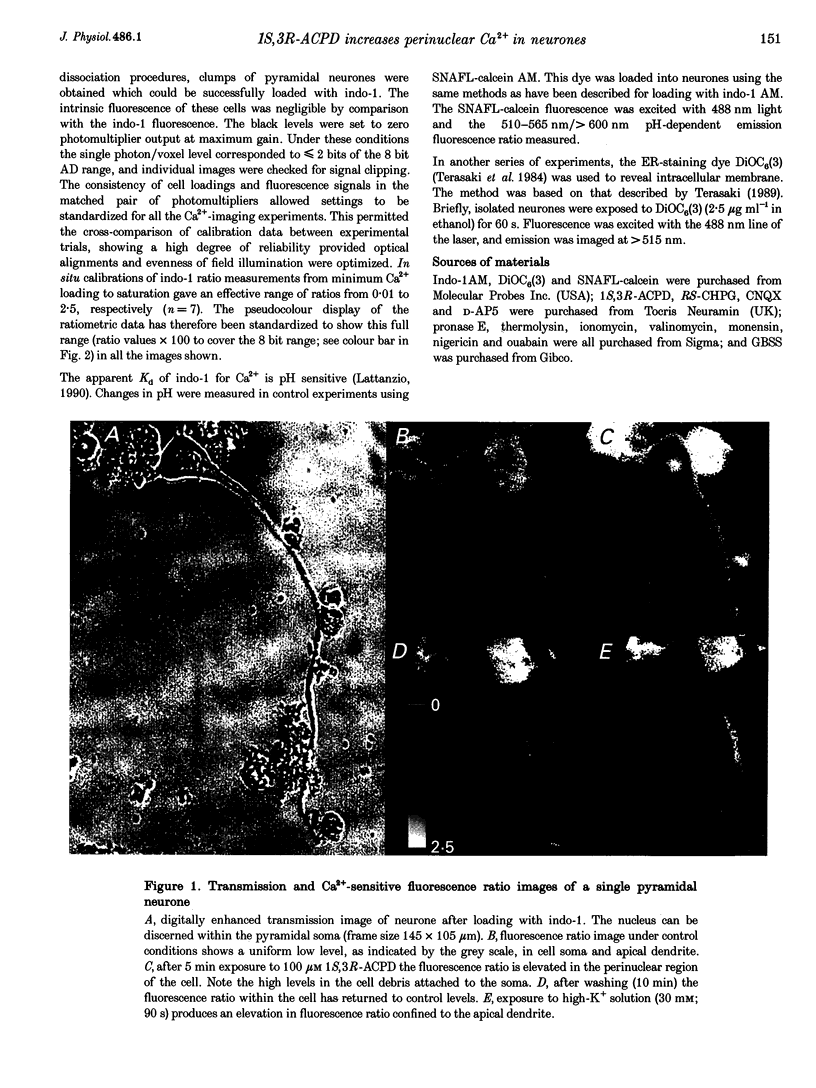
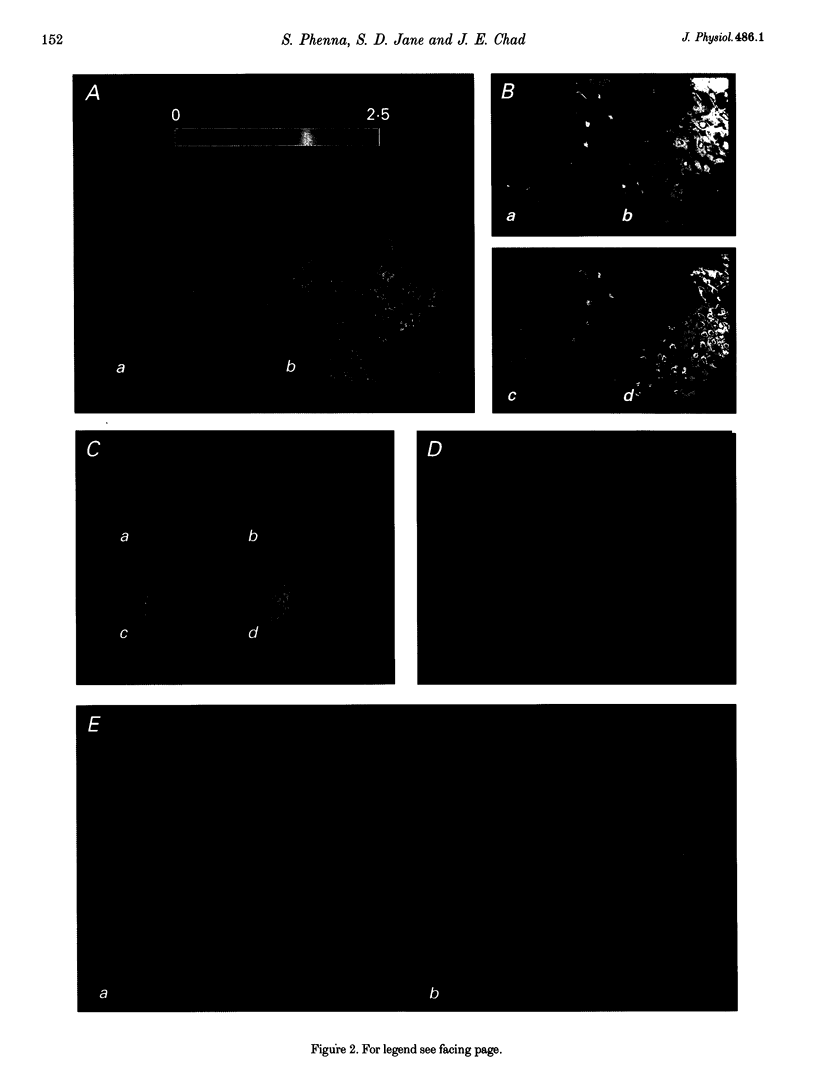
Abstract
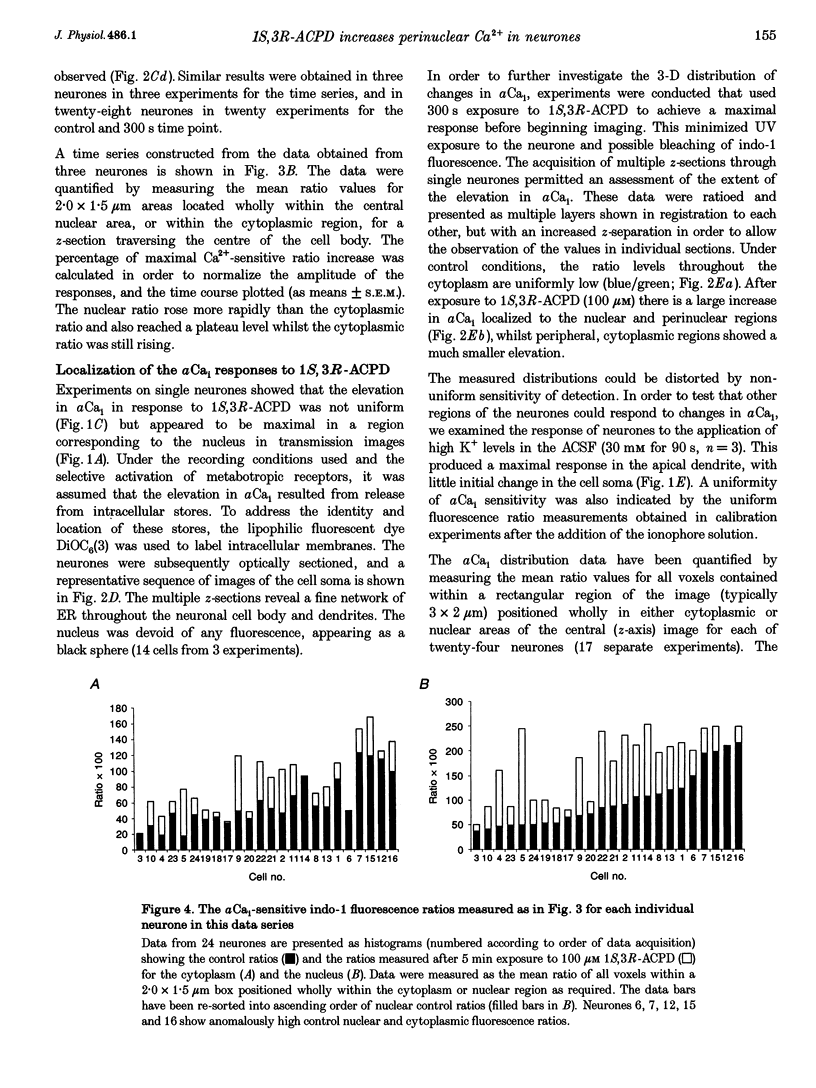
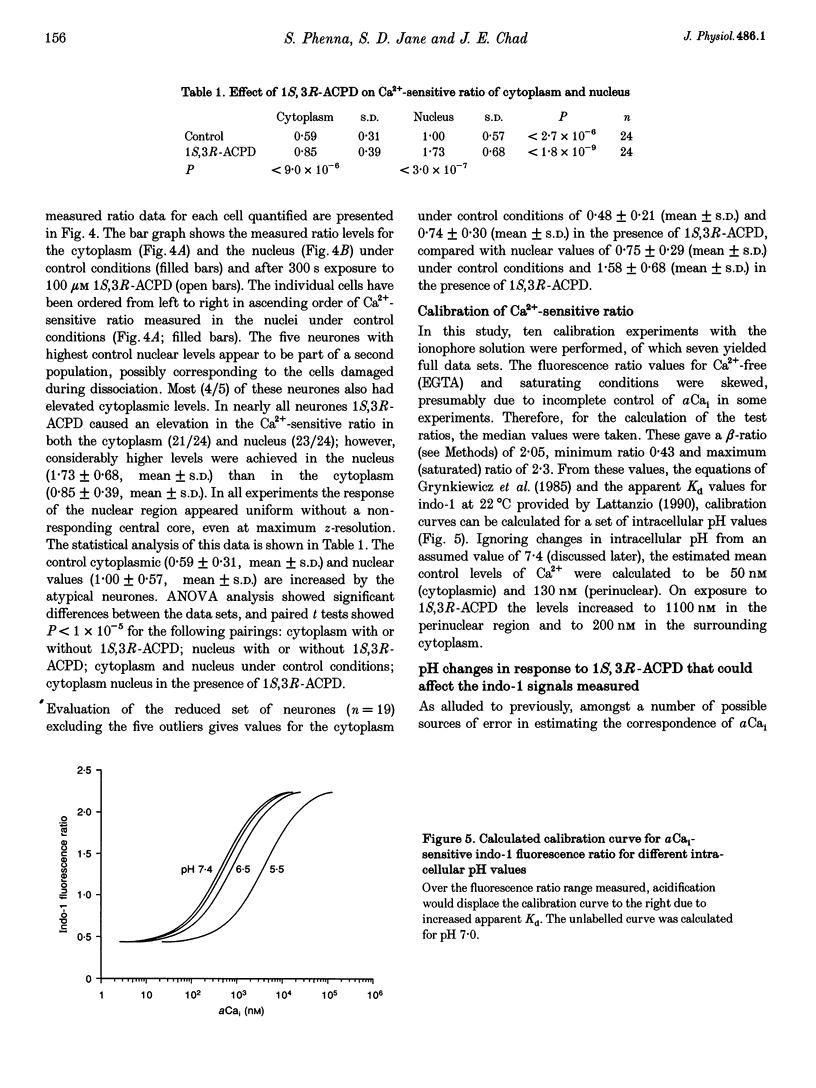
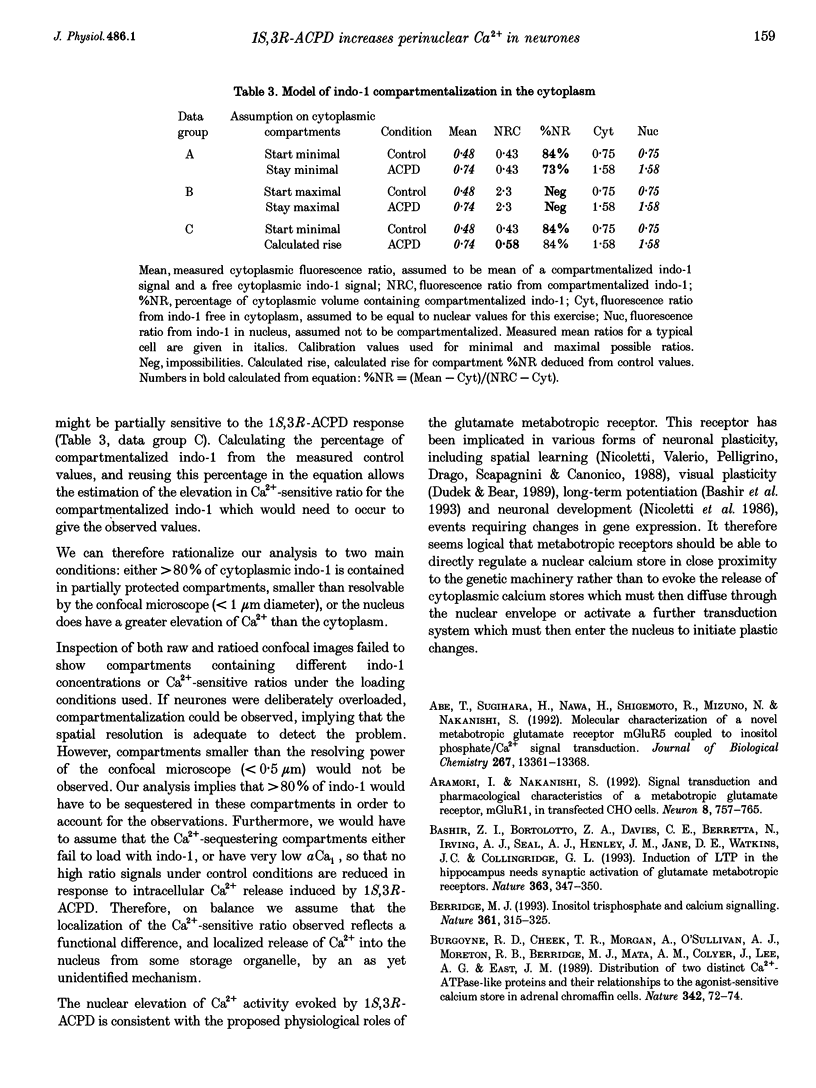
1. The effect of metabotropic glutamate receptor activation on intracellular Ca2+ activity (alpha Cai) of rat hippocampal pyramidal neurones in vitro was examined using ratiometric confocal laser scanning microscopy with the Ca(2+)-sensitive fluorescent probe indo-1 AM. 2. Metabotropic receptors were selectively activated with 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD; 100 microM) in the presence of D-2-amino 5-phosphonovaleric acid (D-APV), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and CdCl2. Most pyramidal neurones (77/84) responded with an elevation in Ca2+ activity, maximal after 3-5 min. Fluorescence ratio responses were concentration dependent (EC50 approximately 10 microM) and were blocked by prior application of the antagonist (RS)-4-carboxy-3-hydroxyphenylglycine (RS-CHPG, 300 microM). 3. Responses to 1S,3R-ACPD (100 microM) also caused acidification of the neurones, from estimated control pH 7.2 to pH 6.6 (measured with the pH-sensitive dye SNAFL-calcein). The correction factor for indo-1 determination of Ca2+ was estimated to be x 1.4. 4. Elevations in alpha Cai were greater within the perinuclear region (> 1000 nM), than in the cytoplasm (approximately 200 nM). This region was devoid of staining by the endoplasmic reticulum staining dye 3,3'-dihexyloxacarbocyanine iodide (DiOC6(3)). 