Abstract
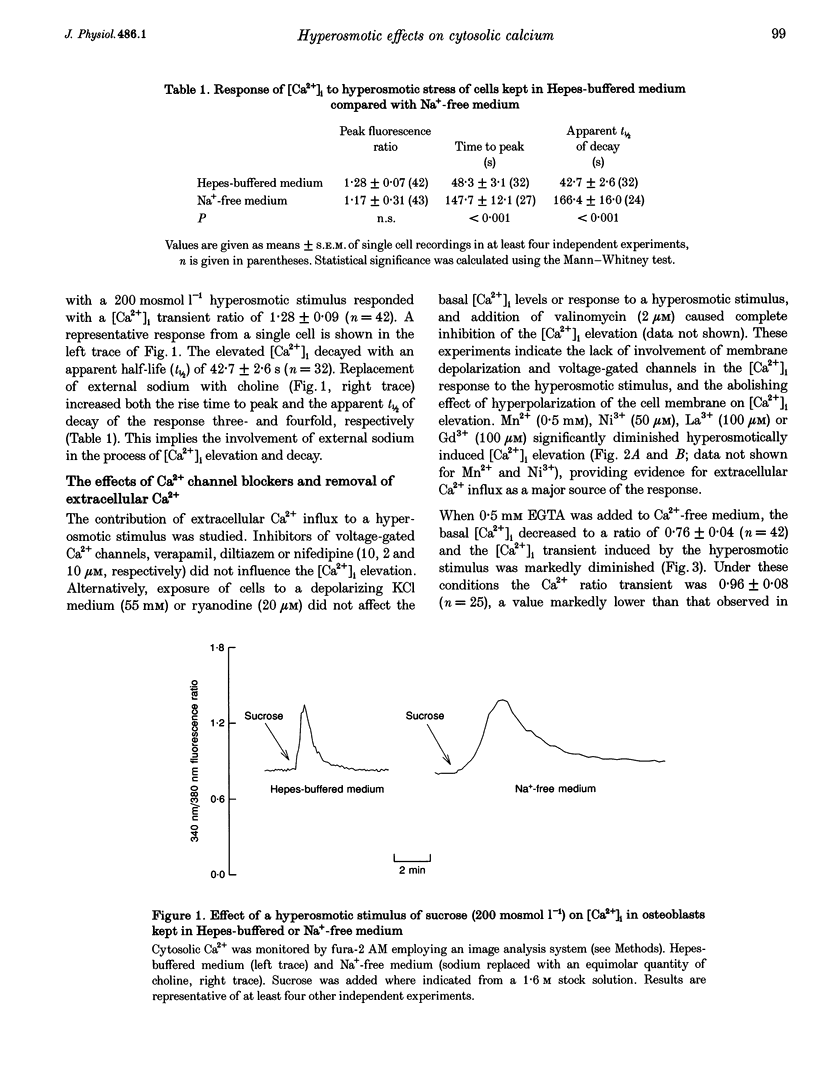
1. The effects of hyperosmotic stress on cytosolic calcium concentration ([Ca2+]i) were studied by ratio image analysis in single cells of an osteoblast-like bone cell line (RCJ 1.20) loaded with fura-2 AM. 2. The ratio (340 nm/380 nm) of steady-state [Ca2+]i in resting osteoblasts kept in Hepes-buffered medium was 0.82 +/- 0.04. A hyperosmotic stimulus (200 mosmol l-1 sucrose) produced a [Ca2+]i transient with a peak ratio of 1.28 +/- 0.09, which decayed with an apparent half-life (t1/2) of 42.7 +/- 2.6 s. 3. The hyperosmotically induced [Ca2+]i transients were insensitive to verapamil, diltiazem or nifedipine, which excludes the involvement of dihydropyridine-sensitive Ca2+ channels in the process. Non-specific Ca2+ channel blockers (Mn2+, Ni2+, La3+ or Gd3+) partially abolished the hyperosmotically induced [Ca2+]i elevation, indicating the contribution of extracellular Ca2+ influx. 4. A hyperosmotic stimulus applied in Ca(2+)-free medium (0.5 mM EGTA) lowered the [Ca2+]i peak to a ratio of 0.96 +/- 0.08 (P < 0.001) compared with a Ca(2+)-containing medium. This suggests that the [Ca2+]i increase is due to extracellular influx, as well as release from an intracellular Ca2+ pool. 5. Application of thapsigargin (0.5 microM), a specific inhibitor of endoplasmic reticulum Ca(2+)-ATPase, in Ca(2+)-free medium caused transient [Ca2+]i elevation to peak ratios of 1.33 +/- 0.09, and completely abolished the [Ca2+]i response to a hyperosmotic stimulus. This implies the existence of a thapsigargin-sensitive intracellular pool of Ca2+ that is mobilized by hyperosmotic stimulus.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

