Summary
Background
Poor prenatal maternal sleep is a pervasive, yet modifiable, health concern affecting maternal and foetal wellbeing. Experimental rodent studies demonstrate that prenatal maternal sleep deprivation affects offspring brain development and leads to adverse outcomes, including increased anxiety-like behaviour. We examined the relation between prenatal maternal sleep quality and neonatal white matter development and subsequent infant negative emotionality.
Methods
Participants included 116 mother-infant (53% female) dyads. Prenatal sleep quality was prospectively assessed three times during gestation (16, 29, and 35 gestational weeks) using the Pittsburgh Sleep Quality Index. Neonatal white matter, as indexed by fractional anisotropy (FA), was assessed via diffusion weighted magnetic resonance imaging. Negative emotionality was measured via behavioural observation and maternal report when the infant was 6-months of age.
Findings
More prenatal sleep problems across pregnancy were associated with higher neonatal FA in the uncinate fasciculus (left: b = 0.20, p = .004; right: b = 0.15, p = .027). Higher neonatal uncinate FA was linked to infant negative emotionality, and uncinate FA partially mediated the association between prenatal maternal sleep and behavioural observation of infant negative emotionality.
Interpretation
Findings highlight prenatal sleep as an environmental signal that affects the developing neonatal brain and later infant negative emotionality.
Funding
National Institutes of Health (R01MH109662, R01HL155744, P50HD103573, K12AR084226, F32 Training fellowships MH125572, HL165844, MH106440, and diversity supplement R01HL155744-01S1).
Keywords: Prenatal sleep, Uncinate fasciculus, Negative emotionality, Diffusion imaging, Intergenerational transmission, Neonatal magnetic resonance imaging
Research in context.
Evidence before this study
New evidence suggests that maternal sleep disturbances during pregnancy are an understudied pathway to poor offspring health. Experimental rodent research demonstrates that sleep disturbances during pregnancy disrupt important processes in offspring brain development and subsequent anxiety-like behaviours. There is critical need for prospective mechanistic human research investigating how prenatal sleep disturbances confer risk in the next generation.
Added value of this study
This study extends prior research on the Developmental Origins of Health and Disease (DOHaD) hypothesis by identifying prenatal maternal sleep as an important factor contributing to intergenerational health outcomes. Results suggest that maternal sleep disturbances during pregnancy may affect offspring mental health through neonatal fronto-limbic circuitry.
Implications of all the available evidence
Prenatal maternal sleep presages infant fronto-limbic circuitry with implications for negative emotionality, a transdiagnostic risk factor for psychopathology. Maternal sleep is a prenatal process that likely contributes to offspring emotional development and may be an avenue for interventions seeking to improve maternal and child health.
Introduction
Rates of sleep disturbances increase during pregnancy,1 and poor prenatal sleep is associated with compromised maternal prenatal mental and physical health.2,3 Notably, little is known about the intergenerational impact of sleep disturbances during pregnancy. The Developmental Origins of Health and Disease (DOHaD) model highlights sensitive periods in early life, such as the prenatal period, as robust contributors to lifespan health.4,5 Consistent with this model, previous research demonstrates intergenerational consequences of poor prenatal maternal sleep on offspring birth outcomes (e.g., preterm birth, low birthweight), above and beyond maternal physical (e.g., body mass index) and mental health (e.g., depression).3,6,7 Emerging evidence also suggests that poor maternal sleep influences child socioemotional development, including negative emotionality,8 which is a transdiagnostic risk factor for later psychopathology.9 The neurobiological pathways by which prenatal maternal sleep may contribute to offspring negative emotionality and vulnerability to subsequent psychopathology remain unknown.
In preclinical models, experimentally induced prenatal maternal sleep deprivation leads to adverse offspring outcomes, such as increased anxiety and depressive-like behaviours.10,11 Further, experimental rodent studies document that prenatal sleep disturbances alter maturation of offspring prefrontal and limbic circuits, thus suggesting a potential mechanistic pathway underlying links between prenatal maternal sleep and offspring outcomes. Specifically maternal gestational sleep deprivation inhibits hippocampal neurogenesis10,11 and affects dendritic spine density within prefrontal regions of the offspring rodent brain lasting into adulthood.12
The developing human brain undergoes incredible growth during pregnancy.13 The exponential and orchestrated maturation of the foetal brain results in the emergence of white matter tracts before birth.13 Such rapid growth renders the brain highly susceptible to intrauterine environmental signals, including variation in maternal-placental-foetal endocrine and immune/inflammatory stress biology.14, 15, 16, 17 Across species, altered maturation of amygdala–prefrontal circuits is a primary consequence of early life stress.18, 19, 20 In humans, the uncinate and cingulum bundle are two primary fronto-limbic tracts that connect regions critical in emotional responsivity and regulation.21, 22, 23 The uncinate fasciculus is a U-shaped fibre connecting the orbitofrontal cortex to the anterior temporal lobe.18 The cingulum bundle similarly connects fronto-temporal regions.24 The uncinate fasciculus and the cingulum promote emotional development, and aberrant maturation within both of these circuits has been linked with compromised cognitive and emotional functioning later in life, including the development of psychopathology.25, 26, 27 Together, these findings support fronto-limbic circuitry as a plausible neurobiological pathway linking prenatal maternal sleep and early offspring negative emotionality.
While the experimental rodent research provides a strong foundation for the links between prenatal maternal sleep and offspring neurocircuit development, the effects of poor prenatal maternal sleep on neonatal white matter microstructure remain largely unknown in humans. Our research group has demonstrated that poor prenatal sleep during pregnancy is associated with increased neonatal hippocampal volume.28 Further, prenatal circadian misalignment assessed with actigraphy is associated with reductions in newborn global grey and white matter volumes.29 Although specific white matter tracts were not assessed, global reductions in white matter support the hypothesis that fronto-limbic tracts known to be influenced by prenatal experiences may be susceptible to prenatal sleep disturbances. These two human studies, alongside more extensive studies with rodents, highlight the potential implications of poor sleep across pregnancy on developing limbic regions and the need for examination of the link between prenatal maternal sleep and maturation of fronto-limbic circuits.
The present study examined the longitudinal relations among prenatal maternal sleep quality, measured across pregnancy, newborn fronto-limbic white matter microstructure, and later infant negative emotionality. Fractional anisotropy (FA), or the fraction of diffusion that is directionally dependent (i.e., anisotropic), is a widely used summary metric that is sensitive to multiple aspects of white matter microstructure. FA was measured within two hypothesis-driven fronto-limbic tracts: the uncinate fasciculus and the cingulum bundle. To probe the specificity of observed associations, two non fronto-limbic control tracts, the corticothalamic-parietal tract and the motor segment of the corpus callosum were also investigated. These two tracts form major white matter pathways that connect motor and somatosensory regions30,31 distinct from fronto-limbic circuitry. Further, as sleep is dynamic over pregnancy32 and sleep at different prenatal timepoints may exert different effects on the offspring brain,28 we first examined links between sleep trajectories and neonatal uncinate and cingulum white matter microstructure, covarying for potential confounding factors, including maternal depression and anxiety, and exposure to stressful life events during pregnancy. We then examined whether neonatal white matter microstructure mediates the associations between prenatal maternal sleep problems and multimodal assessment of infant negative emotionality. Grounded in findings from the experimental rodent literature, we expected that prenatal maternal sleep would longitudinally presage individual differences in offspring fronto-limbic white matter circuitry and subsequent negative emotionality.
Methods
Study overview
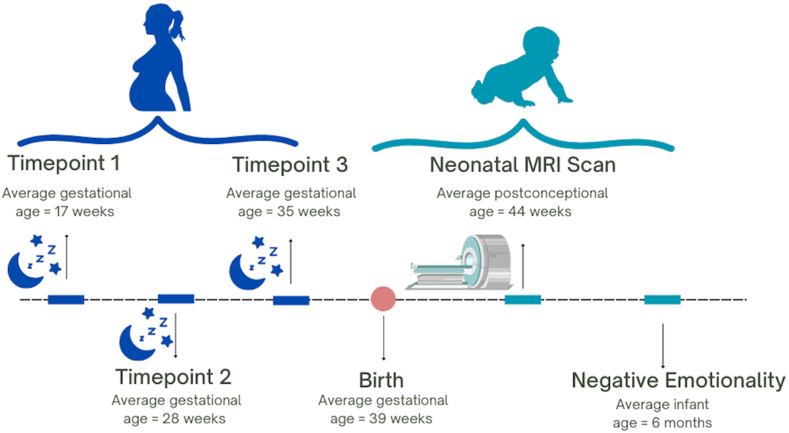
Adult pregnant participants reported on prenatal sleep quality at 16, 29, and 35 gestational weeks. Their offspring's neonatal white matter microstructure was assessed during natural sleep using magnetic resonance imaging (MRI) at around five weeks postnatal age (Mage = 5.1 weeks, SDage = 2.27). Infants were scanned as close to birth as possible to assess the effects of prenatal perturbations to brain development while minimizing the effects of the postnatal environment.28,33 Infant negative emotionality was assessed at six months using both laboratory behavioural observation and maternal report (Mage = 6.1 months, SDage = 0.47). See Fig. 1 for a schematic of the study procedures.
Fig. 1.
Schematic of study timeline.
Ethics
Participants were compensated at each timepoint. Further, written and informed consent was obtained from mothers for themselves and their infant under the University of Denver (956,810) and Colorado Multiple Institutional Review Boards (16–2040).
