Abstract
1. Ozone inhalation causes airway hyper-responsiveness and airway inflammation in dogs. The purpose of this study was to determine whether these effects are associated with increases in oxygen radical production from bronchoalveolar lavage (BAL) cells. 2. Twelve randomly selected dogs were studied twice, 4 weeks apart. On each study day, acetylcholine (ACh) airway responsiveness was measured before and 1 h after ozone (3 p.p.m., 30 min) or dry air inhalation, followed by BAL. The response to ACh was expressed as the concentration causing an increase in lung resistance of 5 cmH2O l-1 s-1 above baseline. Spontaneous and phorbol myristate acetate (PMA) (2.4 mumol l-1)-stimulated oxygen radical release from washed BAL cells (4 x 10(6) cells ml-1) was measured by luminol-enhanced chemiluminescence in a luminometer at 37 degrees C. 3. Ozone inhalation caused airway hyper-responsiveness. The concentration of ACh causing an increase in lung resistance of 5 cmH2O l-1 s-1 (the 'provocative' concentration) fell from 4.68 mg ml-1 (% S.E.M., 1.43) before, to 0.48 mg ml-1 (% S.E.M., 1.60) after ozone (P < 0.0001). Spontaneous chemiluminescence area under the curve (AUC) significantly increased after ozone from 4.08 mV (10 min) (% S.E.M., 1.28) after dry air to 8.25 mV (10 min; % S.E.M., 1.29) after ozone (P = 0.007). Ozone inhalation also increased PMA-stimulated chemiluminescence AUC from 18.97 mV (10 min; % S.E.M., 1.18) after dry air to 144.03 mV (10 min; % S.E.M., 1.45) after ozone (P = 0.0001). The increase in PMA-stimulated chemiluminescence was significantly correlated with ozone-induced ACh airway hyper-responsiveness (r = 0.83, P < 0.001). 4. These results indicate that inhaled ozone increases oxygen radical release from BAL cells and suggest that oxygen radicals are important in causing ozone-induced airway hyper-responsiveness.