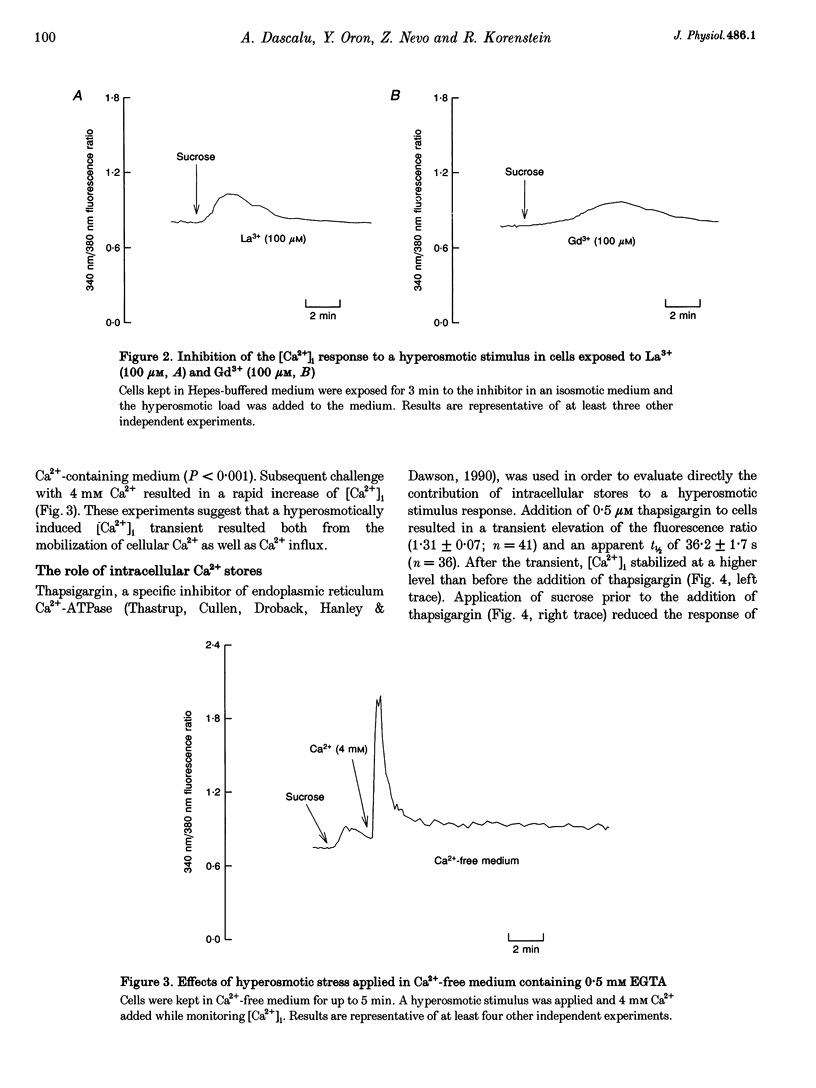


Selected References
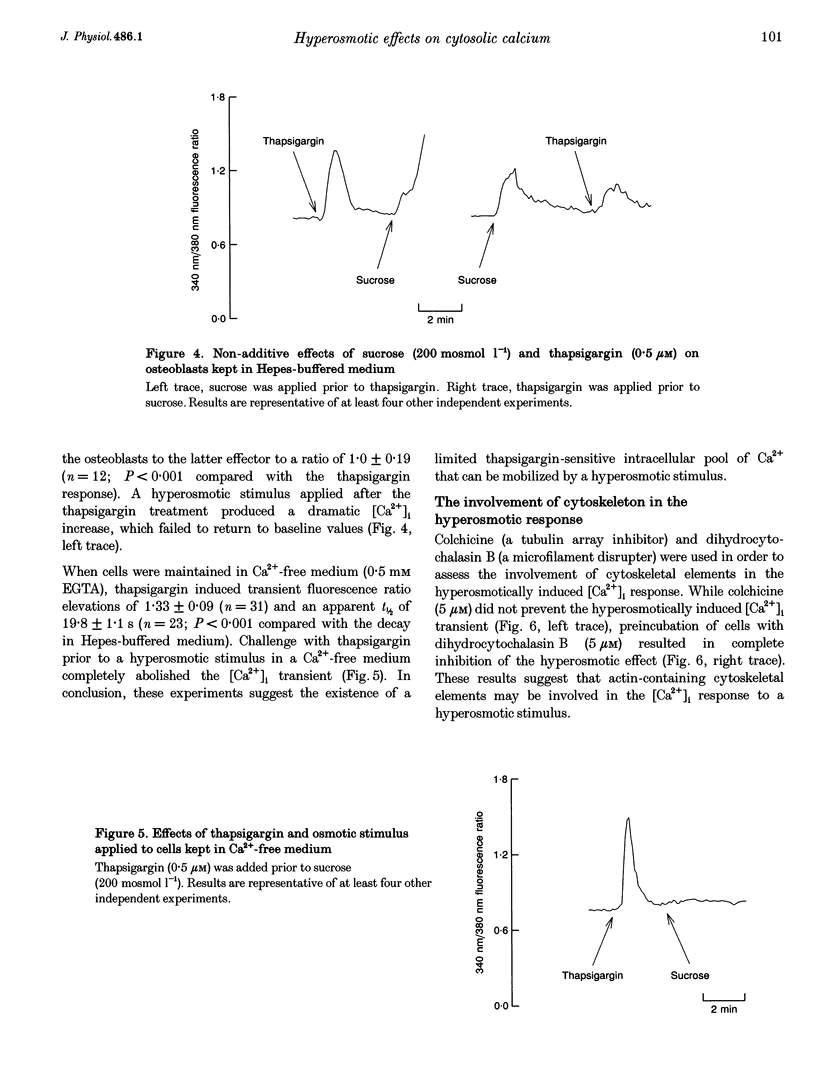
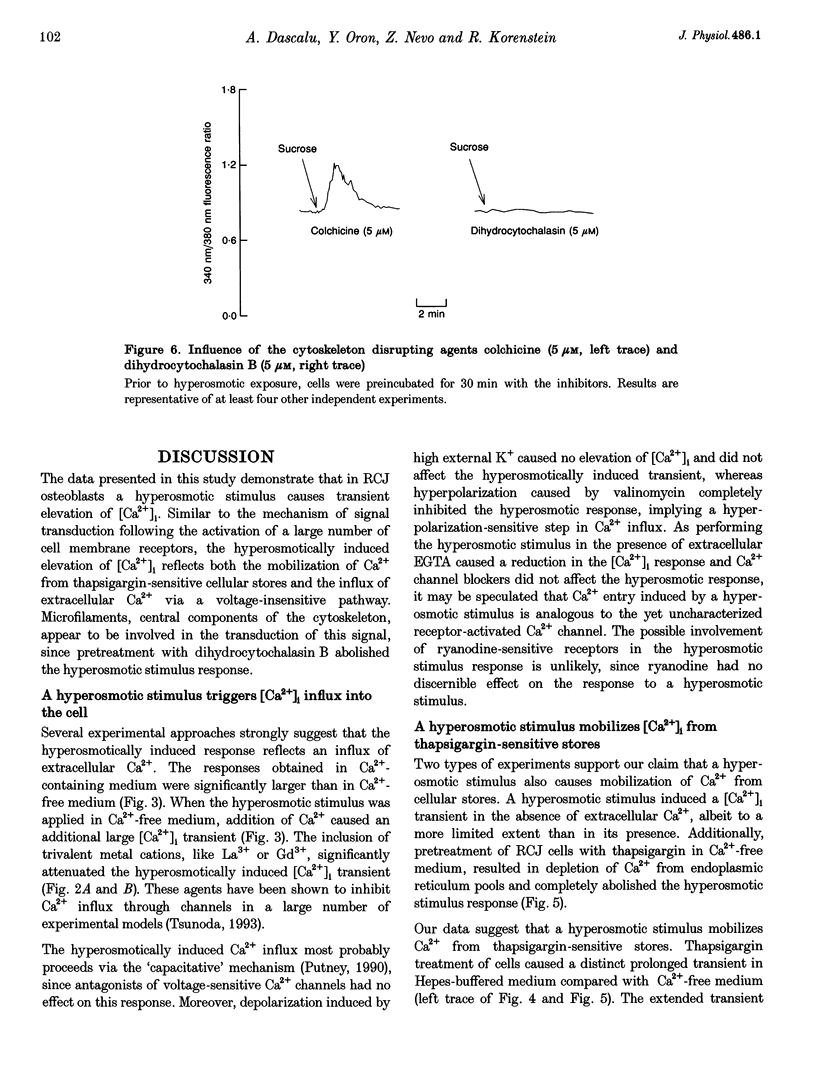
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Smith G. L. The effects of hypertonicity on tension and intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1987 Feb;383:425–439. doi: 10.1113/jphysiol.1987.sp016418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J. E., Heersche J. N., Merrilees M. J., Sodek J. Isolation of bone cell clones with differences in growth, hormone responses, and extracellular matrix production. J Cell Biol. 1982 Feb;92(2):452–461. doi: 10.1083/jcb.92.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busa W. B. Spectral characterization of the effect of viscosity on Fura-2 fluorescence: excitation wavelength optimization abolishes the viscosity artifact. Cell Calcium. 1992 May;13(5):313–319. doi: 10.1016/0143-4160(92)90066-2. [DOI] [PubMed] [Google Scholar]
- Dascalu A., Nevo Z., Korenstein R. Hyperosmotic activation of the Na(+)-H+ exchanger in a rat bone cell line: temperature dependence and activation pathways. J Physiol. 1992 Oct;456:503–518. doi: 10.1113/jphysiol.1992.sp019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens C. J., Gillespie J. I., Greenwell J. R., Hutchinson P. Relationship between intracellular pH (pHi) and calcium (Cai2+) in avian heart fibroblasts. Exp Cell Res. 1990 Mar;187(1):39–46. doi: 10.1016/0014-4827(90)90113-o. [DOI] [PubMed] [Google Scholar]
- Enomoto K., Furuya K., Yamagishi S., Maeno T. Mechanically induced electrical and intracellular calcium responses in normal and cancerous mammary cells. Cell Calcium. 1992 Aug;13(8):501–511. doi: 10.1016/0143-4160(92)90018-n. [DOI] [PubMed] [Google Scholar]
