Abstract
Emerging evidence suggests a broader spectrum of celiac disease (CeD) system involvement, including neurological manifestations. We aimed to conduct a systematic review and meta-analysis of the available evidence from studies assessing the association of cognitive impairment and insomnia with CeD. A total of 259 participants with CeD were included in the studies investigating insomnia and 179 were included in studies investigating cognitive impairment. The overall pooled odds ratio for insomnia in patients with CeD was 1.83 (95% confidence interval, 1.38 to 2.42; I2=0.00%). The present study provides valuable insights into the available evidence from studies investigating cognitive impairment in patients with CeD and our systematic review and meta-analysis revealed a significant association between CeD and insomnia.
Keywords: Celiac disease, Insomnia, Cognitive impairment
INTRODUCTION
Celiac disease (CeD) is a multifaceted autoimmune disorder triggered by the ingestion of gluten-containing cereals such as wheat, barley, and rye that has traditionally been associated with gastrointestinal manifestations.1 However, increasing research has illuminated the intricate relationship between CeD and neurocognitive symptoms, expanding our understanding of the disease beyond its gastrointestinal confines. Recent studies have highlighted the prevalence of neurocognitive symptoms, including cognitive impairment and insomnia, in CeD.2
Similarly, insomnia and neurocognitive impairments tend to be prevalent in a multitude of other autoimmune diseases that can lead to a wide array of psychiatric symptoms, such as mood disorders and cognitive deficits.3 The overlap between neurocognitive function in autoimmune diseases and CeD underscores the interplay between autoimmune diseases and the central nervous system, as both have been associated with a spectrum of neurocognitive symptoms that provide diagnostic challenges.
Traditionally, thought to be limited to Northern and Western Europe, there has been an increase in CeD incidence worldwide, potentially leading to delayed diagnoses. We aimed to explore the evolving landscape of CeD with a specific focus on neurocognitive dimensions, addressing patterns of this autoimmune condition on cognitive function and sleep patterns.
CASE DESCRIPTION
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Multiple databases including PubMed, MEDLINE (OVID), Cochrane, Embase, Scopus, PsycINFO, and Web of Science were searched from inception to November 2023. The protocol of the study was registered under the registration number: INPLASY202410063. We included all published studies that reported the presence of insomnia or any type of cognitive impairment in patients with CeD diagnosis. The search strategy was tailored by a medical librarian (Supplementary Material 1). Two independent reviewers (R.B. and A.G.) extracted data. Disagreements were resolved by a third author (D.A.N.). Pooled prevalence and pooled odds ratio (OR) were calculated after the Freeman-Tukey Double Arcsine Transformation. Heterogeneity was assessed using the Higgins I2 index.
Among 1,775 articles identified, 38 studies were selected for full-text review and seven studies were eligible for inclusion in the final analysis, with perfect agreement between investigators (κ=1.0) (Supplementary Fig. 1).4-10 A total of 259 patients with CeD were included in the studies investigating insomnia and 179 were included in the studies for cognitive impairment, with a predominance of female participants (72.7% among the cognitive impairment studies and 59.63% among the insomnia studies). Most studies included patients with biopsy proven CeD and were conducted in an outpatient setting. Among the studies investigating insomnia in CeD, three utilized a self-reported questionnaire and one the Pittsburgh Sleep Quality Index. Among the studies investigating cognitive impairment, two studies investigated mild cognitive impairment with standardized questionnaires (Subtle Cognitive Impairment Test, Addenbrooke Cognitive Examination-Revised, and INECO Frontal Screening), one study investigated lack of concentration with the Adult ADHD Self-Report Scale Symptoms Checklist and one investigated reaction time using a set of standardized tasks. Additional characteristics of the studies included can be seen in Table 1. The quality of the studies was assessed using the Newcastle-Ottawa scale and a good overall quality rating was registered for the majority of the studies.
Table 1.
