Abstract
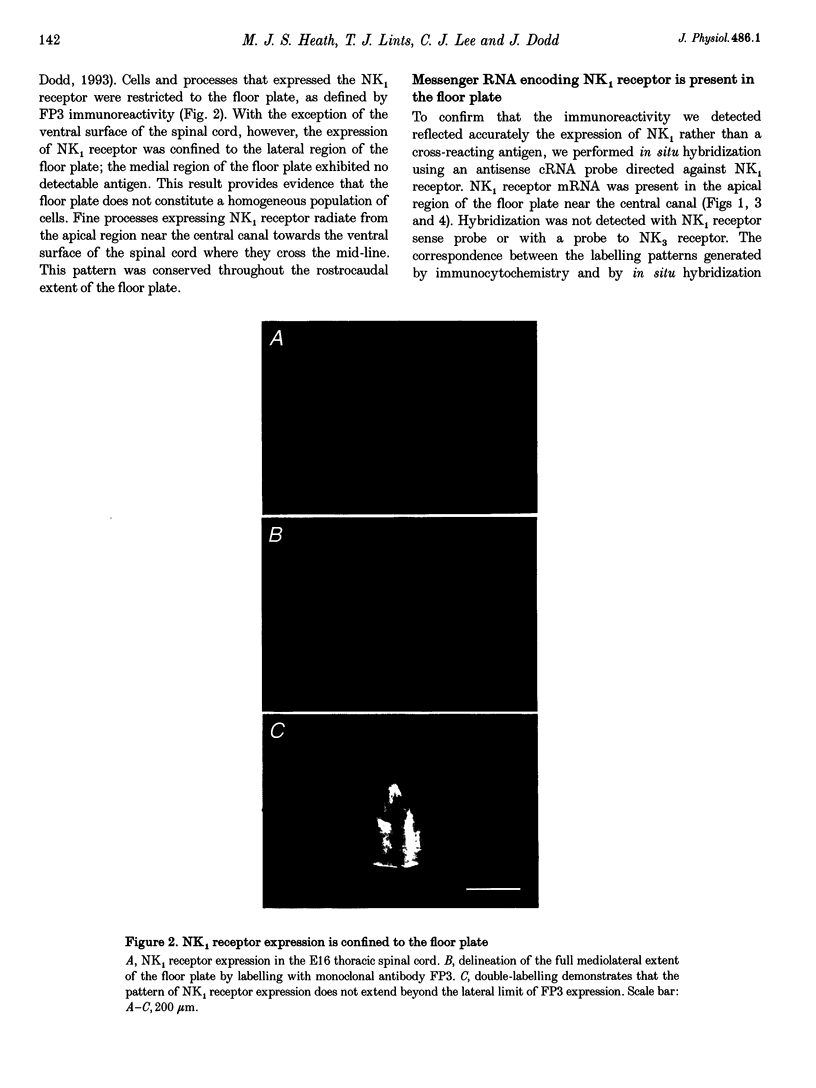
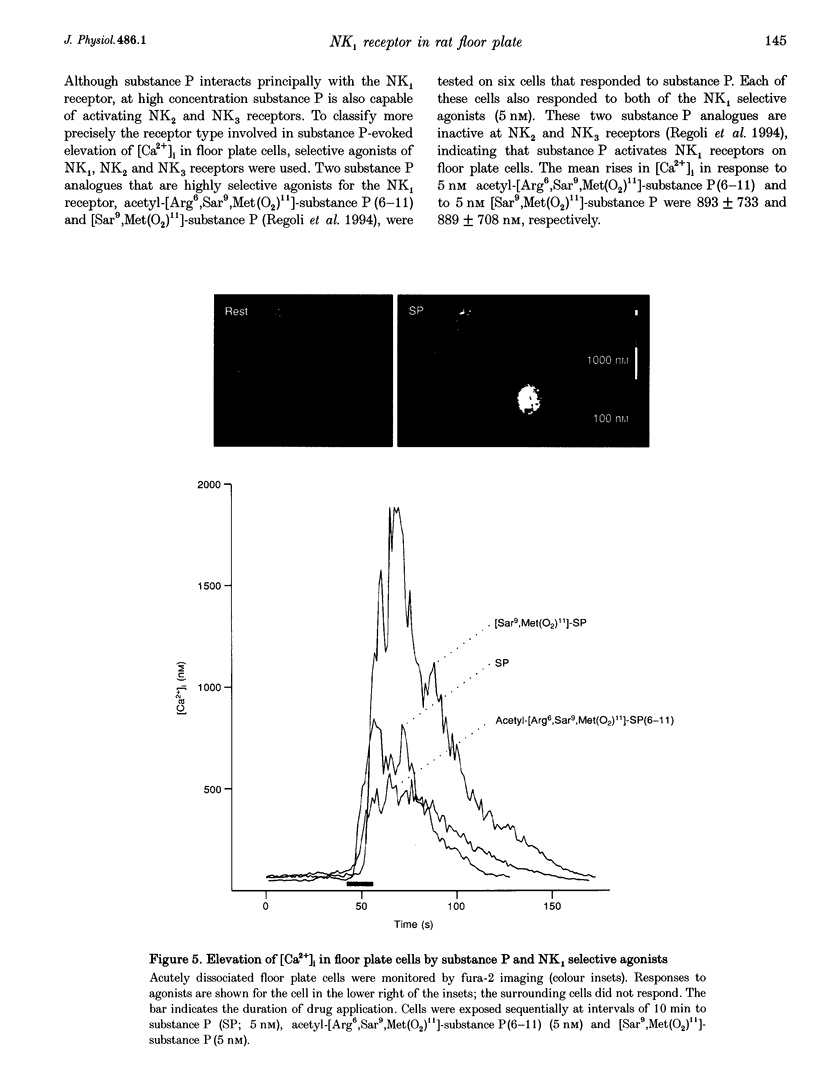
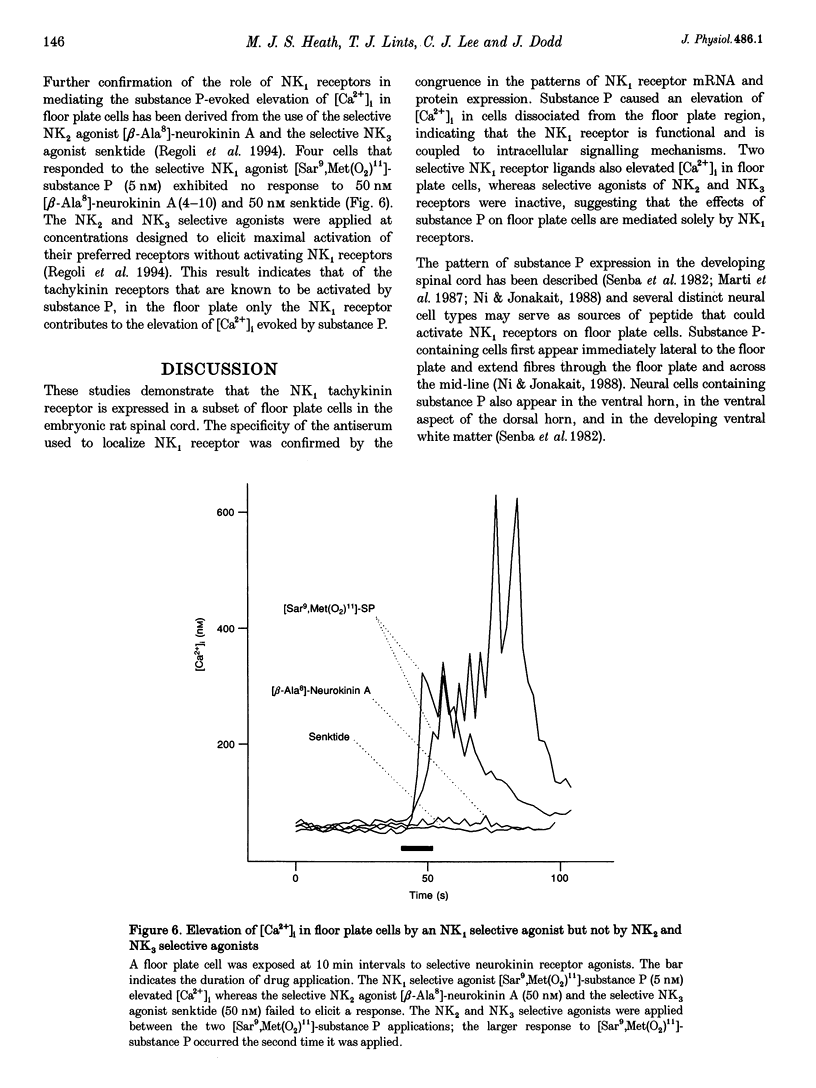
1. The floor plate is a ventral mid-line structure that plays a pivotal role in the organization of the developing vertebrate central nervous system. Previous studies have demonstrated that the floor plate may provide signals that induce neuronal differentiation and guide axons; however, it is not known whether the floor plate can itself respond to signals that derive from surrounding tissue. 2. The peptide substance P is one of the first transmitters to be expressed in the developing spinal cord. To determine whether the floor plate may respond to substance P we have examined the expression of the principal substance P receptor (the tachykinin NK1 receptor) by floor plate cells of the rat embryonic spinal cord using immunocytochemistry, in situ hybridization and fura-2 calcium imaging. 