Abstract
Background
Multimorbidity increases with age, leading to various adverse outcomes, including higher mortality, care dependency, hospitalizations, and healthcare costs. Polydoctoring, managing a patient with multimorbidity by multiple healthcare providers, can be a risk of fragmented care and increased healthcare expenditures. This study aims to identify patient‐related factors contributing to polydoctoring in older adults with multimorbidity.
Methods
This study is a cross‐sectional study using baseline data from the Kawasaki Aging and Wellbeing Project. Participants were residents of Kawasaki City aged 85–89 years, without disability in basic activities of daily living, and being able to visit study site. The regularly visited facilities (RVF) index was employed to quantify polydoctoring. Polydoctoring was defined as having two or more RVFs. Poisson regression analysis was conducted to assess the association between polydoctoring and patient demographics, including types of chronic conditions and socioeconomic factors.
Results
A total of, 968 participants with multimorbidity were analyzed. Increased RVF was significantly associated with eye diseases (rate ratio [RR] 1.27, 95% confidence interval [CI] 1.12–1.44), osteoporosis (RR 1.22, 95% CI 1.08–1.38), prostate diseases (RR 1.22, 95% CI 1.07–1.40), and osteoarthritis (RR 1.16, 95% CI 1.05–1.27). No significant correlation was found with educational status or financial hardship.
Conclusion
The study indicated that certain chronic conditions are linked to increased polydoctoring among multimorbid older adults in Japan. However, most of those conditions are considered to be within a scope of family medicine/general practice. Training general practitioners to manage these conditions could reduce healthcare costs and the treatment burden, indicating a direction for future healthcare policy and medical education.
Keywords: aging society, care fragmentation, multimorbidity, polydoctoring, scope of practice
This study aims to identify patient‐related factors contributing to polydoctoring in older adults in Japan. Polydoctoring was significantly associated with eye diseases, osteoporosis, prostate diseases, and osteoarthritis. No significant correlation was found with educational status or financial hardship.
1. INTRODUCTION
The prevalence of multimorbidity, defined as the concomitant presence of two or more chronic conditions, increases with advancing age. 1 , 2 Multimorbidity is associated with adverse outcomes, such as higher mortality, escalated care dependency, increased incidence of hospitalization, and increased healthcare costs. 3 , 4 , 5 , 6 , 7 Multimorbidity poses a substantial challenge for the healthcare systems in developed nations characterized by aging societies. 8 , 9
Patients with multimorbidity often require care from numerous healthcare providers. This phenomenon, referred to as polydoctoring, entails the management of a single patient by different physicians and healthcare facilities. 10 Inadequate care coordination in polydoctoring can lead to fragmented care delivery, resulting in a higher incidence of hospital admissions, polypharmacy, and an overall increase in healthcare expenditure. 10 , 11 , 12 , 13 Consequently, polydoctoring is now recognized as a pivotal factor in the management of multimorbidity.
Polydoctoring and fragmented care result from the ways patients seek healthcare. This health‐seeking behavior of patients is influenced by a complex interplay of factors including patient characteristics, attributes of the healthcare provider, and the structure of the healthcare system. 14 Japan has a national health insurance system that offers free access to healthcare facilities, allowing patients to choose their healthcare provider. 15 The universal health insurance system coupled with the provision of primary care by specialists, provides a conducive backdrop for the occurrence of polydoctoring. The fragmentation of care for older adults is recognized as a critical issue in healthcare across various nations, and polydoctoring among patients with multimorbidity is expected to provide significant insights for the consideration of healthcare policies internationally. 16 , 17
Previous studies suggest that higher levels of education and income may predispose individuals to prefer specialist consultations; however, the specific characteristics of patients who are at an increased risk of engaging in polydoctoring are not well characterized. 14 , 18 , 19 , 20 Moreover, understanding the specific conditions associated with consulting multiple healthcare facilities is important for policy interventions and capacity building of general practitioners. This study aimed to elucidate the patient‐related factors contributing to polydoctoring among older adults with multimorbidity, thereby addressing a gap in the current understanding of healthcare use patterns within this demographic.
2. METHODS
2.1. Setting, study population, and data collection
This is a cross‐sectional study that used baseline data from the ongoing Kawasaki Aging and Wellbeing Project (KAWP) in Kawasaki City—a major metropolitan area adjacent to Tokyo, Japan, with a population of approximately 1.5 million. 10 , 21 , 22 , 23 , 24 The inclusion criteria for KAWP participants were as follows: (1) residents of Kawasaki City aged 85–89 years; (2) absence of disability in basic activities of daily living; and (3) being able to visit the study site, the Kawasaki Municipal Hospitals independently.
