Abstract
Background
This study aims to evaluate the progression of morphological and functional alterations over time in patients with hypertrophic cardiomyopathy (HCM) using Cardiac Magnetic Resonance (CMR).
Methods
A retrospective analysis was conducted on patients with HCM who underwent serial CMR at 1.5 Tesla. Left ventricular (LV) mass was measured during diastole, including papillary muscles and trabeculae assessment. Appearance of Late Gadolinium Enhancement (LGE) was volumetrically quantified using a 5-standard-deviation (SD) threshold.
Results
Thirty-two patients, with a mean age of 44 ± 16 years (range: 11–70 years), were evaluated after an average follow-up period of 5.2 ± 2.4 years (range: 0.8–9.1 years). Significant increases were observed in LV mass (from 194 ± 56 g to 217 ± 60 g; p = 0.0001), septal wall thickness (from 18 ± 4 mm to 19 ± 4 mm; p = 0.01), LGE mass (from 6 ± 17 g to 8 ± 18 g; p = 0.006), and left atrial volume (from 109 ± 41 ml to 129 ± 40 ml; p = 0.0001). Both left and right ventricular ejection fractions (LVEF and RVEF) significantly decreased over time (LVEF: from 70 ± 9 % to 66 ± 9 %; p = 0.04 and RVEF: from 70 ± 7 % to 67 ± 9 %; p = 0.02). Multivariate regression analysis revealed that HCM mass gain was independently associated with age (B = -0.43; p = 0.02) and LGE mass (B = -0.46; p = 0.02). The median LV mass gain rate in adults was 1.7 g per year/BSA (IQR, 0.6–2.7) compared to 6.0 g per year/BSA (IQR, 0.5–11.6) in adolescents (mean age: 16 years; range: 11–20 years). A positive correlation was found between LV mass and LGE mass (B = 0.55; p = 0.001), while an inverse relationship was observed between LV mass gain and LGE mass gain rates (−0.37; p = 0.03).
Conclusion
The range of morphological changes in HCM seems to reflect an age-related equilibrium between hypertrophy and fibrosis. The extent of changes in LV mass, fibrosis, and functional decline in HCM may help identify patients at risk, emphasizing the importance of ongoing follow-up studies.
Keywords: Hypertrophic Cardiomyopathy (HCM), Cardiac Magnetic Resonance (CMR), Left Ventricular Mass, Late Gadolinium Enhancement (LGE), Prognosis, Outcome
1. Introduction
Hypertrophic Cardiomyopathy (HCM) is characterized by unexplained hypertrophy in at least one segment of the myocardial wall [1], [2], and it can result from a variety of genetic mutations [3], [4]. The phenotypic expression of HCM is highly variable, and the underlying genetic mutations do not reliably predict this variability [5], [6], [7], [8]. Macroscopic presentations differ widely in terms of myocardial hypertrophy severity, the extent and regional distribution of myocardial fibrosis, and the presence or absence of left or, more rarely, right ventricular obstruction. Despite the strong association between the extent of myocardial fibrosis and clinical outcomes in hypertrophic cardiomyopathy, the relationship is not yet fully defined by a single MRI marker or threshold, and the underlying mechanisms, particularly in relation to sudden cardiac death, remain incompletely understood. Additionally, abnormalities in the mitral valve apparatus, left atrial enlargement, myocardial recesses, or apical aneurysms may be observed [1], [2], [3]. The clinical course of HCM is influenced by the timing and progression of these abnormalities, potentially leading to exercise intolerance, sudden cardiac death, or significant functional impairment in a subset of patients [8], [9].
Septal wall thickness, the presence of LGE, left atrial (LA) size, and left ventricular outflow tract (LVOT) obstruction are recognized risk factors for sudden cardiac death in patients with HCM [4], [5], [6], [7], [8], [9], [10]. However, predicting the phenotypic evolution of HCM is clinically challenging due to the reliance on cross-sectional studies [9]. These studies have highlighted an age-related manifestation of the HCM phenotype, typically appearing during the second decade of life, although in some cases it may emerge as late as the sixth or seventh decade [7], [8]. Historical longitudinal data from serial echocardiographic studies have documented significant myocardial growth, including a doubling of myocardial wall thickness in pediatric populations, while hypertrophy remains stable in adults [17]. More recent investigations have shown progressive LGE development in HCM [11]. Nonetheless, detailed information on the age-related progression of the HCM phenotype remains limited.
Cardiovascular magnetic resonance (CMR) imaging is the established gold standard for functional assessment in HCM, offering non-invasive quantification of fibrosis and providing critical insights for differentiating myocardial pathologies [12]. The exceptional reproducibility of quantitative CMR measurements makes it particularly valuable for tracking changes in LV volumes, function, and mass in patients with cardiomyopathies [13]. However, data on age-dependent myocardial mass development and its association with changes in interstitial fibrotic burden are still incomplete [11]. This study aims to evaluate the natural progression of morphological and functional changes in HCM by conducting serial CMR assessments in both adolescent and adult populations, utilizing state-of-the-art quantitative methods.