3. Immunocytochemistry demonstrated selective expression of the NK1 receptor by cells at the ventral mid-line of the spinal cord. Double immunofluorescence labelling with the specific floor plate marker FP3 indicated that NK1 receptor expression is confined to cells in the lateral region of the floor plate. 4. In order to confirm the specificity of the NK1 receptor immunoreactivity we performed in situ hybridization histochemistry using antisense cRNA probes directed against the NK1 receptor. In situ hybridization demonstrated selective expression of NK1 receptor mRNA by floor plate cells. 5. The ontogeny of NK1 receptor protein and mRNA expression in the floor plate was defined. NK1 receptor expression occurred in a rostrocaudal progression that begins at embryonic day 10-11 (E10-E11) and is complete by E12-E14. The restriction of NK1 receptor expression to the lateral part of the floor plate was conserved throughout embryonic development. 6. NK1 receptor signalling was assessed by monitoring substance P-evoked changes in the intracellular concentration of calcium ions ([Ca2+]i) of acutely dissociated cells from the floor plate region. Application of substance P (5 nM) elevated [Ca2+]i in 10% of cells examined. 7. Selective neurokinin agonists were used to identify the receptor subtype involved in the substance P-evoked elevation of [Ca2+]i. Acetyl-[Arg6,Sar9,Met(O2)11]-substance P(6-11) (5 nM) and [Sar9,Met(O2)11]-substance P (5 nM), two highly selective NK1 receptor agonists, both elevated [Ca2+]i in floor plate cells that responded to substance P. [beta-Ala8]-neurokinin A(4-10) (50 nM) and senktide (50 nM), selective agonists respectively of NK2 and NK3 receptors, had no effect on [Ca2+]i.
