Abstract
The intestine and liver share a unique regenerative property that sets them apart from other mammalian visceral organs. The intestinal epithelium exhibits rapid renewal, making it one of the fastest renewing tissues in humans. Under physiological conditions, intestinal stem cells within each intestinal crypt continuously differentiate into the different types of intestinal epithelial cells to maintain intestinal homeostasis. However, when exposed to tissue damage or stressful conditions such as inflammation, intestinal epithelial cells in the gastrointestinal tract exhibit plasticity, allowing fully differentiated cells to regain their stem cell properties. Likewise, hepatic epithelial cells possess a remarkable regenerative capacity to restore lost liver mass through proliferation-mediated liver regeneration. When the proliferation-mediated regenerative capacity is impaired, hepatocytes and biliary epithelial cells (BECs) can undergo plasticity-mediated regeneration and replenish each other. The transition of mammalian liver progenitor cells to hepatocytes/BECs can be observed under tightly controlled experimental conditions such as severe hepatocyte injury accompanied by the loss of regenerative capacity. In this review, we will discuss the mechanism by which cellular plasticity contributes to the regeneration process and the potential therapeutic implications of understanding and harnessing cellular plasticity in the gut and liver.
Keywords: Regeneration, Intestines, Liver, Cellular plasticity
INTRODUCTION
During embryogenesis, various cell types are sequentially differentiated from parent cells to develop functional organs and tissues. However, this cell potency is lost during development, leaving only a limited capacity for regeneration through the self-replication of fully differentiated parenchymal cells within the respective tissues. However, there are anomalies to this custom process, particularly within the endoderm-derived gastrointestinal tissues, such as the liver, pancreas, and intestine, which possess remarkable regenerative capacity. This ability is achieved not only by self-replication but also by cellular dedifferentiation-to-differentiation, a manifestation of cellular plasticity.
Intestinal epithelial cells (IECs) have a unique capacity for renewal in adult organs, with a rapid turnover rate. It is intriguing to note that within each intestinal crypt, stem cells exist and keep differentiating into enteroendocrine cells, goblet cells, and enterocytes, thus replenishing the diverse types of IECs every 4 to 5 days.1 Furthermore, the pancreas, which is intricately intertwined with the gastrointestinal system, exhibits cellular plasticity within its islets to maintain its functionality in different environments, particularly in maintaining glucose homeostasis. Notably, studies have uncovered cases of β-cell dedifferentiation or transdifferentiation from α-cells to β-cells, β-cells to α-cells, and δ-cells to β-cells in various animal models and human ex vivo models, especially under chronic stress conditions.2
Alongside the pancreas, the liver has an extraordinary ability to regenerate. For instance, after surgical resection of approximately 70% of the liver, the liver can restore total lost liver mass. This restoration occurs through the replication of hepatic epithelial cells, specifically hepatocytes (HCs), and biliary epithelial cells (BECs): proliferation-mediated regeneration. Furthermore, in addition to their primary regenerative potential, HCs and BECs, which are derived from a common bipotent lineage known as hepatoblasts during development, substitute the other cell type through interconverting as required to maintain hepatic functions in response to chronic injury: plasticity-mediated regeneration. Nevertheless, it is crucial to acknowledge that this phenomenon is only evident in exceptional situations, such as the deliberate removal of all proliferative cells, serving as a last-ditch effort before complete liver failure, thereby signifying the presence of artificial pathophysiological conditions. In this review, we summarize an updated understanding of the pathophysiology and clinical significance of cellular plasticity-mediated tissue regeneration in the gut and liver.
CELLULAR PLASTICITY-MEDIATED GUT REGENERATION
1. Composition of the intestinal epithelium
The small intestine and colon are major components of the digestive system. In particular, the small intestine plays a crucial role in nutrient absorption, mucosal barrier function, hormone secretion, and the immune system.3-5 The intestinal epithelium, lamina propria, and muscularis mucosa make up the mucosa, the frontline of the gastrointestinal tract, which constitutes the physical and chemical barrier. The intestinal mucosa defends the body from environmental stimuli, such as diet and microbiota. The gastrointestinal tract is the largest compartment of the immune system and the most highly regenerative organ in the body.
The intestinal epithelium is composed of a single layer of IEC. The absorptive epithelium of the small intestine is based on the crypt-villus axis, whereas the colonic epithelium contains only crypts without villi.6 The crypts contain stem cells, Paneth cells, and transit-amplifying cells (progenitor cells). Intestinal stem cells (ISCs) are located at the base of the crypts and are closely related to Paneth cells, which secrete defensins. After dividing the ISCs, the generated transit-amplifying cells continue to divide, differentiate, and migrate towards the villus, where these cells are eventually shed into the lumen.7 The villi of the small intestine contain enterocytes, goblet cells, and enteroendocrine cells. Nutrient-absorbing enterocytes account for more than 90% of the epithelial cells in the small intestine. Goblet cells secrete mucin to form a mucus layer. Besides, hormone-secreting enteroendocrine cells, tuft cells, and other cells are present in the intestinal epithelium.8 The colon has a flat surface epithelium and comprises absorptive colonocytes and goblet cells.6 Notably, Paneth cells are absent in the colon. These diverse cell types interact closely to preserve intestinal homeostasis and maintain intestinal integrity for host defense.
Recent advancements in technology using single-cell RNA sequencing (scRNA-seq) have helped refine the characterization of intestinal cells.9-11 By identifying the transcripts in individual cells, which characterize the differences or similarities in gene expression,12 scRNA-seq allows the assessment of the biological properties of each cell population.
2. Intestinal homeostasis and intestinal cellular plasticity
The intestinal epithelium is constantly exposed to pathogenic environments, such as diet, microbiota, and other harmful agents. To maintain intestinal integrity and homeostasis, rapid and continuous regeneration through the renewal of epithelial cells is essential. The epithelium of the small intestine is composed of repetitive crypt-villus units that are continuously renewed every 3 to 5 days to maintain intestinal homeostasis.7,13
ISCs self-renew by dividing and differentiating into specialized intestinal cells. Therefore, ISCs are critical for normal tissue homeostasis and injury-induced tissue regeneration. In addition to epithelial proliferation, programmed cell death via apoptosis is tightly regulated to maintain intestinal integrity.