5. It is concluded that activation of metabotropic receptors in immature rat hippocampal pyramidal neurones leads to a large increase in perinuclear Ca2+ which would be well positioned to interact with the genome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Sugihara H., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992 Jul 5;267(19):13361–13368. [PubMed] [Google Scholar]
- Aramori I., Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992 Apr;8(4):757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Bashir Z. I., Bortolotto Z. A., Davies C. H., Berretta N., Irving A. J., Seal A. J., Henley J. M., Jane D. E., Watkins J. C., Collingridge G. L. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993 May 27;363(6427):347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Intracellular calcium mobilization by inositol 1,4,5-trisphosphate: intracellular movements and compartmentalization. Cell Calcium. 1993 Mar;14(3):185–200. doi: 10.1016/0143-4160(93)90066-f. [DOI] [PubMed] [Google Scholar]
- Dudek S. M., Bear M. F. A biochemical correlate of the critical period for synaptic modification in the visual cortex. Science. 1989 Nov 3;246(4930):673–675. doi: 10.1126/science.2573152. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Himpens B., De Smedt H., Droogmans G., Casteels R. Differences in regulation between nuclear and cytoplasmic Ca2+ in cultured smooth muscle cells. Am J Physiol. 1992 Jul;263(1 Pt 1):C95–105. doi: 10.1152/ajpcell.1992.263.1.C95. [DOI] [PubMed] [Google Scholar]
