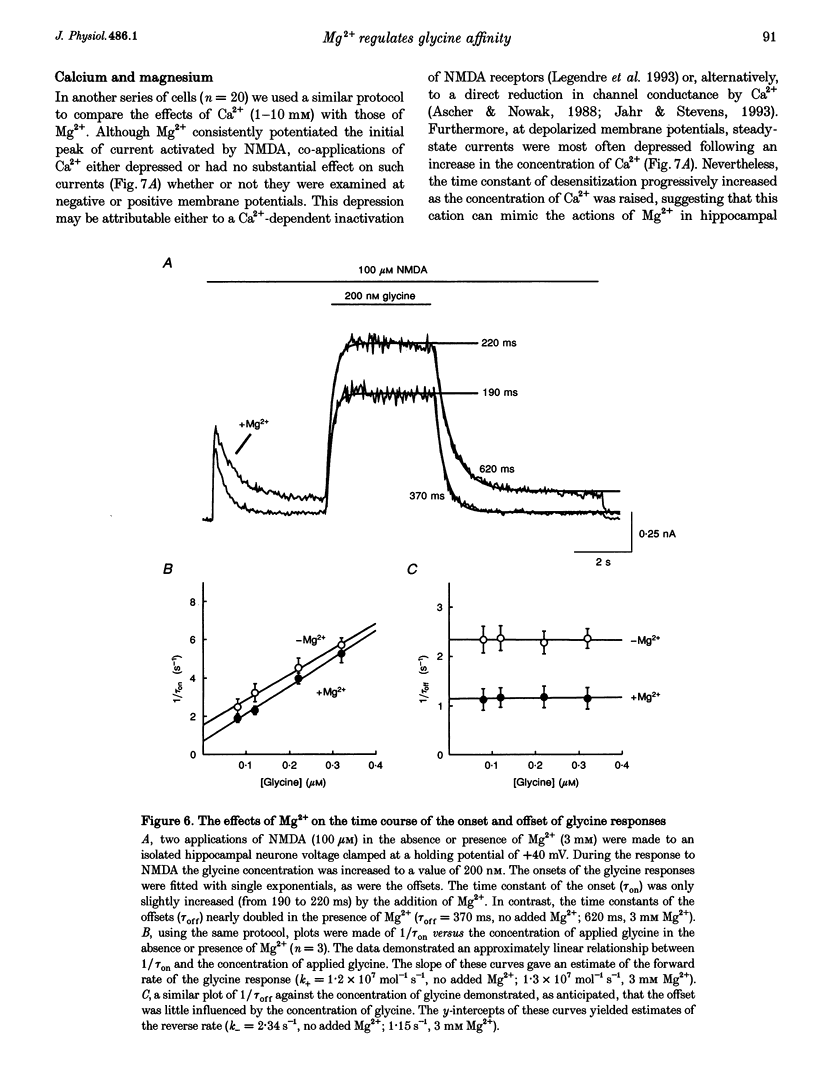
1. The effects of the divalent cation Mg2+ on NMDA currents recorded from cultured fetal mouse and acutely isolated neonatal rat hippocampal neurones were studied using the whole-cell patch-clamp technique. 2. Current-voltage relations were measured in the presence or absence of applied Mg2+ and added glycine. NMDA-evoked currents were studied in the absence or in a low concentration (0.2 mM) of applied Ca2+ in order to minimize Ca(2+)-dependent inactivation of the responses. Mg2+ unexpectedly enhanced NMDA-activated currents at positive membrane potentials. At negative membrane potentials Mg2+ caused a previously characterized voltage-dependent block of inward NMDA-activated currents. 3. The potentiation by Mg2+ of outward currents activated by NMDA was concentration dependent (EC50, approximately 3 mM; Hill coefficient, approximately 2). Mg2+ also reduced the desensitization of the NMDA receptor. The maximal enhancement of steady-state NMDA-activated currents was 2.7-fold and at 6 mM the time constant of desensitization was doubled. 4. Comparisons of concentration-response curves for glycine and 7-chloro-kynurenic acid demonstrated that Mg2+ significantly increased the affinity of the NMDA receptor for glycine. The EC50 for glycine was 380 nM in the absence of Mg2+ and 163 nM in 3 mM Mg2+. Mg2+ had little effect on the forward rate of the glycine response but halved the off-rate (2.34 to 1.15 s-1) and thus similarly reduced the apparent dissociation constant. 5. There was a good correlation between the concentration of extracellular Ca2+ and a reduction in the time constant of the glycine-sensitive component of NMDA receptor desensitization. Ca2+ could enhance these NMDA-activated currents briefly following exposure to high concentrations of Ca2+. These results are consistent with a Ca(2+)-dependent enhancement of the affinity of the NMDA receptor for glycine. 6. Mg2+ can enhance NMDA-mediated currents and reduce desensitization of this receptor by allosterically interacting with the glycine binding site. This interaction may be a key physiological mechanism through which modulation of the NMDA receptor is achieved.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Mayer M. L. Multiple effects of spermine on N-methyl-D-aspartic acid receptor responses of rat cultured hippocampal neurones. J Physiol. 1993 May;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino M., Vicini S. Voltage-dependent block by strychnine of N-methyl-D-aspartic acid-activated cationic channels in rat cortical neurons in culture. Mol Pharmacol. 1988 Aug;34(2):98–103. [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Budai D., Wilcox G. L., Larson A. A. Enhancement of NMDA-evoked neuronal activity by glycine in the rat spinal cord in vivo. Neurosci Lett. 1992 Feb 3;135(2):265–268. doi: 10.1016/0304-3940(92)90452-d. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Schoepfer R., Monyer H., Ruppersberg J. P., Günther W., Seeburg P. H., Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992 Sep 4;257(5075):1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992 Apr 9;356(6369):521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Gu Y., Huang L. Y. Modulation of glycine affinity for NMDA receptors by extracellular Ca2+ in trigeminal neurons. J Neurosci. 1994 Jul;14(7):4561–4570. doi: 10.1523/JNEUROSCI.14-07-04561.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990 Jun;10(6):1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Calcium permeability of the N-methyl-D-aspartate receptor channel in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11573–11577. doi: 10.1073/pnas.90.24.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Equilibrium and kinetic study of glycine action on the N-methyl-D-aspartate receptor in cultured mouse brain neurons. J Physiol. 