Participants
116 pregnant participants and their neonates were included in this study. Participants were drawn from the Care Project, a larger longitudinal study assessing the effect of interpersonal therapy on prenatal maternal depression and offspring health and development.34,35 Individuals randomized to the treatment group of the parent study were not included in current analyses. Study recruitment was primarily from two major medical centres in Denver, Colorado serving ethnic-racially and socioeconomically diverse populations. To promote the recruitment and participation of a sample comparable to the Denver metropolitan area, we provided flexible scheduling (e.g., weekends), daycare assistance, paid transportation, a quarterly project newsletter, and online options (e.g., zoom). At recruitment, inclusion criteria for the overall study was: a) maternal age between 18 and 45, b) singleton pregnancy, c) gestational age <25 weeks, d) English proficiency, e) no current illegal drug or methadone use, f) no major maternal health conditions requiring invasive or intensive treatments (e.g., dialysis, chronic long-term steroid use), g) no current or past symptoms of psychosis or mania based on the Structured Clinical Interview (SCID) for DSM-5. Further, exclusion criteria for this study include: a) miscarriage or foetal demise (n = 2), b) major chromosomal anomalies (n = 1), and c) absence of maternal sleep data (n = 1). Of the 122 newborns who attended an MRI scan appointment, three were not scanned (e.g., did not fall asleep during scanning window), two did not yield imaging data (woke up during scan), and one failed quality control (directional bias within image). The six newborns excluded from analyses did not differ from the 116 participants with respect to maternal age, income, gestational age at birth, and birth weight percentile (all ts < 1.29, all ps > 0.19).
Table 1 provides details on sample characteristics. Pregnant participants were, on average, 30 years old (range 21–41). Participants had a median household income of $79,542, which is lower than the median household income in the county of Denver in 2022 ($88,213).36 Thirty-two percent reported living at or near the federal classification of poverty (less than 200% income-to-needs ratio). Forty-five percent identified as belonging to a marginalized ethnic or racial group. The race and ethnicity of the pregnant participants and their neonates was reported by the pregnant individual and was reflective of the demographics of the Denver metropolitan area.36 Neonates (MGestational Age at Birth = 38.92 weeks, SD = 1.45, range 33.3–41.6) were 52.6% female, 36.8% firstborn, and otherwise healthy at birth.
Table 1.
Sample characteristics (n = 116).
| Maternal characteristics | M (Range) or % |
|---|---|
| Age at enrolment | 30.50 (20–42) |
| Obstetric complications | |
| No complications | 38.3% |
| One complication | 36.5% |
| Two or more complications | 25.2% |
| Annual household income ($) | 79,542 (0–280,001)a,b |
| Household Income-to-needs ratio | 4.19 (0.06–17.0)a,b |
| At or below 200% poverty line | 32.4% |
| Cohabitation status | |
| Cohabitating with partner | 86.7% |
| Living alone | 13.3% |
| Education (highest degree earned) | |
| Less than high school | 2.6% |
| High school | 14.0% |
| Some college | 17.5% |
| Associate degree | 12.3% |
| Bachelor's degree | 31.7% |
| Graduate degree | 21.9% |
| Race and ethnicity | |
| Asian American/Asian | 3.5% |
| African American/Black | 9.7% |
| Hispanic/Latinx | 26.3% |
| Non-Latinx White | 54.3% |
| Native Hawaiian/Pacific Islander | 1% |
| Multiracial/Multiethnic | 5.2% |
| Prenatal substance use (any) | 2.6% |
| Prenatal distress (STAI) | 38.25 (20–77) |
| Prenatal stressful life events (LEC) | 4.49 (0–14) |
| Newborn characteristics | |
| Postconceptional age at MRI (weeks) | 44.05 (41.6–52.3) |
| Age at MRI scan (weeks) | 5.12 (1.9–13.4) |
| Parity (first born) | 36.8% |
| Biological sex at birth | |
| Female | 52.6% |
| Male | 47.4% |
| Race and ethnicity | |
| Asian American/Asian | 3.0% |
| African American/Black | 6.0% |
| Hispanic/Latinx | 25.0% |
| Non-Latinx White | 49.1% |
| Native Hawaiian/Pacific Islander | 1.4% |
| Multiracial/Multiethnic | 15.5% |
| Birth outcome | |
| Gestational age at birth (weeks) | 38.92 (33.3–41.6) |
| Birth weight percentile | 46.39 (1–96) |
| Study variables | |
| Maternal sleep (PSQI) at 1st timepoint | 6.53 (1–17) |
| Maternal sleep (PSQI) at 2nd timepoint | 6.78 (1–18) |
| Maternal sleep (PSQI) at 3rd timepoint | 7.65 (1–19) |
| Motion | 7.36 (0–33) |
| Uncinate FA | |
| Left | 0.26 (0.21–0.34) |
| Right | 0.26 (0.21–0.32) |
| Cingulum FA | |
| Left | 0.25 (0.16–0.30) |
| Right | 0.25 (0.16–0.31) |
| Infant Negative Emotionality (IBQ) | 3.10 (1.53–6.75) |
| Infant Negative Emotionality (Lab-TAB) | |
| Facial Aversion | 1.81 (0–4) |
| Distress Vocalizations | 1.14 (0–5) |
Median reported.
An outlier for income (i.e., SD ≥ 5 above the mean) was converted to the value 3 SDs above the mean, preserving its rank as the highest value.
Measures
Maternal self-report measures
Prenatal sleep
Prenatal maternal sleep quality was collected using the Pittsburgh Sleep Quality Index (PSQI).37 The PSQI is a 19-item questionnaire comprised of seven subscales (sleep latency, sleep duration, sleep disturbances, sleep medication, subjective sleep quality, sleep efficiency, and daytime dysfunction), on a 0–3 scale. The subscales scores are summed to form total subjective sleep quality score from zero to 21 with higher scores indicating poorer sleep quality or more sleep problems.37 On total sleep quality, a score higher than five characterizes poor sleep.38 The PSQI possesses good validity and internal consistency (α = 0.83) within prenatal populations37,39,40 and has been previously validated with wearable devices.41 In our sample, one participant (0.8%) had missing sleep data at the first assessment, six (5%) were missing sleep at the second, and 12 (10%) were missing sleep data at the third prenatal assessment. Little's missing completely at random test demonstrated that data were missing at random (MAR; X2 (7) = 11.62, p = .114). Sixty-five percent of pregnant individuals were identified as poor sleepers (PSQI score >5) early in pregnancy, and this percentage increased to 70.7% in mid and late pregnancy.
Prenatal stressful life events
The Life Events Checklist (LEC-5)42 was used as a measure of objective stress exposure during pregnancy. The LEC is a 17-item questionnaire that assesses exposure to traumatic events, including assault and life-threatening conditions. Pregnant participants were asked if, during their current pregnancy,(1) the event happened to them,(2) witnessed it (3) learned about it happening to someone close to them,(4) happened as part of their job,(5) they were not sure or (6) the event was not applicable to them. Items were added to yield a sum score (0–17), with higher scores indicating greater stress exposure during pregnancy. The LEC-5 possesses good test–retest reliability (r = 0.82).43
Prenatal anxiety
The 20-item state subscale of the State-Trait Anxiety Inventory (STAI)44 was used as a measure of distress during pregnancy given previous research demonstrating that the factor structure reflects high-order negative affectivity.45 Items are endorsed on a 4-point Likert scale, with higher scores indicating more distress during pregnancy. The STAI has been previously validated and utilized within pregnant populations.33,46 In our sample, the STAI possessed excellent internal consistency (α = 0.96).
Prenatal depression
The Edinburgh Postnatal Depression Scale (EPDS)47 was used as a measure of prenatal depression. The EPDS is a widely used measure of depression symptoms with prenatal populations.48 Pregnant participants endorsed depression symptoms on a 4-point Likert scale, with higher scores indicating greater levels of depression. The EPDS possesses good internal consistency and validity.47 In our sample, the EPDS possessed good internal consistency (α = 0.91).
Magnetic resonance imaging acquisition and processing
Infants were scanned unsedated during natural sleep following the same scanning protocol as described in Demers et al., 2021. A Siemens Skyra 3T MRI system equipped with a 20-channel head coil at the Brain Imaging Centre at the University of Colorado Anschutz Medical Campus was used.
Diffusion tensor images were obtained using a simultaneous multislice sequence (SMS; TR = 6100 ms, TE = 60, FOV = 220, matrix size = 128 × 128; 50 axial slices with 2.0 mm thickness; PE direction = AP) for the uncinate fasciculus and the hippocampal component of the cingulum (See Supplement Figure S1). Diffusion MRI data were acquired with three diffusion weightings (b-values) (b = 300, 800, 2000 s/mm2), with 10, 30, and 64 unique gradient directions per respective shell (104 gradient directions total). In addition, 18 interspersed b = 0 s/mm2 images were acquired as a baseline. The total acquisition time was 7 min (multiband acceleration 3, echo time (TE)/TR 92/3600 ms).
