Abstract
According to Chinese Medicine system, Chinese honeysuckle have a lot of valuable metabolites which have a various potential like anti-inflammatory action, antidiabetic, analgesics, antipyretic, eliminates pollutants etc. According to Chinese Medicinal System, the plant is mentioned to cure various diseases like peptic ulcer, diabetes, inflammation etc. It is frequently given for patients with colds that include fever, headaches, and sore throats, but it may also be used to help people who are overheated or under stress chill down. Total 72 articles were found out by the help of online database including Google Scholar, Scopus, PubMed, and Web of Science and 63 had selected. Different keywords like Chinese honeysuckle, Qusqualis indica phytochemistry, pharmacology and toxicology were used to searched the content. The purpose of this review is to summarize the previously reported phytochemicals, pharmacological status of the chosen Chinese plant species. Our findings indicate that Chinese honeysuckle contains a diverse array of bioactive compounds, including flavonoids and terpenoids. Moreover, previous studies have demonstrated that extracts and isolated constituents exhibit significant pharmacological activities and other therapeutic properties. Chinese honeysuckle is a rich source of bioactive compounds that can be incorporated into human diets in low doses and hold potential for treating a range of medical conditions, from minor to severe. This review aims to encourage further research on this plant species, particularly focusing on its toxicity and bioactivity.
Keywords: Chinese medicine system Chinese honeysuckle, Pharmacological potential, Metabolites, Antioxidant, Chinese medicine
Graphical Abstract
Highlights
-
•
According to Chinese Medicine system, Chinese honeysuckle have a lot of valuable metabolites which have a various potential like anti-inflammatory action, antidiabetic, analgesics, antipyretic, eliminates pollutants etc.
-
•
Total 72 articles were found out by the help of online database including Google Scholar, Scopus, PubMed, and Web of Science and 63 had selected. Different keywords like Chinese honeysuckle, Qusqualis indica phytochemistry, pharmacology and toxicology were used to searched the content.
-
•
The purpose of this review is to summarize the previously reported phytochemicals, pharmacological status of the chosen Chinese plant species.
-
•
Our findings indicate that Chinese honeysuckle contains a diverse array of bioactive compounds, including flavonoids and terpenoids. Moreover, previous studies have demonstrated that extracts and isolated constituents exhibit significant pharmacological activities and other therapeutic properties.
1. Introduction
Chinese honeysuckle (Quisqualis indica), is a member of the Combretecea family and is also known as Rangoon creeper. It is grown throughout Africa, the Indo-Malay area, and primarily in India [1]. In general, it needs a location with direct sunshine, frequent watering to keep the soil wet, and a stand to support the vine as it grows. Any plant has to have access to fundamental necessities with well-maintained conditions, such as sunshine, water, fertilizer, etc., in order to develop properly [2]. In the past, leaves and root fluids and extracts were used as anthelmintics and to treat flatulence. Boils and ulcers can be treated externally with a leaf infusion [3], [4].
The 365 drugs (dried parts of medicinal plants) treated in the Chinese book "Pen T'Sao," which was written by Emperor Shen Nung around 2500 BC, include rhei rhizoma, camphor, Theae folium, Podophyllum, the great yellow gentian, ginseng, jimson weed, cinnamon bark, ephedra, and many more [5], [6], [7]. The World Health Organization reports that 80 % of people in developing nations rely on traditional medical systems to treat illnesses when they first arise, whereas the Indian Materia medica contains roughly 2000 natural medicines, nearly all of which are drawn from various traditional systems and folklore customs. Four hundred of these medications that come from the old system come from minerals and animals, while the remaining medications come from vegetables. Traditional medicine has a long history in India, and it has been thriving in many other nations as well [8]. Despite the fact that therapeutic plants have been used for thousands of years in one way or another, it is also known as the "Botanical Garden of the World." Global statistics have unequivocally demonstrated that more than 3/4 of the world's 5 billion inhabitants are dependent on traditional remedies derived from plants since they cannot afford the goods of the pharmaceutical industry in the West [9], [10]. It is estimated that at least 25 % of all contemporary medications originate from medicinal plants, either directly or indirectly, with the main method being the fusion of contemporary technology with traditional knowledge. This number could reach 60 % in the case of some drug classes, such as anticancer and antibacterial medications. Thus, from a medical and financial standpoint, it is imperative that the safety and effectiveness of herbal products and pharmaceutical preparations be thoroughly evaluated scientifically [11], [12], [13].
Based on a review of the literature, it is determined that there is a dearth of comprehensive scientific information on Quisqualis indica (Q. indica). The information that has been gathered could be used as a foundation for future research projects, as well as a supporting source for studies on the plant's anatomy and physiology, formulation development, monograph preparation, and potential uses as a medicine. Q. indica, a robust climber and ligneous vine belonging to the Combretaceae family, can grow up to 8 m in height. It is called Rangoon creeper in common parlance. It is grown throughout India and is native to Africa and the Indo-Malaysinian region [14]. It is an evergreen vine that grows quickly and needs strong support. If given the right growing conditions, it can grow rather out of control, but it doesn't need deep, anchoring roots [15]. In general, it needs full daylight, frequent irrigation to maintain the soil's moisture content, and a support stand for the vine to grow on. Every plant needs basic necessities with well-maintained conditions, such as sunlight, water, and fertilizer, for healthy growth [16]. The highly recognized red rangoon creeper is an African native that was brought to the tropics as a well-liked decorative. Botanically referred to as Q. indica, the creeper is frequently observed growing over compound walls or as a hedge plant. The name Quisqualis, which refers to the plant's varied habit and colors, is derived from the Malay word "Udani." The term "indica" in the species name means "from India" [17], [18].
2. Methodology
We searched the literature using Google Scholar, Scopus, PubMed, and Web of Science to find works that covered the knowledge of Quisqualis indica from 2000 to 2024. Keywords like, Quisqualis indica and phytochemistry, Chinese honeysuckle or Rangoon creeper and lower urinary tract symptoms (LUTS)., Quisqualis indica and benign prostatic hyperplasia, Quisqualis indica Linn and antioxidant, Quisqualis indica and traditional medicine system, Quisqualis indica and pharmacological properties, Quisqualis indica and antibacterial activity, Q. indica and anti-inflammatory activity, Quisqualis indica and immunomodulatory action, Quisqualis indica Linn and anti-diabetic, Quisqualis indica Linn and genotoxicity, Q. indica and antiulcer activity etc were included in the search. Out of the 200 articles that were retrieved, 100 items were taken into consideration. A compilation of relevant works focusing solely on Quisqualis indica was attempted. Such scientific and molecular data has not yet been published in any publications. This review helps with the creation of the plant monograph in accordance with the Chinese medicine system and offers up many new avenues for future studies.
3. Botanical aspects
One of the genera Quisqualis's most striking tropical vines is Q. indica. It comes in a few different variations, each distinguished by the color of its flowers and the size of its leaves. Although it can grow up to 21 m in the wild, in cultivation it usually reaches a length of 2–9 m. a huge, shrubby, woody climber that can be trained to be a specimen shrub while still reaching over pergolas, trellises, etc. When growth conditions are favorable, it usually sports stunningly beautiful, luxuriant, fresh green leaves on cascading branches with a profusion of axillary and terminal drooping racemose inflorescences. Oblong to elliptic, 7–15 cm long, with a rounded base and acuminate tip, the leaves have a pronounced venation. They are polar opposites and straightforward. In the tropics, it constantly and abundantly blooms throughout the year. Both the Thai hybrid and the original Rangoon Creeper, which has thorny stems and blooms red solitary flowers, have the extra benefit of nighttime seductive smell. Over the course of three days, the pendulous trumpet-shaped blossoms in the gorgeously colored flower clusters display different stages of color on a single flower stalk. The blooms open white, change pink, and end up deep pink, bright red, or reddish purple. Its fruit is 2.5–3 cm long, narrowly ellipsoidal, and has five acute longitudinal angles, or wings. The pentagonal, black seeds are 12–15 mm in length and resemble a fruit shell. The ellipsoidal fruit, about 30–35 mm in length, has five noticeable wings. When the fruit ripens, it tastes like almonds [19], [20], [22]. Rangoon creeper is mostly an attractive plant, but for a very long time it was used as traditional medicine because of its phytoconstituents. It could be used alone or in combination with other complementary components. Typically, the leaves, flowers, seeds, fruits, and roots of these plants are the portions that are traditionally employed. These components have certain active chemicals that provide specific pharmacological activity, but they should be taken under professional supervision because they can also cause adverse effects like headache or stomachache, particularly when consumed frequently or freshly [21], [22], [23]. It is an evergreen vine that doesn't need deep, anchoring roots, but it can grow fairly out of control in its ideal growing location due to its aggressive growth and requirement for strong support. It is widely disseminated around the world, with particular emphasis on China, the Philippines, Bangladesh, Myanmar, and Malaysia. It is currently also commonly grown as a decorative plant in most gardens in India. Dispersed throughout the Philippines are 1) secondary woods and swamps. 2) Decorated with flowers in mind. 3) Occurs from India to Malaya as well. 4) Introduced in the majority of tropical nations [21]. Generally speaking, it needs full sun, frequent irrigation to keep the soil damp, and a support stand to allow the vine to grow on. Any plant that wants to grow properly needs to be given the necessities, such sunlight, water, fertilizer, and well-maintained conditions.