There were two main modes of data collection in the KAWP. First, face‐to‐face assessments were conducted by a multidisciplinary team comprising physicians, nurses, pharmacists, and psychologists. These assessments included physical measurements, psychological evaluations, and blood tests. Second, medical and long‐term care insurance claims data were analyzed to examine the utilization patterns of medical and caregiving services.
In March 2017, we began by identifying 12,906 potential participants using the basic resident registration and long‐term care insurance databases in Kawasaki city. We then sent participation invitations to 9976 individuals. Out of these, 1464 eligible residents responded, indicating their interest in joining the study. Over the period from March 2017 to December 2018, we successfully enrolled 1026 independent older adults into the KAWP study. Among the 1026 participants of KAWP, individuals who had consented to the use of their medical and caregiving insurance claims data and had two or more chronic conditions were included in the present study. (Figure 1) Missing data on medical history were treated as absent, and other missing values were not imputed in this study. Instances with unknown data of medical history constituted 175 out of 17,424 condition‐person records, representing approximately 1%.
FIGURE 1.
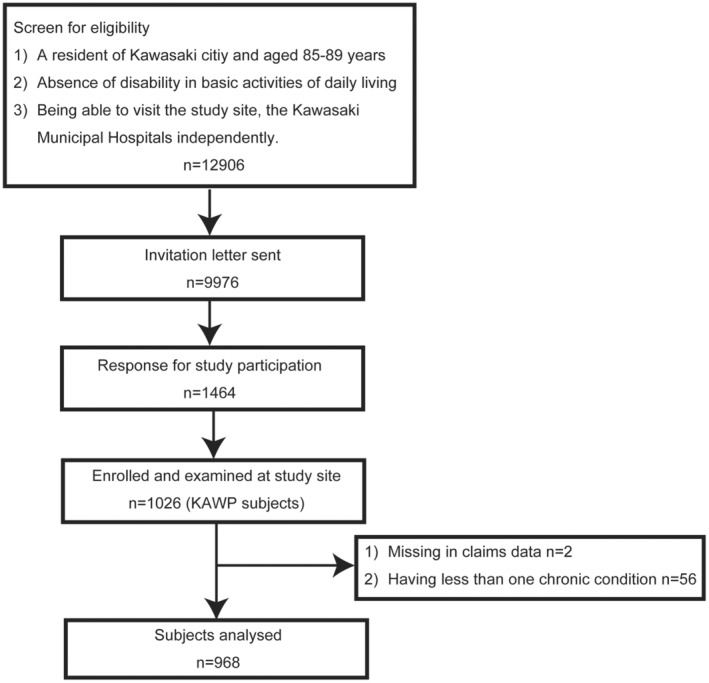
Flow diagram of participant selection. This diagram illustrates the participant selection process for the study. KAWP, Kawasaki Aging and Wellbeing Project.
2.2. Measure
The study employed the Regularly Visited Facilities (RVF) index to measure polydoctoring among multimorbid patients. 10 RVF were defined as healthcare facilities meeting the following two criteria within a year's healthcare claims data: (1) presence of claims data for over three times a year and (2) a minimum of a six‐month interval between the first and last claims data. An RVF score of 0 indicates no regular healthcare facility visits, whereas a score of 1 indicates attendance at a single healthcare institution. This study measured RVF over 1 year following the initiation of the baseline survey. The definition of polydoctoring and the cutoff for RVF have not yet been established. However, in this study, polydoctoring was defined as an RVF of two or more, as in our previous research. 10
The covariates selected for this study were as follows: presence or absence of each of the 18 chronic conditions; gender; frailty; educational background; and financial hardship. Previous literature has indicated that educational background and financial status are associated with specialist consultations. 18 , 19 Frailty is associated with increased healthcare utilization, particularly higher rates of specialist clinic visits. 25 , 26 Therefore, we included these covariates in the model. Due to the relatively homogeneous age range of the study population (85–89 years), age was not included as a covariate to avoid the potential risk of overfitting. Chronic conditions were categorized into the following 18 chronic conditions as our previous study: cerebrovascular disease, cardiac disease, hypertension, diabetes, dyslipidemia, respiratory disease, gastrointestinal disease, renal disease, prostate disease, thyroid disease, Parkinson's disease, connective tissue disease, eye disease, osteoporosis, osteoarthritis, hyperuricemia, malignancy, and dementia. 10 , 23 The presence of chronic diseases was determined through physician‐conducted interviews of the participants. Frailty was assessed through face‐to‐face surveys based on the revised Japanese version of the cardiovascular health study (J‐CHS) criteria. 27 Frailty was treated as a binary variable (frail vs. robust/prefrail) to avoid overfitting risk in our limited sample size. Educational status was measured as completed years of schooling, dividing participants into two groups: those with a high school education or higher (≥12 years) and those with less than a high school education (<12 years), in accordance with previous studies. 19 , 28 Regarding financial status, this study employed the measurement of self‐reported financial hardship. Financial hardship can be conveniently measured using a single question, and its association with health status has been demonstrated. 29 , 30 Financial hardship was measured by the 5‐point Likert scale question, “How does your household manage its monthly finances?” Participants who answered “struggling very much” or “struggling somewhat” were classified as experiencing financial hardship.
