Abstract
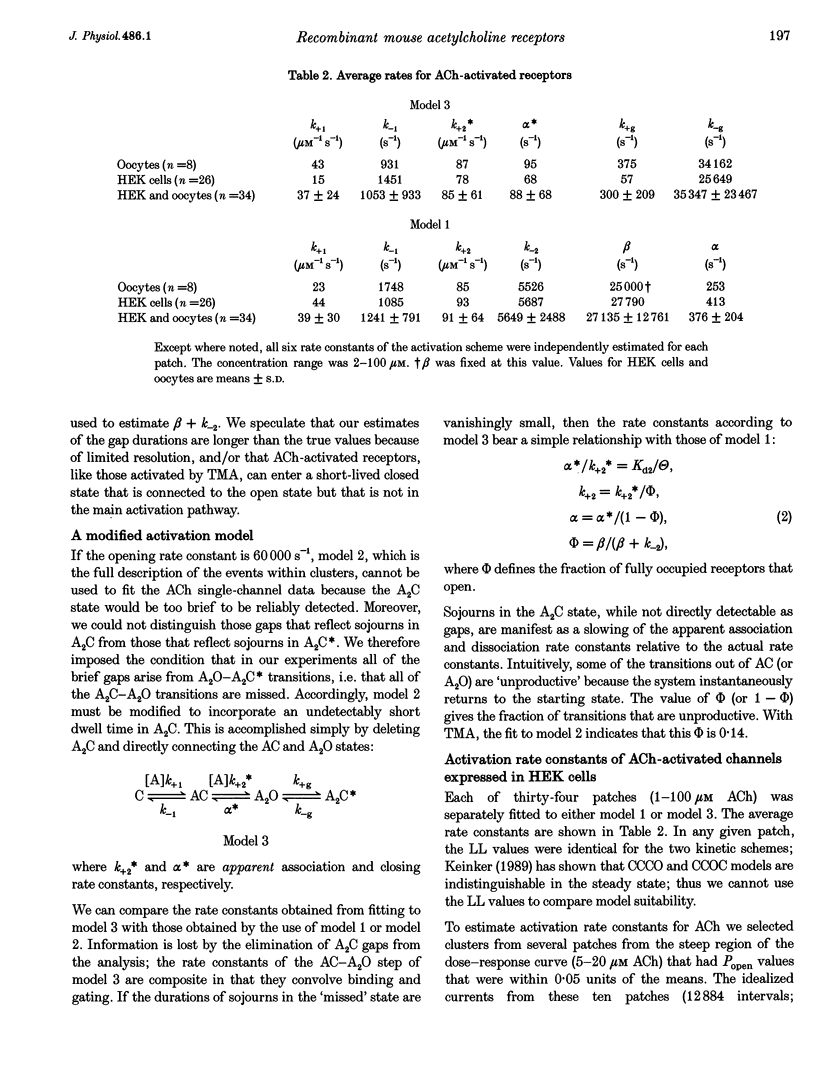
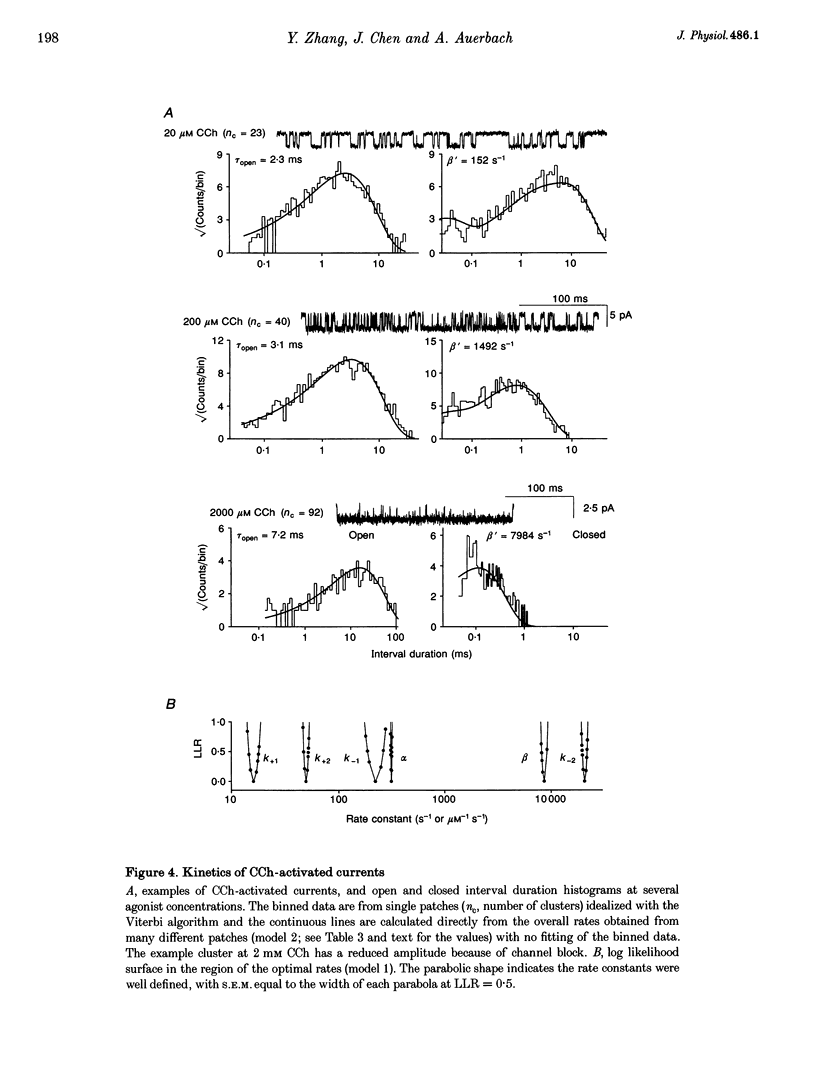
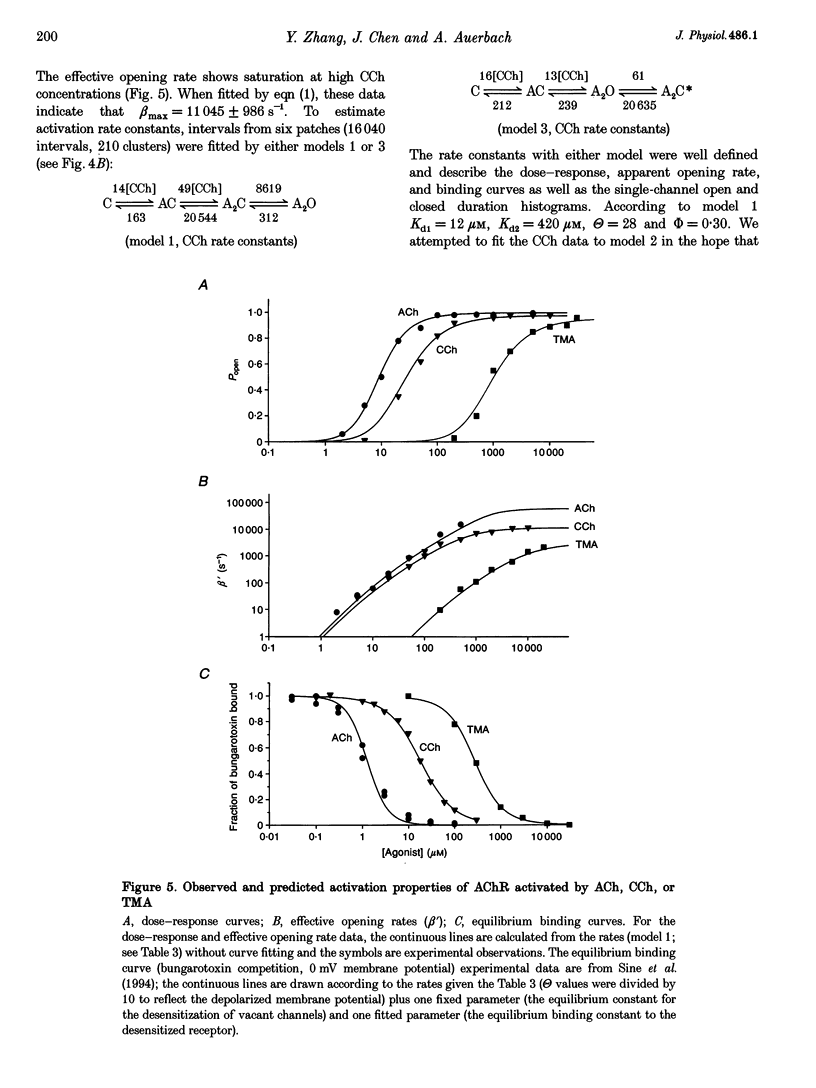
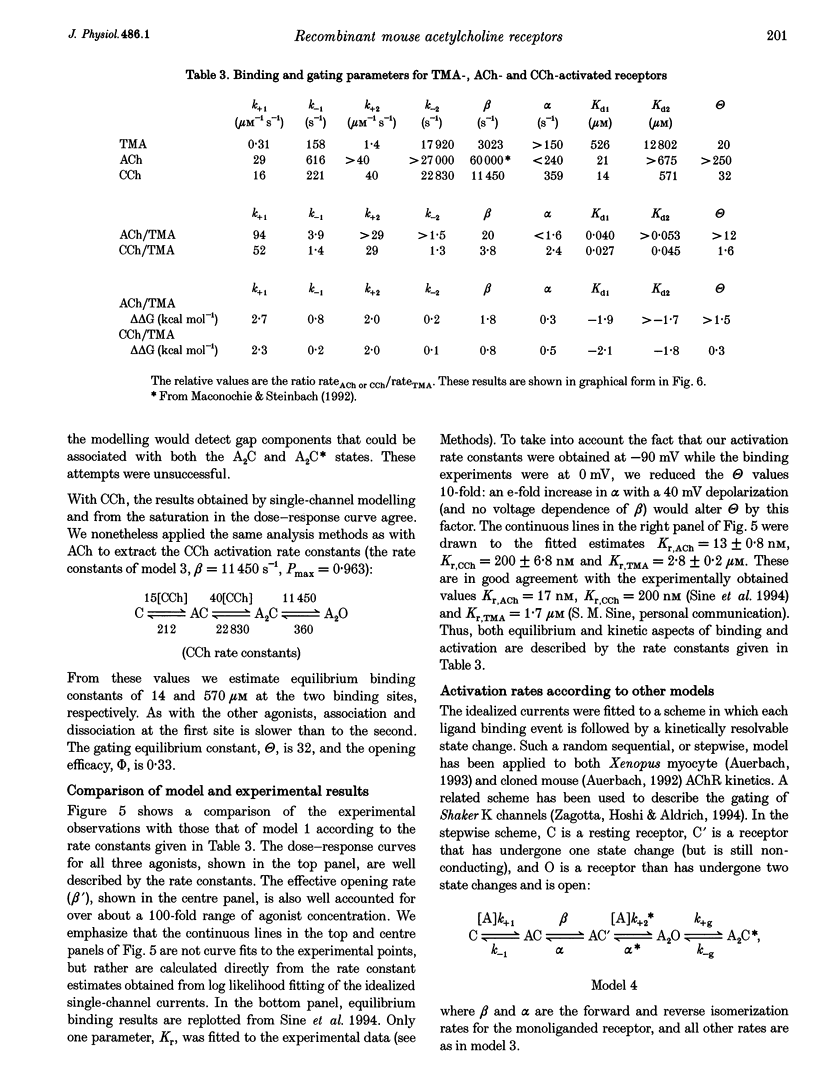
1. The kinetic properties of cloned mouse embryonic nicotinic acetylcholine receptors (AChRs) expressed in HEK 293 cells or Xenopus oocytes were examined using high concentrations of acetylcholine (ACh), carbamylcholine (CCh), or tetramethylammonium (TMA). The rate constants of agonist binding and channel gating were estimated by fitting kinetic models to idealized open and closed intervals over a range of agonist concentrations. 2. Once doubly liganded, TMA-activated receptors open at approximately 3000 s-1. The equilibrium binding constants for TMA are 525 and 12,800 microM. Doubly liganded CCh-activated receptors open at approximately 11,500 s-1; the