4. Phytochemical compounds
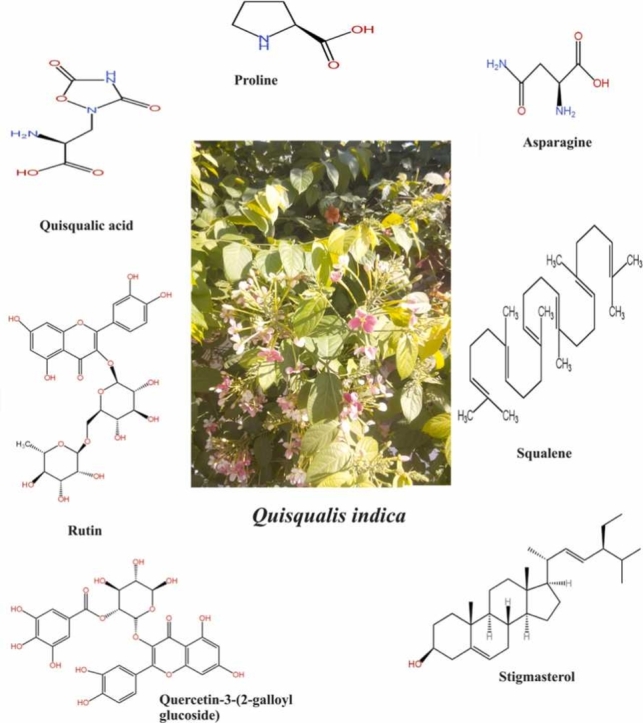
Each plant contains a variety of phytoconstituents, each of which displays a unique pharmacological action and/or toxicity in its different parts. Q. indica is not an exception, exhibiting a wide range of pharmacological activity as a result of the presence of compounds with therapeutic qualities. Q. indica has a variety of phytoconstituents, including as the flavonoid rutin, α-farnesene, the enzymes L-proline and L-asparagine, the agonist quisqualic acid (which binds to both AMPA receptors), the alkaloid trigonelline, and isoenzyme A and isoenzyme B, two distinct forms of cysteine synthase and starch grain. Additionally, rutin and pelargonidin-3-glucoside have been extracted from flowers. Fruits contain levulose, a sugary substance, and cathartic acid, an organic acid. Seeds contain a fixed oil that contains the neuroexcitatory amino acid quisqualic acid along with linoleic, oleic, palmitic, stearic, and arachidic acids, a sterol, and an anthelmintic alkaloid [24], [25], [26], [27], [28], [29], [30].
5. Taxonomic classification [31], [32], [33], [34]
Kingdom: Plantae
Division: Mangoliophyta
Class: Mangoliophyta
Order: Myrtales
Family: Combretaceae
Genus: Combretum
Species: Combretum indicum
Bionomial name:Combretum indicum (L.) De Filipps
Synonym:Quisqualis indica L.
6. Vernacular names
China: Chinese honeysuckle
English: Rangoon creeper
Hindi: Madhumalti
Marathi: Rangoonvel, Madhumalati, Vilayati Chameli
Gujarathi: Barmasivel
Manipuri: Parijat
Telugu: Radha Manoharam
Bengali: Malati, Madhumalati
Common names
Rangoon Creeper, Drunken Sailor, Akar Dani, Akar Suloh, Ara Dani, Akar Pontianak, Red Jasmine.
7. Therapeutic and pharmacological activities of Q. indica
Phytochemicals isolated from various plant parts or combined with other substances to treat a variety of illnesses, including rickettsia, urinary tract infections, body aches, toothaches, stomach pains, colds, and gastric disorders. An agonist for the α-amino-3-hydroxy-5-methyl4-isoxazolepropionic acid (AMPA) glutamate receptor in the brain, quisqualic acid is a compound found in the seeds of Q. indica (Table 1) [35].
Table 1.
| S. No. | Metabolite name | Molecular Formula | Molecular structure |
|---|---|---|---|
| 1 | Quisqualic acid | C5H7N3O5 | 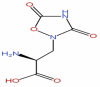 |
| 2 | Trigonelline | C7H7NO2 | 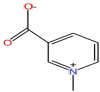 |
| 3 | Proline | C5H9NO2 | 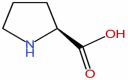 |
| 4 | Asparagine | C4H8N2O3 | 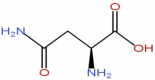 |
| 5 | Rutin | C27H30O16 | 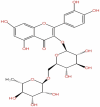 |
| 6 | Pelargonidin−3-glucoside | C21H21ClO10 | 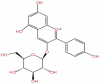 |
| 7 | Linoleic acid | C18H32O2 | 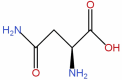 |
| 8 | Palmitic acid | C16H32O2 | |
| 9 | Stearic acid | C18H36O2 | |
| 10 | Linoleic acid | C18H32O2 | 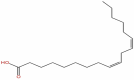 |
| 11 | Oleic acid | C18H34O2 |  |
| 12 | Arachidic acid | C20H40O2 | |
| 13 | Sterol | C17H28O | 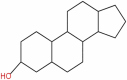 |
| 14 | Alpha Farnesene | C15H24 | 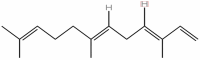 |
| 15 | Heptadecane | C17H36 | |
| 16 | Methyl Palmitate | C17H34O2 | |
| 17 | Methyl linoleate | C19H34O2 | 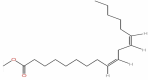 |
| 18 | Phytol | C20H40O | 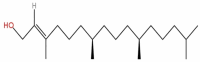 |
| 19 | Methyl stearate | C19H38O2 | |
| 20 | Squalene | C30H50 | 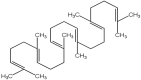 |
| 21 | Penta triacontane | C35H72 | |
| 22 | Tetra teraoctane | ||
| 23 | Vitamin E acetate | C31H52O3 | 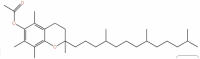 |
| 24 | Stigmasterol | C29H48O | 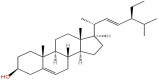 |
| 25 | 1-chloro heptacosane | C27H55Cl | |
| 26 | Methyl linolelaidate | C19H34O2 | |
| 27 | Diethyl phthalate | C12H14O4 | 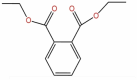 |
| 28 | Viridiflorol | C15H26O | 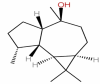 |
| 29 | Cycloartenol acetate | C32H52O2 | 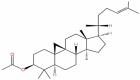 |
| 30 | Dodecane | C12H26 | |
| 31 | Octacosane | C28H58 | |
| 32 | 6-methyloctadecane | C19H40 | |
| 33 | Pentadecane | C15H32 | |
| 34 | Trans-linalool oxide | C10H18O2 | 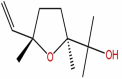 |
| 35 | Quinoline−4-carbonitrile | C10H6N2 | 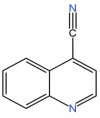 |
| 36 | Methyl benzoate | C8H8O2 | 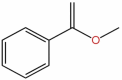 |
| 37 | 2,2,6-trimethyl−6-vinyl-tetrahydropyran−3-ol | C10H18O2 | 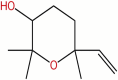 |
| 38 | Crotanecine | C18H13NO3 | 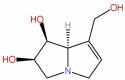 |
| 39 | N-tert-but | 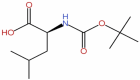 |
|
| 40 | Retronecine | C8H13NO2 | 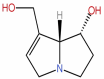 |
| 41 | Fenapanil | C16H19N3 | 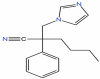 |
| 42 | Ketotifen | C19H19NOS | 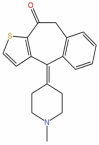 |
| 43 | Neotussilagine | C10H17NO3 | 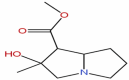 |
| 44 | Stigmatellin Y | C29H40O6 | 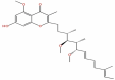 |
| 45 | Geranylfarnesyl diphosphate | C25H41O7P2 | |
| 46 | Luteolin | C15H10O6 | 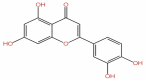 |
| 47 | Myricitrin | C21H20O12 | 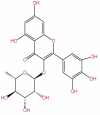 |
| 48 | Clocortolone Pivalate | C27H36ClFO5 | 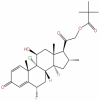 |
| 49 | Kaempferol | C15H10O6 | 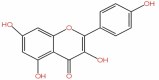 |
| 50 | Genistein | C15H10O5 | 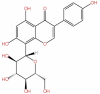 |
| 51 | Quercetin−3-(2-galloyl glucoside) | C28H24O16 | 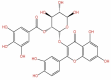 |
| 52 | Punicacortein B | C27H22O18 | 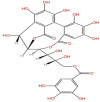 |
| 53 | Gallic acid | C7H6O5 | 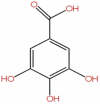 |
| 54 | Salicylic acid | HOC6H4COOH | 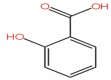 |
| 55 | Chorismic acid | C10H10O6 | 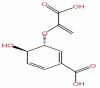 |
7.1. Anti-inflammatory activity
Inflammation is a normal, protective response to tissue injury caused by physical trauma, noxious chemicals or microbiologic agents. Inflammation is defined as a local response to cellular injury that is marked by capillary dilatation, leukocyte infiltration, redness, heat, pain, swelling and often loss of function and that serves as a mechanism initiating the elimination of noxious agents and damaged tissue. Inflammation is of two types, acute inflammation a short term process which appears within few minutes and chronic inflammation a long term process. Anti-inflammatory action is considered to be inhibition of PG synthesis particularly it inhibit the COX at the site of injury, as the decrease the prostaglandin E2 and prostacyclin reduces vasodilation and indirectly, oedema. Accumulation of inflammatory cells is not reduced that it does not depress the production of other mediators like leukotrines, PAF, cytokines, etc so there are many targets for anti-inflammatory action. The acetic acid-induced vascular permeability and cotton pellet granuloma model demonstrate the anti-inflammatory properties of the hydroalcoholic extract of Q. indica. Polyphenols and flavonoids were found, according to the phytochemicals study. Because they prevent the creation of prostaglandins, the polyphenols have strong anti-inflammatory effects. Therefore, bradykinin and the polyphenols' ability to suppress PG production are responsible for the anti-inflammatory properties of the hydroalcoholic extract of Q. indica. [36], [37].