Full text
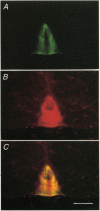
PDF




Images in this article
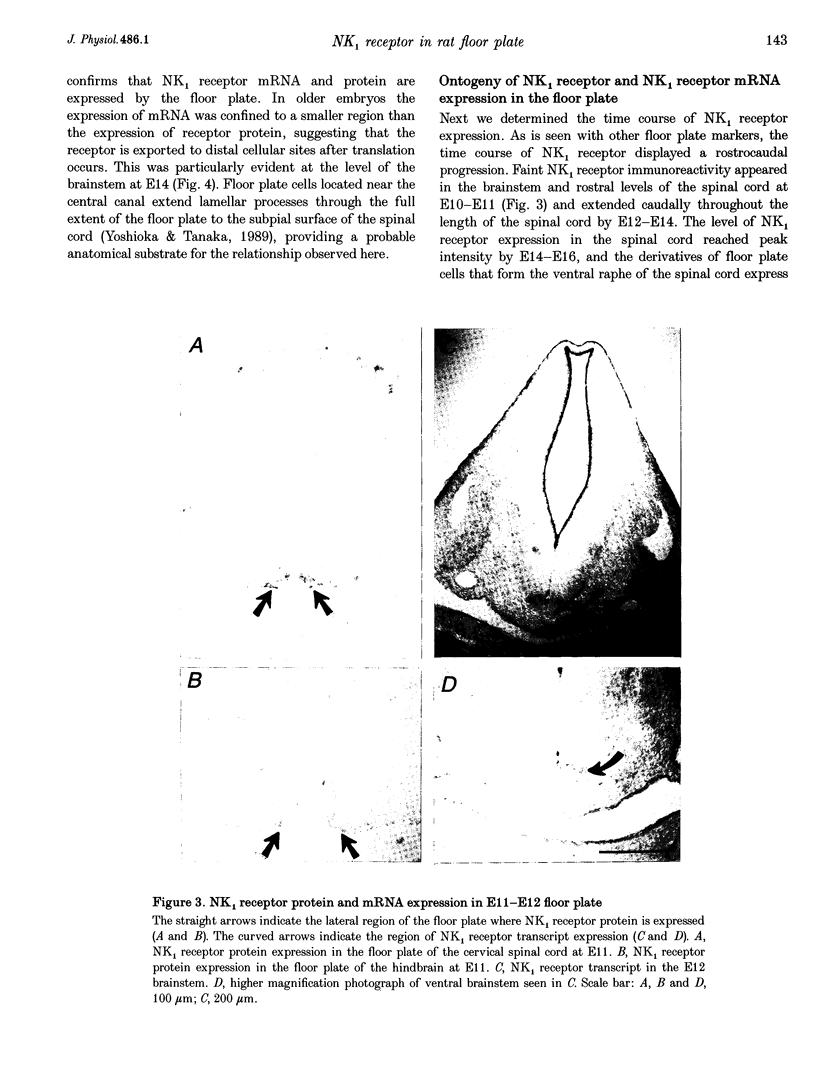
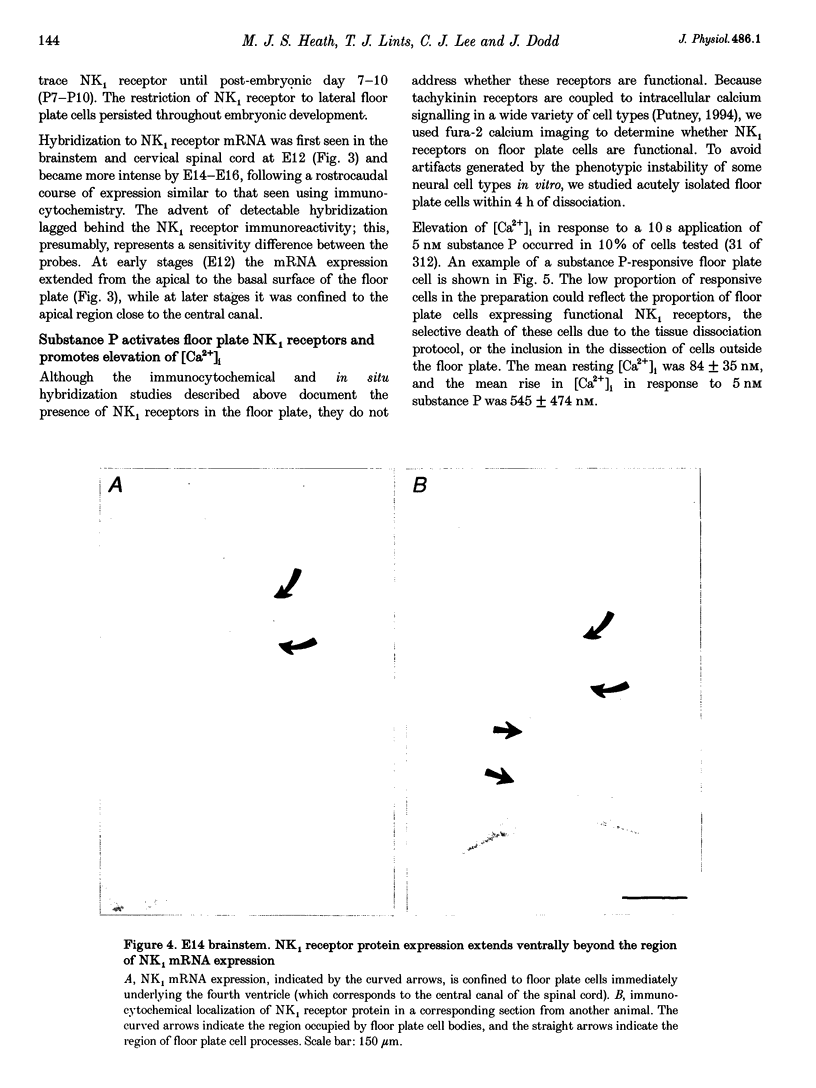
Selected References
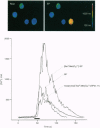
These references are in PubMed. This may not be the complete list of references from this article.
- Bovolenta P., Dodd J. Guidance of commissural growth cones at the floor plate in embryonic rat spinal cord. Development. 1990 Jun;109(2):435–447. doi: 10.1242/dev.109.2.435. [DOI] [PubMed] [Google Scholar]
- Bovolenta P., Dodd J. Perturbation of neuronal differentiation and axon guidance in the spinal cord of mouse embryos lacking a floor plate: analysis of Danforth's short-tail mutation. Development. 1991 Oct;113(2):625–639. doi: 10.1242/dev.113.2.625. [DOI] [PubMed] [Google Scholar]
- Campbell R. M., Peterson A. C. Expression of a lacZ transgene reveals floor plate cell morphology and macromolecular transfer to commissural axons. Development. 1993 Dec;119(4):1217–1228. doi: 10.1242/dev.119.4.1217. [DOI] [PubMed] [Google Scholar]
- Charlton C. G., Helke C. J. Ontogeny of substance P receptors in rat spinal cord: quantitative changes in receptor number and differential expression in specific loci. Brain Res. 1986 Sep;394(1):81–91. doi: 10.1016/0165-3806(86)90084-2. [DOI] [PubMed] [Google Scholar]
- Cholewinski A. J., Hanley M. R., Wilkin G. P. A phosphoinositide-linked peptide response in astrocytes: evidence for regional heterogeneity. Neurochem Res. 1988 Apr;13(4):389–394. doi: 10.1007/BF00972490. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hatta K. Role of the floor plate in axonal patterning in the zebrafish CNS. Neuron. 1992 Oct;9(4):629–642. doi: 10.1016/0896-6273(92)90027-b. [DOI] [PubMed] [Google Scholar]
- Heath M. J., Womack M. D., MacDermott A. B. Substance P elevates intracellular calcium in both neurons and glial cells from the dorsal horn of the spinal cord. J Neurophysiol. 1994 Sep;72(3):1192–1198. doi: 10.1152/jn.1994.72.3.1192. [DOI] [PubMed] [Google Scholar]
- Hershey A. D., Dykema P. E., Krause J. E. Organization, structure, and expression of the gene encoding the rat substance P receptor. J Biol Chem. 1991 Mar 5;266(7):4366–4374. [PubMed] [Google Scholar]