There are two distinct ISC populations in the crypt: the principal population comprises crypt base columnar cells marked with leucine-rich repeat-containing G-protein-coupled receptor 5-positive (LGR5+) and the second population comprises B-cell-specific Moloney murine leukemia virus insertion site 1 (Bmi 1) cells, at the +4 position, which are quiescent stem cells.14,15 LGR5+ ISCs are long-lived, multipotent stem cells that are primarily responsible for the renewal of epithelial cells.13,16,17 The differentiation processes from LGR5+ ISCs to the different kinds of epithelial cells are tightly controlled to maintain intestinal homeostasis.
The ISC niche, a stromal microenvironment that supports stem cells, is a critical component for the regulation of the behavior of ISCs not only during intestinal homeostasis but also during tissue repair.18 The intestinal niche produces several ligands and soluble cytokines, chemokines, and growth factors that are critical for the self-renewal and proliferation of ISCs.
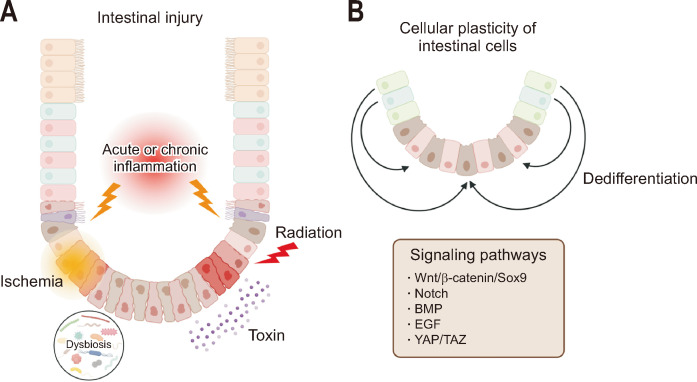
The intestinal epithelium is easily affected by acute injuries, such as ischemia, infection, and radiation and chronic disorders, such as chronic inflammatory bowel disease (IBD). In cases of intestinal epithelial damage and loss of LGR5+ ISCs, active regenerative responses occur to restore the stem cell compartment, and quiescent ISCs are selectively mobilized to repopulate the crypt.13,14,19 In addition to quiescent ISCs, diverse differentiated cells, including Paneth and enteroendocrine cells, are also involved in these regeneration processes. Notably, several different cells can acquire stem cell peculiarity to restore the epithelial cells and can dedifferentiate into LGR5+ ISCs to replenish the stem cell proliferation through a niche-induced conversion.20-22 This process of dedifferentiation of IECs to ISCs is called as intestinal cellular plasticity (Fig. 1). These stem cells exhibit fetal-like properties and contribute to wound repair and tissue restoration.22
Fig. 1.
Cellular plasticity of the intestinal epithelium in response to various injuries. (A) The intestinal epithelium can be exposed to many types of intestinal injury, including inflammation, dysbiosis, ischemia, radiation and toxins, resulting in the loss of intestinal stem cells. (B) Different cells can acquire stem cell capacity (dedifferentiation) through several pathways to repair injured tissue. Wnt, wingless-related integration site; SOX9, SRY (sex-determining region Y protein)-box transcription factor 9; BMP, bone morphogenetic protein; EGF, epidermal growth factor; YAP, yes-associated protein; TAZ, transcriptional coactivator with PDZ-binding motif. The figure was created using BioRender.com.
The mobilization of different cell populations is likely to occur in an injury-dependent manner.21 Tissue injury-induced inflammation may play a key role in the development of cellular plasticity. However, it is unclear whether intestinal cellular plasticity is a transient process that occurs over a limited time period to compensate for and replenish stem cells, or whether it can persist for a long-term period.
3. Cellular plasticity regulation
Several signaling pathways are involved in the maintenance of ISCs, including the wingless-related integration site (Wnt), Notch, bone morphogenetic protein, and epidermal growth factor (EGF), which are produced by the ISC niche.18 These signaling pathways are tightly networked to maintain the self-renewal of ISCs and differentiation during various conditions, such as homeostasis and repair processes. In particular, the Wnt signaling pathway is the main driving force and essential to maintain epithelial homeostasis through the effects on ISCs.23-25 Wnt signaling is closely related with the accumulation and transcription of β-catenin (Wnt/β-catenin). This pathway is a key regulator of the proliferation of ISCs and mucosal renewal. Wnt ligands are expressed by Paneth cells and other mesenchymal cells, such as fibroblasts. Inhibition of Wnt signaling results in crypt loss.23 Usually, the Wnt signaling pathway is mediated by the Wnt target gene Sox9.26,27 The Notch pathway also regulates the differentiation of ISCs, Paneth cell plasticity, and promotes the absorptive cell fate.28,29 The bone morphogenetic protein pathway is important in the promotion of differentiation of epithelial cells.30 On the contrary, Lrig1 secreted from ISCs negatively regulates the growth of ISCs to maintain the homeostasis.31
Therefore, the balance between stemness and differentiation is well regulated by ISC niche to maintain intestinal homeostasis.
4. Intestinal injury and regeneration
Although continuous efforts are being made to maintain intestinal homeostasis, the intestinal epithelium faces harsh luminal environments, such as dysbiosis, radiation, and acute or chronic inflammation. Under physiological conditions, the intestinal epithelium engages in well-regulated self-renewal and regenerative processes to maintain the integrity of the epithelial barrier. However, breakdown of the integrity of the epithelial barrier may occur due to intestinal dysbiosis, ischemia, or chronic inflammation.32,33 When acute injury occurs in the intestinal epithelium, upregulation of Wnt/β-catenin signaling increases LGR5+ stem cell activity and promotes the tissue regeneration process to repair the injury and disintegrity of the epithelial barrier.34 Nevertheless, sustained and severe injury may result in the loss of several portions of ISCs.