2.3. Statistical analysis
Given the exploratory nature of this study, a sample size calculation was not conducted. Poisson regression analysis was conducted with RVF as the dependent variable. Initial univariate regression analyses for each variable were followed by multivariate regression analysis. Given that the RVF index represents count data, Poisson regression was deemed appropriate for modeling the frequency of events. 31 Gender, educational status, financial hardship, presence of each chronic disease, and frailty were included in the model as binary variables. The variance inflation factors (VIFs) were calculated to ensure the absence of multicollinearity, and the presence of overdispersion was assessed using the Poisson regression model. p < 0.05 was considered indicative of statistical significance for all analyses. Statistical analyses were performed using R version 4.3.1 on RStudio 2023.06.1.
3. RESULTS
Out of 1026 participants, two opted out of using their claims data, and 56 had one or fewer chronic conditions, resulting in a total of 968 participants being included in the analysis. (Figure 1) The characteristics of the study population are summarized in Table 1. Forty‐five percent of the participants had more than 12 years of education, and 19% reported experiencing financial hardship. The average number of concurrent chronic conditions among our cohort was 4.7, with a median RVF index of 2. Polydoctoring, defined as two or more RVFs, occurred in 65.3% of participants. The prevalence of each of the 18 diseases studied is presented in Figure 2. Eye diseases were the most common, followed by hypertension, digestive diseases, dyslipidemia, and osteoarthritis.
TABLE 1.
Characteristics of the study population.
| N | 968 |
|---|---|
| Age (median [IQR]) | 86.00 [85.00, 88.00] |
| Gender, n (%) | 487 (50.3) |
| Male | 481 (49.7) |
| Female | 487 (50.3) |
| Education ≧12 years, n (%) | 436 (45.0) |
| Financial hardship, n (%) | |
| No | 760 (78.5) |
| Yes | 178 (18.4) |
| Missing | 30 (3.1) |
| Frailty, n (%) | |
| Robust | 141 (14.9) |
| Prefrail | 574 (60.6) |
| Frail | 232 (24.5) |
| Missing | 21 (2.2) |
| Number of chronic conditions (median [IQR]) | 5.00 [3.00, 6.00] |
| RVF (median [IQR]) | 2.00 [1.00, 3.00] |
| 0 | 55 (5.7) |
| 1 | 281 (29.0) |
| 2 | 292 (30.2) |
| 3 | 217 (22.4) |
| 4 | 71 (7.3) |
| 5 | 35 (3.6) |
| 6 | 10 (1.0) |
| 7 | 6 (0.6) |
| 8 | 1 (0.1) |
Abbreviations: IQR, interquartile range; RVF, regularly visited facility; SD, standard deviation.
FIGURE 2.
