Abstract
Background
Evidence for the pathogenesis and treatment of postacute coronavirus disease 2019 (COVID‐19) (long COVID) is lacking. As long COVID symptoms are predicted to have an impact on the global economy, clarification of the pathogenesis is urgently needed. Our experiences indicated that some symptoms were complicated by diseases established before the COVID‐19 pandemic.
Methods
Using a retrospective, cross‐sectional study, we aimed to evaluate the diseases complicating long COVID. Using the medical records of patients with confirmed COVID‐19 exhibiting residual symptoms lasting ≥60 days postinfection who visited our clinic in January 2021–February 2023, we investigated the symptoms and diseases observed. We identified diseases that occurred after COVID‐19 and excluded those that were exacerbations of existing diseases.
Results
During the first visit, the most common symptoms reported in a total of 798 patients were fatigue (523 patients), anxiety (349 patients), and lack of motivation (344 patients). Complicating diseases were observed in 452 patients (57%). There were 115, 65, and 60 patients with postural tachycardia syndrome, postural syndrome without tachycardia, and mood disorders, respectively. Some diseases requiring immediate treatment included pulmonary thromboembolism, purulent shoulder arthritis, cerebellopontine angle tumors, myasthenia gravis, and cervical myelopathy.
Conclusion
Not all symptoms that occur after COVID‐19 should be treated as long COVID. Similar to normal medical treatment, a list of differential diagnoses should be maintained based on symptoms to obtain definitive diagnoses.
Keywords: differential diagnosis, long COVID symptoms, pathogenesis, postacute COVID‐19, treatment
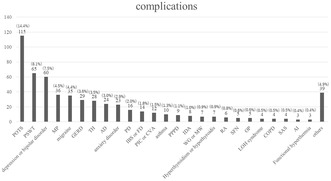
This figure shows all complicated diseases detected by a retrospective chart review of patients with long COVID referred to our hospital. The purpose of this study was to evaluate symptoms and complicated diseases in patients with long COIVD.
1. INTRODUCTION
Novel coronavirus infections have continued to spread worldwide since 2019; there is much evidence for the diagnosis and treatment of the acute phase. 1 However, no diagnostic or therapeutic methods have been established for the postacute symptoms of the novel coronavirus infection (hereafter referred to as “long COVID”). The Centers for Disease Control and Prevention defines long COVID as the onset of symptoms 4 weeks after severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. 2 The World Health Organization defines the condition as (i) typically manifesting 3 months after the onset of COVID‐19, (ii) presenting with symptoms persisting for 2 months or longer, and (iii) not attributable to an alternative diagnosis. Common symptoms include fatigue, shortness of breath, and cognitive dysfunction, all of which affect daily functioning. New‐onset symptoms of long COVID may occur following initial recovery from acute coronavirus disease 2019 (COVID‐19), or symptoms may persist from the initial illness; additionally, they may fluctuate or relapse over time. 3
In 2020, a 6‐month follow‐up report of patients hospitalized because of COVID‐19 in China reported that symptoms after the acute phase developed in 70% of patients 4 ; however, this rate decreased to 50%–70% among hospitalized patients, 10%–30% among nonhospitalized patients, and 10%–12% among vaccinated patients. 5 Subramanian et al. reported that infected patients had increased olfactory dysfunction, shortness of breath, chest pain, fever, hair loss, and sexual dysfunction compared with uninfected patients. 6 Han et al. showed that symptoms such as fatigue, dyspnea, joint pain, depression, insomnia, and memory impairment persisted for 1 year after infection. 7 A systematic review of the time from the onset of COVID‐19 to the appearance of long COVID symptoms has also been published. 8 A German study estimated that the economic loss due to long COVID symptoms was USD 52 billion, and a US study estimated that the direct and indirect costs of the post‐COVID‐19 condition range from USD 14 to 60 billion annually. 9 , 10 Long COVID symptoms are likely to affect the future global situation; therefore, there is an urgent need to elucidate the pathogenesis of this condition and develop treatments.
Several complications that exist in the background of long COVID have been reported. Seeley et al. showed that 79% of patients with long COVID meet the diagnostic criteria of postural orthostatic tachycardia syndrome (POTS). 11 Headache, which often exhibits phenotypes such as migraines and tension‐type headaches, has also been reported to be common, and psychiatric disorders have been reported to newly occur in 16% of those with long COVID. 12 , 13 Nakano et al. reported that of 731 patients, 50 (6.8%) were newly diagnosed with 52 conditions requiring medical intervention. 14 Therefore, attention has been paid to the complications of long COVID. However, to our knowledge, no studies have evaluated the association between long COVID symptoms and underlying complications.