Regeneration is a reversible, active, and dynamic process that requires cellular adaptation between the remaining and newly induced cells. Non-proliferative cells rapidly migrate towards injury sites to seal the damaged epithelium, which is followed by cell proliferation and differentiation. The regeneration of the intestinal epithelium is closely linked with the Hippo signaling pathway, which controls cell proliferation, migration, and cell fate determination.35
The Hippo pathway ends with the phosphorylation of the transcriptional effector yes-associated protein (YAP) and a transcriptional coactivator with PDZ-binding motif (TAZ). YAP is localized in the nucleus of Lgr5+ISCs and plays an important role in intestinal self-renewal. Regenerating crypts showed increased YAP/TAZ levels, and YAP/TAZ inactivation is related with impaired intestinal regeneration. The injured epithelium is reprogrammed into the highly proliferative and primitive epithelium.36 In a dextran sodium sulfate (DSS) murine colitis model, the activation of YAP/TAZ signaling induced a fetal signature in regenerative colonic crypts.37 Increased YAP activity suppresses Wnt signaling and excessive Paneth cell differentiation, leading to the reprogramming of pluripotent fetal-like ISCs, with the restoration of tissue injury.22,38
5. Intestinal regeneration process in IBD
IBD is a chronic idiopathic and progressive inflammatory disorder of the gastrointestinal tract, composed of two distinct diseases: ulcerative colitis and Crohn’s disease.33 The incidence and prevalence of IBD are increasing globally.39,40 Several conditions are involved in the pathogenesis of IBD, including genetic susceptibility, dysregulated immune response, impaired intestinal mucosal barrier system, and environmental factors, such as diet and the microbiota.41-44 With the advancement of novel treatment modalities, such as biologics and small molecules, clinical improvement and remission are achieved in several patients with IBD.45-47 However, persistent ulceration or mucosal lesions due to impaired mucosal integrity are unique clinical features of IBD. Furthermore, “mucosal healing,” a concept of complete restoration of the mucosal structure and function, is recently introduced in the clinical field; nowadays, it is targeted to achieve favorable long-term outcomes in patients with IBD.48
An impaired intestinal mucosal barrier plays a pivotal role in the initiation and aggravation of intestinal inflammation, causing an increase in intestinal permeability and a subsequent increase in bacterial translocation. The intestinal mucosal barrier system is composed of IECs, tight junctions, and adherens junctions (AJ), such as E-cadherin. Epithelial cell damage combined with dysregulation of the intestinal tight junction barrier perturbs the mucosal immune system and induces inflammation, characteristic features of IBD.49,50 Injury and inflammation trigger regeneration of the intestinal epithelial barrier. However, crypt epithelial cells proliferate more slowly in a mouse model after exposure to lipopolysaccharide.51 Moreover, the loss of ISCs resulting from severe and continuous inflammation in patients with IBD may disturb the regeneration of the damaged intestinal epithelium.52 A recent study demonstrated that the transplantation of LGR5+ ISCs attenuates intestinal mucosal injury in murine DSS colitis. Therefore, stem cell therapy has gained attention for improving the healing of the injured epithelium.
6. Cell therapy in IBD
Several studies have demonstrated that hematopoietic stem cells and mesenchymal stem cells (MSCs) may have some beneficial effect on a particular population in patients with Crohn’s disease.53-57 Especially, allogeneic bone-marrow-derived MSC therapy for perianal fistula in Crohn’s disease patients has been proven to be a safe and effective modality.58 MSCs are pluripotent stem cells possessing self-renewal ability, and the therapeutic effects of MSCs are mainly carried out through angiogenesis, tissue repair, and immunomodulation.59,60 In the DSS-colitis model, endoscopic injections of MSCs and MSC spheroids into the inflamed colon area attenuate the inflammation and increase the levels of interferon-gamma, indoleamine 2,3-dioxygenase, and interleukin-10.60 However, the effect of a single MSC treatment is transient, and further studies are needed to optimize MSCs therapy.
With the advancement of technology for the growth of IEC lines, the concept of transplantation of ex vivo cultured ISCs, that is “organoids,” can be possible.61-63 Usually, LGR5+ ISCs are used to make the intestinal organoid, and single LGR5+ ISCs can build the intestinal crypt-villus units.64 Organoids may help in the promotion of the regenerative process of the damaged intestinal epithelium. In a DSS colitis model, organoids transplanted into the rectal ulcer were shown to integrate into the surrounding tissues and constitute a single-layered epithelium. Therefore, transplanted organoids can form self-renewing crypts with normal function.65
These findings suggest that organoid engraftment was successful and contributed to the regeneration of the damaged intestinal epithelium. Therefore, organoids are possible candidates for the treatment of severe ulcerative lesions in patients with IBD.66
The establishment of intestinal organoids depends on the successful reconstitution of the stem cell niche, which possesses several growth factors, including Wnt, noggin, and EGF.67
Recently, the success of patient-derived organoids has also been reported.68 ISCs can be collected from the intact area of patients with IBD through endoscopic biopsy and then expanded in vitro to make patient-derived organoids. These patient-derived organoids can be transplanted into a damaged site through an endoscopic delivery system or a sheet-type device.69
CELLULAR PLASTICITY-MEDIATED LIVER REGENERATION
1. BEC/LPC-drived HC repopulation
The liver possesses an extraordinary regenerative capacity that is largely based on the self-replication/proliferation of two parenchymal cells, HCs and BECs. This proliferation-mediated liver regeneration has been extensively investigated in both acute and chronic injury settings using various experimental animal models. In the case of acute injury, either a 2/3 partial hepatectomy or the administration of acute toxic drugs triggers a robust proliferative response in HCs, aiming to replenish the depleted HC population. However, the fidelity of liver regeneration has come under scrutiny, as the exceptional proliferative capacity of HCs may be compromised under certain circumstances. The first experimental evidence of BEC/liver progenitor cell (LPC)-to-HC conversion was reported in the rat model. To impede the proliferation of HCs during acute liver injury situations, 2-acetylaminofluorene (2-AAF) was administered to rats with 2/3 partial hepatectomy. Consequently, the liver manifested the emergence of a ductular response, the expansion of BECs in peri-portal areas (zone1), with the expression of not only KRT8 and KRT19 but also vimentin and alpha-fetoprotein in these BECs, termed “oval cells.” Subsequently, these cells transitioned from their LPC phenotype and simultaneously expressed the mature HC marker albumin, indicating conversion to the HC fate.70,71
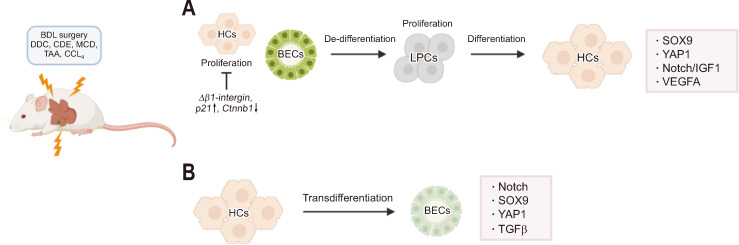
In mice, the Kaestner group demonstrated the differentiation of hepatic stem cells into HCs within the context of hepatic injury models, such as those induced by bile bile duct ligation (BDL) surgery, 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet, or choline-deficient, ethionine-supplemented (CDE) diet models (Fig. 2A). This groundbreaking revelation was achieved by hepatic stem cell lineage tracing using the Foxl1-Cre reporter strains.72-74 Notably, Foxl1, which is predominantly found in a specific population of subepithelial fibroblasts inhabiting the ISC niche,75 exhibited a remarkable surge in expression levels proximal to or within the portal region amidst the injury setting.