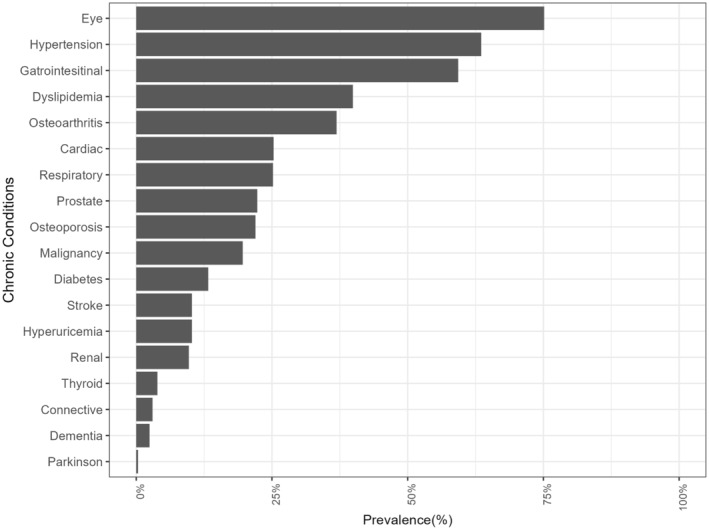
Prevalence of each chronic condition among analyzed participants. This figure illustrates the prevalence of various chronic diseases within the analyzed cohort. Past medical history was obtained not from claims data but through direct interviews by physician. Each chronic disease is represented on the horizontal axis, while the vertical axis displays the prevalence rate (percentage).
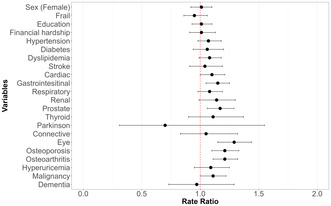
Univariate Poisson regression analysis of factors associated with RVF is depicted in a forest plot in Figure 3. RVF was significantly associated with eye diseases (rate ratio [RR] 1.29, 95% confidence interval [CI] 1.15–1.44), osteoporosis (RR 1.21, 95% CI 1.10–1.33), osteoarthritis (RR 1.21, 95% CI 1.11–1.32), prostate diseases (RR 1.17, 95% CI 1.06–1.29), and gastrointestinal diseases (RR 1.15, 95% CI 1.05–1.25).
FIGURE 3.
Result of univariate Poisson regression analysis for higher regularly visited facility (RVF). This figure presents the outcome of a univariate Poisson regression analysis, where the RVF is the dependent variable. The horizontal axis depicts the rate ratio for an increment of one unit in the RVF attributed to each factor in unadjusted analysis.
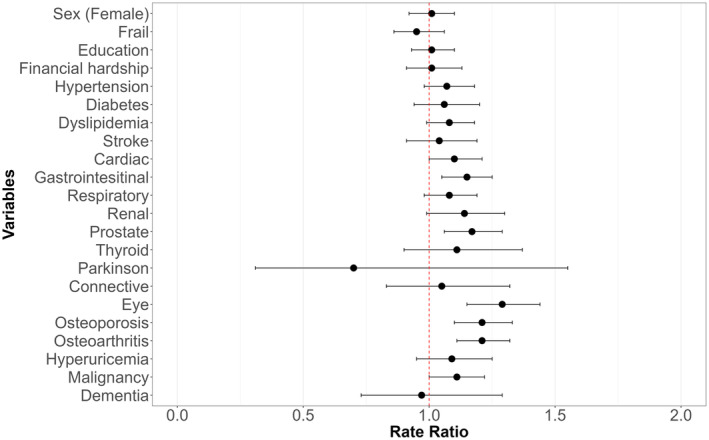
The results of the multivariate Poisson regression analysis are presented in a forest plot in Figure 4. All VIFs of the multivariate model were below 5, indicating that multicollinearity was not observed in our analysis. In this analysis, RVF showed a significant association with eye diseases (RR 1.27, 95% CI 1.12–1.44), osteoporosis (RR 1.22, 95% CI 1.08–1.38), prostate diseases (RR 1.22, 95% CI 1.07–1.40), and osteoarthritis (RR 1.16, 95% CI 1.05–1.27). RVF showed no significant association with educational status (RR 1.03, 95% CI 0.93–1.14) or financial hardship (RR 0.98, 95% CI 0.87–1.11).
FIGURE 4.
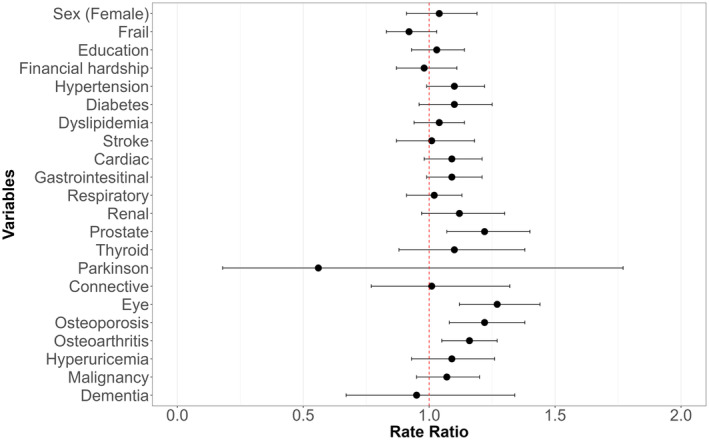
Result of multivariate Poisson regression analysis for higher regularly visited facility (RVF). This figure delineates the results from a multivariate Poisson regression analysis with the RVF as the dependent variable. This adjusted model includes gender, frailty, education, financial hardship, and the presence of each condition. The horizontal axis indicates the rate ratios that represent the expected change in the RVF for each increment of one in the associated factors, while controlling for all other variables in the model.