2. Methods
2.1. Study population and design
Patients with HCM, who had undergone a series of at least two CMR examinations in our institution between 2006 and 2016, with an interval of one year between the examinations, were enrolled for a retrospective analysis. HCM was diagnosed in patients with an otherwise unexplained myocardial wall thickness ≥ 15 mm, or ≥ 13 mm in first degree relatives of patients with HCM, according to European Society of Cardiology (ESC) guidelines [2]. The study population was initially referred to us for a cardiologic work-up of suspected HCM. Clinical data was obtained from patients’ records. Blood samples were drawn on each day of every CMR examination. N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and cardiac troponin T (TnT) were obtained using a commercially available, fully automated analyzer method based on a sandwich immunoassay (ELECSYS, Roche Diagnostics, Mannheim, Germany). The use of de-identified patient data for research purposes was approved by the institution’s ethics committee. The study procedures were in accordance to the Declaration of Helsinki.
2.2. CMR acquisition protocol and image analysis
CMR examinations were performed on a 1.5 Tesla clinical scanner (Achieva, Philips Healthcare, Best, The Netherlands) equipped with a 32-channel cardiac phased array receiver coil. Cine images were obtained using a breath-hold segmented-k-space balanced fast-field echo sequence (steady-state free precession, SSFP), employing retrospective ECG- or pulse oximetric gating with 35 phases per cardiac cycle. Typical CMR imaging parameters were the following: field of view 350 × 350 mm2, repetition time/echo time 2.8/1.4 ms, acquired voxel size 2.2 × 2.2 × 8 mm3, flip angle 60°, reconstructed voxel size 1.3 × 1.2 × 8 mm3. Slice thickness for LGE imaging was 6 to 8 mm. Data were analyzed by a single examiner blinded to the patients’ clinical status. Image analysis was performed with commercially available post-processing software (cvi42™ v5.5, Circle Cardiovascular Imaging, Calgary, Canada). Ventricular volumes and LV mass were derived from measurements in cine images from contiguous SSFP short axis stacks. Endocardial contours were detected by a semi-automatic threshold selection including trabecular tissue and papillary muscles in the LV mass. LV epicardial contours were traced manually in enddiastole for calculation of LV mass. Left atrial volumetry was acquired by area-length method. Absolute and relative LGE mass were measured using a 5-standard-deviation (SD) threshold in reference to visually unenhanced myocardium [14].
2.3. Statistical methods
Statistical analyses were performed with MedCalc™(MedCalc Software version 17, Ostend, Belgium). Normal distribution was assessed using the Kolmogorow-Smirnow-Test. Continuous variables were expressed as mean ± standard deviation. Group differences for normal distributed continuous variables were tested using the paired t-test. Continuous variables without normal distribution were tested for group differences using the nonparametric Wilcoxon test. The association of clinical, imaging and serological parameters was analyzed by methods of linear regression. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Study population
A total of 32 patients with HCM were included in the study, with a predominance of males (n = 26; 81 %) aged between 11 and 70 years (mean age: 44 ± 16 years). These patients underwent serial CMR examinations over an average follow-up period of 5.2 ± 2.4 years. A subset of these patients presented with left ventricular outflow tract (LVOT) obstruction (n = 3) or midventricular obstruction (n = 1). The peak pressure gradients in these obstructive cases were measured at 100 ± 16 mmHg via echocardiography. The distribution of myocardial hypertrophy was predominantly septal (n = 26), with some cases being diffuse (n = 5) or apical (n = 1). Baseline characteristics of the study population are detailed in Table 1.
Table 1.
Baseline characteristics of study population.
| Parameter (n = 32) | |
|---|---|
| Age [yrs] | 44 ± 16 (range, 11–70) |
| Follow Up [yrs] | 5.2 ± 2.4 (range, 0.8–9.1) |
| BMI [kg/m2] | 27 ± 4 (range, 19–37) |
| BSA [m2] | 2.0 ± 0.2 (range, 1.6–2.5) |
| Male [n] | 26 (81 %) |
| HOCM [n (%)] | 4 (13 %) |
| HOCM ΔpRest, [mmHg] | 39 ± 21 (range, 16–60) |
| HOCM ΔpValsalva, [mmHg] | 100 ± 16 (range, 72–110) |
Values are presented as mean ± standard deviation with minimum to maximum range.
BMI = body mass index; BSA = body surface area; HOCM = hypertrophic obstructive cardiomyopathy; ΔpRest/ΔpValsalva = peak intraventricular pressure gradient at rest / Valsalva maneuver measured by continuous wave Doppler echocardiography.