Full text
PDF

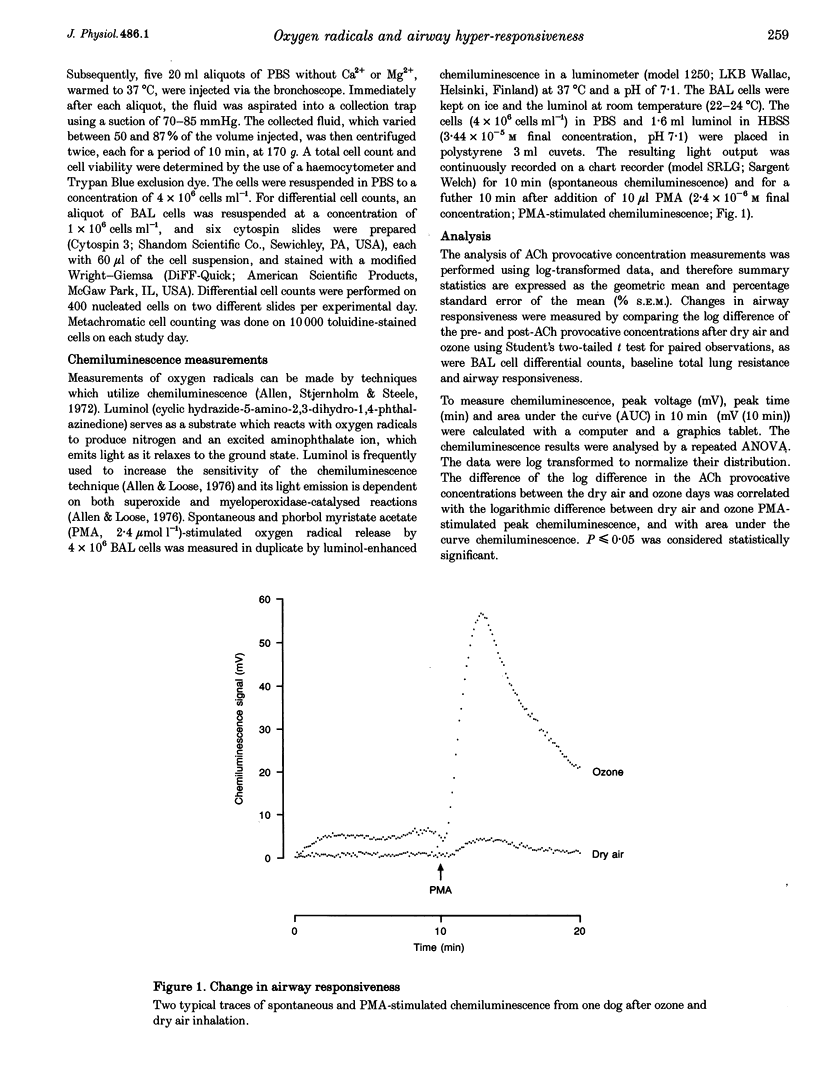
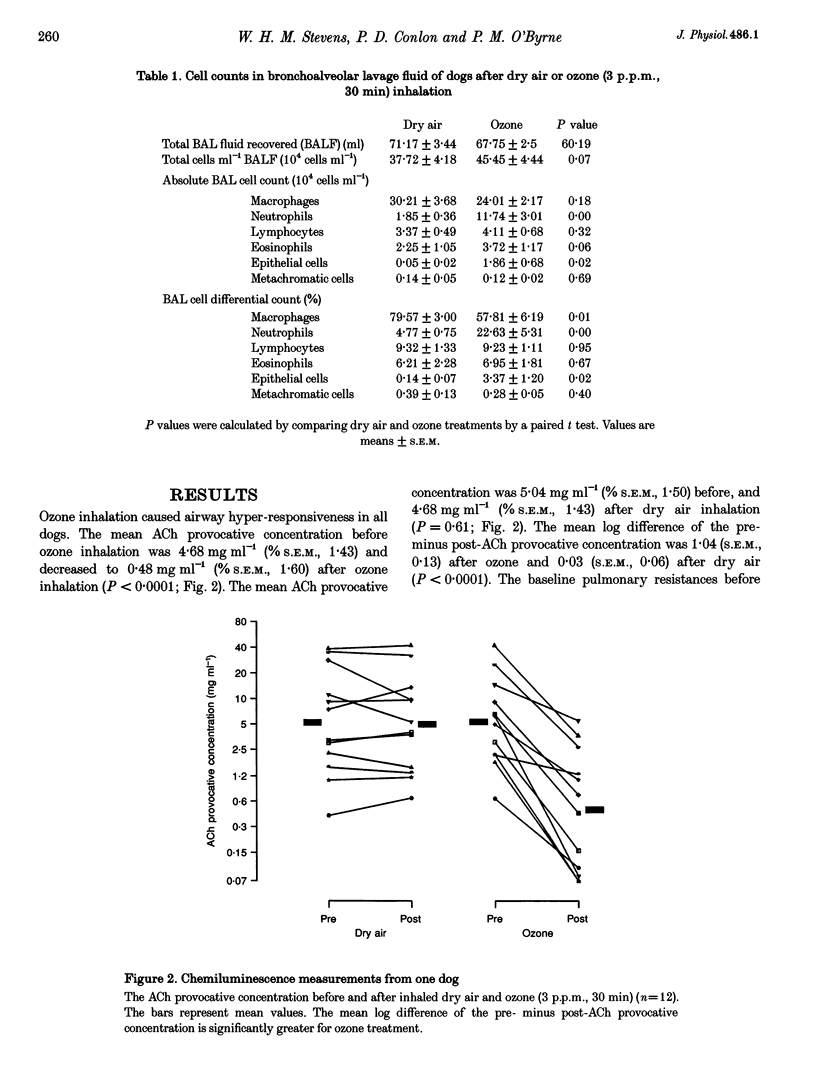
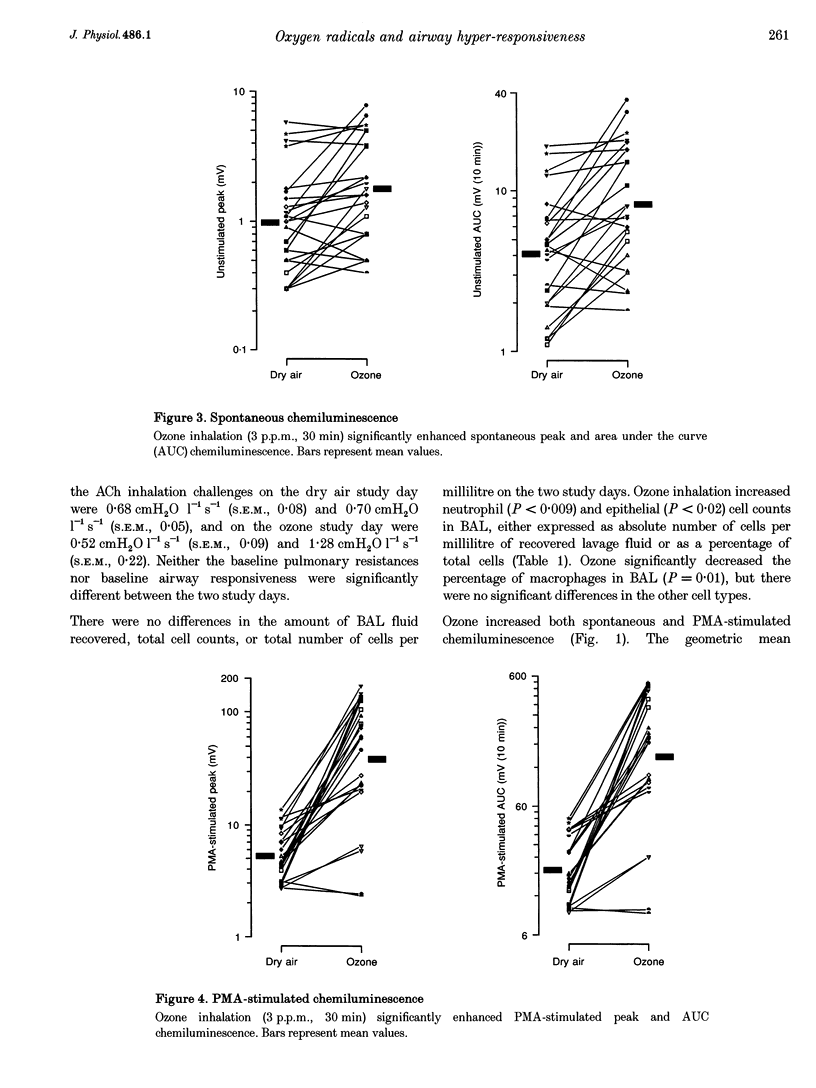
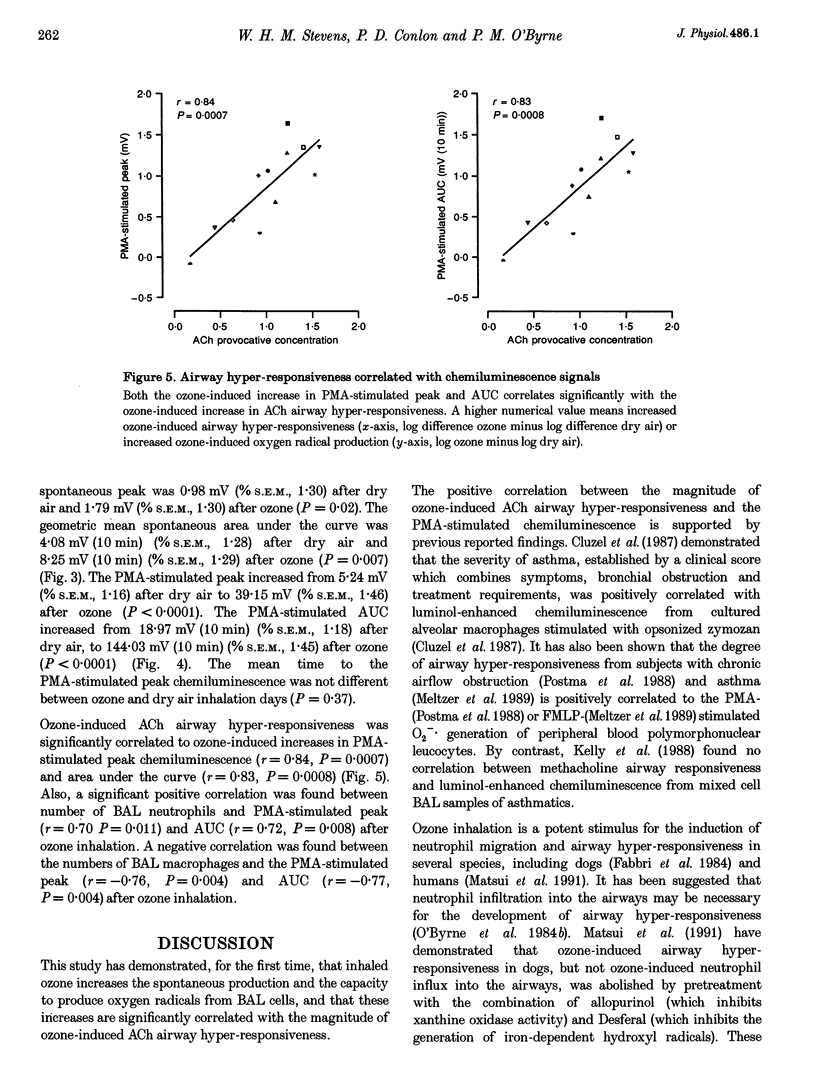



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Brown K. A. The polymorphonuclear cell in rheumatoid arthritis. Br J Rheumatol. 1988 Apr;27(2):150–155. doi: 10.1093/rheumatology/27.2.150. [DOI] [PubMed] [Google Scholar]
- Calhoun W. J., Bush R. K. Enhanced reactive oxygen species metabolism of airspace cells and airway inflammation follow antigen challenge in human asthma. J Allergy Clin Immunol. 1990 Sep;86(3 Pt 1):306–313. doi: 10.1016/s0091-6749(05)80092-2. [DOI] [PubMed] [Google Scholar]
- Cluzel M., Damon M., Chanez P., Bousquet J., Crastes de Paulet A., Michel F. B., Godard P. Enhanced alveolar cell luminol-dependent chemiluminescence in asthma. J Allergy Clin Immunol. 1987 Aug;80(2):195–201. doi: 10.1016/0091-6749(87)90129-1. [DOI] [PubMed] [Google Scholar]
- Cohen D. S., Palmer E., Welch W. J., Sheppard D. The response of guinea pig airway epithelial cells and alveolar macrophages to environmental stress. Am J Respir Cell Mol Biol. 1991 Aug;5(2):133–143. doi: 10.1165/ajrcmb/5.2.133. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., McDonnell W. F., Mann R., Becker S., House D. E., Schreinemachers D., Koren H. S. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol. 1991 Jan;4(1):72–81. doi: 10.1165/ajrcmb/4.1.72. [DOI] [PubMed] [Google Scholar]