We established an outpatient clinic specializing in long COVID patients in January 2021. Our outpatient clinic is staffed by five general practitioners with ≥5 postgraduate years of experience in the Department of General Medicine who have much experience in evaluating biological, psychological, and social problems. Patients present with a wide variety of complaints, and approximately 1000 have been treated to date and evaluated for complications in collaboration with other departments. This study aimed to evaluate the relationship between symptoms and complications of long COVID and to assist in the future care of patients with this condition.
2. METHODS
2.1. Study design and setting
This descriptive study was conducted between January 18, 2021, and February 13, 2023, at the outpatient clinic of St. Marianna University Hospital “our hospital,” located in Kawasaki. The long COVID outpatient clinic treats patients suspected of having COVID‐19 sequelae who are referred from other clinics or hospitals. We have provided information on the hospital website about specialized long COVID outpatient services and accept referrals not only from neighboring clinics but also from distant locations. The patients are initially evaluated by a generalist, and when necessary, we collaborate with various specialized departments.
2.2. Participants
The included patients had a SARS‐CoV‐2 infection confirmed using an antigen or polymerase chain reaction (PCR) test and had residual symptoms lasting at least 60 days postinfection. At the initial consultation, a questionnaire was completed where the patients indicated the symptoms they had. 23 symptom categories were evaluated using the questionnaire, including low‐grade fever, fatigue, dyspnea, cough, taste disorder, smell disorder, hair loss, sore throat, joint pain, numbness of the limbs, muscle pain, headache, dizziness, chest pain, palpitations, nausea, diarrhea, lack of motivation, insomnia, anxiety, depressed mood, forgetfulness, and skin symptoms. Patients were asked to mark “〇” in the questionnaire for symptoms they were aware of. If the patient had symptoms of fatigue, dyspnea, or palpitations, an orthostatic test was performed to assess autonomic dysfunction. When a patient was severely depressed, as in the case of a patient with premeditated ideation of death, we referred them to a psychiatrist. From March 11 to May 30, 2023, the five authors performed a chart review of all eligible patients and evaluated the complicating diseases for which the consulting physician had a diagnosis. Diagnoses of psychiatric complications were made using the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.
We defined diseases as complicating if they were recognized disease concepts before the COVID‐19 pandemic but manifested as new‐onset symptoms following COVID‐19 (e.g., absence of migraine before COVID‐19, but emergence of new‐onset migraine postinfection).
If their preexisting disease worsened (e.g., if they had a migraine in their preexisting history and if their migraine worsened after infection), we excluded those diseases. We also investigated the relationship between the symptoms reported at the initial visit and complicating diseases. This retrospective, cross‐sectional study was conducted using medical records, and we attempted to prevent recall and observer biases. For each case, a different physician from the one who actually examined the patient reviewed the contents of the medical record and assessed the presence or absence of complications.
2.3. Ethical considerations and approval
This study's protocol was approved by the Ethics Committee of Osaka Metropolitan University (Approval Number: 2020–003).
2.4. Consent to participate
Adult (≥18 years old) and pediatric patients and their parents provided written informed consent for initial and follow‐up data collection as part of routine treatment. Informed consent was obtained from patients who visited our hospital, before the study was approved by the ethics committee using an online opt‐out form.
3. RESULTS
3.1. Patient characteristics
A total of 800 patients were included in this study. Of these, 798 patients were analyzed, after excluding two patients for whom antigen and PCR tests were not performed at the time of the interview. The included patient sample comprised 371 men (46.5%) and 427 women (53.5%) and had a median age of 42 (29–51) years. Figure 1 shows the age distribution of the patients. The time of onset of the patients' novel coronavirus infection was from January 10, 2020, to November 19, 2022. In total, 145 (18.2%) had complications of pneumonia during the acute phase. The median interval from COVID‐19 onset to the initial hospital visit was 128 (87–196.5) days. The median interval of attendance at our hospital from the initial visit to the time of chart review was 121.5 (28–241) days.
FIGURE 1.
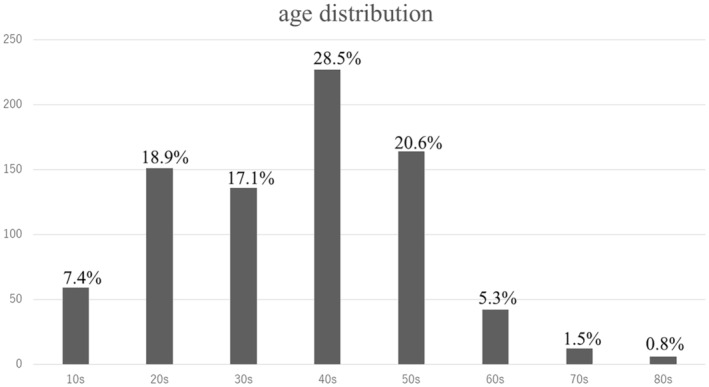
Age distribution of the patients.