Within the category of eye diseases, the most‐prevalent condition was allergic conjunctivitis (28.4%), followed by hyperopic astigmatism (21.7%), cataract (20%), myopic astigmatism (15.9%), and glaucoma (14.1%). Within the category of prostate diseases, 98.1% of the cases had benign prostatic hyperplasia (BPH).
4. DISCUSSION
This study indicated that among very old patients with multimorbidity, the presence of eye diseases, osteoporosis, prostate diseases, and osteoarthritis is associated with an increased tendency to visit multiple healthcare facilities regularly. This trend likely reflects the structure of primary care in Japan, where these conditions are typically managed by the respective specialists.
Eye diseases often require the specialized care of an ophthalmologist; however, the most common condition in this dataset was allergic conjunctivitis. It is conceivable that non‐ophthalmologist primary care physicians can manage mild cases of allergic conjunctivitis. Furthermore, with regard to prostate diseases, over 90% of cases were BPH, which is also a common condition that is amenable to management by non‐urologists, especially if the treatment is pharmacological. Osteoporosis and osteoarthritis are also prevalent diseases, and their pharmacological treatment is generally considered within the scope of primary care physicians. Indeed, guidelines of the American Academy of Family Physicians and the Royal College of General Practice include allergic conjunctivitis, BPH, osteoporosis, and osteoarthritis within the scope of practice for family physicians and general practitioners. 32 , 33 The objectives for general medicine training in Japan also include the management of these conditions, and it is anticipated that general practitioners will manage these conditions in the future. 34
Increased specialist consultations can increase the treatment burden on patients. 20 Furthermore, polydoctoring is a risk factor for polypharmacy and increased healthcare costs. 10 Indeed, comprehensive care provision by primary care physicians is more economically desirable provided that it does not compromise patient outcomes. 10 Enhancing the capacity of primary care physicians to provide safe and effective care for these diseases can reduce the treatment burden of older adults with multimorbidities.
Some reports suggest that patients with certain conditions, such as heart disease and diabetes, may experience better outcomes under the care of specialists than generalists. Conversely, the efficacy of care by specialists and generalists is comparable for some diseases, and the care provided by generalists may even be superior to that provided by specialists in certain contexts. 35 Regarding BPH, several reports have indicated that generalists may be less proactive than urologists in conducting investigations and prescribing medications. 36 , 37 , 38 However, the impact of this difference in approach on patient outcomes remains unclear. For osteoporosis, reports suggest that primary care physicians may opt for treatment choices that are more considerate of costs, patient frailty, and potential medication complications than orthopedic specialists; however, the impact of these preferences on patient outcomes is also unclear. 39 In the case of osteoarthritis, while there is a paucity of studies comparing the care provided by generalists to that provided by specialists, one study indicated minimal difference in functional outcomes among them. 40 , 41 Even for these common diseases, there is still limited knowledge about the impact of care provided by generalists versus specialists on patient outcomes. Further research is warranted to investigate the advantages of comprehensive care provision by generalists supplemented with interventions to bridge gaps versus exclusive specialist care.
Contrary to prior studies suggesting that higher education and financial status promote specialist consultations, no significant association between polydoctoring and educational status or financial hardship was observed in the present study. 18 , 19 This may be influenced by Japan's universal health coverage, which allows equal access to healthcare regardless of financial status. This could diminish the impact of financial hardship on the decision to seek care from multiple providers. Previous studies have measured direct income values, whereas this study assesses subjective financial hardship, which may also contribute to the differences in results. Due to the limited sample size, we used a binary categorization of education level (≥12 years and <12 years). This approach may have limited our ability to detect differences based on education levels. Additionally, the homogeneity of the study's age group and urban setting might not reflect broader societal trends, as healthcare utilization in this demographic could be less affected by socioeconomic factors compared to other age groups or settings.