Fig. 2.
Hepatobiliary plasticity in response to liver insults: a mouse model study. Under various liver insults, hepatocytes (HCs) and biliary epithelial cells (BECs) undergo cell fate conversion. (A) Severe liver insults, along with the genetically induced impairment of HC proliferation, trigger BECs to dedifferentiate into liver progenitor cells (LPCs; bipotent stem cells). Following LPC proliferation, these cells differentiate into HCs. (B) HCs can transdifferentiate into BECs, a process influenced by the induction of biliary factors, which plays a pivotal role in initiating and responding to cell fate conversion. BDL, bile duct ligation; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; CDE, choline-deficient, ethionine-supplemented; MCD, methionine- and choline-deficient; TAA, thioacetamide; CCl4, carbon tetrachloride; SOX9, SRY (sex-determining region Y protein)-box transcription factor 9; Yap1, yes-associated protein 1; IGF1, insulin-like growth factor 1; VEGFA, vascular endothelial growth factor A; TGFβ, transforming growth factor β. The figure was created using BioRender.com.
After their experimental evidence, there have been efforts to authenticate the viability of LPCs as a potential source for HCs in diseased livers using diverse Cre-reporter lines. This has been achieved through the utilization of BEC-specific inducible Cre systems, such as Sox9-CreERT2, OPN-CreERT2, CK19-CreERT2, and others. The labeling efficiency in BECs by these BEC-specific Sox9-, OPN-, and CK19-CreERT2 shows 32%, 99.9%, and 97%, respectively, after tamoxifen injection.76,77 In the absence of tamoxifen, 2% and 13% of BECs were labeled by OPN- and CK19-CreERT2, respectively. However, the efficacy of these inducible systems for incidental labeling of LPCs remains controversial due to the lack of validated LPC-specific markers/strains and an overlap with BEC markers. It is worth noting that the majority of biliary-specific markers are falsely activated in HCs upon injury, which has led to conflicting results and ongoing discussions regarding the technical aspects of these studies. For instance, under CCl4 and CDE injuries, 1.5% to 5.6%, 46.5% to 75.9%, and 1.6% to 2.0% of HCs were labeled by OPN-, Sox9-, and CK19-CreERT2, respectively.78 In light of the numerous publications demonstrating the leakiness and randomness of the labeling efficiency of the respective Cre systems through meticulous examination by multiple research groups, caution must be exercised when estimating the fate of LPCs through lineage tracing studies. Furthermore, the recent development of sophisticated dual labeling systems to combine concomitant tracing of HC and BEC lineage thereby providing positive and negative tracing, especially by exploiting the nearly 100% tropism of the adeno-associated virus serotype 8 (AAV8) system to HC will be required to appropriately estimate the real contribution of BEC/LPC population in HC repopulations.
Based on the current understanding, the transition of mammalian BEC/LPCs into HCs can be observed under tightly controlled experimental conditions: (1) intense injury to HCs combined with the genetic silencing of the entire HC proliferative capacity; (2) intense injury to HCs combined with the pan-HC induced by forced oncogene expression; or (3) extremely long exposure to liver injury until pan-HC loses its regenerative capacity. These experimental scenarios can be likened to patients with end-stage decompensated liver failure who are awaiting liver transplantation. Notably, murine LPC/BEC-to-HC conversion models exhibit high mortality, reflecting these clinical conditions. Using these models, several molecular regulators have been identified that facilitate or suppress the conversion of LPCs to HCs have been identified. However, the critical molecular switch that initiates this conversion remains elusive, and its discovery may hold substantial clinical significance.
As a regenerative medicine, turning on and/or boosting this alternative regenerative process has been deemed the optimal approach for patients suffering from decompensated organ failure. However, a safe and effective treatment regimen for this purpose remains elusive, primarily due to our limited understanding of the intricate molecular mechanisms involved in this process. Furthermore, cellular plasticity has been implicated in the development and progression of cancer, where abnormal cellular reprogramming and transdifferentiation can contribute to malignant growth. Gaining insight into the underlying mechanisms of cellular plasticity is crucial for unraveling the complexities of developmental processes and to develop innovative therapeutic strategies for diseases such as cancer.