3.2. Symptoms at the initial visit
Figure 2 shows all symptoms declared at the initial visit (multiple answers allowed). Fatigue was the most common (523 patients), followed by anxiety (349 patients) and lack of motivation (344 patients).
FIGURE 2.
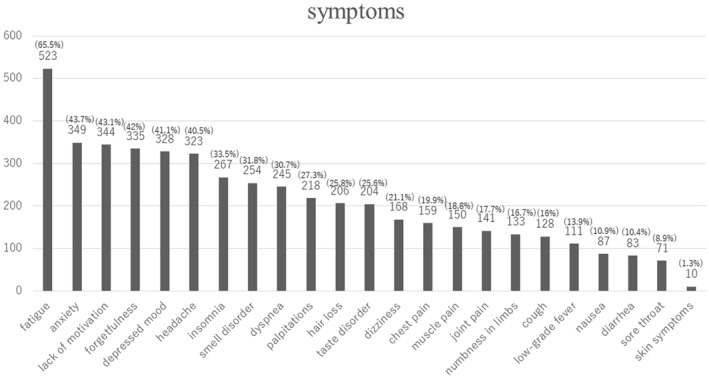
All symptoms declared at the initial visit.
3.3. Complicated diseases
Figure 3 shows all complicated diseases detected by the retrospective chart review (multiple diseases allowed). POTS was the most common (115 patients), followed by postural syndrome without tachycardia (PSWT, 65 patients) and mood disorder (60 patients). The diagnostic criteria for POTS were as follows: ① increase in HR by ≥30 beats/min within 10 min after standing upright in the absence of orthostatic hypotension (i.e., no sustained systolic blood pressure [BP] drop of ≥20 mmHg) and ② frequent symptoms of orthostatic intolerance during standing, with rapid improvement upon return to the supine position. The Schellong test was performed after 5 min of resting in the supine position. Furthermore, the BP and pulse rate were measured in the supine position; immediately after standing up; and after 1, 5, and 10 min. 15 PSWT is a new diagnostic concept proposed by the Canadian Cardiovascular Society and defined as the presence of orthostatic intolerance without meeting the hemodynamic diagnostic criteria for POTS. 16 Complications requiring early emergency hospitalization and surgery after consultation included pulmonary thromboembolism, pyogenic shoulder arthritis, cerebellar pontine angle tumor, and cervical spondylotic myelopathy.
FIGURE 3.
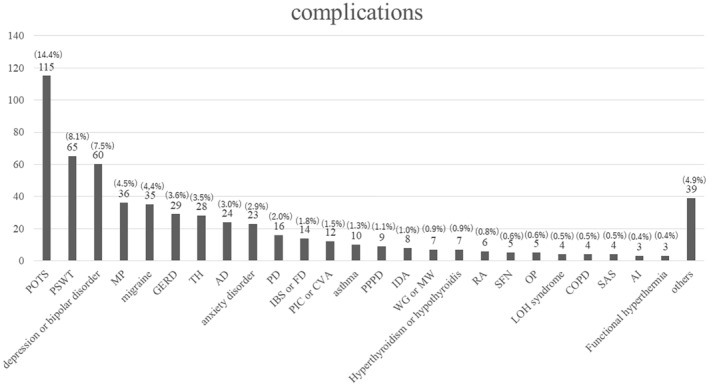
All complicated diseases detected by the retrospective chart review.
3.4. Relationship between initial symptoms and complications
Among patients complaining of fatigue, the accompanying complications included POTS (99), PSWT (58), mood disorders (53), and social anxiety disorders (23). Among patients with complaints of palpitations, POTS (49), PSWT (22), and panic disorder (8) were present as complications. Among those complaining of dyspnea, POTS (50), asthma or chronic obstructive pulmonary disease (COPD) (7), and organizing pneumonia (3) were present as complications. Among patients complaining of cough, postinfectious cough or cough‐variant asthma (12), gastroesophageal reflux disease (10), and bronchial asthma (4) were present as complications. Among patients who complained of headaches, migraine (31) and tension‐type headaches (18) were present as complications. Among those with complaints of dizziness, POTS (38) and persistent postural perceptual dizziness (PPPD) (9) were identified as complications. Among patients with joint pain, 2 were diagnosed with reactive arthritis and 13 with other musculoskeletal problems. Among those complaining of diarrhea, irritable bowel syndrome (IBS) was present in six patients. Among patients with complaints of numbness, small fiber neuropathy (SFN) was diagnosed in five patients. Among those with psychiatric symptoms such as lack of motivation, low mood, anxiety, and insomnia, 60 had unipolar depression or bipolar disorder, 54 had adjustment disorder, 23 had anxiety disorder, and 16 had panic disorder.