- Jessell T. M., Dodd J. Floor plate-derived signals and the control of neural cell pattern in vertebrates. Harvey Lect. 1990;86:87–128. [PubMed] [Google Scholar]
- Marti E., Gibson S. J., Polak J. M., Facer P., Springall D. R., Van Aswegen G., Aitchison M., Koltzenburg M. Ontogeny of peptide- and amine-containing neurones in motor, sensory, and autonomic regions of rat and human spinal cord, dorsal root ganglia, and rat skin. J Comp Neurol. 1987 Dec 15;266(3):332–359. doi: 10.1002/cne.902660304. [DOI] [PubMed] [Google Scholar]
- McCarson K. E., Krause J. E. NK-1 and NK-3 type tachykinin receptor mRNA expression in the rat spinal cord dorsal horn is increased during adjuvant or formalin-induced nociception. J Neurosci. 1994 Feb;14(2):712–720. doi: 10.1523/JNEUROSCI.14-02-00712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKanna J. A., Cohen S. The EGF receptor kinase substrate p35 in the floor plate of the embryonic rat CNS. Science. 1989 Mar 17;243(4897):1477–1479. doi: 10.1126/science.2928781. [DOI] [PubMed] [Google Scholar]
- Narumi S., Fujita T. Stimulatory effects of substance P and nerve growth factor (NGF) on neurite outgrowth in embryonic chick dorsal root ganglia. Neuropharmacology. 1978 Jan;17(1):73–76. doi: 10.1016/0028-3908(78)90176-4. [DOI] [PubMed] [Google Scholar]
- Narumi S., Maki Y. Stimulatory effects of substance P on neurite extension and cyclic AMP levels in cultured neuroblastoma cells. J Neurochem. 1978 Jun;30(6):1321–1326. doi: 10.1111/j.1471-4159.1978.tb10462.x. [DOI] [PubMed] [Google Scholar]
- Ni L., Jonakait G. M. Development of substance P-containing neurons in the central nervous system in mice: an immunocytochemical study. J Comp Neurol. 1988 Sep 22;275(4):493–510. doi: 10.1002/cne.902750403. [DOI] [PubMed] [Google Scholar]
- Placzek M., Jessell T. M., Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993 Jan;117(1):205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- Placzek M., Tessier-Lavigne M., Jessell T., Dodd J. Orientation of commissural axons in vitro in response to a floor plate-derived chemoattractant. Development. 1990 Sep;110(1):19–30. doi: 10.1242/dev.110.1.19. [DOI] [PubMed] [Google Scholar]