A study-specific quality control protocol was applied to all raw DWI data using DTIPrep (www.nitrc.org/projects/dtiprep, version 1.2.10). Slice-wise and gradient-wise artifact detection, eddy current and motion correction were included as part of DTIPrep. For all diffusion tensor analyses, the b = 300 and b = 800 shells (40 gradients total) were used to calculate the diffusion tensor images (DTI) using the weighted least-squares algorithm.49 Lower b-values were employed for calculation of the diffusion tensor given the decreased signal-to-noise and the increased non-Gaussian contribution to the diffusion signal at higher b-value acquisitions.50 As an additional quality control step, interactive tractography was performed in Slicer (http://www.slicer.org) and visually assessed for artifacts undetectable by voxel-wise inspection, such as any consistently observed directional biases. Skull and non-brain tissue were masked using the Brain Extraction Tool (BET)51 on the geometric mean of the DWI image, followed by manual correction, if necessary. Two motion scores were calculated per participant: (a) the number of DWI gradients removed by the DTI Prep preprocessing pipeline, and (b) the number of DWI gradients with significant levels of corrected motion. These two scores were summed to create the single motion artifact covariate used in the association analyses.
A study-specific DTI atlas was created from the diffusion tensor data using the UNC−Utah National Alliance for Medical Image Computing DTI framework.52 Nonlinear, diffeomorphic pair-wise registration was performed to map individual participant DTIs into atlas space, and registration accuracy was visually inspected in DTI-AtlasBuilder to determine if the computed transforms were appropriate. Tracts of interest were determined semi-automatically in this atlas space53 using the tract definition in Short et al.54 Resulting deformation fields were then used to map the atlas fibres into individual participant space, where diffusion tensor metrics were extracted at evenly spaced points (arc lengths) along each fibre tract. As an additional quality control step, individuals were excluded from further association analyses for a given tract if their fractional anisotropy profile was weakly correlated with the population tract average profile (correlation <0.70). A low correlation typically flags poor alignment of the participant's DTI to the atlas across the respective fibre regions. For each participant, the profile of the respective diffusion tensor metric was then averaged along the respective fibre to yield robust tract metric averages for the association analyses.
Infant negative emotionality
Infant negative emotionality was assessed using both maternal report and observations of infant behaviour. Maternal report of infant negative emotionality was collected using the 59-item negative affectivity subscale from the Infant Behaviour Questionnaire (IBQ).55 The IBQ is a standardized 191-item maternal-report questionnaire validated for use with infants and with strong external validity.55,56 Internal consistency for the negative emotionality scale in the current sample was α = .85. Ten participants (9%) were missing infant behaviour questionnaire data.
Infant negative emotionality additionally was assessed using a standardized and validated measure from the Laboratory Temperament Assessment Battery, the Robot Behavioural Task.57 Videos were coded for intensity of facial negative affectivity (0–4) and distress vocalizations (0–5) in response to a robot that moves and makes noises. Scores across facial and vocalization intensity were standardized and averaged to create a composite score of infant negative emotionality, with higher scores indicating higher negative emotionality. Twenty percent of videos were coded independently by two coders and interrater reliability for facial negative affectivity and distress vocalization scores was 0.94. Of 116 participants, 39 were missing data on negative emotionality from the Robot Task. Little's missing at random test demonstrated that parent report and behavioural observation of negative emotionality data were missing at random (X22 = 2.81, p = 0.245).
Pregnancy and birth outcomes
Gestational age at birth, obstetric complications, newborn sex at birth, birth weight percentile, and parity were obtained via medical charts. Gestational age at birth (GAB) was computed using the American College of Obstetricians and Gynaecologists (ACOG) guidelines to derive estimated date of delivery. Estimated date of delivery was based on embryo measurements from an early ultrasound (before 14 weeks gestation) and/or the date of the last menstrual period as obtained from medical records.58 Additionally, neonatal postconceptional age was calculated by adding GAB and weeks from birth to MRI scan, thus accounting for pre and postnatal brain growth. Further, obstetric complications were calculated by summing the number of pregnancy-related complications present in the current pregnancy, including prenatal infection, pregnancy-induced hypertension, gestational diabetes, oligohydramnios, polyhydramnios, preterm labour, vaginal bleeding, placenta previa, and anaemia.59 Additionally, birth weight percentile was calculated considering GAB and newborn sex.
Statistics
Statistical power
As prior studies linking prenatal sleep and offspring white matter in humans are not available, effects sizes for power analyses were derived from previous research with other prenatal factors and offspring white matter microstructure,33 and two studies on prenatal maternal sleep and offspring neurodevelopment.28,29 Effect sizes ranged from small to medium indicating that with 116 dyads, we have 74.3–98.9% power to detect an effect size of 0.01–0.38 at α = 0.05.
Missing data
Given Little's test suggesting that our prenatal sleep and infant negative emotionality data were missing at random, multiple imputation was employed to address missing data using the following list of auxiliary or predictor variables: prenatal income-to-needs ratio, cohabitation status and education, prenatal depression, parity, sex at birth, birthweight percentile, age at six-month assessment, and whether children turned 6 months at the time of the COVID-19 shutdown.
Covariates
Following best practices in infant neuroimaging studies, postconceptional age at scan (defined as gestational length plus postnatal age), motion during acquisition, and sex at birth were included in all analyses.33,60 We additionally examined birth weight percentile, prenatal income-to-needs ratio, parity, cohabitation status, maternal education and maternal postnatal sleep quality as potential covariates using correlations with tract FA across the uncinate and the cingulum, as well as infant negative emotionality (alpha <0.05; See Table 2). A one-way ANOVA was conducted to test obstetric complications (zero, one, two or more) as a potential covariate. Analyses revealed that only posconceptional age at scan and income-to-needs ratio were associated with newborn uncinate fasciculus microstructure (FA; all rs > −0.19, all ps < 0.05) and infant negative emotionality (r = −0.26, p = 0.008) and was therefore retained as a covariate in all analyses.
Table 2.
Bivariate correlations of potential covariates with uncinate and cingulum white matter fractional anisotropy.
| Left Uncinate (FA) | Right Uncinate (FA) | Left Cingulum (FA) | Right Cingulum (FA) | |
|---|---|---|---|---|
| PCA at MRI | 0.73∗∗ | 0.74∗∗ | 0.52∗∗ | 0.49∗∗ |
| Motion | −0.10 | −0.08 | 0.03 | −0.03 |
| Sex at birtha | 0.07 | 0.05 | 0.13 | 0.12 |
| BWP | 0.15 | 0.13 | −0.02 | −0.17 |
| Income-to-needs ratio | −0.19∗ | −0.22∗ | −0.11 | −0.13 |
| Birth orderb | 0.16 | 0.11 | 0.09 | 0.15 |
| Cohabitation status | −0.07 | −0.05 | −0.11 | −0.13 |
| Maternal education | −0.13 | −0.16 | −0.11 | −0.11 |
| Postnatal Sleep | 0.16 | 0.19 | −0.003 | −0.02 |
Note. ∗p < .05, ∗∗p < .01. FA, Fractional Anisotropy; PCA, Postconceptional Age; MRI, Magnetic Resonance Imaging; BWP, Birthweight Percentile; INR, Income-to-needs ratio.
Sex coded as 0 = female, 1 = male.
Birth order coded as 0 = primiparous, 1 = multiparous.
Additional analyses covarying for prenatal stressful life events, maternal anxiety, and depression were conducted to test the effect of maternal sleep quality during pregnancy on neonatal neurocircuitry above and beyond these factors.
Trajectories of prenatal maternal sleep and neonatal uncinate and cingulum white matter microstructure
Generalized linear mixed models (GLMMs), were used to test the links between trajectories of prenatal sleep quality and neonatal uncinate fasciculus and cingulum microstructure using the lmer and glmer packages in R-studio.
GLMMs allow linear and nonlinear data to be nested within individuals and investigated at multiple, hierarchical levels.61 GLMMs also are relatively stable when conducting analyses with longitudinal data that contains robust heterogeneity in timing of repeated assessments. Prenatal maternal sleep quality was regressed on weeks of gestation to test linear and quadratic trajectories for model fit. Our GLMMs were centred at the first measurement timepoint, eight gestational weeks. The best fit of prenatal sleep trajectory was determined by comparing linear and quadratic model fit. If linear slope and quadratic curve estimates were significant, a difference in deviance score estimate was calculated by subtracting the deviance score from the linear and quadratic models. The alpha level of this difference deviance score was then identified using a critical value chi-square distribution table and the difference in degrees of freedom between the linear and the quadratic model. Once trajectory fit was determined, level two predictors, including neonatal uncinate fasciculus and cingulum tract FA and covariates, were added to the GLMMs. False discovery rate (FDR) correction (q = 0.0375) was used to correct for multiple comparisons across bilateral uncinate and cingulum (four tracts, treating left and right separately). For tracts that passed FDR correction, we then examined axial diffusivity (AD; diffusivity along the main fibre) and radial diffusivity (RD; diffusivity perpendicular to the main fibre orientation) to further investigate specific white matter microstructure metrics that might be driving overall differences in FA.
Trajectories of prenatal maternal sleep and white matter microstructure of two control tracts: corticothalamic and corpus callosum
Identical generalized linear mixed models were used to examine associations between sleep and two non-prefrontal-limbic tracts: bilateral corticothalamic-parietal and motor segment of the corpus callosum.
Sensitivity analyses
Sensitivity analyses were conducted excluding pregnant individuals with prenatal substance use exposure (n = 3) and preterm infants (n = 6 born <37 gestational weeks).
Neonatal uncinate fasciculus as pathway linking prenatal maternal sleep and infant negative emotionality
Tests of indirect effects were employed to evaluate whether total poor sleep quality exposure (average across prenatal timepoints) indirectly predicted multimodal assessment of infant negative emotionality through neonatal white matter microstructure. Follow up analyses on timing effects were then conducted to test whether prenatal maternal sleep early, mid, and late in pregnancy were indirectly associated with infant negative emotionality via neonatal white matter microstructure. Mediation analyses62 were conducted using SPSS version 28 if significant associations (alpha <0.05) were found between prenatal maternal sleep quality and neonatal white matter tracts.