- Irving A. J., Schofield J. G., Watkins J. C., Sunter D. C., Collingridge G. L. 1S,3R-ACPD stimulates and L-AP3 blocks Ca2+ mobilization in rat cerebellar neurons. Eur J Pharmacol. 1990 Sep 21;186(2-3):363–365. doi: 10.1016/0014-2999(90)90462-f. [DOI] [PubMed] [Google Scholar]
- Krause K. H. Ca(2+)-storage organelles. FEBS Lett. 1991 Jul 22;285(2):225–229. doi: 10.1016/0014-5793(91)80806-e. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr The effects of pH and temperature on fluorescent calcium indicators as determined with Chelex-100 and EDTA buffer systems. Biochem Biophys Res Commun. 1990 Aug 31;171(1):102–108. doi: 10.1016/0006-291x(90)91362-v. [DOI] [PubMed] [Google Scholar]
- Malviya A. N., Rogue P., Vincendon G. Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9270–9274. doi: 10.1073/pnas.87.23.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor D., Moran N., Segal M. Interactions among calcium compartments in C6 rat glioma cells: involvement of potassium channels. J Physiol. 1994 Jul 15;478(Pt 2):251–263. doi: 10.1113/jphysiol.1994.sp020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. J., Wiegmann T. B., Welling L. W., Chronwall B. M. Rapid simultaneous estimation of intracellular calcium and pH. Methods Cell Biol. 1994;40:183–220. doi: 10.1016/s0091-679x(08)61115-2. [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Iwakabe H., Akazawa C., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993 Jun 5;268(16):11868–11873. [PubMed] [Google Scholar]