Characteristics of the Included Studies
| First author (year) | Type of sleep disturbance | Diagnosis of insomnia | Diagnosis of CeD | No. of CeD | Insomnia in CeD, No. (%) | No. of controls | Insomnia in controls, No. (%) | Main results of the study |
|---|---|---|---|---|---|---|---|---|
| Insomnia in CeD | ||||||||
| Croall (2020)4 | Insomnia | Self-reported to question: "trouble falling or staying asleep?" | Hospital statistics (records of ICD-10) | 104 | 62 (59.7) | 195 | 93 (47.6) | Impairment in reaction time and worsened mental health in celiac patients compared to controls |
| Wang (2022)7 | Insomnia | Self-reported sleep disorders | Serological and/or histological diagnosis | 50 | 23 (46.0) | 2,834 | 771 (27.2) | Compared to controls with other GI complaints, patients with CeD had increased prevalence of sleep disorders |
| Zylberberg (2017)9 | Insomnia | Questionnaire: length of sleep, trouble sleeping, sleep disorders | Chart review | 75 | 28 (37.3) | 15,809 | 4,327 (27.4) | No increased risk of depression and insomnia in participants with CeD compared with a group of nonaffected controls |
| Zingone (2010)8 | Insomnia | Pittsburgh Sleep Quality Index (PSQI) | Serological and histological diagnosis | 30 | 15 (50.0) | 30 | 7 (23.3) | Celiac patients exhibited higher PSQI scores than healthy volunteers |
| Author (year) | Type of cognitive impairment | Diagnosis of cognitive impairment | Diagnosis of CeD | No. of CeD | Cognitive impairment in CeD, No. (%) | No. of controls | Cognitive impairment in controls, No. (%) | Main results of the study |
|---|---|---|---|---|---|---|---|---|
| Cognitive impairment in CeD | ||||||||
| Croall (2020)4 | Delayed reaction time | Reaction time (mean response time across 12 rounds of a snap-like card game) | Hospital statistics (records of ICD-10) | 104 | NA (only listed the mean time of group) | NA | NA | Impairment in reaction time and worsened mental health in celiac patients compared to controls |
| Lichtwark (2014)5 | Subtle cognitive impairment | Subtle Cognitive Impairment Test | Duodenal biopsy | 11 | 3 (27.3) | NA | NA | Adherence to gluten-free diet in newly diagnosed CeD leads to improved cognitive performance |
| Longarini (2019)6 | Mild cognitive impairment | Addenbrooke Cognitive Examination-Revised | Serological and histological diagnosis | 33 | 3 (9.1) | 17 | 0 | Celiac patients had similar cognitive performance and anxiety |
| Kristensen (2019)8 | Lack of concentration | Adult ADHD Self-Report Scale Symptoms Checklist (ASRS) | Serological and histological diagnosis | 31 | 11 (35.4) | 60 | 5 (8.3) | Celiac disease patients presented more inattention symptoms compare to controls and differences disappeared on a gluten-free diet |
CeD, celiac disease; ICD-10, International Classification of Diseases, tenth revision; GI, gastrointestinal; NA, not available.
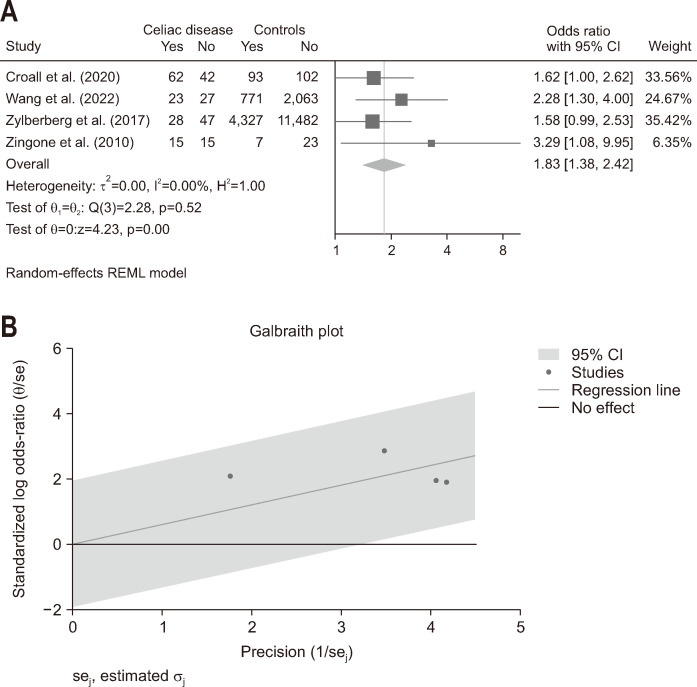
The overall pooled OR for insomnia in patients with CeD was 1.83 (95% confidence interval, 1.38 to 2.42), I2=0.00% with low heterogeneity among the studies (Fig. 1). Notably, a pooled OR could not be calculated for the cognitive impairment due to lack of controls.