Sex differences
Since environmental inputs can affect the foetus in a sex-specific manner,63 we explored sex at birth as a potential moderator of the associations between prenatal maternal sleep quality and neonatal white matter. Sex differences were analysed using hierarchical regressions in SPSS version 28 if significant associations (alpha <0.05) were found between prenatal maternal sleep quality and neonatal white matter tracts.
Role of funders
The funding agencies of this study played no role in study design, data collection, data analysis, interpretation, or writing of this manuscript.
Results
Prenatal maternal sleep quality and neonatal uncinate fasciculus and cingulum
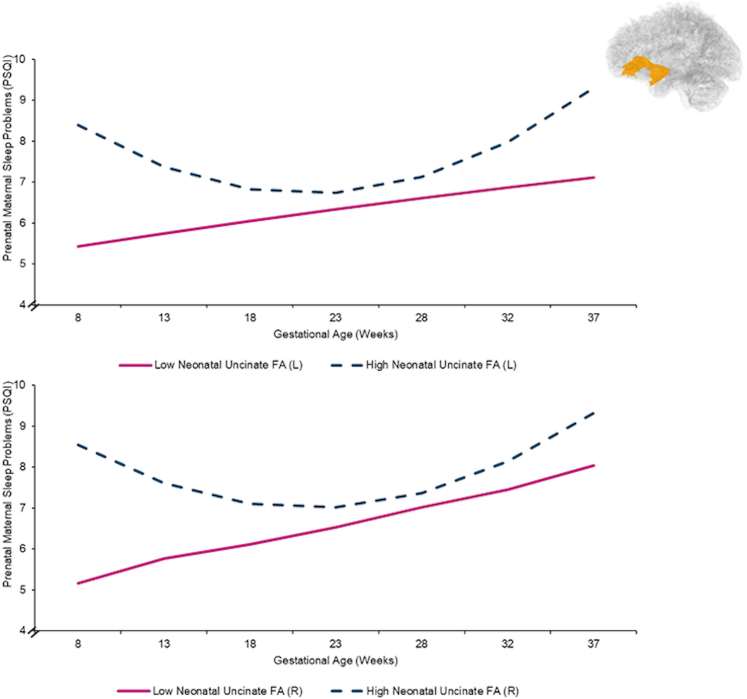
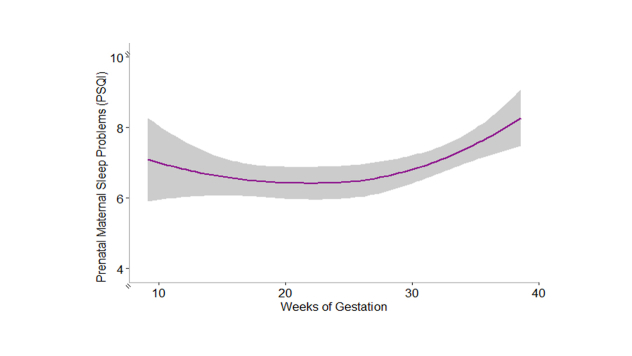
Trajectories of prenatal maternal sleep quality were best modelled via accelerating quadratic growth curves (specifically, model fit indices reveal that a quadratic function best fits the data ΔX2 (1) = 1626.3–1613.1 = 13.2, p = 0.0003, GLMM; See Supplement Table S1), such that sleep problems worsened later in gestation (See Supplement Table S1 & Supplement Figure S2). Poorer prenatal maternal sleep quality across gestation was associated with higher bilateral neonatal uncinate fasciculus FA, after inclusion of covariates (e.g., income-to-needs ratio; left: b = 0.20, p = .004; right: b = 0.15, p = .027, GLMM; See Table 3 and Fig. 2). Follow-up analyses to determine if AD or RD were associated with poor prenatal sleep revealed that prenatal maternal sleep quality was linked to neonatal bilateral RD in the uncinate fasciculus, but not AD (See Supplement Table S2). The trajectory of prenatal sleep quality was not significantly associated with bilateral neonate cingulum FA (left: b = 0.03, p = .31; right: b = 0.11, p = .18, GLMM; See Table 3).
Table 3.
Generalized linear mixed models of maternal sleep quality across gestation and neonatal uncinate fasciculus and cingulum fractional anisotropy (FA).
| Fixed effects | Left Uncinate |
Right Uncinate |
||||||
|---|---|---|---|---|---|---|---|---|
| b estimate | SE | T | p | b estimate | SE | T | p | |
| Uncinate on intercept of prenatal sleep | 61.75∗ | 24.48 | 2.52 | 0.01 | 72.26∗∗ | 25.28 | 2.86 | 0.005 |
| Motion | −0.08∗∗ | 0.03 | −2.71 | 0.01 | −0.08∗∗ | 0.03 | −2.76 | 0.006 |
| PCA at MRI | 0.03 | 0.23 | 0.13 | 0.90 | −0.10 | 0.22 | −0.47 | 0.64 |
| Income-to-needs ratio | −0.21∗∗ | 0.09 | −2.35 | 0.02 | −0.23∗ | 0.09 | −2.64 | 0.01 |
| Sex at birth | −0.01 | 0.61 | −0.03 | 0.98 | −0.21 | 0.58 | −0.36 | 0.72 |
| Uncinate on linear rate of change of prenatal sleep | −6.56∗∗ | 2.30 | −2.86 | 0.005 | −5.51∗ | 2.42 | −2.28 | 0.02 |
| Uncinate on quadratic rate of change of prenatal sleep | 0.20∗∗ | 0.07 | 2.92 | 0.004 | 0.15∗ | 0.07 | 2.21 | 0.03 |
| Fixed effects | Left Cingulum |
Right Cingulum |
||||||
|---|---|---|---|---|---|---|---|---|
| b estimate | SE | T | p | b estimate | SE | T | p | |
| Cingulum on intercept of prenatal sleep | 17.34 | 25.91 | 0.67 | 0.50 | 40.82 | 24.85 | 1.64 | 0.10 |
| Motion | −0.09∗∗ | 0.03 | −2.78 | 0.006 | −0.09∗∗ | 0.03 | −2.82 | 0.005 |
| PCA at MRI | 0.19 | 0.18 | 1.04 | 0.30 | 0.23 | 0.18 | 1.28 | 0.20 |
| Income-to-needs ratio | −0.22∗∗ | 0.09 | −2.42 | 0.02 | −0.23∗ | 0.09 | −2.52 | 0.01 |
| Sex at birth | −0.11 | 0.62 | −0.18 | 0.86 | −0.15 | 0.61 | −0.24 | 0.81 |
| Cingulum on linear rate of change of prenatal sleep | −1.10 | 2.85 | −0.39 | 0.70 | −4.62 | 2.71 | −1.71 | 0.09 |
| Cingulum on quadratic rate of change of prenatal sleep | 0.03 | 0.08 | 0.31 | 0.76 | 0.11 | 0.08 | 1.35 | 0.18 |
Note: ∗p < .05, ∗∗< 0.01, ∗ ∗∗p < .001. PCA, Postconceptional age; MRI, Magnetic resonance imaging.
Fig. 2.
Trajectories of prenatal maternal sleep quality by neonatal uncinate fasciculus fractional anisotropy (FA). Scores >5 on the PSQI indicate poor sleep quality. Data were analyzed continuously and are split +/− 1 SD from the mean FA for visualization purposes only. Topographical figure of the uncinate also included.
Additional analyses covarying for prenatal exposure to stressful life events, anxiety, and depression revealed that associations between prenatal maternal sleep quality and neonatal uncinate fasciculus FA persisted in the presence of these covariates (See Table 4).
Table 4.
Generalized linear mixed models of maternal sleep quality across gestation and neonatal uncinate fasciculus covarying for prenatal stressful life events, anxiety, and depression.