3.2. Phenotypic changes in HCM
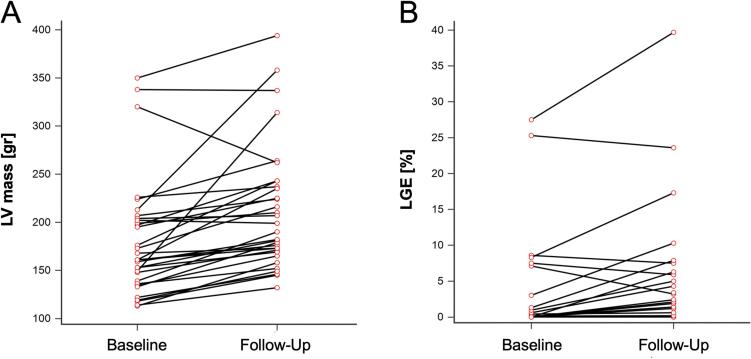
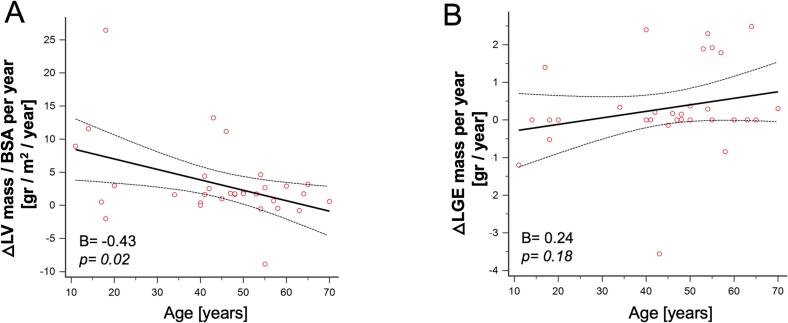
Over the follow-up period, the functional status of patients, as measured by the New York Heart Association (NYHA) class, showed a slight but statistically significant worsening (p = 0.02), though this did not coincide with significant alterations in TNT or NT-proBNP levels. Morphological and functional changes observed between baseline and follow-up CMR are summarized in Table 2. Most patients (78 %; n = 25) exhibited an increase in left ventricular (LV) mass, while the remaining patients (22 %; n = 7) either showed no change or a reduction, as depicted in Fig. 1A. The mean LV mass demonstrated a 12 % increase, rising from 194 ± 56 g at baseline to 217 ± 60 g at follow-up (p = 0.0001). This increase was paralleled by a significant rise in LV papillary muscle mass, from 4 ± 2 g to 5 ± 2 g (p = 0.03), and an increase in the wall thickness of both the septum (from 18 ± 4 mm to 19 ± 4 mm; p = 0.01) and posterior wall (from 8 ± 3 mm to 9 ± 3 mm; p = 0.02). The median LV mass gain rate in adults (mean age: 51 ± 9 years; range: 34–70) was 1.7 g per year/BSA (IQR, 0.6–2.7), which was significantly lower compared to the rate observed in adolescents (mean age: 16 ± 3 years; range: 11–20), where the gain was 6.0 g per year/BSA (IQR, 0.5–11.6) (p < 0.01). The age-related trend in LV mass gain is illustrated in Fig. 2A. Additionally, a significant increase in left atrial (LA) end-systolic volume was observed, from 109 ± 41 ml to 129 ± 40 ml (p < 0.01).
Table 2.
Changes of Morphology and Function over time in Hypertrophic Cardiomyopathy. The study cohort presented with a significant gain of left ventricular mass, fibrosis and left atrial volume after a mean follow up duration of 5.2 ± 2.4 years. These findings were accompanied by a decrease of left and right ventricular ejection fraction and an impact on NYHA functional class.
| Parameter (n = 32) | Baseline | Follow Up | Δchange | p |
|---|---|---|---|---|
| Heart rate [bpm] | 60 ± 8 | 62 ± 10 | n.s. | |
| NYHA class | 1 (1–1) | 1 (1–2) | + 0.3 | 0.02 |
| hs-TNT [ng/l] | 11 ± 8 | 19 ± 11 | n.s. | |
| NT-proBNP [ng/l] | 861 ± 1332 | 563 ± 720 | n.s. | |
| LV septal wall thickness [mm] | 18 ± 4 | 19 ± 4 | + 1.0 | 0.01 |
| LV posterior wall thickness [mm] | 8 ± 3 | 9 ± 3 | + 0.8 | 0.02 |
| LV Mass [gr] | 194 ± 56 | 217 ± 60 | + 23 | 0.0001 |
| LV papillary muscle mass [gr] | 4 ± 2 | 5 ± 2 | + 0.9 | 0.03 |
| LVEDV [ml] | 156 ± 36 | 152 ± 34 | n.s. | |
| LVESV [ml] | 46 ± 14 | 51 ± 16 | n.s. | |
| LVEF [%] | 70 ± 9 | 66 ± 9 | − 3.7 | 0.04 |
| MAPSE [mm] | 11 ± 3 | 12 ± 3 | n.s. | |
| LA volume [ml] | 109 ± 41 | 129 ± 40 | + 20 | 0.0001 |
| LGE mass [gr] | 6.0 ± 17 | 7.8 ± 18 | + 1.8 | 0.006 |
| RVEDV [ml] | 152 ± 42 | 146 ± 42 | n.s. | |
| RVESV [ml] | 47 ± 20 | 49 ± 21 | n.s. | |
| RVEF [%] | 70 ± 7 | 67 ± 9 | − 3.2 | 0.02 |
| TAPSE [mm] | 22 ± 5 | 22 ± 5 | n.s. |
Values are presented as mean ± standard deviation or median with interquartile range.