equilibrium binding constants for this agonist are 14 and 570 microM. If we assume that doubly liganded, ACh-activated receptors open at 60,000 s-1, then the equilibrium binding constants for ACh are 20 and > 650 microM, similar to those for CCh. For all three agonists the higher affinity site both binds and releases agonists more slowly than does the lower affinity site. 3. ACh and CCh bind to the two sites equally rapidly, at approximately 2 x 10(7) and 4 x 10(7) M-1 s-1 at the first and second binding sites, respectively. Compared with ACh, the TMA association rate is approximately 100 times slower at the first binding site, and approximately 30 times slower at the second binding site. These results indicate that at both binding sites the association rate of TMA is not limited by diffusional or steric factors. 4. All three agonists dissociate from the receptor binding sites at similar rates. The dissociation rate for all agonists was approximately 40 times slower at the first binding site than at the second. These results suggest that the interaction of the quarternary amine moiety with the receptor determines the rate of release of the agonist, and that the nature of this interaction is quite different at the two binding sites. 5. Although the channel opening rates for the three agonists varied approximately 20-fold, the channel closing rates were not strongly agonist dependent, and varied less than 3-fold. We speculate that the ester moiety of the agonist promotes both rapid binding and fast opening of the ligand receptors, and that interactions of the quarternary amine moiety of the agonist with the receptor determine the channel closing rate constant.