Research has demonstrated the efficacy of Q. indica extracts in lowering tissue inflammation by reducing edema and swelling brought on by inflammation in animal models. It is useful for treating inflammatory diseases such arthritis, skin inflammations, and muscle soreness because of its anti-edematous qualities.
7.2. Anti-pyretic activity
Fever, or pyrexia, arises during infection due to the generation of pyrogens. This process begins when interleukin-1 (IL-1) releases prostaglandins (PGs) in the central nervous system (CNS), which then elevate the hypothalamic set point for temperature regulation, leading to a fever. One such prostaglandin is synthesized when endogenous fever-inducing agents, such as cytokines, are released from white blood cells activated by infection, hypersensitivity, malignancy, or inflammation. Fever is a common medical sign, defined by an increase in body temperature above the normal range of 36.5–37.5 °C (98–100 °F), due to a higher set point in the body's temperature regulation. This higher set point results in increased muscle tone and shivering. Despite the rising body temperature, individuals often feel cold until the new, elevated temperature is reached, at which point they feel warm. The antipyretic properties of the methanolic leaf extract of the Q. indica plant against the rat's Brewer's yeast-induced pyrexia model were thoroughly studied. The plant's methanolic extract, administered at dose levels of 100 mg/kg and 200 mg/kg, demonstrated capable, strong, and comparable outcomes, endorsing Q. indica as a potential species of antipyretic plant [38].
The main reason for its importance in lowering fever is because of the bioactive substances. They have the ability to reduce inflammation and provide analgesia. The compounds flavonoids, saponins, tannins, and alkaloids found in Q. indica are well-known for their therapeutic uses. These chemicals have antipyretic properties because they can decrease inflammation and prevent the release of pyrogens, which are substances that cause fevers. The antipyretic effect is associated with the inhibition of prostaglandin synthesis. Additionally, it may improve the body's ability to dissipate heat through vasodilation and increased perspiration, which would aid in the lowering of fever.
7.3. Immunomodulatory activity
Strong immunostimulants, such as the hydroalcoholic extract of Q. indica flower extract, can activate both specific and non-specific immunological processes. Phagocytosis's function is to eliminate pathogens, foreign objects, and damaged or dead cells. The majority of immunomodulators are thought to target macrophages, which are important because they ingest infections or foreign substances and trigger the innate immune response. When compared to control group 28, the phagocytic index of Q. indica flower extract (150 mg/kg) and 100 mg/kg showed a substantial (p<0.05) increase [39].
The plant is abundant in substances with immunomodulatory qualities, including as flavonoids, tannins, alkaloids, and saponins. Depending on what the body requires, these bioactive molecules can either boost or decrease immunological responses, assisting in the preservation of immune homeostasis. Q. indica contains flavonoids and polyphenols that have been shown to have immune-boosting and antioxidant properties. These properties strengthen the immune system's capacity to fight pathogens while lowering damaging oxidative stress.
Q. indica's antioxidant qualities are crucial in preventing oxidative damage to immune cells. immunological cells and tissues may occasionally sustain harm from reactive oxygen species (ROS), which are created during immunological reactions. The antioxidants in the plant aid in the neutralization of ROS, protecting immune cell integrity and enhancing activity.
7.4. Anti-staphylococcal activity
An antibiotic against staphylococcal infection can be obtained from the Q. indica stem bark extract that was macerated with methanol and then sequentially solvent-solvent partitioned with n-hexane, carbon tetrachloride, and chloroform [40]. The formula, which expresses the effectiveness of the individual antibiotics as a "Therapeutic Index" (TI),
| TI= LD50/MIC |
which determines the toxicity of antibiotics to animals in vivo (LD50 = a dose that causes 50 % of test animals to die in mg/kg/body weight) and to microorganisms in vitro (MIC = Minimal Inhibitory Concentration in ppm) [41].
It is well known that S. aureus produces biofilms, which are bacterial shields against immune system responses and medications. Treatment for infections is hampered by these biofilms. There is potential for Q. indica to interfere with the production of biofilms, hence improving the efficacy of antibiotics and the immune system in getting rid of bacterial colonies. Treating chronic or resistant S. aureus infections requires this anti-biofilm capability.
7.5. Antioxidant activity
As a result of its redox characteristics, the methanolic plant extract Q. indica exhibited 95 % antioxidant activity. This allowed the extract to function as reducing agents by scavenging free radicals like peroxide, hydroperoxide, or lipid peroxyl and thereby inhibiting the oxidative mechanisms that cause degenerative diseases. The results of this investigation indicate that the chloroform soluble fraction of the methanolic extract of Q. indica (stem bark), in particular, has considerable antioxidant activity [42]. The phosphomolybdenum method was used to calculate the total antioxidant capacity based on a spectrophotometric experiment. The phosphate in the sample combined with the additional molybdenum to create a complex, which was then reduced in an aqueous sulfuric acid media by thiourea.
| Percentage of inhibition: Control OD (A0) - Sample OD (A1) × 100 |
The antioxidant activity of Q. indica using dot-blot DPPH staining assays, phosphomolybdenum, and 1,1′-diphenyl-2-picraylhydrazyl free radical (DPPH) assays, as well as reducing power antioxidant assays. DPPH's free radical scavenging concentrations (SC50) against ascorbic acid as standard (SC50 = 7.45) ranged from 24.38 to 72.10 μg/ml; petroleum ether and dichloromethane (CH2Cl2) showed little activity, but the ethyl acetate fraction showed the highest activity [43]. Q. indica flower's aqueous extract has the strongest DPPH radical-scavenging action. Flowers from Q. indica have a strong hydroxyl radical scavenging activity and a low nitric oxide scavenging activity in their ethanol extract [44]. Q. indica leaf's ethyl acetate extract demonstrated the highest hydroxyl radical scavenging activity, while the aqueous extract demonstrated the best nitric oxide and hydrogen peroxide scavenging properties [45].