- Duddridge M., Ward C., Hendrick D. J., Walters E. H. Changes in bronchoalveolar lavage inflammatory cells in asthmatic patients treated with high dose inhaled beclomethasone dipropionate. Eur Respir J. 1993 Apr;6(4):489–497. [PubMed] [Google Scholar]
- Engels F., Oosting R. S., Henricks P. A., Nijkamp F. P. The involvement of reactive hydroxyl radicals in Haemophilus influenzae-induced deterioration of guinea pig lung beta-adrenergic receptor function. Agents Actions. 1986 Jan;17(3-4):403–404. doi: 10.1007/BF01982665. [DOI] [PubMed] [Google Scholar]
- Fabbri L. M., Aizawa H., Alpert S. E., Walters E. H., O'Byrne P. M., Gold B. D., Nadel J. A., Holtzman M. J. Airway hyperresponsiveness and changes in cell counts in bronchoalveolar lavage after ozone exposure in dogs. Am Rev Respir Dis. 1984 Feb;129(2):288–291. [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman M. J., Fabbri L. M., O'Byrne P. M., Gold B. D., Aizawa H., Walters E. H., Alpert S. E., Nadel J. A. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am Rev Respir Dis. 1983 Jun;127(6):686–690. doi: 10.1164/arrd.1983.127.6.686. [DOI] [PubMed] [Google Scholar]
- Holtzman M. J., Fabbri L. M., Skoogh B. E., O'Byrne P. M., Walters E. H., Aizawa H., Nadel J. A. Time course of airway hyperresponsiveness induced by ozone in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1983 Oct;55(4):1232–1236. doi: 10.1152/jappl.1983.55.4.1232. [DOI] [PubMed] [Google Scholar]
- Jones G. L., Lane C. G., Manning P. J., O'Byrne P. M. Role of the parasympathetic nervous system in airway hyperresponsiveness after ozone inhalation. J Appl Physiol (1985) 1987 Sep;63(3):1174–1179. doi: 10.1152/jappl.1987.63.3.1174. [DOI] [PubMed] [Google Scholar]