| Fixed effects | Left Uncinate |
Right Uncinate |
||||
|---|---|---|---|---|---|---|
| b estimate | SE | T | b estimate | SE | T | |
| Model 1: Prenatal Stressful Life Events | ||||||
| Uncinate on intercept of prenatal sleep | 60.71∗ | 24.35 | 2.49 | 72.81∗∗ | 25.30 | 2.88 |
| Motion | −0.07∗ | 0.03 | −2.24 | −0.08∗ | 0.03 | −2.54 |
| PCA at MRI | 0.03 | 0.23 | −0.13 | −0.11 | 0.22 | −0.49 |
| Income-to-needs ratio | −0.24∗ | 0.09 | −2.64 | −0.25∗∗ | 0.09 | −2.87 |
| Sex at birth | −0.11 | 0.60 | −0.18 | −0.27 | 0.58 | −0.47 |
| Stressful life events | 0.12 | 0.09 | 1.23 | 0.13 | 0.09 | 1.37 |
| Uncinate on linear rate of change of prenatal sleep | −6.23∗∗ | 2.30 | −2.84 | −5.52∗ | 2.42 | −2.28 |
| Uncinate on quadratic rate of change of prenatal sleep | 0.20∗∗ | 0.07 | 2.88 | 0.15∗ | 0.07 | 2.19 |
| Model 2: Prenatal Anxiety | ||||||
| Uncinate on intercept of prenatal sleep | 60.73∗∗ | 22.85 | 2.66 | 73.69∗∗ | 23.80 | 3.10 |
| Motion | −0.07∗ | 0.03 | −2.49 | −0.07∗∗ | 0.03 | −2.66 |
| PCA at MRI | −0.09 | 0.21 | −0.46 | −0.25 | 0.19 | −1.30 |
| Income-to-needs ratio | −0.09 | 0.08 | −1.06 | −0.11 | 0.08 | −1.39 |
| Sex at birth | −0.21 | 0.53 | −0.39 | −0.41 | 0.51 | −0.80 |
| Prenatal anxiety | 0.11∗∗∗ | 0.02 | 5.53 | 0.11∗∗∗ | 0.02 | 5.63 |
| Uncinate on linear rate of change of prenatal sleep | −6.41∗∗ | 2.30 | −2.79 | −5.34∗ | 2.42 | −2.21 |
| Uncinate on quadratic rate of change of prenatal sleep | 0.20∗∗ | 0.07 | 2.85 | 0.15∗ | 0.07 | 2.16 |
| Model 3: Prenatal Depression | ||||||
| Uncinate on intercept of prenatal sleep | 61.56∗∗ | 22.65 | 2.72 | 73.53∗∗ | 23.78 | 3.09 |
| Motion | −0.08∗∗ | 0.03 | −2.73 | −0.07∗∗ | 0.03 | −2.78 |
| PCA at MRI | −0.07 | 0.021 | −0.34 | −0.22 | 0.19 | −1.174 |
| Income-to-needs ratio | −0.01 | 0.08 | −0.08 | −0.03 | 0.08 | −0.39 |
| Sex at birth | −0.16 | 0.51 | −0.30 | −0.23 | 0.49 | −0.48 |
| Prenatal depression | 0.32∗∗∗ | 0.04 | 7.16 | 0.31∗∗∗ | 0.04 | 7.11 |
| Uncinate on linear rate of change of prenatal sleep | −6.62∗∗ | 2.28 | −2.92 | −5.76∗ | 2.39 | −2.41 |
| Uncinate on quadratic rate of change of prenatal sleep | 0.20∗∗ | 0.07 | 2.95 | 0.16∗ | 0.07 | 2.34 |
Note: ∗p < .05, ∗∗< 0.01, ∗ ∗∗p < .001. PCA, Postconceptional age; MRI, Magnetic resonance imaging.
Sensitivity analyses
Sensitivity analyses demonstrated that removing participants with prenatal substance use (n = 3) or preterm infants (<37 weeks GA; n = 6) did not affect the pattern of the findings and were thus retained in final analyses (See Supplement Tables S3 and S4).
Analyses conducted with two non-frontolimbic tracts, bilateral corticothalamic-parietal and motor segment of the corpus callosum, showed that prenatal maternal sleep quality was not linked with FA within either of these control tracts (See Table 5).
Table 5.
Generalized linear mixed models of maternal sleep quality across gestation and neonatal corticothalamic-parietal and motor segment of the corpus callosum (FA).
| Fixed effects | Left corticothalamic-parietal |
Right corticothalamic-parietal |
Corpus callosum |
||||||
|---|---|---|---|---|---|---|---|---|---|
| b estimate | SE | T | b estimate | SE | T | b estimate | SE | T | |
| Region on intercept of prenatal sleep | −0.91 | 0.03 | −0.04 | −16.10 | 24.20 | −0.67 | 2.49 | 24.45 | 0.10 |
| Motion | −0.09∗ | 0.03 | −2.87 | −0.09∗∗ | 0.03 | −3.30 | −0.07∗ | 0.03 | −2.32 |
| PCA at MRI | 0.33 | 0.22 | 1.52 | 0.40∗ | 0.19 | 2.04 | 0.34 | 0.20 | 1.66 |
| Income-to-needs ratio | −0.22∗ | 0.09 | −2.41 | −0.27∗∗ | 0.09 | −3.12 | −0.24 | 0.009 | −2.64 |
| Sex at birth | 0.09 | 0.61 | 0.14 | −0.20 | 0.57 | −0.34 | 0.13 | 0.62 | 0.21 |
| Region on linear rate of change of prenatal sleep | −3.19 | 2.52 | −1.27 | −2.34 | 2.47 | −0.95 | −1.36 | 2.69 | −0.51 |
| Region on quadratic rate of change of prenatal sleep | 0.11 | 0.07 | 1.50 | 0.08 | 0.07 | 1.09 | 0.04 | 0.08 | 0.55 |
Note: ∗p < .05, ∗∗< 0.01, ∗ ∗∗p < .001. PCA, Postconceptional age; MRI, Magnetic resonance imaging.
Neonatal uncinate fasciculus white matter as mechanism linking prenatal maternal sleep and later infant negative emotionality
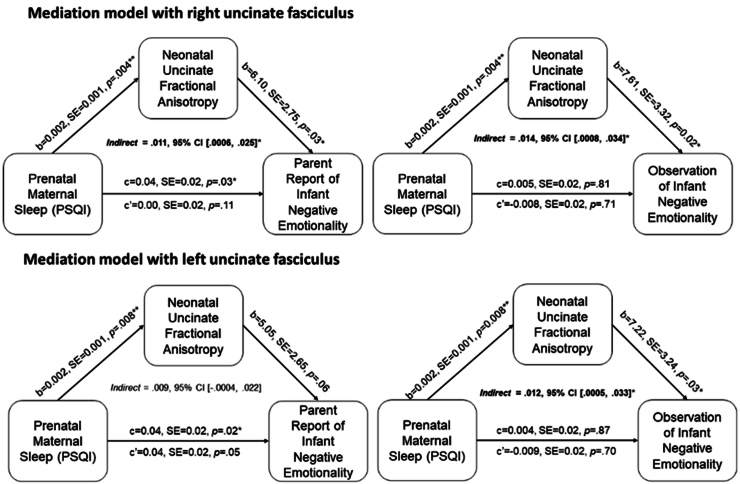
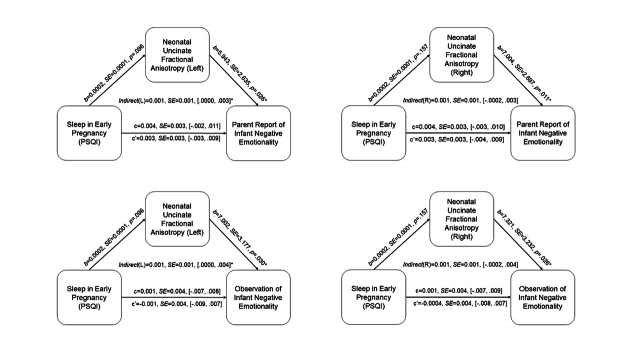
Significant associations were observed between prenatal maternal sleep and neonatal uncinate FA. Thus, mediation analyses were conducted to test the indirect effect of total exposure to prenatal maternal sleep problems (average) on infant negative emotionality through neonatal uncinate FA. Prenatal maternal sleep problems indirectly presaged greater infant negative emotionality (multimodally) via higher bilateral uncinate fasciculus FA (See Fig. 3).
Fig. 3.
Neonatal uncinate fractional anisotropy partially mediates the association between prenatal maternal sleep quality and infant negative emotionality (top row = right uncinate; bottom row = left uncinate).
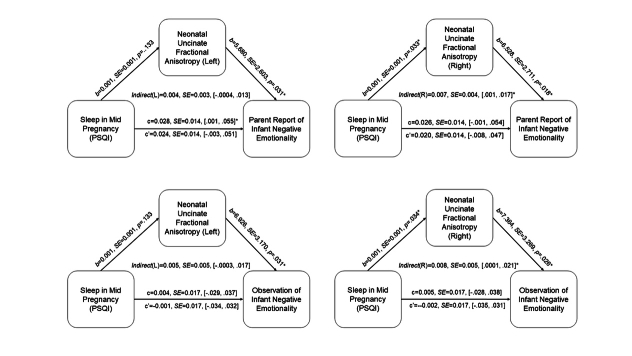
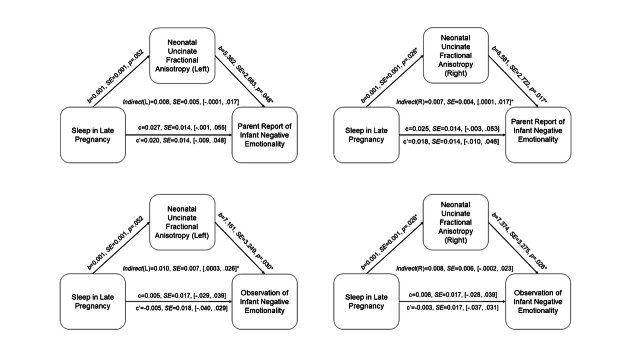
To further address the patterns of associations based on timing of sleep problems during pregnancy, we conducted additional mediation analyses focusing on sleep quality during early, mid, and late pregnancy. No clear pattern based on timing of sleep quality emerged (See Supplement Figures S3–S5).
Sex differences
Regressions were conducted to test whether sex moderated this association between prenatal maternal sleep and neonatal fronto-limbic circuitry. Sex of the infant did not moderate the association between prenatal maternal sleep quality and uncinate (left: b = −0.001, p = .41; right: b = −0.001, p = .25) or cingulum FA (left: b = −0.001, p = .52; right: b = 0.001, p = .61).
Discussion
Maternal sleep quality across gestation portends neonatal fronto-limbic circuit development and subsequent elevations in infants’ negative emotionality. Specifically, elevated sleep problems throughout pregnancy were associated with higher neonatal uncinate fasciculus FA, which in turn, was associated with greater negative emotionality, assessed multimodally, when infants were six months old. Negative emotionality constitutes a transdiagnostic risk phenotype, as infants who are prone to feelings of fear, irritability, and sadness are more susceptible to psychopathology later in life.9 Overall, our findings suggest that prenatal maternal sleep may affect offspring mental health through neonatal fronto-limbic circuitry.