The anti-oxidant activity was measured by Chatterjee et al. (2019) using the disc diffusion method and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) and hydrogen peroxide (H2O2) radical scavenging assay. It was discovered that the aqueous extract of leaves and flowers exhibited greater scavenging activity than the ethyl acetate extract [46].
Q. indica's antioxidant qualities play a major role in delaying the aging process. One of the main causes of wrinkles, elasticity loss, and other symptoms of aging skin is oxidative damage. By neutralizing ROS, the antioxidants in Q. indica can protect skin health and postpone the appearance of aging. The structural protein collagen, which is necessary for the firmness and flexibility of skin, can be stopped from degrading by antioxidants.
7.6. Anti-bacterial activity
The anti-microbial activity of Q. indica flower extracts was studied by Mukherjee et al. (2017) using the agar well diffusion method with different solvents. They discovered that petroleum ether has the best anti-microbial activity against the following microorganisms: Bacillus subtilis (B.subtilis), Escherichia coli (E.coli), Pseudomonas aeruginosa (P. aeruginosa), and Staphylococcus aureus (S. aureus), with MIC values of 27.0, 30.0, 38.0, and 40.0 μg/ml in the presence of phytochemicals like alkaloids and phenol. Using an agar well diffusion assay with petroleum ether, methanol, chloroform, dimethyl sulphoxide, and sterile distilled water extract of Q. indica flower against S. aureus, E. coli, P. aureuginosa, and B. subtilis, they investigated the antibacterial activity. Petroleum ether extract had strong antibacterial action against S. aureus and E. coli thanks to secondary phytochemicals such as alkaloids, steroids, flavonoids, and phenols [47]. Kumar and others (2014). conducted an agar well diffusion assay using Q. indica flower extract and solvents such as methanol, ethanol, and water against a variety of gram positive and gram negative bacteria, including E. coli, B. subtilis, and M. luteus. When Q. indica flower extract was combined with gram-positive M. luteus, it demonstrated a significant zone of inhibition in comparison to ethanol and aqueous extract [48]. Q. indica flower methanol extracts, both in dry and wet form, shown strong antibacterial activity against K. pneumoniae, P. aeruginosa, P. mirabilis, E. coli, Methicillin-Resistant Staphylococcus aureus, and B. subtilis, according to Kiruthika et al.'s 2011 study [49]. The antibacterial activity of an aerial fragment of Q. indica was investigated by Thakur et al. (2017) using solvents such as methanol, ethyl acetate, and hexane against four different bacteria: Staphylococcus pneumoniae, K. pneumoniae, E. coli, and S. aureus. The most effective results were obtained with methanol and hexane extracts against K. pneumoniae and E. coli, whereas S. aureus and E. coli showed the least effective results [50].
Q. indica may make prescription antibiotics work better. Combining the plant extract with conventional antibiotics may enhance treatment results and lower the dosage of synthetic medications required.
When it comes to treating bacterial infections over an extended period of time, natural treatments like Q. indica are a safer option because they usually have less adverse effects than synthetic antibiotics.
By avoiding infections in cuts and abrasions, Q. indica's antibacterial qualities may also aid in the healing of wounds. Topically using the plant extracts may accelerate the healing process.
7.7. Anti-helminthic activity
A class of anti-parasitic medications known as anti-helminthics are used to eradicate parasitic worms without harming the host. Anti-helminthic medications are classified into two categories: broad spectrum and narrow spectrum, based on the extent of their effects [51]. By determining the lethal dose (LD) at LD50 and LD100, Hai et al. (2019) showed the anti-helminthic activity of Q. indica seeds against ascarides, flukes, and E. coli bacteria. Ascariasis resistance was demonstrated by the seed extract with reduced LD50 and LD100 times [52]. When Sarma et al. (2015) examined the anti-helminthic activity of various extracts of Q. indica leaves on Indian earthworms, they used albendazole 60 mg/ml as a standard reference. They discovered that the methanolic extract demonstrated the highest activity when compared to the aqueous extract at the same concentration [53].
Malnutrition, growth retardation, and anemia are linked to helminthic infections, especially in underdeveloped countries and in children. As an anti-helminthic, Q. indica lessens the worm load, facilitating better nutrient absorption and enhancing general health. Especially in disadvantaged populations, eliminating parasites can result in a noticeable improvement in energy levels, immune system performance, and food absorption.
7.8. Anti-diarrhoeal activity
In Albino wistar rats, the anti-diarrheal properties of petroleum ether leaf extract of Q. indica were examined by Singh et al. (2013) utilizing the charcoal-induced gastrointestinal motility test and the castor oil-induced diarrhoea test. In comparison to castor oil, which produced 21.16 mg/kg of total wet faeces, the petroleum ether extract of the plant produced 15.32 and 12.45 mg/kg, and 32.23 % and 27.59 %, respectively, of the inhibition of castor oil-induced diarrhoea. The plant Q. indica has emerged as a potent and similar anti-diarrhoea species because to the presence of phytochemicals such as tri-terpenes, alkaloids, flavonoids, sterols, and tannins [54].
Additionally, quisqualis indica has anti-secretory qualities, which means it lessens the overabundance of fluid and electrolyte secretion into the intestines, a significant contributing cause to diarrhea. This lessens the frequency and amount of bowel motions while also assisting in the restoration of the gut's natural fluid balance. When toxins or infections cause the intestines to leak too much fluid, as is the situation with secretory diarrhea, this effect is especially helpful.
7.9. Anti-hyperglycemic activity
The anti-hyperglycemic properties of Q. indica leaf ethanolic extract were investigated by Verma et al. (2018). Using Gas Chromatography-Mass Spectrometry (GS-MS) analysis, the ethanolic extract revealed the presence of sufficient amounts of phytochemicals that can inhibit α-amylase and glucosidase, such as phytol, linolenic acid, and penta-decanoic acid [55].
One of the main factors influencing the onset, course, and consequences of diabetes is oxidative stress. Q. indica's flavonoids and other components have antioxidant qualities that may help lower oxidative stress in the body and shield insulin-producing beta cells in the pancreas from harm. The plant may help sustain appropriate insulin function and glucose metabolism by lowering oxidative damage, which would obliquely support its hypoglycemic effects.
Q. indica may promote peripheral tissue uptake of glucose, including the uptake of glucose by fat and muscle cells. This action lowers the bloodstream's concentration of glucose and aids in controlling blood sugar levels overall. The plant's bioactive substances may activate mechanisms that improve the function of the glucose transporter (GLUT) proteins on cell membranes, which are essential for transferring glucose from the blood into cells.
7.10. Effectiveness of Q. Indica AgNPs against malaria, filariasis vector and zika virus
Govindrajan et al. (2016) used a single pot biogenic synthesis of silver nanoparticles (AgNPs) to assess the biophysical and mosquitocidal activities of Q. indica. Silver nanoparticles have environmentally favorable qualities that make them a potential replacement for pyrethroids, carbamates, and antimicrobial agents [56]. Both microscopic (AFM, SEM, TEM, and EDX) and spectroscopic (UV, FTIR, and XRD) methods were used to characterize AgNPs. Using a microscopic method, the synthesis of polydispersed AgNPs with a spherical form spanning from 1 to 30 nm was demonstrated. XRD was used to determine the crystallized structure. Compared to the targeted mosquito larvae, the non-target aquatic mosquito predators Anisops bouvieri, Diplonychus indicus, and Gambusia affinis were found to be moderately toxic to the AgNPs generated by Q. indica. Anopheles stephensi, Ades aegypti, Culex quinquefasciatus, malaria, arbovirus, and filariasis vector were used to test the acute toxicity of Q. indica extract and AgNPs. The results showed that Q. indica-AgNPs function as an environmentally friendly weapon against Zika virus, malaria, and filariasis vector.
One of the biggest obstacles to vector control is mosquito resistance to traditional chemical insecticides, which is growing. Because quisqualis indica-mediated AgNPs function differently from conventional pesticides, they present a fresh approach to this issue. Because they disrupt cellular membranes, produce reactive oxygen species (ROS), and interfere with enzyme activity, silver nanoparticles harm mosquito larvae and adults both physically and biochemically. This is why they are so powerful against strains of mosquitoes that are resistant to pesticides.