NYHA class = New York Heart Association functional class; hs-TNT = high sensitive Troponin T; NT-proBNP = N-terminal pro brain natriuretic peptide; LV = left ventricle; LVEDV/LVESV = left ventricular enddiastolic/ −systolic volume; LVEF = left ventricular ejection fraction; MAPSE = mitral annular plane systolic excursion; LA = left atrium; LGE = late gadolinium enhancement; RVEDV/RVESV = right ventricular enddiastolic/ −systolic volume; RVEF = right ventricular ejection fraction; TAPSE = tricuspid annular plane systolic excursion.
Fig. 1.
Development of LV mass (A) and LGE (B) between baseline and follow-up CMR examination.
Fig. 2.
Age-dependency of LV mass gain rate (A) and LGE mass gain rate (B).
3.3. Changes in LGE mass
LGE mass remained stable or decreased in 17 patients (53 %) (Fig. 1B). However, when analyzing the entire cohort, a significant increase in LGE mass was observed, from 6 ± 17 g at baseline to 8 ± 18 g at follow-up (p = 0.006). There was no statistically significant difference in the median LGE mass gain rate between adults (0.1 g per year, IQR 0–0.4) and adolescents (0 g per year, IQR −0.5–0) (p = 0.10). The relationship between LGE mass gain rate and age is depicted in Fig. 2B. Both LV ejection fraction (EF) and right ventricular ejection fraction (RVEF) showed a decline over the follow-up period, with LVEF decreasing from 70 ± 9 % to 66 ± 9 % (p = 0.04) and RVEF from 70 ± 7 % to 67 ± 9 % (p = 0.02).
3.4. Predictors of LV mass gain
As shown in Table 3, univariate linear regression analysis identified several clinical imaging and biochemical parameters at baseline CMR as predictors of LV mass gain rate, including age (B = -0.43; p = 0.02), BMI (B = -0.43; p = 0.01), LA diameter (B = -0.37; p = 0.04), and LGE mass (B = -0.39; p = 0.04). However, when these parameters were assessed in a multivariate regression model, only age (B = -0.44; p = 0.02) and LGE mass (B = -0.46; p = 0.02) remained significant predictors.
Table 3.
Predictors of annual left ventricular mass gain () in hypertrophic cardiomyopathy. Univariate linear regression analysis suggested age, BMI, LGE and LA diameter to be predictive for LV mass gain rate. A multivariate regression model revealed, age and LGE mass at the time of baseline CMR as the only independent predictors of LV mass gain rate (R2 = 0.48; p = 0.002).
|
A)Univariate linear regression analysis. | ||||
|---|---|---|---|---|
| Parameter (n = 32) | Beta | R2 | B | p |
| Age [yrs] | −0.43 | 0.18 | −0.16 | 0.02 |
| BMI [m2] | −0.45 | 0.20 | −0.61 | 0.01 |
| LV mass [gr] | −0.33 | 0.11 | −0.04 | 0.06 |
| LGE mass [gr] | −0.39 | 0.15 | −0.14 | 0.03 |
| LA diameter [mm] | −0.37 | 0.14 | −0.30 | 0.04 |
| NT-proBNP [ng/l] | 0.39 | 0.15 | 0.002 | 0.08 |
|
B)Multivariate linear regression model. | |||
|---|---|---|---|
| Parameter (n=32) | Beta | B | p |
| Age [yrs] | −0.44 | −0.17 | 0.02 |
| BMI [m2] | −0.26 | −0.35 | 0.20 |
| LGE mass [gr] | −0.46 | −0.14 | 0.02 |
| LA diameter [mm] | −0.15 | −0.11 | 0.45 |
Beta = standardized regression coefficient; B = non-standardized regression coefficient; CI: confidence interval.
BMI = body mass index; LV mass = left ventricular mass; LGE = late gadolinium enhancement; LA = left atrium; NT-proBNP = N-terminal pro brain natriuretic peptide.