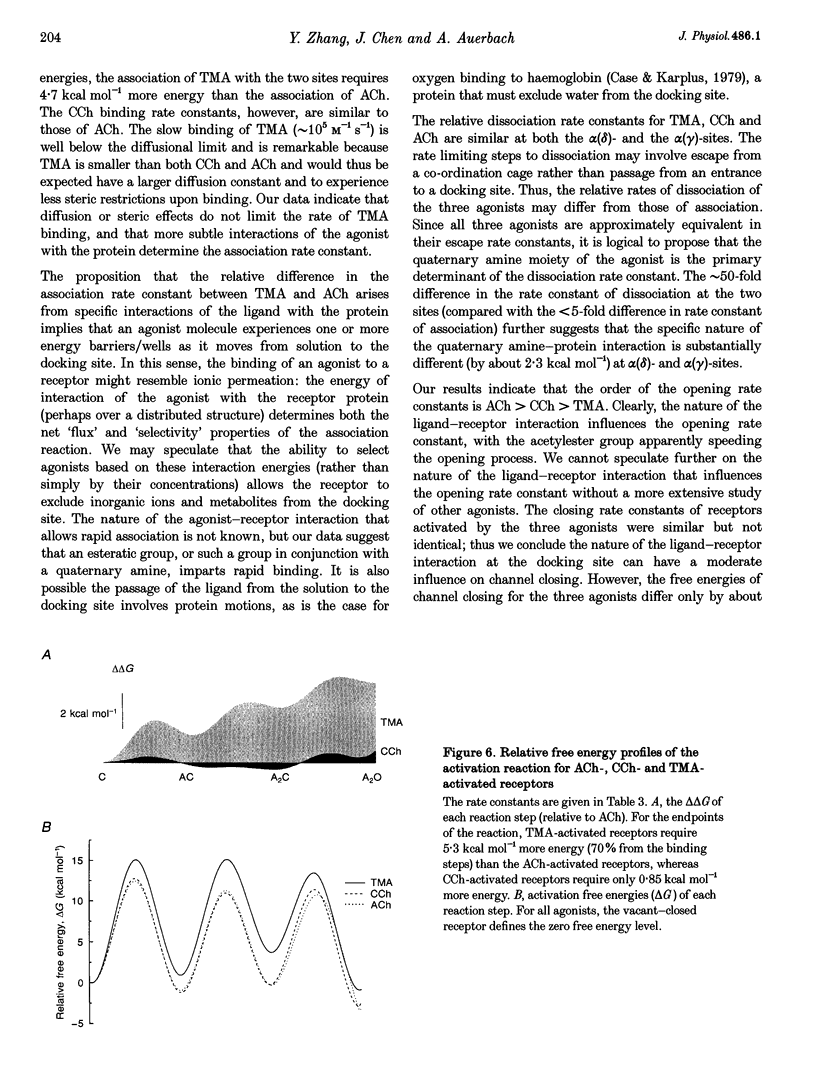
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A. A statistical analysis of acetylcholine receptor activation in Xenopus myocytes: stepwise versus concerted models of gating. J Physiol. 1993 Feb;461:339–378. doi: 10.1113/jphysiol.1993.sp019517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Lingle C. J. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986 Sep;378:119–140. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Flickering of a nicotinic ion channel to a subconductance state. Biophys J. 1983 Apr;42(1):1–10. doi: 10.1016/S0006-3495(83)84362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball F. G., Sansom M. S. Ion-channel gating mechanisms: model identification and parameter estimation from single channel recordings. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):385–416. doi: 10.1098/rspb.1989.0029. [DOI] [PubMed] [Google Scholar]
- Blount P., Merlie J. P. Molecular basis of the two nonequivalent ligand binding sites of the muscle nicotinic acetylcholine receptor. Neuron. 1989 Sep;3(3):349–357. doi: 10.1016/0896-6273(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Case D. A., Karplus M. Dynamics of ligand binding to heme proteins. J Mol Biol. 1979 Aug 15;132(3):343–368. doi: 10.1016/0022-2836(79)90265-1. [DOI] [PubMed] [Google Scholar]
- Chung S. H., Moore J. B., Xia L. G., Premkumar L. S., Gage P. W. Characterization of single channel currents using digital signal processing techniques based on Hidden Markov Models. Philos Trans R Soc Lond B Biol Sci. 1990 Sep 29;329(1254):265–285. doi: 10.1098/rstb.1990.0170. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski C., Kaufmann C., Karlin A. Negatively charged amino acid residues in the nicotinic receptor delta subunit that contribute to the binding of acetylcholine. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6285–6289. doi: 10.1073/pnas.90.13.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys J. 1987 Feb;51(2):255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Shaker potassium channel gating. I: Transitions near the open state. J Gen Physiol. 1994 Feb;103(2):249–278. doi: 10.1085/jgp.103.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Dependence of acetylcholine receptor channel kinetics on agonist concentration in cultured mouse muscle fibres. J Physiol. 1988 Mar;397:555–583. doi: 10.1113/jphysiol.1988.sp017019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2199–2203. doi: 10.1073/pnas.86.7.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienker P. Equivalence of aggregated Markov models of ion-channel gating. Proc R Soc Lond B Biol Sci. 1989 Apr 22;236(1284):269–309. doi: 10.1098/rspb.1989.0024. [DOI] [PubMed] [Google Scholar]