- Katsumata U., Miura M., Ichinose M., Kimura K., Takahashi T., Inoue H., Takishima T. Oxygen radicals produce airway constriction and hyperresponsiveness in anesthetized cats. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1158–1161. doi: 10.1164/ajrccm/141.5_Pt_1.1158. [DOI] [PubMed] [Google Scholar]
- Kelly C., Ward C., Stenton C. S., Bird G., Hendrick D. J., Walters E. H. Number and activity of inflammatory cells in bronchoalveolar lavage fluid in asthma and their relation to airway responsiveness. Thorax. 1988 Sep;43(9):684–692. doi: 10.1136/thx.43.9.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing M. W., Mansour E., Ahmed A., Cortes A., Garcia L., Lauredo I. T., Wanner A., Abraham W. M. Lipid mediators contribute to oxygen-radical-induced airway responses in sheep. Am Rev Respir Dis. 1991 Dec;144(6):1291–1296. doi: 10.1164/ajrccm/144.6.1291. [DOI] [PubMed] [Google Scholar]
- Leikauf G. D., Driscoll K. E., Wey H. E. Ozone-induced augmentation of eicosanoid metabolism in epithelial cells from bovine trachea. Am Rev Respir Dis. 1988 Feb;137(2):435–442. doi: 10.1164/ajrccm/137.2.435. [DOI] [PubMed] [Google Scholar]
- Leikauf G. D., Zhao Q., Zhou S., Santrock J. Ozonolysis products of membrane fatty acids activate eicosanoid metabolism in human airway epithelial cells. Am J Respir Cell Mol Biol. 1993 Dec;9(6):594–602. doi: 10.1165/ajrcmb/9.6.594. [DOI] [PubMed] [Google Scholar]
- Lemen R., Benson M., Jones J. G. Absolute pressure measurements with hand-dipped and manufactured esophageal balloons. J Appl Physiol. 1974 Oct;37(4):600–603. doi: 10.1152/jappl.1974.37.4.600. [DOI] [PubMed] [Google Scholar]