7.11. Assessment of cytotoxic activity of Q. indica copper-nanoparticle on B16F10 melanoma cell
Mukhopadhyay et al. (2018) used copper acetate to biofabricate copper nanoparticles (QCuNPs) in order to analyze the biophysical and cytotoxic activity of Q. indica flower extract. The cytotoxic capability of the nano formulation was assessed in rat B16F10 melanoma cells using the MTT and Lactate dehydrogenase (LDH) assay. Proteomic analysis revealed the quantity of apoptotic and cell cycle arrest protein in the treated sample, while gene transcript analysis revealed the overexpression of cascade dependent and cascade independent (AIF) apoptotic genes [57].
A common model used to research melanoma, an aggressive form of skin cancer, is B16F10 melanoma cells. CuNPs mediated by Q. indica exhibit cytotoxic effect against these cells, indicating their potential for use in cancer therapy. Through the production of reactive oxygen species (ROS), which result in oxidative stress and ultimately apoptosis (programmed cell death), copper nanoparticles cause cytotoxicity in cancer cells. Additionally, the nanoparticles interact with the membranes of cancer cells, impairing their ability to function and ultimately causing cellular damage and death. According to studies, CuNPs exhibit selective cytotoxicity, which is essential for reducing harm to healthy tissue during cancer treatment, and are effective in killing cancer cells at lower levels than healthy cells.
7.12. Efficacy of Q. indica against hyperlipidemia
The hypolipidemic efficacy of a methanolic extract made from Q. indica aerial parts was studied by Sahu et al. (2013). One burning cigarette was placed in a confined chamber to cause passive smoking hyperlipidemia, and the blood serum level was measured at 505 nm in UV light. By lowering blood levels of low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), cholesterol, and triglycerides and increasing levels of high-density lipoprotein (HDL) by blocking lipid peroxidation, methanolic extract has a remarkable effect on the harmful lipid layer in blood serum in a dose-dependent manner [58], [59].
According to some research, extracts from Q. indica may help increase levels of HDL (high-density lipoprotein) while lowering levels of triglycerides, total cholesterol, and low-density lipoprotein (LDL). Maintaining this equilibrium is essential to lower the risk of cardiovascular illnesses. Q. indica may help avoid the development of atherosclerotic plaques in arteries, which are a major cause of heart attacks and strokes, by encouraging healthier lipid profiles.
7.13. Anticancer activity
Using a chromatographic approach, Thomas et al. (2008) and Birgid et al. (2015) identified 25-o-acetyl-23,24-di hydro-cucurbitacin F as a cytotoxic ingredient of Q. indica and they used nuclear magnetic resonance (NMR) and mass spectrometry (MS) to understand its chemical structure. By lowering cell cycle check point regulators cyclin B, cyclin A, CDK 1 and CDK 2, it lowers cell viability by arresting the cell G2/M interface in a dose-dependent way. Furthermore, it was discovered that apoptosis is induced in liposarcoma and rhabdomyosarcoma cells via a caspase-3 dependent pathway [60].
Q. indica's antioxidant activity aids in lowering oxidative stress, which is linked to the advancement of cancer. The plant may help shield cells from DNA damage that could result in cancer by scavenging free radicals.
According to certain research, Q. indica extracts may prevent cancer cells from migrating and invading, two essential stages in the metastatic process. This capacity might reduce the cancer's potential to spread to other tissues.
7.14. Improve benign prostatic hyperplasia
The therapeutic effectiveness of Q. indica extract was investigated by Wijerathane et al. (2017) using a testosterone-induced BPH rat model and the human prostate cancer cell line LNCaP to treat benign prostatic hyperplasia (BPH). Prostate specific antigen (SPA), testosterone propionate (TP), androgen receptor (AR) were added to Q. indica extract-treated cancer cells, and the results demonstrated that Q. indica therapy inhibited TP-induced increases in AR and SPA expression in LNCaP cells. The anti-proliferative and proapototic properties of Q. indica dramatically reduced the expression of 5-α-reductase mRNA, prostate weight, TP-induced prostatic hyperplasis, proliferating cell nuclear antigen (PCNA), and cyclin D in BPH rats [61]. Dee-geon-kim et al. (2020) investigated the effectiveness of Q. indica extract on symptoms related to the lower urinary tract and discovered that it lessened the rise in urethral pressure, which is one of the most noticeable symptoms of an enlarged prostate [62].
According to certain research, plant extracts may have an impact on the hormonal processes that lead to prostate enlargement. If Q. indica has comparable effects, it may be able to control levels of DHT and testosterone, two substances that contribute to the development of BPH.
7.15. Anti-ulcer activity
Ulcers were induced using various methods including pylorus ligation, ethanol, and stress-induced models. Group 1 served as the negative control, while Group 2 (the standard group) received Sucralfate at a dosage of 8.6 mg/kg. Group 3 was administered an aqueous extract of Q. indica (AEQI) at 200 mg/kg, and Group 4 received AEQI at 400 mg/kg. Group 5 was treated with an ethanolic extract of Q. indica (EEQI) at 200 mg/kg, and Group 6 received EEQI at 400 mg/kg. All treatments were given orally twice daily. Upon completion of the treatment course, blood and gastrointestinal samples were collected for biochemical analysis. The mechanism of the extracts was investigated using acetylcholine and histamine drug response curves. The groups treated with the extracts showed a significant reduction in the ulcer index, with the antiulcer effects of AEQI and EEQI being dose-dependent. Hematological, hepatic, and cardiac parameters were largely unaffected by the extracts, although there was a notable increase in high-density lipoprotein levels. The drug response curve analysis indicated that AEQI and EEQI inhibit acetylcholine and histamine. These findings were further supported by histopathological analysis [63].
According to certain research, Q. indica may increase the stomach lining's production of mucus that functions as a barrier against acid and digestive enzymes. To stop ulcers, this mucosal defence is essential. It is possible that Q. indica can control the secretion of stomach acid. It can promote ulcer healing and stop the eroding of the stomach lining by lowering the amount of acid produced excessively.
Drawbacks of Quisqualis indica
-
1.
Aggressive growth and invasiveness
The fast-growing vine Quisqualis indica, sometimes referred to as rangoon creeper, has the potential to become invasive in some areas. Due to its speedy growth, it frequently suffocates neighboring plants and takes over garden areas. In a garden setting, this aggressive behavior might be difficult to regulate. If the plant isn't regularly pruned and maintained, it may take over the landscape and harm other plant species. The plant has the potential to become an invasive species in some areas where it is not native, upsetting local ecosystems by outcompeting native plants for resources like sunlight, water, and nutrients. To keep it from becoming out of hand, gardeners and landscapers must closely monitor and control its development [63].
-
2.
Root invasiveness and structural damage
Apart from its vigorous growth above ground, Q. indica's root system can also be problematic. Strong roots can make the plant invasive, particularly if it is planted close to pavement, walls, or fences. The roots have the ability to pierce structural fissures over time, damaging pavement, walls, and foundations. Because of this, the plant is inappropriate for tiny gardens or places with limited space because of how much its roots can reach below the surface. To prevent any harm, homeowners should plant Q. indica at a safe distance from structures. To stop long-term damage, its root system must be managed via barriers or containment methods [63].
-
3.
High maintenance and pruning requirements
Q. indica needs a lot of care since it grows quickly and is a widespread plant. To keep the plant's growth, form, and spread under control, gardeners must frequently prune it. Pruning keeps the plant from growing too lanky or overgrown and encourages healthy growth and blossoming. Furthermore, because the plant is climbing, it requires support structures like fences or trellises in order to grow appropriately. The plant's aesthetic attractiveness may be diminished if it is neglected and becomes messy. The Q. indica plant might not be the greatest choice for anyone searching for low-maintenance vegetation [63].
-
4.
Sensitivity to frost and cold weather
Q. indica thrives in tropical and subtropical climates, which means it is not frost-tolerant. In areas with cold winters or frost, the plant may die back or struggle to survive outdoors. While it may be possible to grow the plant in containers and bring it indoors during the winter, this adds to the maintenance burden. Its sensitivity to frost limits its cultivation to warmer climates or requires extra care and attention in colder regions. Gardeners in temperate zones may find that they need to provide additional protection, such as covering the plant or moving it to a sheltered location during cold snaps, to ensure its survival [63].
-
5.
Pest and Disease Susceptibility
Despite its relative hardiness, Q. indica is susceptible to a number of pests and diseases. Aphids, mealybugs, and spider mites are common garden pests that can harm the plant's leaves, stems, and flowers; infestations can weaken the plant, reduce its ornamental value, and cause stunted growth if not treated promptly; fungal infections can also arise in humid conditions, especially if the plant's foliage remains wet for extended periods of time. Proper maintenance, monitoring, and pest control are necessary to maintain the plant's health [63].