3.5. Relationship between LV mass and LGE
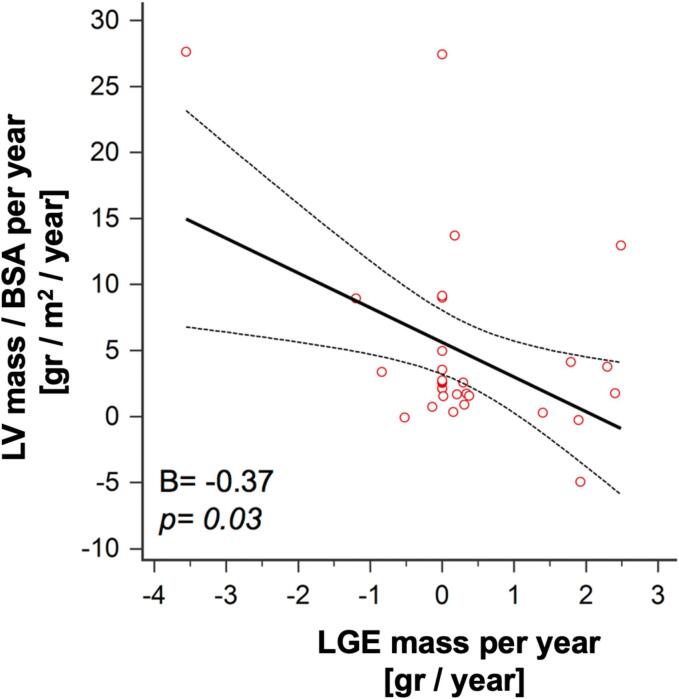
A significant positive correlation was found between LV mass and LGE mass (B = 0.55; p = 0.001). However, the rates of mass gain for LV and LGE were negatively correlated (−0.37; p = 0.03).(See Fig. 3). Fig. 4 illustrates the range of temporal changes observed in HCM, from cases of substantial LV mass gain without LGE (Fig. 4A) to cases where LV mass reduction coincided with increasing LGE mass (Fig. 4B).
Fig. 3.
Relation of LV mass gain rate and LGE mass gain rate.
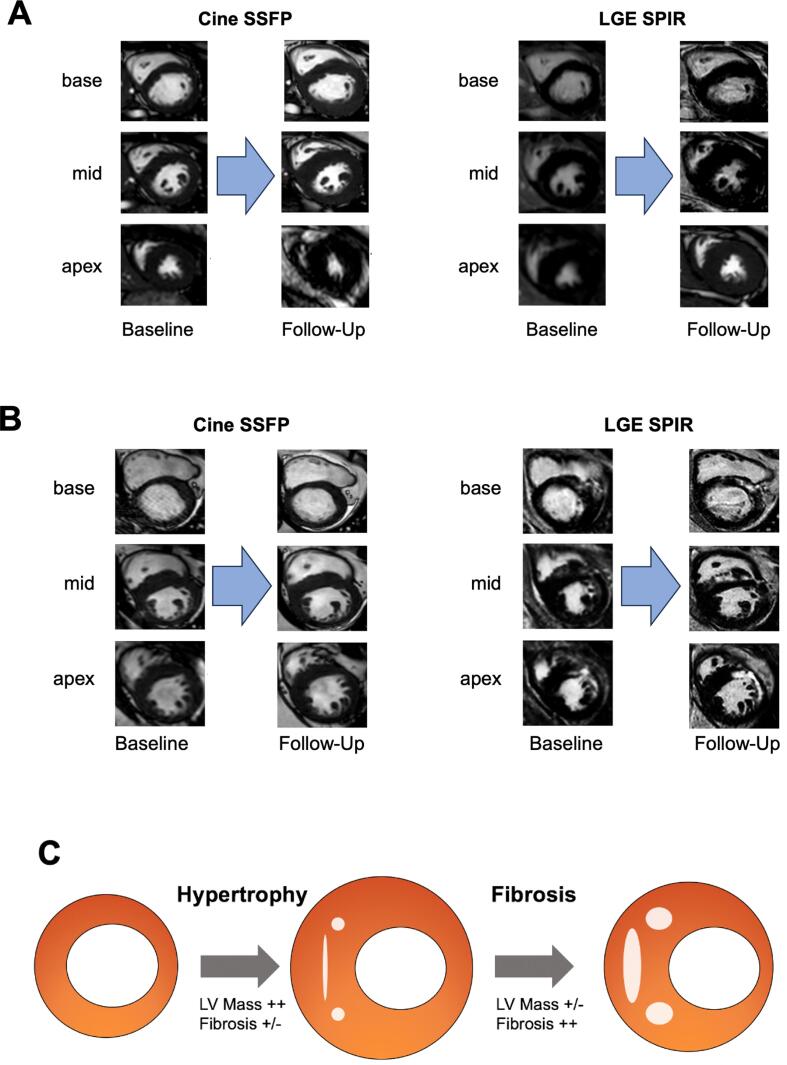
Fig. 4.