Evidence that prenatal maternal sleep problems are associated with increased uncinate fasciculus FA is consistent with previous human research indicating that development of the uncinate fasciculus is susceptible to early life signals.18, 19, 20 Experimental evidence in rats is also consistent with these findings, highlighting increases in offspring synaptic density following prenatal maternal sleep deprivation.12 As FA increases on average across development,13 it has been hypothesized that higher FA following exposure to adversity may reflect accelerated maturation.64 Prior research has found evidence of accelerated white matter maturation (higher FA) in fronto-limbic circuitry in infants of mothers exposed to distress during pregnancy,33 and higher uncinate fasciculus FA in response to early life adversity. In addition to FA, volumetric studies provide evidence for accelerated maturation in response to prenatal adversity65 including poor maternal sleep.28 While helping to meet challenging environmental demands in the immediate short term, such accelerated development may shorten important periods of developmental plasticity and increase vulnerability to subsequent psychopathology.18,64 Consistent with this possibility, we find that increases in uncinate FA were associated with elevated negative emotionality in infancy. As negative emotionality is a risk factor for development of psychopathology, such heightened maturation of the uncinate associated with poor prenatal sleep may contribute to increased vulnerability to mental illness later in life.
Notably, prenatal maternal sleep quality was associated with neonatal uncinate FA after covarying for mental health (anxiety and depression) and exposure to stressful life events. These results suggest that impacts of prenatal maternal sleep are independent of maternal mental health and are consistent with prior evidence that prenatal sleep disturbances precede increases in depression symptoms in pregnancy.66
Poor maternal sleep was related to increased neonatal uncinate FA but not cingulum FA. This finding is consistent with several studies indicating that the uncinate fasciculus may be more specifically associated with early life adversity19 and prenatal influences.67 The uncinate develops around 13 weeks, followed by the cingulum, which can be traced after 17 weeks.68 White matter tracts continue to develop along differing time courses postnatally.13 It is plausible that disparate sensitive periods exist for the uncinate fasciculus and the cingulum, and that effects of prenatal sleep on maturation of the cingulum may emerge later in development.
The present investigation focused on two primary fronto-limbic circuits known both to be affected by early life experiences67,69 and linked to negative emotionality.25 To further probe the specificity of these associations, we examined white matter microstructure of the motor segment corpus callosum and corticothalamic-parietal tracts as two other tracts without a priori reasons to hypothesize links to prenatal sleep or infant negative emotionality. In contrast to our focus on negative emotionality, disruptions in white matter microstructure within these two tracts are typically associated with motor and cognitive deficiencies.70,71 Our analyses tested specific hypotheses regarding fronto-limbic tracts, there is, however, evidence that brain development is broadly interconnected, with most circuits showing some correlation in development.72, 73, 74 It is plausible that the effects of poor prenatal sleep extend beyond the uncinate and the hippocampal cingulum, which is an important direction for future research to investigate.
Prenatal maternal sleep problems presaged greater negative emotionality at six months through changes in neonatal uncinate FA. Notably, these longitudinal associations were corroborated across multimodal assessments of infant's negative emotionality, including both observational and maternal report. Negative emotionality is an early emerging transdiagnostic risk factor for diverse physical and mental health outcomes.75 Previous work has linked negative emotionality with structural differences within the uncinate.25,76 Building on this association, our results showed that prenatal maternal sleep problems were linked to later infant negative emotionality indirectly through uncinate white matter microstructure. This evidence that prenatal maternal sleep may sculpt the foetal brain is consistent with previous findings linking prenatal maternal sleep and offspring hippocampal volume.28 Prenatal maternal sleep and early white matter maturation of the uncinate are linked to offspring cognitive and emotional functions,8,25 suggesting that neonatal uncinate white matter may be one pathway underlying the association between prenatal maternal sleep and infant negative emotionality.
Increases in uncinate FA was also accompanied by a reduction in RD; however, no differences were observed in AD. Although it is challenging to discern the biological underpinnings of differences in diffusion metrics, RD (the diffusivity perpendicular to the main fibre) primarily indexes axonal myelination,77 while AD (the diffusivity along the main fibre) is thought to reflect information about axonal fibre organization, integrity and density.77,78 Thus, processes related to myelination may be susceptible to poor prenatal sleep. RD findings are relatively uncommon in neonatal studies due to its inherently low signal-to-noise ratio at such a young age. Thus, detection of reductions in RD in this study suggests that this may potentially be a strong effect. Our findings supporting links with RD and not AD suggest that differences in myelination could be a primary factor underlying this association; however, reductions in RD have also been shown to index axon composition and density,79 therefore it is also possible that these alternative underlying processes may also be affected. Further, the specific mechanisms through which prenatal sleep alters these processes is unknown. It is plausible that maternal biology, such as changes in hypothalamic-pituitary-adrenal (HPA) and placental axis functioning, and immune and inflammatory markers play a key role as they are linked to both prenatal sleep health and offspring neurodevelopment.80, 81, 82, 83
Study findings need to be considered in light of both strengths and limitations. A key strength of this study is the prospective and longitudinal design, including the repeated assessment of sleep to capture the dynamic nature of sleep across pregnancy. However, no clear pattern based on timing of sleep quality across pregnancy emerged. In support of the ubiquity of sleep difficulties, over two-thirds of individuals in the current study were classified as poor sleepers. Sleep health is a multidimensional construct, and a limitation of the present investigation is that only subjective report of sleep quality was reported in this study. Multiple modalities to assess sleep exist (e.g., polysomnography, actigraphy, and self-report), and yet objective and subjective sleep are modestly correlated in clinical and nonclinical samples.84,85 Future studies should incorporate prospective and longitudinal assessment of multiple sleep parameters such as sleep onset latency, sleep midpoint, and between-night variability, using objective measures such as via actigraphy. An additional limitation is that maternal sleep disturbances were not examined prior to conception and thus, future research investigating the relation between preconception maternal sleep problems and neonatal white matter is needed. Postnatal maternal sleep was tested as a covariate and findings revealed that it did not correlate with neonatal white matter microstructure. Another strength of this study is that the effects of prenatal maternal sleep on offspring neurobiology remained after covarying for socioeconomic status, maternal prenatal stressful life events, prenatal anxiety and depression, and postnatal maternal sleep. However, it is possible that other factors beyond maternal sleep quality may influence infant brain development.86 For example, epidemiological studies show evidence of racial and ethnic disparities in sleep health during pregnancy.87 Future studies should address this limitation and investigate the prospective and longitudinal associations between prenatal sleep health and other contextual factors, including considerations of social determinants of prenatal sleep.
Study findings are consistent with the accelerated maturation hypothesis. Neonates were scanned shortly after birth to assess the effects of prenatal influences while minimizing the interacting effects of the postnatal environment. However, a limitation of the present findings is that neonatal white matter microstructure was only assessed once in this study. As white matter tracts continue to develop in the early postnatal period,13 future studies should implement repeated assessment of diffusion imaging early in life to characterize developmental trajectories of FA in association with prenatal sleep quality. Future analyses using advanced dMRI techniques, such as neurite orientation dispersion and density imaging (NODDI)88 may be beneficial to further characterize the dynamic patterns of microstructural maturation beyond the diffusion metrics (FA, RD, and AD) investigated within the current study. Finally, in contrast to prior studies indicating sex-specific responses to early adversity,63 we did not find sex moderation for the association between prenatal sleep quality and neonatal white matter. Our study was likely underpowered to detect sex moderation, and continued work is needed to address this limitation and elucidate sex-specific responses to the prenatal environment.
Findings from the present study support prenatal sleep health as a robust signal with implications for offspring white matter maturation and infant negative emotionality. These findings highlight the importance of considering prenatal sleep health as a potential factor sculpting child neurodevelopment and demonstrate the need for prenatal interventions to improve sleep during pregnancy.34,89,90
Contributors
Nevarez-Brewster, Demers, Hankin, and Davis conceptualized this study. Hankin and Davis acquired funding for the data collection and management of this study. Nevarez-Brewster, Demers, Deer, Hankin, Styner, and Davis analysed and interpreted the data. Nevarez-Brewster, Demers and Deer accessed and verified the underlying data of this study prior to manuscript submission. Nevarez-Brewster, Demers, Deer, Gallop, Styner and Davis drafted the manuscript. Styner, Bagonis, Al-Ali, and Hoeflich Haase pre-processed imaging data. All authors contributed to editing the manuscript and approved the final manuscript.
Data sharing statement
Data and code used for analyses can be made available by contacting the investigator with a written proposal with a plan of how the data will be used (e.g., meta-analysis).
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research is supported by the National Institutes of Health: R01MH109662 [EPD, BLH], R01HL155744 [EPD, BLH], P50HD103573 [MAS], K12AR084226 [CHD], F32 Training fellowships MH125572 [CHD], HL165844 [LD], MH106440 [MMB], and diversity supplement R01HL155744-01S1 [EPD, Recipient: MNB].
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105384.
Contributor Information
Melissa Nevarez-Brewster, Email: melissa.nevarezbrewster@du.edu.
Catherine H. Demers, Email: catherine.demers@cuanschutz.edu.
Appendix A. Supplementary data
Supplementary Figure S1.
Supplementary Figure S2.
Supplementary Figure S3.
Supplementary Figure S4.