Some limitations of this study should be considered while interpreting the results. First, there is a potential for information bias, as data on chronic diseases were obtained through physician‐conducted interviews. Participants' misunderstanding of their medical conditions may lead to inaccuracies in the reported prevalence and details of chronic conditions. Second, this study focused on an urban area and the findings may not be directly applicable to rural regions with limited access to healthcare facilities. Previous studies have found differences in specialist consultations between urban and rural areas. 14 , 18 , 20 Urban areas generally have a higher density of specialists and better access to medical facilities, and urban residents have a greater tendency for seeking specialist care. Future research should encompass both urban and rural regions on a nationwide scale to provide more robust insights. Furthermore, the voluntary nature of participation in this study may have introduced an element of participation bias, limiting its generalizability to the broader population. Additionally, polydoctoring was measured quantitatively using the RVF in this study, without considering qualitative aspects or instances where patients visit multiple departments within the same medical facility. 10 For example, a patient may visit different internal medicine departments within the same institution. This limitation might affect the interpretation of our results, as it does not fully capture the complexity of healthcare‐seeking behaviors. However, this is one of the first studies to describe the healthcare‐seeking behaviors of older adults with multimorbidity, particularly in relation to polydoctoring, offering vital insights for more efficient care strategies for this population.
5. CONCLUSION
Eye diseases, osteoporosis, prostate diseases, and osteoarthritis increase the likelihood of patients regularly visiting multiple healthcare facilities in Japan, where organ‐specific specialists predominantly deliver primary care. Although this study is situated within the context of Japan's healthcare system, its findings on the determinants of polydoctoring have broader implications. The interplay between patient behavior and healthcare system structure that encourages multiple consultations can inform healthcare system designs in other countries. Thus, identifying the specific conditions and patient demographics linked to polydoctoring can aid in developing more integrated care approaches, both within Japan and potentially in other healthcare systems facing similar issues. Training of general practitioners to cover these conditions, which are within the scope of general medicine training, can help reduce healthcare costs and the treatment burden on patients. Insights from this study can help inform future healthcare policy and medical education.
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ETHICS STATEMENT
Ethics approval statement: This study was approved by the ethics committee of Keio University School of Medicine (ID: 20160297).
PATIENT CONSENT STATEMENT
All participants signed an informed consent statement prior to participation in the study.
CLINICAL TRIAL REGISTRATION
This study is registered in the University Hospital Medical Information Network Clinical Trial Registry as an observational study (ID: UMIN000026053).
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI Grant Number 21K10356 and 24K13322.
Ando T, Sasaki T, Abe Y, Nishimoto Y, Hirata T, Haruta J, et al. Determinants of polydoctoring among multimorbid older adults; a cross‐sectional study in an urban area of Japan. J Gen Fam Med. 2024;25:376–383. 10.1002/jgf2.728
REFERENCES
- 1. Aramrat C, Choksomngam Y, Jiraporncharoen W, Wiwatkunupakarn N, Pinyopornpanish K, Mallinson PAC, et al. Advancing multimorbidity management in primary care: a narrative review. Prim Health Care Res Dev. 2022;23:e36. 10.1017/S1463423622000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross‐sectional study. Lancet. 2012;380(9836):37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 3. Nunes BP, Flores TR, Mielke GI, Thumé E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta‐analysis. Arch Gerontol Geriatr. 2016;67:130–138. 10.1016/j.archger.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 4. Ryan A, Wallace E, O'Hara P, Smith SM. Multimorbidity and functional decline in community‐dwelling adults: a systematic review. Health Qual Life Outcomes. 2015;13:168. 10.1186/s12955-015-0355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigues LP, de Oliveira Rezende AT, Delpino FM, Mendonça CR, Noll M, Nunes BP, et al. Association between multimorbidity and hospitalization in older adults: systematic review and meta‐analysis. Age Ageing. 2022;51(7):afac155. 10.1093/ageing/afac155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. 10.1001/archinte.162.20.2269 [DOI] [PubMed] [Google Scholar]