- Matsui S., Jones G. L., Woolley M. J., Lane C. G., Gontovnick L. S., O'Byrne P. M. The effect of antioxidants on ozone-induced airway hyperresponsiveness in dogs. Am Rev Respir Dis. 1991 Dec;144(6):1287–1290. doi: 10.1164/ajrccm/144.6.1287. [DOI] [PubMed] [Google Scholar]
- Mehlman M. A., Borek C. Toxicity and biochemical mechanisms of ozone. Environ Res. 1987 Feb;42(1):36–53. doi: 10.1016/s0013-9351(87)80005-1. [DOI] [PubMed] [Google Scholar]
- Meltzer S., Goldberg B., Lad P., Easton J. Superoxide generation and its modulation by adenosine in the neutrophils of subjects with asthma. J Allergy Clin Immunol. 1989 May;83(5):960–966. doi: 10.1016/0091-6749(89)90112-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Leikauf G. D., Aizawa H., Bethel R. A., Ueki I. F., Holtzman M. J., Nadel J. A. Leukotriene B4 induces airway hyperresponsiveness in dogs. J Appl Physiol (1985) 1985 Dec;59(6):1941–1946. doi: 10.1152/jappl.1985.59.6.1941. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Walters E. H., Aizawa H., Fabbri L. M., Holtzman M. J., Nadel J. A. Indomethacin inhibits the airway hyperresponsiveness but not the neutrophil influx induced by ozone in dogs. Am Rev Respir Dis. 1984 Aug;130(2):220–224. doi: 10.1164/arrd.1984.130.2.220. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Walters E. H., Gold B. D., Aizawa H. A., Fabbri L. M., Alpert S. E., Nadel J. A., Holtzman M. J. Neutrophil depletion inhibits airway hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis. 1984 Aug;130(2):214–219. doi: 10.1164/arrd.1984.130.2.214. [DOI] [PubMed] [Google Scholar]