Supplementary Figure S5.
References
- 1.Mindell J.A., Cook R.A., Nikolovski J. Sleep patterns and sleep disturbances across pregnancy. Sleep Med. 2015;16(4):483–488. doi: 10.1016/j.sleep.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 2.González-Mesa E., Cuenca-Marín C., Suarez-Arana M., et al. Poor sleep quality is associated with perinatal depression. A systematic review of last decade scientific literature and meta-analysis. J Perinat Med. 2019;47(7):689–703. doi: 10.1515/jpm-2019-0214. [DOI] [PubMed] [Google Scholar]
- 3.Cai S., Tan S., Gluckman P.D., et al. Sleep quality and nocturnal sleep duration in pregnancy and risk of gestational diabetes mellitus. Sleep. 2017;40(2) doi: 10.1093/sleep/zsw058. [DOI] [PubMed] [Google Scholar]
- 4.Barker D.J.P. In utero programming of chronic disease. Clin Sci. 1998;95(2):115–128. [PubMed] [Google Scholar]
- 5.Gluckman P.D., Hanson M.A. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 6.Adler I., Weidner K., Eberhard-Gran M., Garthus-Niegel S. The impact of maternal symptoms of perinatal insomnia on social-emotional child development: a population-based, 2-year follow-up study. Behav Sleep Med. 2021 May 4;19(3):303–317. doi: 10.1080/15402002.2020.1746661. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y., Huang J., Baker P.N., Liao B., Yu X. The prevalence and associated factors of prenatal depression and anxiety in twin pregnancy: a cross-sectional study in Chongqing, China. BMC Pregnancy Childbirth. 2022;22(1):877. doi: 10.1186/s12884-022-05203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciciolla L., Addante S., Quigley A., Erato G., Fields K. Infant sleep and negative reactivity: the role of maternal adversity and perinatal sleep. Infant Behav Dev. 2022;66 doi: 10.1016/j.infbeh.2021.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahey B.B., Class Q.A., Zald D.H., Rathouz P.J., Applegate B., Waldman I.D. Prospective test of the developmental propensity model of antisocial behavior: from childhood and adolescence into early adulthood. J Child Psychol Psychiatry. 2018;59(6):676–683. doi: 10.1111/jcpp.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q., Peng C., Wu X., Chen Y., Wang C., You Z. Maternal sleep deprivation inhibits hippocampal neurogenesis associated with inflammatory response in young offspring rats. Neurobiol Dis. 2014;68:57–65. doi: 10.1016/j.nbd.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Peng Y., Wang W., Tan T., et al. Maternal sleep deprivation at different stages of pregnancy impairs the emotional and cognitive functions, and suppresses hippocampal long-term potentiation in the offspring rats. Mol Brain. 2016;9(1):17. doi: 10.1186/s13041-016-0197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderplow A.M., Kermath B.A., Bernhardt C.R., et al. A feature of maternal sleep apnea during gestation causes autism-relevant neuronal and behavioral phenotypes in offspring. PLoS Biol. 2022;20(2) doi: 10.1371/journal.pbio.3001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmore J.H., Knickmeyer R.C., Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19(3):123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scorza P., Doyle C., Monk C. In: Prenatal stress and child development. Wazana A., Székely E., Oberlander T.F., editors. Springer International Publishing; Cham: 2021. Prenatal programming in the fetus and placenta; pp. 53–88. [cited 2024 Feb 16] [DOI] [Google Scholar]
- 15.Glover V., O'Donnell K.J., O'Connor T.G., Fisher J. Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology-A global perspective. Dev Psychopathol. 2018;30(3):843–854. doi: 10.1017/S095457941800038X. [DOI] [PubMed] [Google Scholar]
- 16.Sandman C.A., Curran M.M., Davis E.P., Glynn L.M., Head K., Baram T.Z. Cortical thinning and neuropsychiatric outcomes in children exposed to prenatal adversity: a role for placental crh? Aust J Pharm. 2018;175(5):471–479. doi: 10.1176/appi.ajp.2017.16121433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demers C.H., Aran Ö., Glynn L.M., Davis E.P. Prenatal stress and child development. Springer; 2021. Prenatal programming of neurodevelopment: structural and functional changes; pp. 193–242. [Google Scholar]
- 18.Gee D.G., Gabard-Durnam L.J., Flannery J., et al. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granger S.J., Glynn L.M., Sandman C.A., et al. Aberrant maturation of the uncinate fasciculus follows exposure to unpredictable patterns of maternal signals. J Neurosci. 2021;41(6):1242–1250. doi: 10.1523/JNEUROSCI.0374-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tottenham N., Galván A. Stress and the adolescent brain: amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev. 2016;70:217. doi: 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Incapacity to control emotion in major depression may arise from disrupted white matter integrity and OFC-amygdala inhibition - zheng - 2018 - CNS Neuroscience & Therapeutics - Wiley Online Library. https://onlinelibrary-wiley-com.du.idm.oclc.org/doi/10.1111/cns.12800 [cited 2024 Feb 16]. Available from: [DOI] [PMC free article] [PubMed]
- 22.Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008 Sep;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Von Der Heide R.J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(Pt 6):1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bubb E.J., Metzler-Baddeley C., Aggleton J.P. The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev. 2018;92:104–127. doi: 10.1016/j.neubiorev.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banihashemi L., Bertocci M.A., Alkhars H.M., et al. Limbic white matter structural integrity at 3 months prospectively predicts negative emotionality in 9-month-old infants: a preliminary study. J Affect Disord. 2020;273:538–541. doi: 10.1016/j.jad.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanel D., Vanes L.D., Pecheva D., et al. Neonatal white matter microstructure and emotional development during the preschool years in children who were born very preterm. eNeuro. 2021;8(5) doi: 10.1523/ENEURO.0546-20.2021. https://www.eneuro.org/content/8/5/ENEURO.0546-20.2021 [cited 2022 Nov 28] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X.H., Wang Y., Wang D.F., et al. White matter microstructural abnormalities and their association with anticipatory anhedonia in depression. Psychiatry Res Neuroimaging. 2017;264:29–34. doi: 10.1016/j.pscychresns.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Nevarez-Brewster M., Demers C.H., Mejia A., et al. Longitudinal and prospective assessment of prenatal maternal sleep quality and associations with newborn hippocampal and amygdala volume. Dev Cogn Neurosci. 2022;58 doi: 10.1016/j.dcn.2022.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyniak C., Whalen D., Luby J., et al. Sleep and circadian rhythms during pregnancy, social disadvantage, and alterations in brain development in neonates. Dev Sci. 2023;27 doi: 10.1111/desc.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cappe C., Rouiller E.M., Barone P. In: The neural bases of multisensory processes. Murray M.M., Wallace M.T., editors. CRC Press/Taylor & Francis; Boca Raton (FL): 2012. Cortical and thalamic pathways for multisensory and sensorimotor interplay.http://www.ncbi.nlm.nih.gov/books/NBK92866/ [cited 2024 Feb 16]. (Frontiers in Neuroscience). Available from: [PubMed] [Google Scholar]
- 31.Wahl M., Ziemann U. The human motor corpus callosum. Rev Neurosci. 2008;19(6):451–466. doi: 10.1515/revneuro.2008.19.6.451. [DOI] [PubMed] [Google Scholar]
- 32.Nevarez-Brewster M., Aran Ö., Narayan A.J., et al. Adverse and benevolent childhood experiences predict prenatal sleep quality. Adv Res Sci. 2022;4:391–402. doi: 10.1007/s42844-022-00070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demers C.H., Bagonis M.M., Al-Ali K., et al. Exposure to prenatal maternal distress and infant white matter neurodevelopment. Dev Psychopathol. 2021;33(5):1526–1538. doi: 10.1017/s0954579421000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis E.P., Hankin B.L., Swales D.A., Hoffman M.C. An experimental test of the fetal programming hypothesis: can we reduce child ontogenetic vulnerability to psychopathology by decreasing maternal depression? Dev Psychopathol. 2018;30(3):787–806. doi: 10.1017/S0954579418000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis E.P., Demers C.H., Deer L., et al. Impact of prenatal maternal depression on gestational length: post hoc analysis of a randomized clinical trial. eClinicalMedicine. 2024;72 doi: 10.1016/j.eclinm.2024.102601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bureau UC Census.gov. American Community Survey 5-Year Data (2009-2022) https://www.census.gov/data/developers/data-sets/acs-5year.html [cited 2024 Aug 20] Available from:
- 37.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 38.Ong J.C., Cardé N.B., Gross J.J., Manber R. A two-dimensional approach to assessing affective states in good and poor sleepers. J Sleep Res. 2011;20(4):606–610. doi: 10.1111/j.1365-2869.2011.00907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu C., Gelaye B., Zhong Q.Y., Enquobahrie D.A., Frederick I.O., Williams M.A. Construct validity and factor structure of the Pittsburgh Sleep Quality Index among pregnant women in a Pacific-Northwest cohort. Sleep Breath. 2016;20(1):293–301. doi: 10.1007/s11325-016-1313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skouteris H., Wertheim E.H., Germano C., Paxton S.J., Milgrom J. Assessing sleep during pregnancy: a study across two time points examining the Pittsburgh sleep quality index and associations with depressive symptoms. Wom Health Issues. 2009;19(1):45–51. doi: 10.1016/j.whi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Forner-Cordero A., Umemura G.S., Furtado F., Gonçalves B. da SB. Comparison of sleep quality assessed by actigraphy and questionnaires to healthy subjects. Sleep Sci. 2018;11(3):141–145. doi: 10.5935/1984-0063.20180027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weathers F.W., Blake D.D., Schnurr P.P., Kaloupek D.G., Marx B.P., Keane T.M. 2013. The life events checklist for DSM-5 (LEC-5) – standard.https://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp Available from: [Google Scholar]