4. DISCUSSION
Of the 798 patients referred to our clinic for long COVID, 452 had complicating diseases. Among those with a complaint of fatigue, POTS was the most common complication, followed by PSWT, mood disorders, anxiety disorders, and thyroid diseases. Palpitations and dyspnea were also common in patients with POTS. Postinfectious cough and cough‐variant asthma were the most common among cough symptoms, and migraine and tension‐type headaches were the most common among headache symptoms. POTS was also commonly associated with dizziness, followed by PPPD, whereas musculoskeletal problems were commonly associated with arthralgia. Diarrhea was associated with IBS complications, while numbness was associated with SFN. Psychopsychiatric complaints were often complicated with mood and adjustment disorders.
4.1. Fatigue
POTS and PSWT were frequently associated with complaints of fatigue. POTS is a form of orthostatic intolerance caused by abnormal autonomic responses that lead to excessive tachycardia without hypotension upon standing. Orthostatic symptoms include lightheadedness, blurred vision, tunnel vision, palpitations, tremulousness, and weakness. Other symptoms included fatigue, exercise intolerance, hyperventilation, shortness of breath, anxiety, chest pain, nausea, acral coldness or pain, concentration difficulties, and headache. 15 These symptoms are similar to those of long COVID. POTS has been reported to occur after viral infections, and cases of POTS following COVID‐19 have also been reported. 17 Factors contributing to the development of POTS after COVID‐19 are hypovolemia, neurotropism, inflammation, and autoimmunity. 18 Treatment strategies for POTS include sufficient fluid and salt intake, wearing elastic stockings, and the use of beta‐blockers and alpha‐1 adrenergic receptor agonists. 19 , 20 These treatments may relieve POTS symptoms. Consequently, patients can be informed that, in POTS pathology, standing alone raises the pulse rate, and physical activity causes tachycardia, which in turn causes fatigue; this would help them understand their condition and alleviate their anxiety. Ormiston et al. reported that 2%–14% of survivors of COVID‐19 develop POTS, 9%–61% experience POTS‐like symptoms, and long‐term COVID‐19 patients should be evaluated for POTS. 21 Patients with fatigue and anxiety after COVID‐19 often have limited employment and schoolwork. 22 Psychogenic assessment for mental illness as a factor in fatigue must also focus on underlying social factors.
Among those who complained of fatigue, six patients had hypothyroidism or hyperthyroidism. Yanachkova et al. reported persistent abnormal thyroid function and the development of subacute thyroiditis due to autoantibody production after COVID‐19. 23 Appropriate treatment for abnormal thyroid function may lead to symptomatic improvement, and it is necessary to evaluate thyroid function in patients with long COVID who complain of fatigue.
4.2. Palpitations and dyspnea
Complications observed with POTS included palpitations in 49 patients and dyspnea in 50 patients. The Shellong test should be performed for palpitations and dyspnea that newly appear after COVID‐19, considering the possibility of POTS. Another common complication associated with palpitations is panic disorder, which is important for diagnosis and treatment because the symptoms interfere with daily life. In addition to POTS, asthma, COPD, and organizing pneumonia were observed among those with dyspnea. Lee et al. reported six cases of new‐onset asthma among 394 patients after COVID‐19 and stated that asthma should be considered a differential diagnosis when respiratory symptoms persist after infection. 24 In contrast, although there have been many reports of patients with COPD experiencing exacerbations triggered by COVID‐19, 25 there have been no reports of new‐onset COPD following COVID‐19. This is thought to be because COPD is a chronic disease caused mainly by smoking, and COVID‐19 is not a factor in its development. The newly diagnosed COPD at our hospital was thought to be an exacerbation of previously undiagnosed COPD triggered by COVID‐19. In addition, persistent inflammation of the lungs due to severe coronavirus pneumonia is known to induce organizing pneumonia. 26 When a patient with severe pneumonia due to the COVID‐19 complains of dyspnea after physical activity, respiratory function tests and imaging studies should be performed to exclude the possibility of organizing pneumonia.