-
6.
Potential Toxicity and Allergic Reactions
Despite being used in traditional medicine and being harmless in moderation, Q. indica seeds are known to contain substances that can be harmful if ingested in excessive amounts, especially by young children. If the plant is cultivated in a place where kids or pets may get to it, there could be a risk. Furthermore, when exposed to the pollen or blossoms of the plant, some people may develop allergic responses, which can cause symptoms including skin irritation or breathing problems. This means that the placement of the plant is crucial, particularly in houses where there are sensitive people living there [63].
-
7.
Environmental Water Requirements
For areas that are vulnerable to drought or water scarcity, Q. indica's hydration requirements may pose a disadvantage. For the plant to retain its lush foliage and copious flowering, continuous watering is necessary, especially during dry times. As a result, it is less appropriate for places where conserving water is important. Given that Q. indica's water requirements can make garden maintenance more difficult during times of little rainfall, drought-tolerant plants would be a better option in these circumstances. Q.indica is nevertheless a well-liked plant despite these disadvantages because of its attractiveness and aroma. However, when considering whether to include it in a garden, one must take into account its propensity for invasiveness, high maintenance requirements, and environmental limits [63].
-
8.
Pharmacognostic features of Q. indica
Flowers are fragrant, tubular, showy, first white, then becoming red, reddish-purple or orange, exhibiting the range of colors in clusters, on the same flower stalk. Eisikowitch and coworkers proved that the flowering period of every flower is near 3 days where as at the night time the flowers are deled by hawk moths while the red flowers are neglected by night visitors. During the day, pink and red flowers are visited by a wide range of visitors: solitary bees, honeybees, flies, and sunbirds. Pollen grains germinate well on the stigmatic fluid during the first few hours, but germination is reduced during the day. Pollen tubes do not penetrate into the style, and seeds are not produced in Israel. Nectar flow begins at flower dehiscence, reaches its peak at early morning, and then is absorbed by the flower. During the first hours of blooming, the flower is typically "hawk moth" but, by the next morning, attracts visitors other than hawk moths. Fruit is narrowly ellipsoid, 2.5–3 centimeters long, with five, sharp, longitudinal angles or wings. Seeds are pentagonal and black. Fresh leaves of Q. indica are dark green in color, compound, alternate arrangement, 8–10 pairs of veins are present, slightly crenate to entire margin, acuminate apex, ventral surface is smooth, dorsal surface is rough, pinnate venation, base symmetrical ovate shaped, bitter taste and odorless. Mayank and coworkers investigated the microscopic characters of leaf (through midrib and lamina) showed the following characters like presence of various areas such as upper epidermis, lower epidermis, parenchymatous cells, colenchymatous cells trichomes, xylem and phloem. Upper epidermis is uniseriate without intracellular spaces except stomata, slightly curvier, covered with cuticle. Single layer of a single palisade layer with 4–5 layers of spongy collenchyma just below the upper epidermis and above lower. Whole of midrib filled with collenchyma with different types of trichomes like covering, glandular. Midrib is almost triangular shows the presence of endodermal layer, it is a single layered, surrounds with vascular bundle, packed with starch grains. Endodermis covers vascular bundle and contains a number of starch grains. Leaf surface (upper & lower surface) study shows the presence of epidermal cells, parasitic stomata, and subsidiary cells. Powder microscopy proved the presence of vessels, covering trichome, glandular trichome calcium oxalate crystals, epidermal cells, parasitic stomata [68], [69].
-
9.
Toxicological profile of Q. indica
Jeong-Won Kim and team were studied the toxicological profile of potential repeated dose toxicity and genotoxicity of a standardized Q. indica L. seed ethanolic extract were determined under good laboratory practice conditions. Sprague–Dawley (SD) rats were used in this study at doses of 500, 1000, and 2000 mg/kg/day for 13 consecutive weeks. The genotoxicity of plant was determined with a standard battery of genotoxicity test, including an in vitro bacterial reverse mutation test, in vitro chromosomal aberration test and an in vivo micronucleus test. After 13 weeks of repeated dose of plant extract by oral administration, there was no treatment related adverse clinical sign including food consumption, organ weights, and histopathological findings or significant decrement in bodyweight and no-observed-adverse-effect was higher than 2000 mg/kg in both male and female SD rats. No target organs were identified. In addition, no evidence of genotoxicity was detected based on results from the bacterial reverse mutation test, chromosomal aberration test, and micronucleus test. Based on results of this study, Q. indica could be safely used in food and medical products within the tested dose range [70].
Samu M and coworkers investigated the different extracts of Q. indica linn. (leaves, flower, stem and roots) like petroleum ether, ethyl acetate, 80 % ethanol and water were tested for cytotoxic activity on L269cells using the MTT assay. MTT assay was used to evaluate the reduction of viability of cell cultures in the presence and absence of the extracts. Cell viability was inhibited to different extents by the extracts. Ethanolic extract of leaves and aqueous extract of flowers were not cytotoxic at 500 μg/ml. Ethanolic extract of stem and aqueous extract of roots exhibited weak cytotoxic activity. Petroleum ether extract of flowers, ethyl acetate extract leaves and flowers and ethanolic extract of flowers showed stronger cytotoxic activity [71].
8. Conclusion
The review of Q. indica highlights its broad spectrum of pharmacological activities, making it a valuable candidate for various therapeutic applications, particularly within the framework of the Chinese medicinal system. The plant exhibits significant antiulcer properties, as demonstrated by its efficacy in pylorus ligation, ethanol-induced, and stress-induced ulcer models, with both aqueous (AEQI) and ethanolic extracts (EEQI) reducing ulcer indices in a dose-dependent manner. Additionally, Q. indica has shown inhibitory effects on acetylcholine and histamine, contributing to its antiulcer potential. Beyond its gastrointestinal benefits, Q. indica also possesses anti-inflammatory, antioxidant, and antimicrobial activities, aligning well with the holistic and preventive approaches of traditional Chinese medicine. The plant's extracts have not shown adverse effects on hematological, hepatic, or cardiac parameters, although they positively influence high-density lipoprotein levels. These findings underscore the importance of further research into the bioactive compounds of Q. indica and their mechanisms of action. By integrating Q. indica into the Chinese medicinal system, there is potential to enhance treatment options for a variety of conditions, leveraging its multifaceted pharmacological properties.
Future perspective
The promising pharmacological profile of Chinese honeysuckle opens up several avenues for future research and development. Key areas for further exploration include:
Mechanistic Studies: Detailed investigations into the molecular mechanisms underlying the antiulcer, anti-inflammatory, antioxidant, and antimicrobial effects are essential. This can help identify specific bioactive compounds responsible for these activities and their targets within the human body.
Clinical Trials: While preclinical studies provide a strong foundation, clinical trials are necessary to confirm the efficacy and safety of Q. indica extracts in human populations. These studies should focus on various dosages, formulations, and treatment durations to optimize therapeutic protocols.
Toxicological Assessments: Comprehensive toxicological studies are needed to establish the long-term safety profile of Chinese honeysuckle. These should include evaluations of its potential for toxicity, genotoxicity, and any adverse effects on major organ systems.
Standardization of Extracts: Developing standardized extracts of Q. indica with consistent bioactive compound concentrations will be crucial for ensuring reproducibility and efficacy in both research and therapeutic applications.
Formulation Development: Innovative formulations, such as nanoparticles, sustained-release systems, and combination therapies, should be explored to enhance the bioavailability and therapeutic effects of extracts.
Integration into Traditional Medicine: Given its alignment with the principles of traditional Chinese medicine (TCM), efforts should be made to integrate Chinese honeysuckle into TCM practices. This could involve collaboration with TCM practitioners to develop new therapeutic protocols that incorporate Q. indica.
Expanding Therapeutic Applications: Beyond its current known uses, research should explore other potential therapeutic applications, such as in metabolic disorders, neurodegenerative diseases, and cancer. Investigating its synergistic effects with other medicinal plants could also yield new treatment strategies.
Sustainable Cultivation Practices: To support the increased demand for Q. indica, sustainable cultivation practices need to be developed. This includes optimizing growing conditions, harvesting techniques, and post-harvest processing to ensure a reliable supply of high-quality plant material. By addressing these future prospects, Chinese honeysuckle can be effectively developed into a versatile and valuable medicinal resource, contributing significantly to modern healthcare and traditional medicinal systems alike.