1992 Sep;455:339–365. doi: 10.1113/jphysiol.1992.sp019305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate-activated channels. Biophys J. 1990 May;57(5):1085–1090. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawajiri S., Dingledine R. Multiple structural determinants of voltage-dependent magnesium block in recombinant NMDA receptors. Neuropharmacology. 1993 Nov;32(11):1203–1211. doi: 10.1016/0028-3908(93)90014-t. [DOI] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., Kashiwabuchi N., Mori H., Sakimura K., Kushiya E., Araki K., Meguro H., Masaki H., Kumanishi T., Arakawa M. Molecular diversity of the NMDA receptor channel. Nature. 1992 Jul 2;358(6381):36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Legendre P., Rosenmund C., Westbrook G. L. Inactivation of NMDA channels in cultured hippocampal neurons by intracellular calcium. J Neurosci. 1993 Feb;13(2):674–684. doi: 10.1523/JNEUROSCI.13-02-00674.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J. Spermine regulates N-methyl-D-aspartate receptor desensitization. Neuron. 1992 Feb;8(2):343–352. doi: 10.1016/0896-6273(92)90300-3. [DOI] [PubMed] [Google Scholar]
- Loo P. S., Braunwalder A. F., Lehmann J., Williams M., Sills M. A. Interaction of L-glutamate and magnesium with phencyclidine recognition sites in rat brain: evidence for multiple affinity states of the phencyclidine/N-methyl-D-aspartate receptor complex. Mol Pharmacol. 1987 Dec;32(6):820–830. [PubMed] [Google Scholar]
- MacDonald J. F., Mody I., Salter M. W. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol. 1989 Jul;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Porietis A. V., Wojtowicz J. M. L-Aspartic acid induces a region of negative slope conductance in the current-voltage relationship of cultured spinal cord neurons. Brain Res. 1982 Apr 8;237(1):248–253. doi: 10.1016/0006-8993(82)90575-3. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Benveniste M., Patneau D. K., Vyklicky L., Jr Pharmacologic properties of NMDA receptors. Ann N Y Acad Sci. 1992 May 11;648:194–204. doi: 10.1111/j.1749-6632.1992.tb24538.x. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mori H., Masaki H., Yamakura T., Mishina M. Identification by mutagenesis of a Mg(2+)-block site of the NMDA receptor channel. Nature. 1992 Aug 20;358(6388):673–675. doi: 10.1038/358673a0. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perouansky M., Yaari Y. Kinetic properties of NMDA receptor-mediated synaptic currents in rat hippocampal pyramidal cells versus interneurones. J Physiol. 1993 Jun;465:223–244. doi: 10.1113/jphysiol.1993.sp019674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumain R., Heinemann U. Stimulus- and amino acid-induced calcium and potassium changes in rat neocortex. J Neurophysiol. 1985 Jan;53(1):1–16. doi: 10.1152/jn.1985.53.1.1. [DOI] [PubMed] [Google Scholar]
- Rajdev S., Reynolds I. J. Effects of monovalent and divalent cations on 3-(+)[125I]iododizocilpine binding to the N-methyl-D-aspartate receptor of rat brain membranes. J Neurochem. 1992 Apr;58(4):1469–1476. doi: 10.1111/j.1471-4159.1992.tb11366.x. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J. [3H]CGP 39653 binding to the agonist site of the N-methyl-D-aspartate receptor is modulated by Mg2+ and polyamines independently of the arcaine-sensitive polyamine site. J Neurochem. 1994 Jan;62(1):54–62. doi: 10.1046/j.1471-4159.1994.62010054.x. [DOI] [PubMed] [Google Scholar]
- Salt T. E. Modulation of NMDA receptor-mediated responses by glycine and D-serine in the rat thalamus in vivo. Brain Res. 1989 Mar 6;481(2):403–406. doi: 10.1016/0006-8993(89)90823-8. [DOI] [PubMed] [Google Scholar]
- Sather W., Dieudonné S., MacDonald J. F., Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992 May;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. M., Walker V. E., Flynn D. M. Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature. 1989 Mar 30;338(6214):422–424. doi: 10.1038/338422a0. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]