2. HC-drived BEC repopulation
In contrast to the BEC-to-HC transition, the HC-to-BEC transition occurs under much more permissive experimental conditions.79-82 Upon administration of CCl4, DDC, damage-associated molecular pattern molecules, or BDL surgery, either transplanted or intrinsic HCs labeled by the HC-specific Cre system undergo transdifferentiation into BECs (Fig. 2B).78,83,84 Mechanistic investigations have revealed the essential role of biliary-specific transcriptional regulators, including Notch, Sox9, and the Hippo-YAP1 cascade. Notably, the overexpression of the active form of YAP1 in HCs triggers their dedifferentiation to a ductal fate.85 In this process, the Notch-Sox9 axis has been identified as functional downstream of the YAP1/TEAD complex, implying the essential roles and crosstalk of biliary-specific transcription factors in the commitment of hepatic cell fate in the diseased liver. In contrast to the prevailing view that Notch signaling is indispensable for HC to BEC transdifferentiation, the Willenbring group has identified an alternative pathway in this cellular fate conversion.86 Using the Alagille syndrome mouse model, where the intrahepatic peripheral bile ducts development failure was induced with Alb-Cre;Rbpj(f/f);Hnf6(f/f), they revealed that transforming growth factor β (TGFβ) signaling drives Notch-independent de novo bile duct formation. This finding has clinical impact on Alagille syndrome patients, especially those with genetic JAG1 (ligand of Notch receptor) deficiency. Furthermore, the HC-derived BECs were further confirmed by a comprehensive scRNA-seq comparing HCs, HC-derived BECs, and native BECs, which revealed a significant enrichment of Notch and TGFβ signaling exclusively in HC-derived BECs.
The chromatin accessibility analysis revealed an open chromatin state at the binding sites of HC-specific factors, such as HNF4a, CEBP, and FOXA in HCs. Conversely, the chromatin accessibility in HC-derived BECs mimicked the chromatin state of BECs, thereby exhibiting an open chromatin state at the binding sites of BEC-specific factors, including TEAD and HNF1b. Moreover, these cells exhibited a closed heterochromatin state for HC-specific factors, indicating extensive chromatin remodeling by various epigenetic regulators during this cellular transformation.87 Furthermore, the association between chromatin accessibility and the process of HC to BEC transdifferentiation is consistent with recent discoveries pertaining to the development of HCs-derived intrahepatic cholangiocarcinoma (iCCA) from HCs.88,89 Recent evidence indicates that the induction of BEC-specific transcription factors and epigenetic regulators plays a crucial role in the transformation of HCs into malignant iCCA. This implies that HCs expressing these factors, which are frequently detected in the livers of patients with cholestasis have the potential to act as a source of a specific subset of clinical iCCA and/or to play a role in the development of newly formed bile ducts. The extent of this contribution depends largely on the pathological condition of the liver, which contains hidden factors that determine the pathophysiological fate of this population.90,91
Additionally, it is worth mentioning that the HC-specific labeling systems currently in use, such as the AAV8, exhibit a particularly high level of reliability and accuracy, with minimal leakage. As a result, the process of HC to BEC transdifferentiation is less controversial and is being studied extensively to elucidate the fundamental mechanisms involved. This research is aimed at identifying pivotal factors that have potential for translation in the context of cholestasis patients who experience severe biliary damage and require the regeneration of new bile ducts and are at risk of developing iCCA.
3. Cell therapy in liver disease
Orthotopic liver transplantation (OLT) is currently the definitive treatment for various end-stage liver diseases. However, due to a significant shortage of healthy donor livers, there is a significant mismatch between the number of organs available and the number of patients on the waiting list, resulting in high mortality rates among those waiting OLT. As a result, cell therapy has emerged as a potential alternative treatment for these patients. The advantages of cell therapy include the potential to use cells from a single donor liver for multiple recipients, the simplicity of cell administration through intravascular catheters rather than complex surgery, and the ability to use cryopreserved cells to schedule treatments in non-emergency situations. In addition, cell therapy allows for repeated OLT and is considered “reversible” as the native liver remains intact, potentially reducing costs significantly compared to whole-organ OLT.92
However, this approach faces several challenges, including the limited availability of suitable donor livers, difficulties in isolating high-quality cells from these livers, challenges in cryopreserving human liver cells without compromising their viability, low engraftment and proliferation rates of transplanted cells, and the risk of long-term allograft rejection. Moreover, the disparity between animal models and human clinical outcomes is significant, as animal models often do not accurately replicate the prolonged and severe liver injury seen in humans. The primary methods of cell delivery include infusion through the portal vein or hepatic artery, and less commonly, ectopic implantation into the spleen or peritoneum. It is crucial that the transplanted cells reach the liver parenchyma within 24 hours to avoid macrophage clearance.93,94
To alleviate this bottleneck, initial challenges involve providing functional mature HCs that maximize their self-replication ability into liver diseases. Many clinical and preclinical studies have focused on cell therapy to repopulate HCs, including transplantation of mature HC. However, maintaining the functionality of mature HCs in vitro is extremely difficult, and the mammalian liver has a strict metabolic zonation with distinct molecular signatures.95 With the rapid advances in stem cell research for organ regeneration, alternative challenges include numerous trials that have aimed to provide functional HCs derived from various stem cell progenitors.96-99 These efforts have expanded to include different types of stem cells, such as whole bone marrow cells, hematopoietic stem cells, and MSCs.98,96,100-103 The beneficial effects following stem cell injection are still self-replication of HCs rather than due to cellular plasticity or transdifferentiation. Instead, they result from the paracrine effect of cytokines released by the injected cells, which improve the cellular niche and promote regeneration.99,104
The concept of regenerating HCs by reprogramming has been experimentally validated.105 HCs from human cirrhotic livers, which are typically non-proliferative and dysfunctional, were successfully reprogrammed into hepatic progenitor cells in vitro. These progenitor cells were then differentiated back into mature HCs, which not only exhibited functional HC markers, but also demonstrated the ability to proliferate. When transplanted into severe combined immunodeficiency (SCID) mice, these regenerated HCs retained their mature markers and showed no signs of tumor formation. This demonstrates that even end-stage liver HCs can be rejuvenated and restored to functionality.