4.3. Cough
Chronic cough after COVID‐19 is influenced by chronic inflammation and vagal reflexes, and, as with previous coughs after viral infections. Previous coughs after viral infections require treatment with inhaled steroids and common antitussive agents. 27 , 28 However, there is still no evidence of the efficacy of inhaled steroids for chronic cough after COVID‐19. 29
In this study, of 129 patients with complaints of chronic cough, 12 were diagnosed with postinfectious cough or cough‐variant asthma. Chronic cough after COVID‐19 is not a unique condition, but treatment evidence for chronic cough after COVID‐19 needs more research.
4.4. Headache
Headache is the most common neurological symptom after COVID‐19 and has been reported to present with a migraine or tension‐headache‐like phenotype. 12 The symptoms may be exacerbated when there is a preexisting headache, whereas new‐onset headache may occur and is thought to involve sustained activation of the immune system and trigeminal vessels. Headache due to COVID‐19 is common among middle‐aged women. The pain is moderate to severe and may be accompanied by fatigue, cognitive dysfunction, dizziness, insomnia, and olfactory dysfunction. 12
Migraine‐like headaches during the acute phase are associated with symptomatic headaches after infection. 30
Of the 323 patients with headache complaints in this study, 31 were newly diagnosed with migraine and 18 with tension headaches. Rather than treating headache as a generalized symptom of postinfectious headache, an approach should be adopted to address the underlying primary headache.
4.5. Dizziness
According to the self‐reports of 1082 patients in Germany, 60% complained of dizziness, and 30% of tinnitus after COVID‐19, of whom 10% were severe. 31
Of the 168 patients who reported dizziness in our initial questionnaire, 38 had POTS, and 9 had PPPD. PPPD is characterized by floating, unstable, and nonrotational vertigo that persists for >3 months. Symptoms are exacerbated by a standing posture and walking, active or passive body movements, and viewing moving objects or complex visual patterns, which is secondary to some equilibrium disorders, mainly vestibular disease. Organic vestibular or psychiatric disorders may complicate these symptoms but do not explain them. 32 An increase in PPPD in older adult patients with stress since the COVID‐19 pandemic in 2020 has been reported, 33 and PPPD and POTS should be included in the differential diagnosis as factors for dizziness in long COVID.
4.6. Arthralgia and myalgia
Arthralgia and myalgia are reportedly present in 25% and 20%, respectively, of postillness symptoms. 34
In our study, 141 and 150 patients exhibited arthralgia and myalgia, respectively, during the initial interview. Of the patients who reported arthralgia, two were diagnosed with reactive arthritis, and 13 had musculoskeletal problems. In their review of arthritis after COVID‐19, Jacopo et al. suggested that inflammatory arthritis may be a new entity of musculoskeletal disease temporarily associated with postinfection, and the course of the disease and its response to treatment needs to be determined in prospective studies. 35
4.7. Diarrhea
Although persistent gastrointestinal symptoms after COVID‐19 have not been well documented, Joseph et al. reported that 21 of 48 hospitalized patients (43.8%) still had IBS‐like abdominal pain and indigestion 6 months later. 36 Other reports have shown an increase in gastrointestinal disorders, such as reflux esophagitis, peptic ulcers, acute pancreatitis/cholecystitis, and functional dyspepsia after COVID‐19. 37 IBS was present in six patients in this study who complained of diarrhea. Proper diagnosis of IBS is important because treatment can lead to early improvement of symptoms.
4.8. Numbness
Sensory symptoms in patients with long COVID are described as burning, tingling, numbness, itching, crawling pain, electric shock, and tightness. These symptoms tend to be symmetrical and length‐dependent, with a polyneuropathic pattern, often more symptomatic in the hands and feet, with diffuse sensory abnormalities. However, nerve conduction studies are usually not abnormal in many patients. SFN is suspected in such cases and has been reported after COVID‐19. 38 , 39
A skin biopsy is necessary to diagnose SFN, but we were unable to perform skin biopsies. Therefore, we diagnosed SFN in patients with complaints of numbness in the absence of abnormalities in nerve conduction velocity, magnetic resonance imaging of the head and spine, and blood tests. SFN should be listed as a differential diagnosis in cases of limb numbness after COVID‐19.
4.9. Complications of psychogenic psychiatric disorders
In this study, psychogenic psychiatric disorders, such as mood, adjustment, generalized anxiety, and panic disorders, were common.