Spectrum of morphological Development in HCM. A 18-year-old female with non-obstructive HCM presenting with LV mass gain from 146 gr to 306 gr after an interval of 3.8 years without LGE. B 53-year-old male with non-obstructive HCM showed a loss of LV mass from 220 gr to 199 gr within 5.8 years whereas LGE mass increased in that time interval from 5.7 gr to 16.7 gr. C Illustration of the suggested spectrum of development in HCM. Predominantly young patients presented with extensive LV mass gain with low or non-evident LGE progression, whereas mainly older patients showed sustained LV mass with increasing LGE mass.
3.6. Subgroup analysis regarding functional status
A subgroup analysis based on NYHA class provided further insight into the relationship between functional status and the progression of both left ventricular (LV) mass and late gadolinium enhancement (LGE) mass in hypertrophic cardiomyopathy (HCM) patients. Our findings revealed that patients in Subgroup A2 (NYHA 2–4) exhibited a significantly greater increase in LGE mass compared to those in Subgroup A1 (NYHA 0–1), with LGE mass increasing by 4.6 g per year in Subgroup A2 versus 0.9 g per year in Subgroup A1 (p < 0.01). Conversely, LV mass increase was more pronounced in Subgroup A1, with a gain of 36.7 g per year compared to 22.2 g per year in Subgroup A2, although this difference was not statistically significant (p = 0.32).
3.7. Subgroup analysis regarding LGE mass progression
The subgroup analysis of patients with stable or decreasing LGE mass (Subgroup B1) compared to those with increasing LGE mass (Subgroup B2) provides important context for hypertrophic cardiomyopathy (HCM) progression, suggesting that fibrosis development might be age-related. Although the mean age of patients in Subgroup B1 was lower (42.0 years) than in Subgroup B2 (49.7 years), this difference did not reach statistical significance (p = 0.16), nor did gender distribution vary significantly between the groups. However, we observed a striking inverse relationship between LV mass and LGE mass changes. Patients in Subgroup B1, despite exhibiting stable or decreasing LGE mass, showed a greater increase in LV mass (31.6 g per year) compared to Subgroup B2 (20.6 g per year, p = 0.12).
4. Discussion
This study provides an in-depth analysis of the morphological and functional progression of HCM over time using CMR. The findings include (1) growth rates of left ventricular (LV) mass and LGE mass across adolescents and adults, (2) identification of age and LGE mass as independent predictors of LV mass increase, and (3) an observed inverse relationship between changes in LV mass and LGE mass. To our knowledge, this study is the first to evaluate these relationships in a longitudinal follow-up of HCM, employing a precise quantitative method that incorporates trabecular tissue and papillary muscles in the assessment of LV mass. Including these structures is essential for an accurate quantification of LV hypertrophy, because these structures contribute significantly to the overall myocardial mass and can impact the quantification of hypertrophy and fibrosis, particularly in HCM [21]. This technique therefore improves upon prior studies, which often excluded or inconsistently included trabecular tissue and papillary muscles, leading to variability in results; by incorporating these structures, our study offers a more holistic and anatomically accurate assessment.
The morphological changes observed in septal wall thickness, LV mass, LGE, and left atrial (LA) enlargement during the natural course of HCM have significant implications for individual risk stratification concerning life-threatening arrhythmic events [4], [5], [6], [7], [8], [9], [10]. Although mean LV and LGE masses increased over time, a subset of patients exhibited either a reduction or no change in these parameters, highlighting the heterogeneous nature of HCM. This variation underscores the need for future research to determine whether serial CMR examinations can enhance risk stratification. Additionally, the observed increase in LV mass may correlate with a heightened risk of LV outflow tract (LVOT) obstruction and subsequent congestive heart failure [18], [19]. Consequently, the prediction of disease progression, particularly the expected rate of change, should inform the clinical scheduling of follow-up visits and imaging studies.
The significant LV mass gain noted in adolescents aligns with previous findings from serial M−mode echocardiography studies [17]. However, a novel finding of this study is that LV mass increased in most cases, regardless of age. The underlying pathophysiology remains speculative, but factors such as fluctuating growth hormone levels during adolescence and gene-dosage effects have been implicated in the severity and timing of disease onset [20].