- 7. Glynn LG, Valderas JM, Healy P, Burke E, Newell J, Gillespie P, et al. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Fam Pract. 2011;28(5):516–523. 10.1093/FAMPRA/CMR013 [DOI] [PubMed] [Google Scholar]
- 8. Skou ST, Mair FS, Fortin M, Guthrie B, Nunes BP, Miranda JJ, et al. Multimorbidity. Nat Rev Dis Primers. 2022;8(1):48. 10.1038/s41572-022-00376-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–439. 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 10. Ando T, Sasaki T, Abe Y, Nishimoto Y, Hirata T, Haruta J, et al. Measurement of polydoctoring as a crucial component of fragmentation of care among patients with multimorbidity: cross‐sectional study in Japan. J Gen Fam Med. 2023;24(6):343–349. 10.1002/jgf2.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schrag D, Xu F, Hanger M, Elkin E, Bickell NA, Bach PB. Fragmentation of care for frequently hospitalized urban residents. Med Care. 2006;44(6):560–567. 10.1097/01.mlr.0000215811.68308.ae [DOI] [PubMed] [Google Scholar]
- 12. Kaneko M, Shinoda S, Shimizu S, Kuroki M, Nakagami S, Chiba T, et al. Fragmentation of ambulatory care among older adults: an exhaustive database study in an ageing city in Japan. BMJ Open. 2022;12(8):1–6. 10.1136/bmjopen-2022-061921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stange KC. The problem of fragmentation and the need for integrative solutions. Ann Fam Med. 2009;7(2):100–103. 10.1370/afm.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forrest CB, Nutting PA, Von Schrader S, Rohde C, Starfield B. Primary care physician specialty referral decision making: patient, physician, and health care system determinants. Med Decis Making. 2006;26(1):76–85. 10.1177/0272989X05284110 [DOI] [PubMed] [Google Scholar]
- 15. Kato D, Ryu H, Matsumoto T, Abe K, Kaneko M, Ko M, et al. Building primary care in Japan: literature review. J Gen Fam Med. 2019;20(5):170–179. 10.1002/JGF2.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akintayo‐Usman NO. Fragmentation of care: a major challenge for older people living with multimorbidity. Geriatr Gerontol Aging. 2021;15:e0210030. 10.53886/gga.0210030 [DOI] [Google Scholar]
- 17. Clarfield AM, Bergman H, Kane R. Fragmentation of care for frail older people— an international problem. Experience from three countries: Israel, Canada, and the United States. J Am Geriatr Soc. 2001;49(12):1714–1721. 10.1046/J.1532-5415.2001.49285.X [DOI] [PubMed] [Google Scholar]
- 18. Xakellis GC. Are patients who use a generalist physician healthier than those who seek specialty care directly? Fam Med. 2005;37(10):719–726. [PubMed] [Google Scholar]
- 19. Suominen‐Taipale AL, Koskinen S, Martelin T, Holmen J, Johnsen R. Differences in older adults' use of primary and specialist care services in two Nordic countries. Eur J Public Health. 2004;14(4):375–380. 10.1093/EURPUB/14.4.375 [DOI] [PubMed] [Google Scholar]
- 20. Messi M, Mueller Y, Haller DM, Zeller A, Neuner‐Jehle S, Streit S, et al. A cross‐sectional study of swiss ambulatory care services use by multimorbid patients in primary care in the light of the Andersen model. BMC Fam Pract. 2020;21(1):150. 10.1186/s12875-020-01221-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka A, Arai Y, Hirata T, Abe Y, Oguma Y, Urushihara H. Effects of polypharmacy and anticholinergic/sedative drugs on the physical/cognitive/mental related outcomes of community‐dwelling elderly people: the Kawasaki wellbeing project. Nihon Ronen Igakkai Zasshi. 2019;56(4):504–515. 10.3143/geriatrics.56.504 [DOI] [PubMed] [Google Scholar]