Gasnier et al. followed‐up 177 patients admitted to intensive care because of COVID‐19. A total of 115 (65%) patients had at least one psychiatric disorder. New‐onset psychiatric disorders were observed in 29 patients (16.4%), including 24 (13.6%) with major depressive episodes, 20 (11.3%) with anxiety disorders, 20 (11.3%) with PTSD7, 20 (11.3%) with anxiety disorders, 7 (3.9%) with PTSD, and 9 (5.1%) at risk for suicide. 13
Early intervention for psychiatric symptoms in patients with long COVID, including selective serotonin reuptake inhibitor prescription, improves prognosis; however, in case of treatment resistance, patients should be promptly referred to a psychiatrist for systematic evaluation of psychoneurotic disorders and suicide risk. 40
It is important to always keep in mind the possibility of psychogenic psychiatric disorders, as well as to administer the usual medical care. Furthermore, prompt collaboration with a psychiatrist is encouraged when the likelihood of psychogenic psychiatric disorder is high or when the patient is refractory to treatment.
When caring for patients who complain of various symptoms that appear after COVID‐19, it is necessary to list possible differential diagnoses based on symptoms and to accurately diagnose complications as usually performed for patients who are not infected with COVID‐19.
4.10. Limitations
This cross‐sectional single‐center study was conducted at a university hospital. In Japan, there are few university hospitals specializing in long COVID; this may have caused a selection bias. Our outpatient clinic required face‐to‐face consultations, and we were unable to evaluate patients with severe long COVID who were unable to leave their homes. Further patient studies are required to confirm the generalizability of our findings. Diagnostic bias may also factor into these findings because the method of diagnosing complications was based solely on the judgment of one person who reviewed the medical records. The symptoms identified in this study were those reported at the time of initial consultation, and some patients subsequently developed new symptoms that led to the discovery of complications. Therefore, it is possible that the symptoms and complications did not match. In the future, it will be necessary to investigate the chronological changes in complications further by dividing them into those that were present from the beginning and those that emerged during the course of the disease. Additional research is planned to determine whether treatment of complications improves the course of symptoms after the disease.
It has been reported that COVID‐19 vaccination decreases the risk of long COVID. Conversely, multiple COVID‐19 increase its risk. 41 , 42 However, this study did not evaluate the vaccination status and multiple infections in all subjects. The onset of COVID‐19 was from January 10, 2020, to November 19, 2022. In Japan, vaccination began on May 24, 2021; by January 14, 2022, 78% of all Japanese had received two vaccine doses. 43 In total, 528 (66.2%) of the patients in this study were infected with COVID‐19 by January 13, 2022, and the other subjects may have developed the disease after receiving two vaccinations. Further investigation is needed to determine the details of long COVID with and without vaccination, the development of complications, and changes in long COVID due to overlapping infections.
5. CONCLUSION
Although the pathogenesis and treatment of long COVID symptoms remain unclear, these symptoms are often associated with diseases for which disease concepts have already been established prior to the COVID‐19 pandemic. When addressing symptoms after COVID‐19, it is important to identify differential diagnoses from symptoms and accurately diagnose complications, as in conventional medicine, rather than lumping all symptoms together as long COVID.
AUTHOR CONTRIBUTIONS
TT and KI helped in conceptualization. YN and KY helped with methodology. MH worked in formal analysis and investigation. TT helped in writing—original draft preparation. KI, KK, YN, and YK helped in writing—review and editing. TT, MH, FH, RH, KI, YI, KK, and YO worked on resources. TM and YO worked on supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest for this article.
ETHICS APPROVAL
This study's protocol was approved by the Ethics Committee of Osaka Metropolitan University (Approval Number: 2020‐003).
CONSENT TO PARTICIPATE
Consent to participate: (≥18 years old) and pediatric patients and their legal guardians provided written informed consent for the collection of initial and follow‐up data as part of the routine treatment. Informed consent was obtained via an opt‐out form on the hospital's website from patients who visited our hospital prior to ethical approval of the study.
ACKNOWLEDGMENTS
This work was supported by the MHLW Research on Emerging and Re‐Emerging Infectious Diseases and Immunization (Program Grant Number JPMH23HA2011).
Tsuchida T, Hirose M, Fujii H, Hisatomi R, Ishizuka K, Inoue Y, et al. Evaluation of diseases complicating long COVID: A retrospective chart review. J Gen Fam Med. 2024;25:324–332. 10.1002/jgf2.716
REFERENCES
- 1. National Institute for Health and Care Excellence . https://www.nice.org.uk/guidance/ng191/resources/covid19‐rapid‐guideline‐managing‐covid19‐pdf‐51035553326. Accessed 13 Oct 2023.
- 2. Centers for Disease Control and Prevention . Long COVID or post‐COVID conditions. https://www.cdc.gov/coronavirus/2019‐ncov/long‐term‐effects/index.html. Accessed Jan 8, 2023.