- Postma D. S., Renkema T. E., Noordhoek J. A., Faber H., Sluiter H. J., Kauffman H. Association between nonspecific bronchial hyperreactivity and superoxide anion production by polymorphonuclear leukocytes in chronic air-flow obstruction. Am Rev Respir Dis. 1988 Jan;137(1):57–61. doi: 10.1164/ajrccm/137.1.57. [DOI] [PubMed] [Google Scholar]
- Roehm J. N., Hadley J. G., Menzel D. B. Oxidation of unsaturated fatty acids by ozone and nitrogen dioxide. A common mechanism of action. Arch Environ Health. 1971 Aug;23(2):142–148. doi: 10.1080/00039896.1971.10665972. [DOI] [PubMed] [Google Scholar]
- Sporn P. H., Peters-Golden M., Simon R. H. Hydrogen-peroxide-induced arachidonic acid metabolism in the rat alveolar macrophage. Am Rev Respir Dis. 1988 Jan;137(1):49–56. doi: 10.1164/ajrccm/137.1.49. [DOI] [PubMed] [Google Scholar]
- Strausz J., Müller-Quernheim J., Steppling H., Ferlinz R. Oxygen radical production by alveolar inflammatory cells in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1990 Jan;141(1):124–128. doi: 10.1164/ajrccm/141.1.124. [DOI] [PubMed] [Google Scholar]
- Ward C., Kelly C. A., Stenton S. C., Duddridge M., Hendrick D. J., Walters E. H. The relative contribution of bronchoalveolar macrophages and neutrophils to lucigenin- and luminol-amplified chemiluminescence. Eur Respir J. 1990 Oct;3(9):1008–1014. [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Human bronchoalveolar lavage cells and luminol-dependent chemiluminescence. J Clin Pathol. 1981 Feb;34(2):167–171. doi: 10.1136/jcp.34.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]