Our study further suggests that higher LV mass is associated with an increased burden of regional myocardial fibrosis [11]. Intriguingly, the rate of LV mass gain appears to inversely correlate with changes in myocardial fibrotic burden. This observation supports a spectrum of HCM progression, ranging from substantial LV mass growth without evident fibrosis to the development of extensive collagen deposits and a subsequent decline in LV mass (Fig. 4C). These findings reinforce a model of continuous stages in HCM progression, previously proposed in cross-sectional studies but not yet confirmed by longitudinal data [19]. According to this model, HCM may evolve from a non-hypertrophic or classic phenotype (Stage I and II) to adverse remodeling and overt dysfunction (Stage III and IV), where myocardial hypertrophy is predominant in the earlier stages, and progressive fibrosis and functional impairment characterize the later stages. Microvascular dysfunction and consequent ischemia are believed to play a crucial role in myocardial fibrosis pathogenesis [20]. Histological evidence of flow-limiting microvascular remodeling and perfusion deficits has been found in areas of myocardial fibrosis, independent of the extent of regional hypertrophy [15], [16]. Whether these findings represent triggers or consequences of fibrosis remains unclear. However, ischemic injury due to relative coronary insufficiency, followed by replacement fibrosis, aligns with our observations of an inverse progression between LV mass and fibrosis. These results contrast with a previous study that reported a positive correlation between LV mass and fibrotic burden [11]. The differences between these studies may be attributed to varying follow-up periods or the exclusion of papillary muscles and trabecular tissue in LV mass assessment in the earlier research.
The subgroup analysis regarding functional status underscores the distinct pathophysiological processes occurring in HCM: while early-stage patients (NYHA 0–1) may exhibit more pronounced LV hypertrophy, advanced-stage patients (NYHA 2–4) tend to develop greater myocardial fibrosis, as reflected by LGE accumulation. This aligns with previous studies, including the work by Todiere et al. 2012, which demonstrated that patients with worsened functional status tend to accumulate more myocardial fibrosis, as indicated by LGE [11]. The pattern highlights the dynamic relationship between hypertrophy and fibrosis in HCM progression. The inverse relationship between LV mass increase and LGE mass increase may suggest that, as the disease advances and fibrosis becomes more prominent, the capacity for further hypertrophic growth diminishes. Our findings are consistent with previous literature suggesting that LGE serves as a critical marker of adverse myocardial remodeling and is associated with worse clinical outcomes. Thus, both LV mass and LGE should be considered important parameters in the clinical assessment of HCM patients, offering valuable prognostic information to guide treatment and monitoring strategies. These observations emphasize the importance of integrating serial imaging studies, particularly CMR, to monitor changes in LV mass and LGE over time.
The findings regarding LGE mass progression suggest that in younger patients, hypertrophic remodeling may occur without a concurrent increase in fibrosis, as measured by LGE. This challenges the notion that fibrosis and hypertrophy progress in parallel and highlights that the relationship between these two processes may vary depending on patient age and disease stage. The observation that significant LV mass growth can occur in the absence of substantial LGE accumulation suggests that fibrosis may not always develop linearly with hypertrophy, particularly in the early stages of the disease [15], [16]. This adds nuance to our model of HCM progression, underscoring the complex and non-linear interplay between hypertrophy and fibrosis [20]. As such, our findings emphasize the importance of considering both LV mass and LGE dynamics over time in the clinical assessment of HCM, as younger patients may experience marked hypertrophic growth without a corresponding increase in fibrosis, which may influence their long-term prognosis and management [19].
This study has some limitations. (1) The study cohort may be subject to selection bias, as most patients were referred for diagnostic evaluation of symptomatic HCM, potentially leading to an overrepresentation of severe disease cases, particularly among younger patients. (2) The small cohort size may limit the statistical power to detect other significant relationships between imaging-derived parameters and serum or clinical biomarkers. (3) We did not evaluate changes using recently introduced cardiac mapping techniques, including extracellular volume measurements, to maintain a short examination time and ensure patient compliance. Although they could have provided additional insights into diffuse myocardial structural changes, particularly related to diffuse fibrosis, maintaining a short protocol was critical to ensure the feasibility of longitudinal patient follow-up. (4) The small sample size and limited observation window prevent us from drawing statistically significant conclusions about the association between left ventricular (LV) mass gain, late gadolinium enhancement (LGE) mass increase, and mortality in hypertrophic cardiomyopathy (HCM). Larger studies, such as the one conducted by Green et al. (2012) are necessary to establish robust mortality data in this population [10].
5. Conclusions
In patients with HCM, the phenotypic changes in adolescents are predominantly characterized by significant myocardial mass gain, whereas older patients exhibit more adverse remodeling, with increased myocardial fibrosis and, in some cases, a reduction in myocardial mass. These data suggest an age-related inverse relationship between myocardial mass gain and the progression of replacement fibrosis. They were made possible by the use of advanced techniques for LV mass assessment, which allowed us to gain novel insights that, in part, challenge previous observations. We recommend including the papillary muscles in future assessments, as this approach proved essential for obtaining these innovative findings.
Consent for publication
Documentation of consent was waived for this retrospective study, which utilized de-identified patient data.
Ethics approval and consent to participate
The analysis of de-identified patient data was approved by the local institution’s ethics committee. Documentation of consent was waived for this retrospective study.