- 3. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post‐COVID‐19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. 10.1016/s1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2023;401:e21–e33. 10.1016/s0140-6736(23)00810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–146. 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non‐hospitalized adults. Nat Med. 2022;28:1706–1714. 10.1038/s41591-022-01909-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han Q, Zheng B, Daines L, Sheikh A. Long‐term sequelae of COVID‐19: a systematic review and meta‐analysis of one‐year follow‐up studies on post‐COVID symptoms. Pathogens. 2022;11:269. 10.3390/pathogens11020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, et al. Prevalence of post‐acute COVID‐19 syndrome symptoms at different follow‐up periods: a systematic review and meta‐analysis. Clin Microbiol Infect. 2022;28:657–666. 10.1016/j.cmi.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gandjour A. Long COVID: costs for the German economy and health care and pension system. BMC Health Serv Res. 2023;23:641. 10.1186/s12913-023-09601-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mirin AA. A preliminary estimate of the economic impact of long COVID in the United States. Fatigue. 2022;10:190–199. 10.1080/21641846.2022.2124064 [DOI] [Google Scholar]
- 11. Seeley MC, Gallagher C, Ong E, Langdon A, Chieng J, Bailey D, et al. High incidence of autonomic dysfunction and postural orthostatic tachycardia syndrome in patients with long COVID: implications for management and health care planning. Am J Med. 2023;S0002‐9343(23)00402‐3. Epub ahead of print. 10.1016/j.amjmed.2023.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tana C, Bentivegna E, Cho S‐J, Harriott AM, García‐Azorín D, Labastida‐Ramirez A, et al. Long COVID headache. J Headache Pain. 2022;23:93. 10.1186/s10194-022-01450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gasnier M, Choucha W, Radiguer F, Faulet T, Chappell K, Bougarel A, et al. Comorbidity of long COVID and psychiatric disorders after a hospitalisation for COVID‐19: a cross‐sectional study. J Neurol Neurosurg Psychiatry. 2022;93:1091–1098. 10.1136/jnnp-2021-328516 [DOI] [PubMed] [Google Scholar]
- 14. Nakano Y, Sunada N, Tokumasu K, Honda H, Otsuka Y, Sakurada Y, et al. Occult endocrine disorders newly diagnosed in patients with post‐COVID‐19 symptoms. Sci Rep. 2024;14:5446. 10.1038/s41598-024-55526-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 16. Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, et al. Canadian cardiovascular society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020;36:357–372. 10.1016/j.cjca.2019.12.024 [DOI] [PubMed] [Google Scholar]
- 17. Chadda KR, Blakey EE, Huang CL‐H, Jeevaratnam K. Long COVID‐19 and postural orthostatic tachycardia syndrome‐ is dysautonomia to be blamed? Front Cardiovasc Med. 2022;9:860198. 10.3389/fcvm.2022.860198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID‐19 infection: a case series of 20 patients. Immunol Res. 2021;69:205–211. 10.1007/s12026-021-09185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fu Q, Levine BD. Exercise and non‐pharmacological treatment of POTS. Auton Neurosci. 2018;215:20–27. 10.1016/j.autneu.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller AJ, Raj SR. Pharmacotherapy for postural tachycardia syndrome. Auton Neurosci. 2018;215:28–36. 10.1016/j.autneu.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 21. Ormiston CK, Świątkiewicz I, Taub PR. Postural orthostatic tachycardia syndrome as a sequela of COVID‐19. Heart Rhythm. 2022;19:1880–1889. 10.1016/j.hrthm.2022.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsuchida T, Yoshimura N, Ishizuka K, Katayama K, Inoue Y, Hirose M, et al. Five cluster classifications of long COVID and their background factors: a cross‐sectional study in Japan. Clin Exp Med. 2023;23:3663–3670. 10.1007/s10238-023-01057-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yanachkova V, Stankova T, Staynova R. Thyroid dysfunction as a long‐term post‐COVID‐19 complication in mild‐to‐moderate COVID‐19. Biotechnol Biotechnol Equip. 2023;37:194–202. 10.1080/13102818.2023.2170829 [DOI] [Google Scholar]