- Green J., Kleeman C. R. Role of calcium and cAMP messenger systems in intracellular pH regulation of osteoblastic cells. Am J Physiol. 1992 Jan;262(1 Pt 1):C111–C121. doi: 10.1152/ajpcell.1992.262.1.C111. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Dupre A., Rothstein A. Volume regulation by human lymphocytes. Role of calcium. J Gen Physiol. 1982 May;79(5):849–868. doi: 10.1085/jgp.79.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Janmey P. A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Nolte H., Scholübbers J. G., Turner E., Veltel D. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991 Mar;12(2):101–110. doi: 10.1016/0142-9612(91)90186-e. [DOI] [PubMed] [Google Scholar]
- Kazilek C. J., Merkle C. J., Chandler D. E. Hyperosmotic inhibition of calcium signals and exocytosis in rabbit neutrophils. Am J Physiol. 1988 May;254(5 Pt 1):C709–C718. doi: 10.1152/ajpcell.1988.254.5.C709. [DOI] [PubMed] [Google Scholar]
- Loechner K. J., Knox R. J., Connor J. A., Kaczmarek L. K. Hyperosmotic media inhibit voltage-dependent calcium influx and peptide release in Aplysia neurons. J Membr Biol. 1992 May;128(1):41–52. doi: 10.1007/BF00231869. [DOI] [PubMed] [Google Scholar]
- Lupu-Meiri M., Beit-Or A., Christensen S. B., Oron Y. Calcium entry in Xenopus oocytes: effects of inositol trisphosphate, thapsigargin and DMSO. Cell Calcium. 1993 Feb;14(2):101–110. doi: 10.1016/0143-4160(93)90080-p. [DOI] [PubMed] [Google Scholar]