In addition to the experimental evidence, it has been proposed that progenitor-derived HCs repopulate the parenchymal extinction region in cirrhotic livers.106 To bridge the gap between preclinical models and clinical trials, studies have been conducted to assess the clinical potential of this cell therapy. Intriguingly, preclinical models showed that transplanted hepatic progenitor cells contributed to the restoration of liver parenchymal cells.107,108 Furthermore, transplantation of human fetal bile duct stem cells improved two patients with advanced liver cirrhosis without any adverse effects.109 Additionally, HC-like cells derived from human induced pluripotent stem cells showed functionality, suggesting their potential for cell therapy.110
Building on this, recent advances in cell therapy have shown promising results in human liver applications. In one notable study,111 primary human BECs were isolated from various regions of the biliary system, including the intrahepatic bile ducts, common bile duct, and gallbladder. scRNA-seq revealed distinct transcriptomic profiles for BECs from different regions, despite their common markers. These BECs were cultivated into organoids and then transplanted into deceased human donor livers using ex vivo normothermic perfusion, a technique that helps preserve organs and reduce ischemic damage.
Despite the biliary tree's susceptibility to ischemia, which can cause ductal damage, the transplanted BECs organoids successfully engrafted within the intrahepatic biliary tree. Impressively, 40% to 85% of the bile duct cells in these livers were derived from the transplanted organoids. Functional assessments showed that these organoids not only repaired the damaged intrahepatic bile ducts, but also improved bile properties, without differentiating into other hepatic lineages. These findings highlight the potential of using BECs organoids for therapeutic interventions in human livers, particularly under conditions facilitated by normothermic perfusion.
This series of studies not only highlights the regenerative capabilities of reprogrammed HCs, but also illustrates the innovative applications of cell therapy in liver diseases, providing a viable alternative to traditional liver OLT.
CONCLUSION
The liver and gastrointestinal tract are unique in their ability to regenerate, which is achieved not only by self-replication but also by cellular plasticity. Although, the intestinal epithelium is constantly exposed to pathogenic environments, such as diet, microbiota, and other harmful agents, intestinal integrity and homeostasis can be maintained by rapid and continuous regeneration of epithelial cells. However, when severe injury results in the loss of ISCs, the process of dedifferentiation of IECs into ISCs occurs, which have fetal-like properties and contribute to wound repair and tissue restoration. Advance in understanding of intestinal cellular plasticity, new therapeutic modalities, including stem cell therapy, may aid in the treatment of patients with IBD. In addition, organoids are being introduced as a therapeutic tool that can help in the promotion of the regenerative process of the damaged intestinal epithelium.
Despite the remarkable regenerative capacity of the liver, liver failure due to chronic liver disease is the 9th leading cause of death in the United States. The waiting list for liver OLT, the only reliable cure for end-stage liver disease, has been steadily increasing for decades, despite many research advances, such as stem cell therapy.112 Patients with end-stage liver disease are in desperate need of functional HCs and/or BECs. However, the lack of intact cells forces them to enter the cell cycle. Theoretically, the concept of borrowing stem-like cells from each other based on their specific needs, could be an ideal and less aggressive approach to obtaining cells with functional capabilities and alleviating their condition. Recent studies suggest the possibility of innovative applications of cell therapy in advanced liver disease, providing a viable alternative to traditional liver OLT. Further research in this area will undoubtedly reveal additional factors and mechanisms, thereby paving the way for more effective therapeutic interventions in the future.
ACKNOWLEDGEMENTS
Funding was provided by NIH grant 1R01CA258449 to S.K and 1P30DK120531-01 to the Pittsburgh Liver Research Center.
Footnotes
CONFLICTS OF INTEREST
Y.S.K. is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 2.Tanday N, Tarasov AI, Moffett RC, Flatt PR, Irwin N. Pancreatic islet cell plasticity: pathogenic or therapeutically exploitable? Diabetes Obes Metab. 2024;26:16–31. doi: 10.1111/dom.15300. [DOI] [PubMed] [Google Scholar]
- 3.Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 6.Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- 7.Patankar JV, Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol. 2020;17:543–556. doi: 10.1038/s41575-020-0326-4. [DOI] [PubMed] [Google Scholar]
- 8.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Haber AL, Biton M, Rogel N, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grün D, Lyubimova A, Kester L, et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature. 2015;525:251–255. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- 11.Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50:1–14. doi: 10.1038/s12276-018-0071-8.f40e2b868673466fbfe21e5f399da102 [DOI] [Google Scholar]
- 12.Olsen TK, Baryawno N. Introduction to single-cell RNA sequencing. Curr Protoc Mol Biol. 2018;122:e57. doi: 10.1002/cpmb.57. [DOI] [PubMed] [Google Scholar]
- 13.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 14.Tian H, Biehs B, Warming S, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 17.Andersson-Rolf A, Zilbauer M, Koo BK, Clevers H. Stem cells in repair of gastrointestinal epithelia. Physiology (Bethesda) 2017;32:278–289. doi: 10.1152/physiol.00005.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker MR, Patel KK, Stappenbeck TS. The stem cell niche. J Pathol. 2009;217:169–180. doi: 10.1002/path.2474. [DOI] [PubMed] [Google Scholar]