- Nicoletti F., Iadarola M. J., Wroblewski J. T., Costa E. Excitatory amino acid recognition sites coupled with inositol phospholipid metabolism: developmental changes and interaction with alpha 1-adrenoceptors. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1931–1935. doi: 10.1073/pnas.83.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F., Valerio C., Pellegrino C., Drago F., Scapagnini U., Canonico P. L. Spatial learning potentiates the stimulation of phosphoinositide hydrolysis by excitatory amino acids in rat hippocampal slices. J Neurochem. 1988 Sep;51(3):725–729. doi: 10.1111/j.1471-4159.1988.tb01804.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Alho H., Eva C., Fadda E., Costa E. Lesions of putative glutamatergic pathways potentiate the increase of inositol phospholipid hydrolysis elicited by excitatory amino acids. Brain Res. 1987 Dec 8;436(1):103–112. doi: 10.1016/0006-8993(87)91561-7. [DOI] [PubMed] [Google Scholar]
- Okamoto N., Hori S., Akazawa C., Hayashi Y., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J Biol Chem. 1994 Jan 14;269(2):1231–1236. [PubMed] [Google Scholar]
- Palmer E., Monaghan D. T., Cotman C. W. Trans-ACPD, a selective agonist of the phosphoinositide-coupled excitatory amino acid receptor. Eur J Pharmacol. 1989 Aug 3;166(3):585–587. doi: 10.1016/0014-2999(89)90383-x. [DOI] [PubMed] [Google Scholar]