- Parker I., Zhu P. H. Effects of hypertonic solutions on calcium transients in frog twitch muscle fibres. J Physiol. 1987 Feb;383:615–627. doi: 10.1113/jphysiol.1987.sp016432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990 Feb-Mar;11(2-3):85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Reich K. M., Frangos J. A. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol. 1991 Sep;261(3 Pt 1):C428–C432. doi: 10.1152/ajpcell.1991.261.3.C428. [DOI] [PubMed] [Google Scholar]
- Rubin C. T., Lanyon L. E. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985 Jul;37(4):411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- Sandy J. R., Meghji S., Farndale R. W., Meikle M. C. Dual elevation of cyclic AMP and inositol phosphates in response to mechanical deformation of murine osteoblasts. Biochim Biophys Acta. 1989 Feb 9;1010(2):265–269. doi: 10.1016/0167-4889(89)90171-7. [DOI] [PubMed] [Google Scholar]
- Savarese D. M., Russell J. T., Fatatis A., Liotta L. A. Type IV collagen stimulates an increase in intracellular calcium. Potential role in tumor cell motility. J Biol Chem. 1992 Oct 25;267(30):21928–21935. [PubMed] [Google Scholar]
- Sigurdson W. J., Sachs F., Diamond S. L. Mechanical perturbation of cultured human endothelial cells causes rapid increases of intracellular calcium. Am J Physiol. 1993 Jun;264(6 Pt 2):H1745–H1752. doi: 10.1152/ajpheart.1993.264.6.H1745. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda Y. Receptor-operated Ca2+ signaling and crosstalk in stimulus secretion coupling. Biochim Biophys Acta. 1993 Oct 29;1154(2):105–156. doi: 10.1016/0304-4157(93)90008-c. [DOI] [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Yamaguchi D. T., Green J., Kleeman C. R., Muallem S. Characterization of volume-sensitive, calcium-permeating pathways in the osteosarcoma cell line UMR-106-01. J Biol Chem. 1989 Mar 15;264(8):4383–4390. [PubMed] [Google Scholar]