- 19.Yan KS, Chia LA, Li X, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer AR, Brown ME, McGrath PS, Dempsey PJ. Injury-induced cellular plasticity drives intestinal regeneration. Cell Mol Gastroenterol Hepatol. 2022;13:843–856. doi: 10.1016/j.jcmgh.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Sousa E Melo F, de Sauvage FJ. Cellular plasticity in intestinal homeostasis and disease. Cell Stem Cell. 2019;24:54–64. doi: 10.1016/j.stem.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Blanpain C, Fuchs E. Stem cell plasticity: plasticity of epithelial stem cells in tissue regeneration. Science. 2014;344:1242281. doi: 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farin HF, Van Es JH, Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 24.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastide P, Darido C, Pannequin J, et al. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori-Akiyama Y, van den Born M, van Es JH, et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 28.VanDussen KL, Carulli AJ, Keeley TM, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu S, Tong K, Zhao Y, et al. Paneth cell multipotency induced by notch activation following injury. Cell Stem Cell. 2018;23:46–59. doi: 10.1016/j.stem.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosinski C, Li VS, Chan AS, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc Natl Acad Sci U S A. 2007;104:15418–15423. doi: 10.1073/pnas.0707210104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong VW, Stange DE, Page ME, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Lee C, Cadete M, et al. Impaired Wnt/β-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell Death Dis. 2019;10:743. doi: 10.1038/s41419-019-1987-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregorieff A, Wrana JL. Hippo signalling in intestinal regeneration and cancer. Curr Opin Cell Biol. 2017;48:17–25. doi: 10.1016/j.ceb.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 37.Yui S, Azzolin L, Maimets M, et al. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell. 2018;22:35–49. doi: 10.1016/j.stem.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barry ER, Morikawa T, Butler BL, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:56–66. doi: 10.1038/s41575-020-00360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SH. Update on the epidemiology of inflammatory bowel disease in Asia: where are we now? Intest Res. 2022;20:159–164. doi: 10.5217/ir.2021.00115.b758430e5d2f48c9825f8dcc9ae32504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahadevan U, Silverberg MS. Inflammatory bowel disease: Gastroenterology Diamond Jubilee review. Gastroenterology. 2018;154:1555–1558. doi: 10.1053/j.gastro.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383:2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 43.Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. 2017;49:e338. doi: 10.1038/emm.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379–383. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 45.Na SY, Moon W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver. 2019;13:604–616. doi: 10.5009/gnl19019.8e32d3490d954df48dac75f413aa5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vulliemoz M, Brand S, Juillerat P, et al. TNF-alpha blockers in inflammatory bowel diseases: practical recommendations and a user's guide. An update. Digestion. 2020;101 Suppl 1:16–26. doi: 10.1159/000506898. [DOI] [PubMed] [Google Scholar]
- 47.Na SY, Kim YS. Management of inflammatory bowel disease beyond tumor necrosis factor inhibitors: novel biologics and small-molecule drugs. Korean J Intern Med. 2022;37:906–919. doi: 10.3904/kjim.2022.152.7a060b05f4434130990895b12422f17d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010;16:338–346. doi: 10.1002/ibd.20997. [DOI] [PubMed] [Google Scholar]
- 49.Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11.a41e4fa112074f119518acbf246e9a5c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hegyi P, Maléth J, Walters JR, Hofmann AF, Keely SJ. Guts and gall: bile acids in regulation of intestinal epithelial function in health and disease. Physiol Rev. 2018;98:1983–2023. doi: 10.1152/physrev.00054.2017. [DOI] [PubMed] [Google Scholar]
- 51.Geng H, Bu HF, Liu F, et al. In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology. 2018;155:144–155. doi: 10.1053/j.gastro.2018.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamoto R, Watanabe M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig Dis Sci. 2005;50 Suppl 1:S34–S38. doi: 10.1007/s10620-005-2804-5. [DOI] [PubMed] [Google Scholar]
- 53.Burt RK, Traynor A, Oyama Y, Craig R. High-dose immune suppression and autologous hematopoietic stem cell transplantation in refractory Crohn disease. Blood. 2003;101:2064–2066. doi: 10.1182/blood-2002-07-2122. [DOI] [PubMed] [Google Scholar]
- 54.Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA. 2015;314:2524–2534. doi: 10.1001/jama.2015.16700. [DOI] [PubMed] [Google Scholar]
- 55.Forbes GM, Sturm MJ, Leong RW, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical cord mesenchymal stem cell treatment for Crohn's disease: a randomized controlled clinical trial. Gut Liver. 2018;12:73–78. doi: 10.5009/gnl17035.cdb8df039ed94460a567456b74701a52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimizu H, Suzuki K, Watanabe M, Okamoto R. Stem cell-based therapy for inflammatory bowel disease. Intest Res. 2019;17:311–316. doi: 10.5217/ir.2019.00043.7208f76e26ba48739030d12aa0d6f493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnhoorn MC, Wasser MN, Roelofs H, et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn's disease perianal fistulas. J Crohns Colitis. 2020;14:64–70. doi: 10.1093/ecco-jcc/jjz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caplan AI. MSCs: the sentinel and safe-guards of injury. J Cell Physiol. 2016;231:1413–1416. doi: 10.1002/jcp.25255. [DOI] [PubMed] [Google Scholar]
- 60.Barnhoorn M, de Jonge-Muller E, Molendijk I, et al. Endoscopic administration of mesenchymal stromal cells reduces inflammation in experimental colitis. Inflamm Bowel Dis. 2018;24:1755–1767. doi: 10.1093/ibd/izy130. [DOI] [PubMed] [Google Scholar]
- 61.Sato T, Clevers H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 62.Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19:671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 63.Qi D, Shi W, Black AR, et al. Repair and regeneration of small intestine: a review of current engineering approaches. Biomaterials. 2020;240:119832. doi: 10.1016/j.biomaterials.2020.119832. [DOI] [PubMed] [Google Scholar]
- 64.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 65.Yui S, Nakamura T, Sato T, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 66.Okamoto R, Shimizu H, Suzuki K, et al. Organoid-based regenerative medicine for inflammatory bowel disease. Regen Ther. 2020;13:1–6. doi: 10.1016/j.reth.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.VanDussen KL, Marinshaw JM, Shaikh N, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeda M, Kanai N, Kobayashi S, et al. Endoscopic cell sheet transplantation device developed by using a 3-dimensional printer and its feasibility evaluation in a porcine model. Gastrointest Endosc. 2015;82:147–152. doi: 10.1016/j.gie.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 70.Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol. 1997;26:343–352. doi: 10.1016/S0168-8278(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 71.Evarts RP, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson SS. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- 72.Shin S, Upadhyay N, Greenbaum LE, Kaestner KH. Ablation of Foxl1-Cre-labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. Gastroenterology. 2015;148:192–202. doi: 10.1053/j.gastro.