Fig. 1.
Association of insomnia and celiac disease. (A) Forest plot of odd ratio for insomnia, comparing celiac disease patients and healthy controls. (B) Galbraith plot assessing the heterogeneity of the studies. CI, confidence interval.
DISCUSSION
Our study findings notably confirmed a consistent association between CeD and insomnia, demonstrating minimal variation, with a pooled OR of 1.83. This reinforces previous research suggesting a potential link between CeD and insomnia, as individuals diagnosed with CeD have been observed to exhibit a higher prevalence of insomnia in several studies.8,9 On a study comparing sleep quality in patients presenting to a gastroenterology clinic, those with CeD ranked second highest, with 61% reporting poor sleep quality and 35% experiencing clinical insomnia.11 Additionally, research by Mårild et al.11 highlights an increased risk of poor sleep among CeD patients, characterized by the repeated use of prescribed hypnotics. The mechanisms underlying this association remain a subject of investigation, with current hypotheses suggesting that the immune system's response to gluten, gut-brain axis, or nutritional deficiencies might play a role in disrupting sleep patterns.9 Furthermore, there is limited data on the sleep quality of CeD patients compared to other gastrointestinal disorders such as irritable bowel syndrome, inflammatory bowel disease, and gastroesophageal reflux disease.12,13
In addition to the proposed mechanisms of gut-brain axis dysregulation above, several comorbid symptoms such as acid reflux, anxiety, depression, and restless leg syndrome are thought to contribute to the sleep disturbances seen in patients with CeD.9 Sleep disorders are a clinical diagnosis and fall along a spectrum from difficulty falling asleep, staying asleep or having quality sleep and several questionnaires are utilized to assist with the diagnosis. Zingone et al.8 utilized the Pittsburgh Sleep Quality Index questionnaire to demonstrate that patients with CeD, treated or untreated, despite having similar reported sleep duration to healthy controls experience subjectively lower sleep quality, and increased sleep disturbances, increased use of sleep medications and increased daytime dysfunction regardless of gastrointestinal symptom burden suggesting disturbances may contribute to the pathophysiology of CeD.
The cognitive improvements observed on a gluten-free diet, as indicated by Lichtwark et al.,5 hint at potential biological or molecular explanations. Gluten-triggered inflammatory responses may influence neurotransmitter balance, contributing to cognitive manifestations like brain fog. Previous research has established that anti-gliadin antibodies, known to react with brain blood vessels, contribute to an increased risk of dementia in individuals with CeD. Additionally, anti-gliadin antibodies exhibit cross-reactivity with neuronal synapsin 1 leading to altered neurotransmission, suggesting potential disruption to brain functions beyond its impact on vasculature.14 Also, cognitive impairment, often termed “brain fog,” may also be due to elevated cytokine levels, which induce systemic inflammation. These cytokines can breach the blood-brain barrier, facilitating leukocyte entry into the brain, initiating neuronal inflammation, impediments in neural signaling, according to Larochelle et al.15 Further elucidation of these potential biological mechanisms is crucial for guiding targeted interventions in individuals with CeD experiencing cognitive symptoms.
Studies showing improvement in cognitive impairment in patients with CeD following a gluten-free diet further emphasize that understanding the implications of the interrelation between sleep disorders and cognitive impairment with CeD is pivotal.6 Healthcare practitioners are advised to consider screening for sleep disorders and cognitive impairment in individuals diagnosed with CeD. This facilitates the early detection of insomnia-related issues and emphasizes the necessity for ongoing research to inform targeted interventions and improve quality of life of individuals with CeD.
One potential limitation of this study is the absence of a pooled OR calculation for cognitive impairment. This limitation arose due to the absence of control groups in the included studies, which prevented a comparative analysis between CeD patients and non-CeD individuals in terms of cognitive function. To address this gap, future research should prioritize including control groups to enable comparative analyses for cognitive impairment in CeD patients. Additionally, employing standardized assessment tools specifically designed to evaluate cognitive function would enhance the reliability and comparability of findings across studies. Furthermore, longitudinal studies could offer insights into the progression of cognitive impairment in CeD patients over time, shedding light on potential mechanisms and interventions for mitigating cognitive dysfunction in this population.