CRediT authorship contribution statement
Akash Ved: Formal analysis. Yogesh Murti: Data curation. Deepti M Sati: Conceptualization. Anuj Kumar Sharma: Writing – review & editing. Anita Singh: Software. Arpita Singh: Investigation. Sachdev Yadav: Methodology. Mayank Kulshreshtha: Supervision. Karuna Shanker Shukla: Resources. Amit Kumar Nigam: Investigation. Manjul Pratap Singh: Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Prof. L.H. Lash
Data Availability
Data will be made available on request.
References
- 1.Stewart R. Herbalism most common form of medicine available. East Pharm. 1997;475:21. [Google Scholar]
- 2.Kulshreshtha M., Shukla K.S., Tiwari G.A., Singh M.P., Singh A. Pharmacognostical, phytochemical and pharmacological aspects of Quisqualis indica: an update. J. Nat. Sci. Med. 2018;1:41–47. [Google Scholar]
- 3.Joshi S.G. Medicinal plants. First ed. Oxford & IBH Publishing Co Pvt. Ltd; Delhi: 2002. [Google Scholar]
- 4.Kirtikar K.R., Basu B.D. Indian Medicinal plant. Second ed. Allahabad Lalit Mohan Basu; New Delhi: 2006. [Google Scholar]
- 5.Bottcher H. Miracle Drugs 1965 Zagreb Zora:23–139.
- 6.Wiart C. New Jersey Humana Press; 2006. Etnopharmacology of Medicinal Plants; pp. 1–50. [Google Scholar]
- 7.Tucakov J. Healing with Plants – Phytotherapy. Beogr. Cult. 1971:180–900. [Google Scholar]
- 8.Mukerjee P.K. Quality Control of Herbal Drugs 20021st New Delhi Business Horizons Publication:2–24.
- 9.Chaudhri R.D. Herbal Drugs Industry: A Practical approach to Industrial Pharmacognosy 20041st New Delhi Eastren Publisher:1–5.
- 10.Books E.I.R.I. Delhi Published by Engineers India Research Institute; 2009. Handbook of Medicinal and Aromatic Plants Cultivation, Utilization and Extraction Processes; pp. 1–3. [Google Scholar]
- 11.Sucher N.J., Carles M.C., et al. Genome-based approaches to the authentication of medicinal plants. Planta Med. 2008;74(6):603–623. doi: 10.1055/s-2008-1074517. [DOI] [PubMed] [Google Scholar]
- 12.Calixto J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz. J. Med. Biol. Res. 2000;33:179–189. doi: 10.1590/s0100-879x2000000200004. [DOI] [PubMed] [Google Scholar]
- 13.Ernst E., Coon Thompson “Clinical Pharmacological and Therapeutics”, 2001, 70(6): 497-504. [DOI] [PubMed]
- 14.Joshi S.G. Medicinal Plants 20021st New Delhi Oxford and IBH Publishing Co., Pvt.:122–3.
- 15.Shih Chun T.Z. “Stuartexchange” Niyog Niyogan 2011 Art Guild for Education and Communication Foundation Inc.
- 16.Kirtikar K.R., Basu B.D. Indian Medicinal Plant 1987 2nd New Delhi International Book Publishers:86–87.
- 17.http://www.thehindu.com/thehindu/mag/2003/03/16/stories/2003031600300800.htm..
- 18.https://www.florafaunaweb.nparks.gov.sg/special-pages/plant-detail.aspx?id=1497.
- 19.Munir M., et al. Excitotoxic cell death anddelayed rescue in human neurons derived fromNT2 cells. J. Neurosci. 1995;15:7847–7860. doi: 10.1523/JNEUROSCI.15-12-07847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy T.H., Schnaar R.L., et al. Glutamatecytotoxicity in a neuronal cell line is blockedby membrane depolarization. Brain Res. 1988;510(1):155–160. doi: 10.1016/0006-8993(88)91216-4. [DOI] [PubMed] [Google Scholar]
- 21.Shih Chun Tzu “Stuartexchange” niyogniyogan, Art guild for education andcommunication foundation Inc, 2011.
- 22.Kirtikar K.R., Basu B.D. Second ed. Vol. 2. Prashant Gahlot atvalley offset publishers; New Delhi: 2006. p. 1037. (Indian Medicinalplant). [Google Scholar]
- 23.Joshi S.G. Mohan Primlanifor Oxford and IBH publishing Co.pvt. Ltd; New Delhi: 1992. Medicinal plants; p. 141. [Google Scholar]
- 24.Lin Ta. Chen, et al. “Tannin and related compounds from Quisqualis indica. J. Chin. Chem. Soc. 1997;44(2):151–155. 04. [Google Scholar]
- 25.Mayank K., Gunja S., Manjul P.S. Pharmacognostical, anti-oxidant activity and high performance thin layer chromatography studies on leaves of Quisqualis indica linn. Curr. Tradit. Med. 2018 [Google Scholar]
- 26.Kulshreshtha M., Dwivedi H., Singh M.P. Antimicrobial effects of leaves of Indian herbal plants with reference to peptic ulcer. Environ. Dis. 2018;3:18–26. [Google Scholar]
- 27.Shamili, G. and G.R. Santhi. “PHARMACOGNOSTICAL STANDARDAISATION OF THE FLOWERS OF Quisqualis indica.” (2017).
- 28.Mukherjee D., Chandra G. Flower extracts of Quisqualis Indica as novel antibacterial agent against some pathogenic bacteria. Ann. Pharm. Pharm. 2017;2(7):1040. [Google Scholar]
- 29.Barik Braja, Das Shritam, Hussain Tahziba. Pharmacognostic Properties of Quisqualis indica Linn: Against Human Pathogenic Microorganisms: an Insight Review. Eur. J. Med. Plants. 2020;31:87–103. doi: 10.9734/EJMP/2020/v31i2030369. [DOI] [Google Scholar]
- 30.Rout P.K., Kumar P., Rao Y.R., Kumar A., Bawankule D.U., Singh R., Singh K.B., Chanotiya C.S., Naik S.N. A quinoline alkaloid rich Quisqualis indica floral extract enhances the bioactivity. Nat. Prod. Res. 2021 May;35(10):1632–1638. doi: 10.1080/14786419.2019.1634709. Epub 2019 Jul 2. PMID: 31264476. [DOI] [PubMed] [Google Scholar]
- 31.https://florafaunaweb.nparks.gov.sg/special-pages/plant-detail.aspx?id=1497 [Last assessed on 1 february 2017].
- 32.Nadkarni A.K. Indian Materia Medica. Third ed. Popular Prakashan; Mumbai: 2000. pp. 1046–1047. [Google Scholar]
- 33.Kirtikar K.R., Basu B.D. Indian Medicinal Plants. Second ed. Periodical expert book agency; Delhi: 1993. pp. 1037–1038. [Google Scholar]
- 34.The Wealth of India . -VIII: Ph-Re. Council of Scientific & Industrial Research; New Delhi: 2005. pp. 357–358. (A Dictionary of Indian Raw Materials and Industrial Products). [Google Scholar]
- 35.Agarwal A., Prajapati R., Raza S.K., Thakur L.K. GC-MS Analysis and Antibacterial Activity of Aerial Parts of Quisqualis indica Plant Extracts. Indian J. Pharm. Educ. 2017;51:329–336. [Google Scholar]
- 36.Yashraj Yadav, Mohanty P.K., et al. Antiinflammatory activity of hydroalcoholic extract of Quisqualis indica Linn. flower in rats. Int. J. Pharm. Life Sci. 2011;2:977–981. [Google Scholar]
- 37.Raju Gautam, Jachak M.S. Naturally occurring polyphenols with anti-inflammatory activity”. CRlPS. 2007;8(4):20–32. [Google Scholar]
- 38.Singh Nitu, et al. Antipyretic activity of methanolic extract of leaves of Quisqualis indica linn. IJPRD. 2010;2:122–126. [Google Scholar]
- 39.Yashraj Yadav, Mohanty P.K. Evaluation of immunomodulatory activity of hydroalcoholic extract of Quisqualis indica Linn. flower in wistar rats. IJPLS. 2011;2 689-686. [Google Scholar]
- 40.Jahan Fatima N., Rahman Mohammad S. Diphenylpropanoids from Quisqualis indica Linn. and their Anti-staphylococcal Activity. Lat. Am. J. Pharm. 2009;28(2):279–283. [Google Scholar]
- 41.Pauli A. Alternative Antibiotics with Specific Anti-Staphylococcal Activity; International Conference on Emerging Infectious Diseases, Program and Abstracts Book, Atlanta, USA, 2002:136.