- Placzek M., Yamada T., Tessier-Lavigne M., Jessell T., Dodd J. Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate. Dev Suppl. 1991;Suppl 2:105–122. [PubMed] [Google Scholar]
- Quirion R., Dam T. V. Ontogeny of substance P receptor binding sites in rat brain. J Neurosci. 1986 Aug;6(8):2187–2199. doi: 10.1523/JNEUROSCI.06-08-02187.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelink H., Augsburger A., Heemskerk J., Korzh V., Norlin S., Ruiz i Altaba A., Tanabe Y., Placzek M., Edlund T., Jessell T. M. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994 Feb 25;76(4):761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Senba E., Shiosaka S., Hara Y., Inagaki S., Sakanaka M., Takatsuki K., Kawai Y., Tohyama M. Ontogeny of the peptidergic system in the rat spinal cord: immunohistochemical analysis. J Comp Neurol. 1982 Jun 10;208(1):54–66. doi: 10.1002/cne.902080105. [DOI] [PubMed] [Google Scholar]
- Shigemoto R., Yokota Y., Tsuchida K., Nakanishi S. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem. 1990 Jan 15;265(2):623–628. [PubMed] [Google Scholar]
- Tessier-Lavigne M., Placzek M., Lumsden A. G., Dodd J., Jessell T. M. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988 Dec 22;336(6201):775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Vigna S. R., Bowden J. J., McDonald D. M., Fisher J., Okamoto A., McVey D. C., Payan D. G., Bunnett N. W. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J Neurosci. 1994 Feb;14(2):834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Pfaff S. L., Edlund T., Jessell T. M. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1993 May 21;73(4):673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- Yamada T., Placzek M., Tanaka H., Dodd J., Jessell T. M. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991 Feb 8;64(3):635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- Yoshioka T., Tanaka O. Ultrastructural and cytochemical characterisation of the floor plate ependyma of the developing rat spinal cord. J Anat. 1989 Aug;165:87–100. [PMC free article] [PubMed] [Google Scholar]