- Przywara D. A., Bhave S. V., Bhave A., Wakade T. D., Wakade A. R. Stimulated rise in neuronal calcium is faster and greater in the nucleus than the cytosol. FASEB J. 1991 Feb;5(2):217–222. doi: 10.1096/fasebj.5.2.2004666. [DOI] [PubMed] [Google Scholar]
- Schoepp D. D., Conn P. J. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci. 1993 Jan;14(1):13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y., Masu M., Ishii T., Shigemoto R., Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992 Jan;8(1):169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Nomura A., Masu M., Shigemoto R., Mizuno N., Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci. 1993 Apr;13(4):1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M. Fluorescent labeling of endoplasmic reticulum. Methods Cell Biol. 1989;29:125–135. doi: 10.1016/s0091-679x(08)60191-0. [DOI] [PubMed] [Google Scholar]
- Terasaki M., Song J., Wong J. R., Weiss M. J., Chen L. B. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell. 1984 Aug;38(1):101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- White J. G., Amos W. B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987 Jul;105(1):41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Akiyama K., Otsuki S. Hippocampal kindling enhances excitatory amino acid receptor-mediated polyphosphoinositide hydrolysis in the hippocampus and amygdala/pyriform cortex. Brain Res. 1989 Jun 19;490(1):126–132. doi: 10.1016/0006-8993(89)90437-x. [DOI] [PubMed] [Google Scholar]