CRediT authorship contribution statement
Sebastian M. Haberkorn: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Mukaram Rana: Data curation. Vitali Koch: Visualization, Validation. Simon Martin: Visualization, Validation. Thomas Vogl: Writing – review & editing, Validation. David M. Leistner: Writing – review & editing, Validation. Marco M. Ochs: Writing – review & editing, Supervision, Methodology, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank our technologists for their excellent support.
References
- 1.Maron M.S., Maron B.J., Harrigan C., Buros J., Gibson C.M., Olivotto I., et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2009;54:220–228. doi: 10.1016/j.jacc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Rickers C., Wilke N.M., Jerosch-Herold M., Casey S.A., Panse P., Panse N., et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. Am. Heart Association, Inc. 2005;112:855–861. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JM, Villareal RP, Hariharan R, Massumi A, Muthupillai R, Flamm SD. Magnetic resonance imaging of myocardial fibrosis in hypertrophic cardiomyopathy. 2002;29:176–80. [PMC free article] [PubMed]
- 4.Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert E-M, et al. Myocardial Scar Visualized by Cardiovascular Magnetic Resonance Imaging Predicts Major Adverse Events in Patients With Hypertrophic Cardiomyopathy. Journal of the American College of Cardiology [Internet]. Elsevier Inc; 2010;56:875–87. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0735109710019121. [DOI] [PubMed]
- 5.Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, et al. Clinical Profile and Significance of Delayed Enhancement in Hypertrophic CardiomyopathyCLINICAL PERSPECTIVE. Circulation: Heart Failure. American Heart Association, Inc; 2008;1:184–91. [DOI] [PubMed]
- 6.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. Journal of the American College of Cardiology [Internet]. Elsevier Inc; 2010;56:867–74. Available from: doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed]
- 7.Spirito P., Bellone P., Harris K.M., Bernabò P., Bruzzi P., Maron B.J. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. Massachusetts Med. Soc. 2009;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 8.Elliott P.M., Gimeno Blanes J.R., Mahon N.G., Poloniecki J.D., Mckenna W.J. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–424. doi: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 9.Olivotto I., Maron M.S., Autore C., Lesser J.R., Rega L., Casolo G., et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2008;52:559–566. doi: 10.1016/j.jacc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Green J.J., Berger J.S., Kramer C.M., Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. Img. 2012;5:370–377. doi: 10.1016/j.jcmg.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Todiere G., Aquaro G.D., Piaggi P., Formisano F., Barison A., Masci P.G., et al. Progression of myocardial fibrosis assessed with cardiac magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2012;60:922–929. doi: 10.1016/j.jacc.2012.03.076. [DOI] [PubMed] [Google Scholar]
- 12.Assomull R.G., Lyne J.C., Keenan N., Gulati A., Bunce N.H., Davies S.W., et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur. Heart J. 2007;28:1242–1249. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 13.Strohm O, Schulz-Menger J, Pilz B, Osterziel K-J, Dietz R, Friedrich MG. Measurement of left ventricular dimensions and function in patients with dilated cardiomyopathy. Journal of Magnetic Resonance Imaging. John Wiley & Sons, Inc; 2001;13:367–71. [DOI] [PubMed]
- 14.Moravsky G., Ofek E., Rakowski H., Butany J., Williams L., Ralph-Edwards A., et al. Myocardial fibrosis in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. Img. 2013;6:587–596. doi: 10.1016/j.jcmg.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Galati G, Leone O, Pasquale F, Olivotto I, Biagini E, Grigioni F, et al. Histological and Histometric Characterization of Myocardial Fibrosis in End-Stage Hypertrophic Cardiomyopathy: A Clinical-Pathological Study of 30 Explanted Hearts. Circulation: Heart Failure. 2016;9:e003090. [DOI] [PubMed]
- 16.Mohammed S.F., Hussain S., Mirzoyev S.A., Edwards W.D., Maleszewski J.J., Redfield M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haberka M., Starzak M., Smolka G., Wojakowski W., Gąsior Z. Echocardiography and cardiac magnetic resonance in the assessment of left-ventricle remodeling: differences implying clinical decision. J. Clin. Med. 2024;13(6):1620. doi: 10.3390/jcm13061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koren M.J., Devereux R.B., Casale P.N., Savage D.D., Laragh J.H. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann. Intern. Med. 1991;5:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 19.Drazner M.H., Rame J.E., Marino E.K., Gottdiener J.S., Kitzman D.W., Gardin J.M., et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2004;43(12):2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 20.Marian A.J. Molecular genetic basis of hypertrophic cardiomyopathy. Circ. Res. 2021;128(10):1533–1553. doi: 10.1161/CIRCRESAHA.121.318346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Captur G., Muthurangu V., Cook C., Flett A.S., Wilson R., Barison A., et al. Quantification of left ventricular trabeculae using fractal analysis. J. Cardiovasc. Magn. Reson. 2013;15(1):36. doi: 10.1186/1532-429X-15-36. [DOI] [PMC free article] [PubMed] [Google Scholar]