While a significant component of CeD is its gastrointestinal manifestations, increasing data supports neurocognitive symptoms including neurocognitive impairments and insomnia. Further research is needed to further delineate the mechanism by which CeD leads to neurocognitive and sleep disturbances and identify effective treatment options.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl240063.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study conception and design: R.B., S.K., A.G., D.A.N. Data acquisition: D.I.V., R.B., A.G., E.E.M.S., Y.O.A. Data analysis and interpretation: D.A.N., R.B., S.K., D.I.V., M.R. Drafting of the manuscript: R.B., M.R., D.I.V., A.G., E.E.M.S., S.K., Y.O.A. All authors reviewed the results and approved the final version of the manuscript.
REFERENCES
- 1.Catassi C, Verdu EF, Bai JC, Lionetti E. Coeliac disease. Lancet. 2022;399:2413–2426. doi: 10.1016/S0140-6736(22)00794-2. [DOI] [PubMed] [Google Scholar]
- 2.Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54:8–21. doi: 10.1097/MCG.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss DB, Dyrud J, House RM, Beresford TP. Psychiatric manifestations of autoimmune disorders. Curr Treat Options Neurol. 2005;7:413–417. doi: 10.1007/s11940-005-0033-z. [DOI] [PubMed] [Google Scholar]
- 4.Croall ID, Sanders DS, Hadjivassiliou M, Hoggard N. Cognitive deficit and white matter changes in persons with celiac disease: a population-based study. Gastroenterology. 2020;158:2112–2122. doi: 10.1053/j.gastro.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Lichtwark IT, Newnham ED, Robinson SR, et al. Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity. Aliment Pharmacol Ther. 2014;40:160–170. doi: 10.1111/apt.12809. [DOI] [PubMed] [Google Scholar]
- 6.Longarini G, Richly P, Temprano MP, et al. A prospective study on cognitive impairment in middle-aged adults with newly diagnosed celiac disease. J Clin Gastroenterol. 2019;53:290–294. doi: 10.1097/MCG.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Kong WJ, Feng Y, et al. Epidemiological, clinical, and histological presentation of celiac disease in Northwest China. World J Gastroenterol. 2022;28:1272–1283. doi: 10.3748/wjg.v28.i12.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zingone F, Siniscalchi M, Capone P, et al. The quality of sleep in patients with coeliac disease. Aliment Pharmacol Ther. 2010;32:1031–1036. doi: 10.1111/j.1365-2036.2010.04432.x. [DOI] [PubMed] [Google Scholar]
- 9.Zylberberg HM, Demmer RT, Murray JA, Green PH, Lebwohl B. Depression and insomnia among individuals with celiac disease or on a gluten-free diet in the USA: results from a national survey. Eur J Gastroenterol Hepatol. 2017;29:1091–1096. doi: 10.1097/MEG.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristensen VA, Valeur J, Brackmann S, Jahnsen J, Brunborg C, Tveito K. Attention deficit and hyperactivity disorder symptoms respond to gluten-free diet in patients with coeliac disease. Scand J Gastroenterol. 2019;54:571–576. doi: 10.1080/00365521.2019.1608467. [DOI] [PubMed] [Google Scholar]
- 11.Mårild K, Morgenthaler TI, Somers VK, Kotagal S, Murray JA, Ludvigsson JF. Increased use of hypnotics in individuals with celiac disease: a nationwide case-control study. BMC Gastroenterol. 2015;15:10. doi: 10.1186/s12876-015-0236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22:1748–1753. doi: 10.1111/j.1440-1746.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 13.Krystal AD, Prather AA, Ashbrook LH. The assessment and management of insomnia: an update. World Psychiatry. 2019;18:337–352. doi: 10.1002/wps.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanza G, Bella R, Cantone M, Pennisi G, Ferri R, Pennisi M. Cognitive impairment and celiac disease: is transcranial magnetic stimulation a trait d'union between gut and brain? Int J Mol Sci. 2018;19:2243. doi: 10.3390/ijms19082243.e6ba42c1bb13416ab5329bef562d7c87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770–3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.