- 24. Lee H, Kim B‐G, Chung SJ, Park DW, Park TS, Moon J‐Y, et al. New‐onset asthma following COVID‐19 in adults. J Allergy Clin Immunol Pract. 2023;11:2228–2231. 10.1016/j.jaip.2023.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Awatade N, Wark P, Chan A, Mamun SM, Mohd Esa N, Matsunaga K, et al. The complex association between COPD and COVID‐19. J Clin Med. 2023;12:3791. 10.3390/jcm12113791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bazdyrev E, Panova M, Zherebtsova V, Burdenkova A, Grishagin I, Novikov F, et al. The hidden pandemic of COVID‐19‐induced organizing pneumonia. Pharmaceuticals (Basel). 2022;15:1574. 10.3390/ph15121574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharma P, Rai D, Karmakar S, Thakur S, Ameet H, Yadav R, et al. Approach to post COVID‐19 persistent cough: a narrative review. Lung India. 2023;40:149–154. 10.4103/lungindia.lungindia_250_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. García‐Vicente P, Rodríguez‐Valiente A, Górriz Gil C, Márquez Altemir R, Martínez‐Pérez F, López‐Pajaro LF, et al. Chronic cough in post‐COVID syndrome: laryngeal electromyography findings in vagus nerve neuropathy. PLoS One. 2023;18:e0283758. 10.1371/journal.pone.0283758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xie PP, Zhang Y, Niu WK, Tu B, Yang N, Fang Y, et al. Clinical characteristics and effects of inhaled corticosteroid in patients with post‐COVID‐19 chronic cough during the omicron variant outbreak. BMC Pulm Med. 2024;24:156. 10.1186/s12890-024-02937-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garcia‐Azorin D, Layos‐Romero A, Porta‐Etessam J, Membrilla JA, Caronna E, Gonzalez‐Martinez A, et al. Post‐COVID‐19 persistent headache: a multicentric 9‐months follow‐up study of 905 patients. Cephalalgia. 2022;42:804–809. 10.1177/03331024211068074 [DOI] [PubMed] [Google Scholar]
- 31. Degen CV, Mikuteit M, Niewolik J, Schröder D, Vahldiek K, Mücke U, et al. Self‐reported tinnitus and vertigo or dizziness in a cohort of adult long COVID patients. Front Neurol. 2022;13:884002. 10.3389/fneur.2022.884002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Staab JP, Eckhardt‐Henn A, Horii A, Jacob R, Strupp M, Brandt T, et al. Diagnostic criteria for persistent postural‐perceptual dizziness (PPPD): consensus document of the committee for the classification of vestibular disorders of the Bárány society. J Vestib Res. 2017;27:191–208. 10.3233/ves-170622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Guo D, Liu S, Yu A, Sun C, Zhou L. COVID‐19 pandemic impacts on the elderly: the relationship between PPPD and prefrontal alpha rhythm. Int J Neurosci. 2022;134:1–6. 10.1080/00207454.2022.2102978 [DOI] [PubMed] [Google Scholar]
- 34. Swarnakar R, Jenifa S, Wadhwa S. Musculoskeletal complications in long COVID‐19: a systematic review. World J Virol. 2022;11:485–495. 10.5501/wjv.v11.i6.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ciaffi J, Vanni E, Mancarella L, Brusi V, Lisi L, Pignatti F, et al. Post‐acute COVID‐19 joint pain and new onset of rheumatic musculoskeletal diseases: a systematic review. Diagnostics (Basel). 2023;13:1850. 10.3390/diagnostics13111850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cooney J, Appiahene P, Findlay R, Al‐Hillawi L, Rafique K, Laband W, et al. COVID‐19 infection causing residual gastrointestinal symptoms – a single UK centre case series. Clin Med. 2022;22:181–183. 10.7861/clinmed.2021-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu E, Xie Y, Al‐Aly Z. Long‐term gastrointestinal outcomes of COVID‐19. Nat Commun. 2023;14:983. 10.1038/s41467-023-36223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abrams RMC, Zhou L, Shin SC. Persistent post–COVID‐19 neuromuscular symptoms. Muscle Nerve. 2023;68:350–355. 10.1002/mus.27940 [DOI] [PubMed] [Google Scholar]
- 39. Panagiotides NG, Zimprich F, Machold K, Schlager O, Müller M, Ertl S, et al. A case of autoimmune small fiber neuropathy as possible post COVID sequelae. Int J Environ Res Public Health. 2023;20:4918. 10.3390/ijerph20064918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gasnier M, Montani D, Corruble E, Colle R. Psychiatric disorders and long COVID. Respir Med Res. 2022;82:100958. 10.1016/j.resmer.2022.100958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Català M, Mercadé‐Besora N, Kolde R, Trinh NTH, Roel E, Burn E, et al. The effectiveness of COVID‐19 vaccines to prevent long COVID symptoms: staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir Med. 2024;12:225–236. 10.1016/S2213-2600(23)00414-9 [DOI] [PubMed] [Google Scholar]
- 42. Bowe B, Xie Y, Al‐Aly Z. Postacute sequelae of COVID‐19 at 2 years. Nat Med. 2023;29:2347–2357. 10.1038/s41591-023-02521-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. National Institute of Infectious Diseases . https://www.niid.go.jp/niid/images/epi/corona/68/covid19_vaccine_20220121.pdf Accessed 1 June 2024.