- 43.Gray M.J., Litz B.T., Hsu J.L., Lombardo T.W. Psychometric properties of the life events checklist. Assessment. 2004;11(4):330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- 44.Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the state-tait anxiety inventory. [Google Scholar]
- 45.Bados A., Gómez-Benito J., Balaguer G. The state-trait anxiety inventory, trait version: does it really measure anxiety? J Pers Assess. 2010 Nov;92(6):560–567. doi: 10.1080/00223891.2010.513295. [DOI] [PubMed] [Google Scholar]
- 46.Hennessey E.M.P., Swales D.A., Markant J., Hoffman M.C., Hankin B.L., Davis E.P. Maternal anxiety during pregnancy predicts infant attention to affective faces. J Affect Disord. 2024;344:104–114. doi: 10.1016/j.jad.2023.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatr. 1987 Jun;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 48.Noroña-Zhou A., Aran Ö., Garcia S.E., et al. Experiences of discrimination and depression trajectories over pregnancy. Wom Health Issues. 2022;32(2):147–155. doi: 10.1016/j.whi.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvador R., Peña A., Menon D.K., Carpenter T.A., Pickard J.D., Bullmore E.T. Formal characterization and extension of the linearized diffusion tensor model. Hum Brain Mapp. 2005;24(2):144–155. doi: 10.1002/hbm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones D.K., Basser P.J. “Squashing peanuts and smashing pumpkins”: how noise distorts diffusion-weighted MR data. Magn Reson Med. 2004;52(5):979–993. doi: 10.1002/mrm.20283. [DOI] [PubMed] [Google Scholar]
- 51.Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002 Nov;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verde A., Budin F., Berger J.B., et al. UNC-Utah NA-MIC framework for DTI fiber tract analysis. Front Neuroinf. 2014;7:51. doi: 10.3389/fninf.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ngattai Lam P.D., Belhomme G., Ferrall J., Patterson B., Styner M., Prieto J.C. TRAFIC: fiber tract classification using deep learning. Proc SPIE Int Soc Opt Eng. 2018;10574 doi: 10.1117/12.2293931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Short S.J., Jang D.K., Steiner R.J., et al. Diffusion tensor based white matter tract atlases for pediatric populations. Front Neurosci. 2022;16 doi: 10.3389/fnins.2022.806268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gartstein M.A., Rothbart M.K. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav Dev. 2003;26(1):64–86. [Google Scholar]
- 56.Parade S.H., Leerkes E.M. The reliability and validity of the infant behavior questionnaire-revised. Infant Behav Dev. 2008 Dec;31(4):637–646. doi: 10.1016/j.infbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldsmith H.H., Rothbart M.K. Technical Manual, Department of Psychology, University of Wisconsin; Madison, WI: 1996. Prelocomotor and locomotor laboratory temperament assessment Battery, lab-TAB. [Google Scholar]
- 58.ACOG Methods for estimating the due date. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2017/05/methods-for-estimating-the-due-date [cited 2023 Aug 21]. Available from:
- 59.Hobel C.J., Youkeles L., Forsythe A. Prenatal and intrapartum high-risk screening. II. Risk factors reassessed. Am J Obstet Gynecol. 1979;135(8):1051–1056. doi: 10.1016/0002-9378(79)90735-x. [DOI] [PubMed] [Google Scholar]
- 60.Moog N.K., Nolvi S., Kleih T.S., et al. Prospective association of maternal psychosocial stress in pregnancy with newborn hippocampal volume and implications for infant social-emotional development. Neurobiol Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolker B.M., Brooks M.E., Clark C.J., et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24(3):127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 62.Hayes A.F. Partial, conditional, and moderated moderated mediation: quantification, inference, and interpretation. Commun Monogr. 2018;85(1):4–40. [Google Scholar]
- 63.Sandman C.A., Glynn L.M., Davis E.P. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res. 2013;75(4):327–335. doi: 10.1016/j.jpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Callaghan B.L., Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demers C.H., Hankin B.L., Hennessey E.M.P., et al. Maternal adverse childhood experiences and infant subcortical brain volume. Neurobiol Stress. 2022;21 doi: 10.1016/j.ynstr.2022.100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skouteris H., Germano C., Wertheim E.H., Paxton S.J., Milgrom J. Sleep quality and depression during pregnancy: a prospective study. J Sleep Res. 2008;17(2):217–220. doi: 10.1111/j.1365-2869.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 67.Lautarescu A., Bonthrone A.F., Pietsch M., et al. Maternal depressive symptoms, neonatal white matter, and toddler social-emotional development. Transl Psychiatry. 2022;12(1):323. doi: 10.1038/s41398-022-02073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang H., Xue R., Zhang J., et al. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci. 2009;29(13):4263–4273. doi: 10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lean R.E., Smyser C.D., Brady R.G., et al. Prenatal exposure to maternal social disadvantage and psychosocial stress and neonatal white matter connectivity at birth. Proc Natl Acad Sci USA. 2022;119(42) doi: 10.1073/pnas.2204135119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wahl M., Lauterbach-Soon B., Hattingen E., et al. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27(45):12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cappe C., Morel A., Rouiller E.M. Thalamocortical and the dual pattern of corticothalamic projections of the posterior parietal cortex in macaque monkeys. Neuroscience. 2007;146(3):1371–1387. doi: 10.1016/j.neuroscience.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 72.Thompson D.K., Inder T.E., Faggian N., et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage. 2012;59(4):3571–3581. doi: 10.1016/j.neuroimage.2011.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menegaux A., Meng C., Bäuml J.G., et al. Aberrant cortico-thalamic structural connectivity in premature-born adults. Cortex. 2021;141:347–362. doi: 10.1016/j.cortex.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 74.Telford E.J., Cox S.R., Fletcher-Watson S., et al. A latent measure explains substantial variance in white matter microstructure across the newborn human brain. Brain Struct Funct. 2017;222(9):4023–4033. doi: 10.1007/s00429-017-1455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Green C.G., Szekely E., Babineau V., et al. Negative emotionality as a candidate mediating mechanism linking prenatal maternal mood problems and offspring internalizing behaviour. Dev Psychopathol. 2022:1–15. doi: 10.1017/S0954579421001747. [DOI] [PubMed] [Google Scholar]
- 76.Mincic A.M. Neuroanatomical correlates of negative emotionality-related traits: a systematic review and meta-analysis. Neuropsychologia. 2015;77:97–118. doi: 10.1016/j.neuropsychologia.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Winklewski P.J., Sabisz A., Naumczyk P., Jodzio K., Szurowska E., Szarmach A. Understanding the physiopathology behind axial and radial diffusivity changes-what do we know? Front Neurol. 2018;9:92. doi: 10.3389/fneur.2018.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 79.Klawiter E.C., Schmidt R.E., Trinkaus K., et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bublitz M.H., Bourjeily G., D'Angelo C., Stroud L.R. Maternal sleep quality and diurnal cortisol regulation over pregnancy. Behav Sleep Med. 2018;16(3):282–293. doi: 10.1080/15402002.2016.1210147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davis E.P., Head K., Buss C., Sandman C.A. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology. 2017;75:56–63. doi: 10.1016/j.psyneuen.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwon H.K., Choi G.B., Huh J.R. Maternal inflammation and its ramifications on fetal neurodevelopment. Trends Immunol. 2022;43(3):230–244. doi: 10.1016/j.it.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okun M.L. Sleep disturbances and modulations in inflammation: implications for pregnancy health. Soc Personal Psychol Compass. 2019;13(5):19. doi: 10.1111/spc3.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Etain B., Krane-Gartiser K., Hennion V., Meyrel M., Scott J. Do self-ratings of the Pittsburgh Sleep Quality Index reflect actigraphy recordings of sleep quality or variability? An exploratory study of bipolar disorders versus healthy controls. J Sleep Res. 2022;31(3) doi: 10.1111/jsr.13507. [DOI] [PubMed] [Google Scholar]
- 85.Grandner M.A., Kripke D.F., Yoon I.Y., Youngstedt S.D. Criterion validity of the Pittsburgh sleep quality index: investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–136. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demers C.H., Hankin B.L., Haase M.H., et al. Maternal adverse childhood experiences and infant visual-limbic white matter development. J Affect Disord. 2024;367:49–57. doi: 10.1016/j.jad.2024.08.146. [DOI] [PubMed] [Google Scholar]
- 87.Lucchini M., O'Brien L.M., Kahn L.G., et al. Racial/ethnic disparities in subjective sleep duration, sleep quality, and sleep disturbances during pregnancy: an ECHO study. Sleep. 2022;45(9) doi: 10.1093/sleep/zsac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- 89.Hankin B.L., Demers C.H., Hennessey E.M.P., et al. Effect of brief interpersonal therapy on depression during pregnancy: a randomized clinical trial. JAMA Psychiatr. 2023;80(6):539–547. doi: 10.1001/jamapsychiatry.2023.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bei B., Pinnington D.M., Shen L., et al. A scalable cognitive behavioural program to promote healthy sleep during pregnancy and postpartum periods: protocol of a randomised controlled trial (the SEED project) BMC Pregnancy Childbirth. 2019;19(1):254. doi: 10.1186/s12884-019-2390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.