- 22. Arai Y, Oguma Y, Abe Y, Takayama M, Hara A, Urushihara H, et al. Behavioral changes and hygiene practices of older adults in Japan during the first wave of COVID‐19 emergency. BMC Geriatr. 2021;21(1):137. 10.1186/S12877-021-02085-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ando T, Nishimoto Y, Hirata T, Abe Y, Takayama M, Maeno T, et al. Association between multimorbidity, self‐rated health and life satisfaction among independent, community‐dwelling very old persons in Japan: longitudinal cohort analysis from the Kawasaki ageing and well‐being project. BMJ Open. 2022;12(2):e049262. 10.1136/BMJOPEN-2021-049262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurata H, Meguro S, Abe Y, Sasaki T, Asakura K, Arai Y, et al. Dietary protein intake and all‐cause mortality: results from the Kawasaki aging and wellbeing project. BMC Geriatr. 2023;23(1):479. 10.1186/s12877-023-04173-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ge L, Yap CW, Heng BH, Tan WS. Frailty and healthcare utilisation across care settings among community‐dwelling older adults in Singapore. BMC Geriatr. 2020;20(1):389. 10.1186/s12877-020-01800-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li CM, Lin CH, Li CI, Liu CS, Lin WY, Li TC, et al. Frailty status changes are associated with healthcare utilization and subsequent mortality in the elderly population. BMC Public Health. 2021;21(1):645. 10.1186/s12889-021-10688-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Satake S, Arai H. The revised Japanese version of the cardiovascular health study criteria (revised J‐CHS criteria). Geriatr Gerontol Int. 2020;20(10):992–993. 10.1111/GGI.14005 [DOI] [PubMed] [Google Scholar]
- 28. Kern LM, Rajan M, Colantonio LD, Reshetnyak E, Ringel JB, Muntner PM, et al. Differences in ambulatory care fragmentation by race. BMC Health Serv Res. 2021;21(1):1–10. 10.1186/s12913-021-06133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marshall GL, Tucker‐Seeley R. The association between hardship and self‐rated health: does the choice of indicator matter? Ann Epidemiol. 2018;28(7):462–467. 10.1016/j.annepidem.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Homer K, Taylor J, Miller A, Pickett K, Wilson L, Robson J. Making ends meet – relating a self‐reported indicator of financial hardship to health status. J Public Health. 2023;45(4):888–893. 10.1093/pubmed/fdad161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coxe S, West SG, Aiken LS. The analysis of count data: a gentle introduction to poisson regression and its alternatives. J Pers Assess. 2009;91(2):121–136. 10.1080/00223890802634175 [DOI] [PubMed] [Google Scholar]
- 32. Royal Colleg of General Practice . GP curriculum: clinical topic guides. [cited 2024 Feb 28]. Available from: https://www.rcgp.org.uk/mrcgp‐exams/gp‐curriculum/clinical‐topic‐guides
- 33. American Academy of Family Physician . Curriculum guidelines. Published November 15, 2019. [cited 2024 Feb 28]. Available from: https://www.aafp.org/students‐residents/residency‐program‐directors/curriculum‐guidelines.html
- 34. Japanese Medical Specialty Board . Standard for general medicine specialty training programs. [cited 2024 Feb 28]. Available from: https://jbgm.org/
- 35. Smetana GW, Landon BE, Bindman AB, Burstin H, Davis RB, Tjia J, et al. A comparison of outcomes resulting from generalist vs specialist Care for a Single Discrete Medical Condition: a systematic review and methodologic critique. Arch Intern Med. 2007;167(1):10–20. 10.1001/ARCHINTE.167.1.10 [DOI] [PubMed] [Google Scholar]
- 36. Miner MM. Primary care physician versus urologist: how does their medical management of LUTS associated with BPH differ? Curr Urol Rep. 2009;10(4):254–260. 10.1007/s11934-009-0042-7 [DOI] [PubMed] [Google Scholar]
- 37. Han LC, Kim SP, Gross CP, Ross JS, van Houten HK, Smaldone MC, et al. Association of physician specialty and medical therapy for benign prostatic hyperplasia. Med Care. 2014;52(2):128–136. 10.1097/MLR.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 38. Wei JT, Miner MM, Steers WD, Rosen RC, Seftel AD, Pasta DJ, et al. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol. 2011;186(3):971–976. 10.1016/j.juro.2011.04.081 [DOI] [PubMed] [Google Scholar]
- 39. Simonelli C, Killeen K, Mehle S, Swanson L. Barriers to osteoporosis identification and treatment among primary care physicians and orthopedic surgeons. Mayo Clin Proc. 2002;77(4):334–338. 10.4065/77.4.334 [DOI] [PubMed] [Google Scholar]
- 40. Anderson JJ, Ruwe M, Miller DR, Kazis L, Felson DT, Prashker M. Relative costs and effectiveness of specialist and general internist ambulatory care for patients with 2 chronic musculoskeletal conditions. J Rheumatol. 2002;29(7):1488–1495. [PubMed] [Google Scholar]
- 41. Mazzuca SA, Brandt KD, Katz BP, Dittus RS, Freund DA, Lubitz R, et al. Comparison of general internists, family physicians, and rheumatologists managing patients with symptoms of osteoarthritis of the knee. Arthritis Care Res. 1997;10(5):289–299. 10.1002/art.1790100503 [DOI] [PubMed] [Google Scholar]