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shin S, Walton G, Aoki R, et al. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev. 2011;25:1185–1192. doi: 10.1101/gad.2027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sackett SD, Li Z, Hurtt R, et al. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology. 2009;49:920–929. doi: 10.1002/hep.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aoki R, Shoshkes-Carmel M, Gao N, et al. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol. 2016;2:175–188. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 77.Lesaffer B, Verboven E, Van Huffel L, et al. Comparison of the Opn-CreER and Ck19-CreER drivers in bile ducts of normal and injured mouse livers. Cells. 2019;8:380. doi: 10.3390/cells8040380.92621a6794e94aaf8c029bbd47c489fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yanger K, Zong Y, Maggs LR, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishikawa Y, Doi Y, Watanabe H, et al. Transdifferentiation of mature rat hepatocytes into bile duct-like cells in vitro. Am J Pathol. 2005;166:1077–1088. doi: 10.1016/S0002-9440(10)62328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Michalopoulos GK, Bowen WC, Mulè K, Lopez-Talavera JC, Mars W. Hepatocytes undergo phenotypic transformation to biliary epithelium in organoid cultures. Hepatology. 2002;36:278–283. doi: 10.1053/jhep.2002.34858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–1713. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nagahama Y, Sone M, Chen X, et al. Contributions of hepatocytes and bile ductular cells in ductular reactions and remodeling of the biliary system after chronic liver injury. Am J Pathol. 2014;184:3001–3012. doi: 10.1016/j.ajpath.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Sekiya S, Suzuki A. Hepatocytes, rather than cholangiocytes, can be the major source of primitive ductules in the chronically injured mouse liver. Am J Pathol. 2014;184:1468–1478. doi: 10.1016/j.ajpath.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 85.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schaub JR, Huppert KA, Kurial SN, et al. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature. 2018;557:247–251. doi: 10.1038/s41586-018-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim M, Delgado E, Ko S. DNA methylation in cell plasticity and malignant transformation in liver diseases. Pharmacol Ther. 2023;241:108334. doi: 10.1016/j.pharmthera.2022.108334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu S, Molina L, Tao J, et al. NOTCH-YAP1/TEAD-DNMT1 axis drives hepatocyte reprogramming into intrahepatic cholangiocarcinoma. Gastroenterology. 2022;163:449–465. doi: 10.1053/j.gastro.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y, Zhuo S, Zhou Y, et al. Yap-Sox9 signaling determines hepatocyte plasticity and lineage-specific hepatocarcinogenesis. J Hepatol. 2022;76:652–664. doi: 10.1016/j.jhep.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshii D, Shimata K, Yokouchi Y, et al. SOX9 contributes to the progression of ductular reaction for the protection from chronic liver injury. Hum Cell. 2022;35:721–734. doi: 10.1007/s13577-022-00683-8. [DOI] [PubMed] [Google Scholar]
- 91.Han X, Wang Y, Pu W, et al. Lineage tracing reveals the bipotency of SOX9+ hepatocytes during liver regeneration. Stem Cell Reports. 2019;12:624–638. doi: 10.1016/j.stemcr.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: from liver transplantation to cell factory. J Hepatol. 2015;62:S157–S169. doi: 10.1016/j.jhep.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 93.Tsolaki E, Yannaki E. Stem cell-based regenerative opportunities for the liver: state of the art and beyond. World J Gastroenterol. 2015;21:12334–12350. doi: 10.3748/wjg.v21.i43.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anantharaju A, Van Thiel DH. Liver transplantation for alcoholic liver disease. Alcohol Res Health. 2003;27:257–268. doi: 10.26226/morressier.58eb975cd462b80290b4fe54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ko S, Russell JO, Molina LM, Monga SP. Liver progenitors and adult cell plasticity in hepatic injury and repair: knowns and unknowns. Annu Rev Pathol. 2020;15:23–50. doi: 10.1146/annurev-pathmechdis-012419-032824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alison MR, Poulsom R, Jeffery R, et al. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 97.Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–384. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 99.Snykers S, Vanhaecke T, Papeleu P, et al. Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol Sci. 2006;94:330–341. doi: 10.1093/toxsci/kfl058. [DOI] [PubMed] [Google Scholar]
- 100.Bird TG, Lu WY, Boulter L, et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci U S A. 2013;110:6542–6547. doi: 10.1073/pnas.1302168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fallowfield JA, Mizuno M, Kendall TJ, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 103.Thomas JA, Pope C, Wojtacha D, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 104.Zhang S, Chen L, Liu T, et al. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A. 2012;18:1352–1364. doi: 10.1089/ten.tea.2011.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyoshi T, Hidaka M, Miyamoto D, et al. Successful induction of human chemically induced liver progenitors with small molecules from damaged liver. J Gastroenterol. 2022;57:441–452. doi: 10.1007/s00535-022-01869-5. [DOI] [PubMed] [Google Scholar]
- 106.Stueck AE, Wanless IR. Hepatocyte buds derived from progenitor cells repopulate regions of parenchymal extinction in human cirrhosis. Hepatology. 2015;61:1696–1707. doi: 10.1002/hep.27706. [DOI] [PubMed] [Google Scholar]
- 107.Lu WY, Bird TG, Boulter L, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Susick R, Moss N, Kubota H, et al. Hepatic progenitors and strategies for liver cell therapies. Ann N Y Acad Sci. 2001;944:398–419. doi: 10.1111/j.1749-6632.2001.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 109.Cardinale V, Carpino G, Gentile R, et al. Transplantation of human fetal biliary tree stem/progenitor cells into two patients with advanced liver cirrhosis. BMC Gastroenterol. 2014;14:204. doi: 10.1186/s12876-014-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takayama K, Akita N, Mimura N, et al. Generation of safe and therapeutically effective human induced pluripotent stem cell-derived hepatocyte-like cells for regenerative medicine. Hepatol Commun. 2017;1:1058–1069. doi: 10.1002/hep4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sampaziotis F, Muraro D, Tysoe OC, et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371:839–846. doi: 10.1126/science.aaz6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang YH, Chen EQ. Mesenchymal stem cell therapy in acute liver failure. Gut Liver. 2023;17:674–683. doi: 10.5009/gnl220417.3d81c73e12e245f886dcabfff7105654 [DOI] [PMC free article] [PubMed] [Google Scholar]