- Lingle C. J., Maconochie D., Steinbach J. H. Activation of skeletal muscle nicotinic acetylcholine receptors. J Membr Biol. 1992 Mar;126(3):195–217. doi: 10.1007/BF00232318. [DOI] [PubMed] [Google Scholar]
- Marshall C. G., Ogden D., Colquhoun D. Activation of ion channels in the frog endplate by several analogues of acetylcholine. J Physiol. 1991 Feb;433:73–93. doi: 10.1113/jphysiol.1991.sp018415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo D., Brehm P. Modal shifts in acetylcholine receptor channel gating confer subunit-dependent desensitization. Science. 1993 Jun 18;260(5115):1811–1814. doi: 10.1126/science.8511590. [DOI] [PubMed] [Google Scholar]
- Pedersen S. E., Cohen J. B. d-Tubocurarine binding sites are located at alpha-gamma and alpha-delta subunit interfaces of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2785–2789. doi: 10.1073/pnas.87.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Claudio T. Gamma- and delta-subunits regulate the affinity and the cooperativity of ligand binding to the acetylcholine receptor. J Biol Chem. 1991 Oct 15;266(29):19369–19377. [PubMed] [Google Scholar]
- Sine S. M., Claudio T., Sigworth F. J. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J Gen Physiol. 1990 Aug;96(2):395–437. doi: 10.1085/jgp.96.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of residues that determine curare selectivity. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9436–9440. doi: 10.1073/pnas.90.20.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Quiram P., Papanikolaou F., Kreienkamp H. J., Taylor P. Conserved tyrosines in the alpha subunit of the nicotinic acetylcholine receptor stabilize quaternary ammonium groups of agonists and curariform antagonists. J Biol Chem. 1994 Mar 25;269(12):8808–8816. [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of acetylcholine receptors on clonal mammalian BC3H-1 cells by high concentrations of agonist. J Physiol. 1987 Apr;385:325–359. doi: 10.1113/jphysiol.1987.sp016496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli G. F., McLaughlin J. T., Jurman M. E., Hawrot E., Yellen G. Mutations affecting agonist sensitivity of the nicotinic acetylcholine receptor. Biophys J. 1991 Sep;60(3):721–727. doi: 10.1016/S0006-3495(91)82102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J Gen Physiol. 1994 Feb;103(2):321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]