- 42.Kaisar Md. Abul, Islam Mohammad Rashedul, et al. Total Phenolic Content, Free Radical Scavenging Activity and Reducing Power of Quisqualis indica Linn. Dhaka Univ. J. Pharm. Sci. 2009;8(2):173–175. [Google Scholar]
- 43.Abd El-Rahman A.A.A., Abd E.I.A., Refahy L.A., El-Shazly M.A. Total phenolic content, cytotoxic and anti-oxidant activities of Quisqualis indica (Linn.) growing in Egypt. Der. Pharma Chem. 2016;8(3):53–59. [Google Scholar]
- 44.Afify A.E.-M.R.R., Hassan H.M.M. Free radical scavenging activity of three different flowers-Hibiscus rosa-sinensis, Quisqualis indica and Senna surattensis. Asian Pac. J. Trop. Biomed. 2016;6(9):771–777. [Google Scholar]
- 45.Bose A., Bose S., Maji S., Chakraborty P. Free radical scavenging property of Quisqualis indica. Int. J. Biomed. Pharm. Sci. 2009;3(1):1–4. [Google Scholar]
- 46.Dutta A., Biswas S., Biswas M., Ghosh P., Ghosh C., Das S., Chatterjee S. Phytochemical screening, anti-oxidant and anti-microbial activity of leaf, stem and flower of Rangoon creeper: a comparative study. J. Med. Plants Stud. 2019;7(2):123–130. [Google Scholar]
- 47.Mukherjee D., Chandra G. Flower Extracts of Quisqualis Indica as Novel anti-bacterial agent against some pathogenic bacteria. Ann. Pharmacol. Pharmaceu. 2017;2(4):1040–1047. [Google Scholar]
- 48.Kumar M., Gitika Sharma A. In vitro antibacterial activity of flower extracts of Quisqualis indica Linn. against gram positive and gram negative bacteria. Int. J. Adv. Pharm., Biol. Chem. 2014;3(3):781–785. [Google Scholar]
- 49.Kiruthika K.A., Jaisheeba A.A., Sornaraj R. Evaluation of anti-bacterial activity of some selected angiosperm flower extract. Int. J. Chem. Tech. Res. 2011;3(4):1945–1951. [Google Scholar]
- 50.Agarwal A., Prajapati R., Raza S.K., Thakur L.K. GC-MS Analysis and anti-bacterial activity of aerial parts of Quisqualis indica plant extracts. Ind. J. Pharm. Edu. Res. 2017;51(2):329–336. [Google Scholar]
- 51.Das P., Sinhababu S.P., Dam T. Screening of anti-helminthic effects of Indian plant extracts: a preliminary report. J. Alt. Complement. Med. 2006;12(3):299–301. doi: 10.1089/acm.2006.12.299. [DOI] [PubMed] [Google Scholar]
- 52.Hai N., Atsushi M., Nguyen H.T.T., Nguyen T.V. A study on anit-helmintic and antibacterial effects of extracts from Chinese honeysuckle (Quisqualis indica L.) seeds and areca (Areca catechu) nuts. Asian J. Pharm. Clin. Res. 2019;12(6):88–92. [Google Scholar]
- 53.Sarma S.K., Srinivasan R., Kumar R., Nagajyothi D., Prabhavathi V., Bai M.S. Evaluation of anti-helminthic activity of leaves of Quisqualis indica L. World J. Pharma. Pharm. Sci. 2015;4(04):819–824. [Google Scholar]
- 54.Singh N., Mohan G., Sharma R.K., Gnaneshwari D. Evaluation of antidiarrhoeal activity of Quisqualis indica L. leaves. Ind. J. Nat. Prod. Res. 2013;4(2):155–160. [Google Scholar]
- 55.Verma J., Arora D., Singh A. Evaluation of anti-hyperglycaemic potential of the ethanolic leaf extract of Quisqualis indica. Biosci. Biotechnol. Res. Comm. 2018;11(2):324–334. [Google Scholar]
- 56.Govindarajan M., Vijayan P., Kadaikunnan S., Alharbi N.S., Benelli G. One-pot biogenic fabrication of silver nanocrystals using Quisqualis indica: effectiveness on malaria and Zika virus mosquito vectors, and impact on non-target aquatic organisms. J. Photochem. Photobiol. B: Biol. 2016;162:646–655. doi: 10.1016/j.jphotobiol.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 57.Mukhopadhyay R., Kazi J., Debnath M.C. Synthesis and characterization of copper nanoparticles stabilized with Quisqualis indica extract: evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed. Pharmacother. 2018;97:1373–1385. doi: 10.1016/j.biopha.2017.10.167. [DOI] [PubMed] [Google Scholar]
- 58.Jyoti S., Pushpendra K.P., Balkrishna D. Effects of methanolic extracts of Quisqualis indica (Aerial Parts) on passive smoking induced hyperlipidemia in rats. J. Pharm. Technol. 2013;3:26–29. [Google Scholar]
- 59.Efferth T., Kahl S., Paulus K., Adams M., Rauh R., Boechzelt H. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese materia medica with activity against tumor cells. Mol. Cancer Ther. 2008;7:152–161. doi: 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- 60.Lohberger B., Kretschmer N., Bernhart E., Rinner B., Stuendl N., Kaltenegger H., Kahl S., Bauer R., Leithner A. 25-O-acetyl-23,24- dihydro-cucurbitacin F induces cell cycle G2/M arrest and apoptosis in human soft tissue sarcoma cells. J. Ethnopharmacol. 2015;164:265–272. doi: 10.1016/j.jep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 61.Ub Wijerathne C., Park H.S., Jeong H.Y., Song J.W., Moon O.S., Seo Y.W. Quisqualis indica improves benign prostatic 103 hyperplasia by regulating prostate cell proliferation and apoptosis. Biol. Pharm. Bull. 2017;40:2125–2133. doi: 10.1248/bpb.b17-00468. [DOI] [PubMed] [Google Scholar]
- 62.Kim D.G., Kwon H.J., Lim J.H., Kim J.H., Lee K.P. Quisqualis indica extract ameliorates low urinary tract symptoms in testosterone propionate-induced benign prostatic hyperplasia rats. Lab. Anim. Res. 2020;36(1):1–10. doi: 10.1186/s42826-020-00059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayank K., Rajaneesh K.C., Supriya R., Karuna S.S., Anita S., Manjul P.S. Pharmacological Investigation on Unravelling Mechanism of Action of Quisqualis indica Leaves for Predicted Treatment of Peptic Ulcer Disease. Curr. Funct. Foods. 2023;1(2) e200323214728. [Google Scholar]
- 64.Srivastava Pragati, Murti Yogesh, Panjwani Dharamveer, Kulshreshtha Mayank. Anti-diabetic potential of Aconitum ferox roots. Pharmacol. Res. - Mod. Chin. Med. December 2023;9 [Google Scholar]
- 65.Jyoti S., Pushpendra K.P., Balkrishna D. Effects of Methanolic Extracts of Quisqualis indica (Aerial Parts) on Passive Smoking Induced Hyperlipidemia in Rats. J. Pharm. Technol. 2013;3(1):26–29. [Google Scholar]
- 66.Marimuthu G., Periasamy V., Shine K., Naiyf S., Alharbib G.B. One-pot biogenic fabrication of silver nanocrystals using Quisqualis indica: effectiveness on malaria and Zika virus mosquito vectors, and impact on non-target aquatic organisms. J. Photochem. Photobiol. B: Biol. 2016;162:646–655. doi: 10.1016/j.jphotobiol.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal A., Prajapati R., Raza S.K., Thakur L.K. GC-MS analysis and antibacterial activity of aerial parts of Quisqualis indica plant extracts. Indian J. Pharm. Educ. Res. 2017;51(2):329–336. [Google Scholar]
- 68.http://www.stuartxchange.com/Niyog.html [Last assessed on 21 September 2024].
- 69.Eisikowitch D., Rotem R. Flower orientation and color change in Quisqualis indica and their possible role in pollinator partitioning. Bot. Gaz. 1987;148(2):175–179. [Google Scholar]
- 70.Kim J.W., Kim H., Park H., Yoon J.S., Kim M.I., Ko J.W., Kim T.W. Repeated oral dose toxicity and genotoxicity of a standardized Quisqualis indica extract. Toxicol. Res. 2022;38(4):577–589. doi: 10.1007/s43188-022-00140-6. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samu A.M., Jose J., Thomas T. Cytotoxic activity of crude extracts from Quisqualis indicaLinn. (Combreteceae) IJDDR. 2013;4(3):49–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


